Abstract
Objective:
Rates of alcohol dependence are elevated in women with eating disorders who engage in binge eating or compensatory behaviors compared with women with eating disorders who do not report binge eating or compensatory behaviors and with healthy controls. Alcohol dependence, binge eating, and compensatory behaviors are heritable; however, it is unclear whether a shared genetic liability contributes to the phenotypic association among these traits, and little information exists regarding this shared liability in men. We investigated genetic and environmental correlations among alcohol dependence, binge eating, and compensatory behaviors in male and female twins.
Method:
Participants included 5,993 same- and opposite-sex twins from the Australian Twin Registry who completed a modified version of the Semi-Structured Assessment for the Genetics of Alcoholism that assessed lifetime alcohol dependence and binge eating as defined in the Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised. Compensatory behaviors were assessed via a general health questionnaire in women only. Biometrical twin models estimated genetic and environmental influences on alcohol dependence, binge eating, and compensatory behaviors.
Results:
In women, the multivariate twin model suggested that additive genetic and nonshared environmental effects influenced alcohol dependence, binge eating, and compensatory behaviors, with heritability estimates ranging from 38% to 53%. The best-fitting sex-limitation model was a common effects model that equated all genetic and nonshared environmental influences in men and women. The heritability estimates were 50% and 38% for alcohol dependence and binge eating, respectively. Overall, there were significant genetic correlations between alcohol dependence and binge eating, alcohol dependence and compensatory behaviors, and binge eating and compensatory behaviors.
Conclusions:
These findings indicate that common genetic factors may underlie the vulnerability to alcohol dependence and the liability to binge eating and compensatory behaviors.
Prior studies have suggested an association between alcohol use disorders and eating disorders in women. For example, within a population of women meeting criteria for alcohol dependence from the Collaborative Study of the Genetics of Alcoholism, lifetime rates of bulimia nervosa were ∼6% in women—a rate greater than what would be expected in the general population (Schuckit et al., 1996). In addition, among women seeking treatment for alcohol abuse and dependence, 36% reported binge eating and 26% met diagnostic criteria for a probable eating disorder (Peveler and Fairburn, 1990). Within the eating disorder population, rates of alcohol abuse and dependence are higher in women who have an eating disorder compared with women who do not. Women with a binge–purge form of eating disorder (bulimia nervosa or anorexia nervosa) have higher rates of alcohol abuse and dependence (22%–35%) than do women with the restricting type of anorexia nervosa (∼9%) (Krug et al., 2009; Root et al., 2010). These rates of alcohol abuse and dependence among women with an eating disorder are compared to 6% in women without a history of an eating disorder (Root et al., 2010). Thus, it is possible that binge eating and/or inappropriate compensatory behaviors (including purging [self-induced vomiting and laxative/diuretic use] and nonpurging [fasting, excessive exercise, and strict dieting] behaviors) may be instrumental in explaining the association between alcohol use disorders and eating disorders (Corte and Stein, 2000; Gadalla and Piran, 2007; Krug et al., 2009; Wiederman and Pryor, 1996).
Family and twin studies have provided important insights into the etiology of comorbid alcohol dependence and eating disorders, even though there are inconsistencies in the findings. In general, family studies have suggested that the two disorders are transmitted independently in families (Bulik, 1987; Kaye et al., 1996; Lilenfeld et al., 1998; Schuckit et al., 1996; von Ranson et al., 2003). In other words, there is little to no genetic or shared environmental overlap between alcohol dependence and bulimia nervosa or between alcohol dependence and anorexia nervosa. Twin studies have provided additional evidence on this etiology by partitioning the phenotypic variance of alcohol dependence and bulimia nervosa into genetic and environmental effects.
Although the heritabilities of alcohol dependence and bulimia nervosa (and bulimia symptoms, such as binge eating and compensatory behaviors) have individually been studied extensively in adults, with heritabilities ranging from 50% to 64% for alcohol dependence (Dick et al., 2009; Heath et al., 1997; McGue, 1999) and 28% to 83% for bulimia nervosa, binge eating, and compensatory behaviors (Thornton et al., 2011), only three twin studies have investigated the potential overlap of genetic and environmental effects for alcohol dependence and bulimia nervosa. Although one of these studies (Kendler et al., 1995) found little evidence for shared genetic risk factors between alcoholism and bulimia nervosa, which corroborates the family studies, the other two studies reported significant genetic correlations between alcohol problems and bulimia symptoms, ranging from .31 to .53 (Baker et al., 2010; Slane et al., 2012). Furthermore, the former, but not latter, study also found overlapping non-shared environmental influences (i.e., environmental effects that contribute to differences among members of the same family).
Discrepancies in findings from family and twin studies could be attributable to the small sample size when examining clinical disorders, especially for eating disorders, or the use of clinical diagnoses rather than symptoms of these disorders. In light of these inconsistent results, more work is needed to elucidate the relationship between alcohol dependence and bulimia symptoms.
To better understand the relation between alcohol dependence and bulimia symptoms, two important questions need to be answered. First, although it has been speculated that binge eating and/or compensatory behaviors are instrumental in explaining the association between alcohol use disorders and eating disorders (Corte and Stein, 2000; Gadalla and Piran, 2007; Krug et al., 2009; Wiederman and Pryor, 1996), few studies have directly tested this hypothesis, and none did so from a genetic perspective. (Notably, however, Slane et al., 2012, investigated overlapping genetic and environmental influences between problem alcohol use and binge eating and between problem alcohol use and compensatory behaviors.) Second, it is unknown whether findings from previous studies in all-female samples extrapolate to men. Compared with women, men have been shown to have higher rates of alcohol dependence (Hasin et al., 2007; Teesson et al., 2006) but similar rates of lifetime binge eating (4% compared with 4.9% in women) (Hudson et al., 2007) and levels of clinical impairment for binge eating (Striegel et al., 2012). Heritability estimates for alcohol dependence (Heath et al., 1997; Knopik et al., 2004) and bulimia symptoms (Reichborn-Kjennerud et al., 2003) have been reported for men and women, yet no study has examined overlapping genetic and environmental effects for these traits in men. This additional information will yield important insights into whether binge eating and compensatory behaviors explain part of the association between alcohol dependence and eating disorders in women, as well as the genetic and environmental architecture of comorbid alcohol dependence and binge eating among men.
The primary aim of this study was to evaluate whether the familial influences between alcohol dependence and binge eating and between alcohol dependence and compensatory behaviors are similar in female twin pairs. (Because of study limitations, data on compensatory behaviors are only available from women in this sample [see the Method section].) A secondary aim of this study was to investigate whether there are shared familial influences between alcohol dependence and binge eating in men and women. Results from this study will be informative for understanding the etiology of comorbid alcohol dependence and bulimia symptoms and their associated negative sequelae.
Method
Participants
Participants included 5,993 same- and opposite-sex twins, born between 1892 and 1964, who were part of the 1981 cohort of the Australian Twin Registry (Hansell et al., 2008; Heath et al., 1997; Martin et al., 1985a, 1985b). Overall, there were 637 male (401 monozygotic [MZ] and 236 dizygotic [DZ]), 1,480 female (940 MZ and 540 DZ), and 603 opposite-sex (DZO) complete twin pairs for which both co-twins chose to participate in the study. Data from an additional 158 same-sex male (81 MZ and 77 DZ), 219 same-sex female (115 MZ and 104 DZ), and 176 DZO (51 men and 125 women) twins who had missing co-twin information were also included in the analysis. Between 1980 and 1982, individuals completed a mailed general health questionnaire (Wave 1) and then completed a follow-up self-report questionnaire between 1988 and 1990 (Wave 2). Between 1992 and 1993, twins were given a diagnostic telephone interview (Wave 3), which included measures of alcohol use, abuse, and dependence, as well as anorexia and bulimia symptoms and disorders. The mean age of men at Wave 3 was 42.69 years (SD = 11.81; range: 28–89), and the mean age for women was 44.74 years (SD = 12.60; range: 27–90). All participants gave informed consent, and the study protocol was approved by all relevant institutional review boards.
Measures
Alcohol dependence.
At Wave 3, individuals were given a modified version of the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA-OZ) (Bucholz et al., 1994) to assess alcohol abuse and dependence as defined in the Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised (DSM-III-R; American Psychiatric Association, 1987), as well as anorexia and bulimia nervosa. We operationalized a quantitative lifetime measure of alcohol dependence using a factor analysis of the nine dependence items, stratified by gender, to compute a factor score. (We called this measure the alcohol dependence factor score [AD-FS].) This measure was developed to increase statistical power to detect associations in genomic studies of alcoholism (Heath et al., 2011). In men and in women, a one-factor solution provided the best fit to the data, with standardized Cronbach’s α of .71 and .73, respectively (results available on request). Before analyses, AD-FS was divided into seven categories with approximately equal sample sizes among categories to include in twin-modeling procedures. Loss of precision using this factor score compared with a continuous measure of alcohol dependence symptom counts would be expected to be negligible.
Binge eating.
Binge eating was assessed from three questions in the SSAGA-OZ interview at Wave 3: “Has there ever been a time in your life when you went on eating binges—eating a large amount of food in a short period of time (usually less than 2 hours)?”; “Did you go on eating binges as often as twice a week for at least 3 months?”; and “During these binges, were you afraid you could not stop eating or that your eating was out of control?” Individuals who did not endorse the first question were not asked the second question, and those not endorsing the second question were not asked the third question. Based on their interview responses, women were placed into one of three groups, where 0 indicated no binge eating, 1 indicated binge eating less than two times per week for at least 3 months, and 2 indicated binge eating at least twice a week for 3 months. Although we could distinguish between individuals reporting binge eating meeting the frequency criterion with and without loss of control, the number was low (with loss of control: n = 93; without loss of control: n = 29); therefore, these individuals were combined to comprise the latter binge eating group. Because the number of men endorsing binge eating who met the frequency criterion was small, it was not possible to create a three-level variable for men. Thus, a two-level variable was used, representing either no binge eating versus possible binge eating. Although diagnoses were available for anorexia and bulimia nervosa, the number of individuals meeting diagnostic criteria was too low in the sample for meaningful inclusion in biometrical twin analyses (anorexia nervosa: n = 1 man and 36 women; bulimia nervosa: n = 8 men and 70 women).
Compensatory behaviors.
Compensatory behaviors were assessed only in women via a general health questionnaire at Wave 2. Unlike the SSAGA-OZ interview at Wave 3, in which participants who did not endorse binge eating (87.35%) were not asked questions regarding compensatory behaviors, these items were asked of all female participants in the questionnaire. Thus, responses were asked of all women regardless of whether they endorsed binge eating, which is important to assess in light of recent work identifying individuals who use compensatory behaviors without engaging in binge eating (Keel et al., 2005; Wade, 2007). Overall, 3,495 (89.45%) of 3,907 women who completed the Wave 3 interview also completed the questionnaire at Wave 2. The behaviors assessed were “starvation,” “self-induced vomiting,” “excessive exercise,” “laxative” use, “fluid tablet” use, and “slimming tablet” use. Lifetime compensatory behaviors was coded as a three-level variable, where 0 indicated no behaviors, 1 indicated using only one of these behaviors, and 2 indicated using two or more compensatory behaviors as a means to control weight. A more detailed description of the binge eating and compensatory behavior items, as well as the eating disorder assessments from the Australian Twin Registry, can be found elsewhere (Wade et al., 1996, 2006).
Zygosity determination
Zygosity was determined based on two items assessed at Wave 1: (a) Participants provided a response to the question, “As children, were you and your twin mistaken by people who knew you?” and (b) after receiving a brief description of differences between non-identical and identical twins, participants provided a self-report of being “an identical twin” or “a non-identical twin” (Heath et al., 1995). These items have greater than 95% agreement with blood typing (Martin and Martin, 1975). To further validate the accuracy of the self-report questions, 347 twin pairs from the Australian Twin Registry were genotyped at nine microsatellite polymorphisms plus the sex marker amelogenin. Results confirmed the accuracy of our zygosity assignment to be 100% in these individuals (Hansell et al., 2008).
Statistical analyses
Twin correlations and biometrical model fitting were conducted in Mx (Neale and Cardon, 1992; Neale et al., 2006). Individuals who had missing data for AD-FS, binge eating, or compensatory behaviors were not included in the twin analyses. A test of bivariate normality for twin pair data on AD-FS, binge eating, and compensatory behaviors was conducted separately for each variable to support the use of normal liability threshold models: within each sex, the models provided a nonsignificant p value (all p values > .05), supporting this approach. Age-dependent thresholds were included as definition variables and were estimated in Mx to allow for different phenotypic endorsement rates per age group. Because of the wide age range (27–90 years) and to avoid the strong assumption regarding linear effects of age, we categorized age into approximately equal quartiles (i.e., 28–33 years, 34–39 years, 40–46 years, and 47 years or older for men and 27–34 years, 35–41 years, 42–52 years, and 53 years or older for women).
We evaluated the contribution of additive genetic (A; sum of the effects of multiple genes affecting a trait), shared environmental (C; environmental effects that make family members similar), and nonshared environmental (E; environmental effects that account for differences among family members) influences on AD-FS, binge eating, and compensatory behaviors. Measurement error is included in the nonshared environmental influence estimate. In addition, we examined the additive genetic (rg), shared environmental (rc), and nonshared environmental (re) correlations between AD-FS and bulimia symptoms.
Before performing multivariate and bivariate model fitting, we can compare the MZ correlation (rMZ) to the DZ correlation (rDZ) to estimate shared environmental influences for the traits. If the twin correlations are identical, there is evidence for shared environment and little evidence for genetic effects; if the rDZ is greater than half the rMZ, there is evidence for shared environmental influences. If the rMZ is greater than the rDZ, there is evidence for genetic influences on the trait. In the univariate case, nonshared environmental influences are present if the rMZ is less than one. In the bivariate case, nonshared environmental influences are present if the MZ cross-trait correlation is less than the phenotypic correlation.
For multivariate and bivariate model fitting, multiple submodels of the full ACE model were fitted to the data that constrained A or C to zero. Standard chi-square difference tests (Δχ2) (Neale et al., 2006) were used to compare the fit of nested models. P values less than .05 for Δχ2 indicated a poorer fit to the data compared with the full (or sub-) model it was tested against, suggesting that the parameter(s) could not be dropped from the model without resulting in a significant decrement in model fit. Akaike’s Information Criterion (AIC; Akaike, 1987) was used to compare nonnested models. Lower AIC values indicated better-fitting and more parsimonious models.
An important strength of this study is the inclusion of opposite-sex twin pairs because it allows for an investigation of both the qualitative and quantitative (Genotype × Sex interaction) effects for AD-FS and binge eating between men and women. Qualitative effects investigate whether the same genes and environments for these traits are involved in both sexes. Quantitative effects, on the other hand, evaluate whether the magnitude of genetic and environmental influences on AD-FS and binge eating are similar between sexes. Three sex-limitation models that simultaneously examine qualitative and quantitative effects were tested. First, a general sex-limitation model was examined in which there were different magnitudes of genetic effects for men and women, as well as different genes influencing the liability to AD-FS, binge eating, and the correlation between both traits. The second model was a common effects model, which allowed for different magnitudes of genetic effects while specifying that the same genes influence the variance and covariance of AD-FS and binge eating. Finally, we tested a scalar sex-limitation model, which specified the same magnitudes of genetic effects and the same genes, but allowed for different variances. Both the common effects and scalar models are nested under the general sex-limitation model.
Results
Descriptive information
There were a total of 2,086 men and 3,907 women in this sample. Twenty-one individuals (7 men and 14 women) had missing data for alcohol dependence, 18 individuals (7 men and 11 women) had missing data for binge eating, and 402 women had missing data for compensatory behaviors. Four hundred and ninety-one (23.61%) men and 234 (6.01%) women met criteria for lifetime alcohol dependence. Two hundred and twenty-four (10.77%) men reported any history of binge eating compared with 493 (12.65%) women. Of the women, 122 (3.13%) reported binge eating twice a week for at least 3 months, and 371 (9.52%) reported binge eating less than twice a week for 3 months. In addition, 2,399 women (68.45%) reported never engaging in compensatory behaviors as a means to control body weight, 619 (17.66%) reported using one behavior, and 487 women (13.89%) reported using two or more compensatory behaviors in their lifetime.
Phenotypic and twin correlations in men and women
In men, the phenotypic correlation between AD-FS and binge eating was .19 (95% CI [.11, .27]). Among women, the phenotypic correlation between AD-FS and binge eating was .20 [.13, .25], between AD-FS and compensatory behaviors it was .22 [.17, .28], and between binge eating and compensatory behaviors it was .46 [.40, .52]. Point estimates for twin correlations in men and/or women (Table 1) generally suggested that the rMZ was greater than the rDZ separately for AD-FS, binge eating, and compensatory behaviors, consistent with genetic influences contributing to each trait. For the bivariate models, the rMZ and rDZ were similar in women, suggesting evidence for shared environmental influences contributing to the covariance between AD-FS and binge eating. In men, the rMZ was greater than the rDZ, again suggesting that genetic influences were contributing to the covariance between AD-FS and binge eating. In addition, the rDZO was similar to the rDZ for men and women, indicating that there was little evidence for sex differences in genetic effects. However, the 95% CIs were wide (some included zero), suggesting that the true values could be smaller or larger than reported here.
Table 1.
Polychoric twin correlations for alcohol dependence factor score (AD-FS), binge eating (BE), and compensatory behaviors (CB)
| Variable | rMZM [95% CI] | rDZM [95% CI] | rMZF [95% CI] | rDZF [95% CI] | rDZO [95% CI] |
| AD-FS | .47 [.37, .56] | .14 [.00, .29] | .52 [.44, .58] | .19 [.07, .30] | .22 [.10, .33] |
| BE | .37 [.10, .60] | .43 [.14, .66] | .40 [.26, .53] | .11 [.00, .27] | .12 [.00, .33] |
| CB | – | – | .50 [.41,.58] | .30 [.16, .42] | – |
| AD-FS and BE | .11 [-.03, .24] | .02 [-.15, .18] | .10 [.01, .20] | .11 [.01, .20] | .02 [-.10, .14] |
| AD-FS and CB | – | – | .17 [.09, .24] | .03 [.00, .12] | – |
| BE and CB | – | – | .30 [.20, .39] | .04 [.00, .15] | – |
Notes: rMZM = monozygotic twin correlation in males; rDZM = dizygotic twin correlation in males; rMZF = monozygotic twin correlation in females; rDZF = dizygotic twin correlation in females; rDZO = dizygotic opposite-sex twin correlation; CI = confidence interval. Compensatory behaviors were not asked in men.
Multivariate twin model of AD-FS, binge eating, and compensatory behaviors among women
We first examined a model allowing for all additive genetic, shared environmental, and nonshared environmental parameter estimates to be estimated as free parameters, as well as all genetic and environmental correlations. This model yielded nonsignificant parameter estimates (as assessed by point estimates and 95% CIs) for all shared environmental effects, although significant additive genetic parameters were detected. Consequently, we fitted a second model that allowed only for additive genetic and nonshared environmental effects, as well as their respective correlations. This model (AIC = -3716.57, -2LL = 13649.43, df = 8683) provided a better fit to the data compared with the full ACE model, Δχ2(6) = 3.59, p = .73.
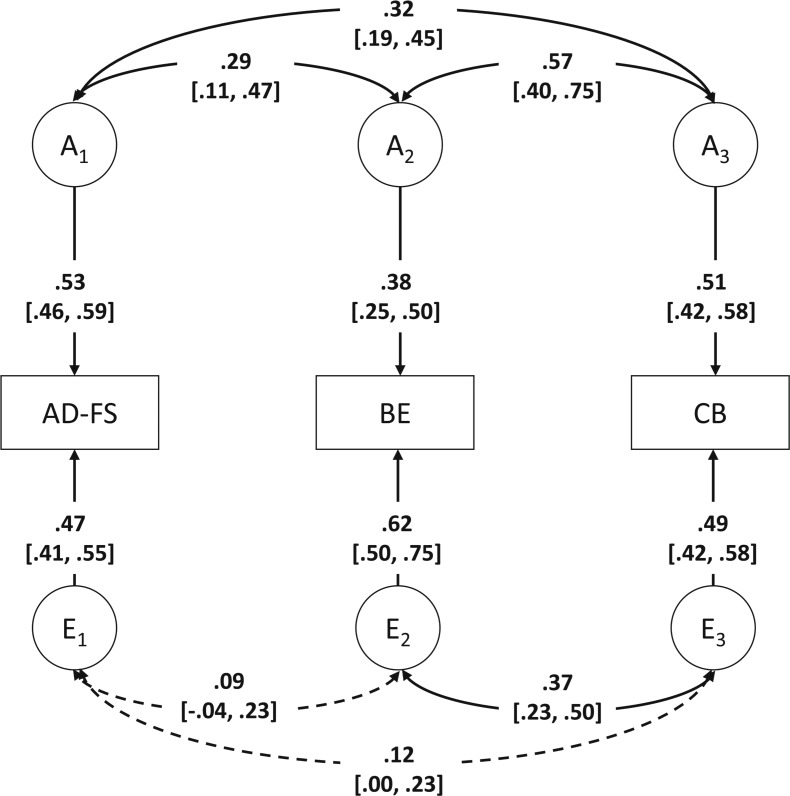
Results from the multivariate model that allowed for additive genetic and nonshared environmental effects, and their correlations, to be freely estimated are shown in Figure 1. Path coefficients and correlations that could be dropped from the model are indicated by dotted lines, whereas path coefficients and correlations that could not be dropped from the model are indicated by solid lines. The heritability of AD-FS was 53% [46%, 59%]. For binge eating and compensatory behaviors, additive genetic effects accounted for 38% [25%, 50%] and 51% [42%, 58%] of the phenotypic variance, respectively. The remaining variance for all three traits was attributable to nonshared environmental effects, with point estimates ranging from 47% to 62%. There was a significant genetic correlation between AD-FS and binge eating (rg = .29 [.11, .47]), AD-FS and compensatory behaviors (rg = .32 [.19, .45]), and binge eating and compensatory behaviors (rg = .57 [.40, .75]). The nonshared environmental correlation was only significant between binge eating and compensatory behaviors (re = .37 [.23, .50]).
Figure 1.
Best-fitting multivariate model in women. AD-FS = alcohol dependence factor score; BE = binge eating; CB = compensatory behaviors; A = additive genetic effects; E = nonshared environmental effects. This model only includes same-sex female twin pairs, as CB were not asked in men. Dotted arrows represent path coefficients/correlations that could be dropped from the model, whereas solid arrows represent path coefficients/correlations that could not be dropped from the model; 95% confidence intervals are presented in brackets.
Bivariate sex-limitation model of AD-FS and binge eating
We first compared the ACE general sex-limitation model (Model 1) to the CE (Model 2) and AE (Model 3) general sex-limitation models (Table 2). Model 2 provided a significant decrement in fit compared with Model 1, Δχ2(6) = 24.21, p < .001, whereas Model 3 provided a more parsimonious fit to the data than Model 1, Δχ2(6) = 2.54, p = .86. Therefore, the AE general sex-limitation model was retained for further comparisons of the sex-limitation models.
Table 2.
Bivariate sex-limitation model results
| Model no. | Model | Model fit |
Comparative fit |
||||
| -2LL | df | AIC | Δχ2 | Δdf | p | ||
| ACE Model | |||||||
| 1 | General | 18,740.59 | 10,665 | -2,589.41 | – | – | – |
| CE Model | |||||||
| 2 | General | 18,764.80 | 10,671 | -2,577.20 | – | – | – |
| AE Models | |||||||
| 3 | General | 18,743.13 | 10,671 | -2,598.87 | – | – | – |
| 4a | Common effects | 18,744.17 | 10,674 | -2,603.83 | 1.04 | 3 | .79 |
| 4ab | Equate BE a2 | 18,744.22 | 10,675 | -2,605.78 | 0.05 | 1 | .82 |
| 4bb | Equate AD-FS a2 | 18,744.23 | 10,675 | -2,605.77 | 0.06 | 1 | .81 |
| 4cb | Equate both a2 | 18,744.29 | 10,676 | -2,607.71 | 0.12 | 2 | .94 |
| 4db | Equate both a2, rg | 18,745.40 | 10,677 | -2,608.60 | 1.23 | 3 | .75 |
| 5a | Scalar | 18,840.52 | 10,678 | -2,515.48 | 97.93 | 7 | <.001 |
Notes: No. = number; -2LL = -2 log-likelihood; df = degrees of freedom; AIC = Akaike’s Information Criterion; Δχ2 = chi-square difference test; Δdf = change in degrees of freedom; ACE Model = model allowing for additive genetic, shared environmental, and nonshared environmental influences; CE Model = model allowing for shared and nonshared environmental influences; AE Model = model allowing for additive genetic and nonshared environmental influences; BE = binge eating; a2 = additive genetic influences; AD-FS = alcohol dependence factor score; rg = additive genetic correlation. The general sex-limitation model allows for different magnitudes of genetic effects for men and women, as well as different genes influencing the liability to AD-FS, BE, and the correlation between both traits. The common effects model allows for different magnitudes of genetic effects while specifying that the same genes influence the variance and covariance of AD-FS and BE. The scalar sex-limitation model specifies the same magnitudes of genetic effects and the same genes, but allows for different variances. Both the common effects and scalar models are nested under the general sex-limitation model.
Denotes a model whose fit statistics were compared with Model 3.
Denotes a model whose fit statistics were compared with Model 4. The best-fitting model is indicated by bold type.
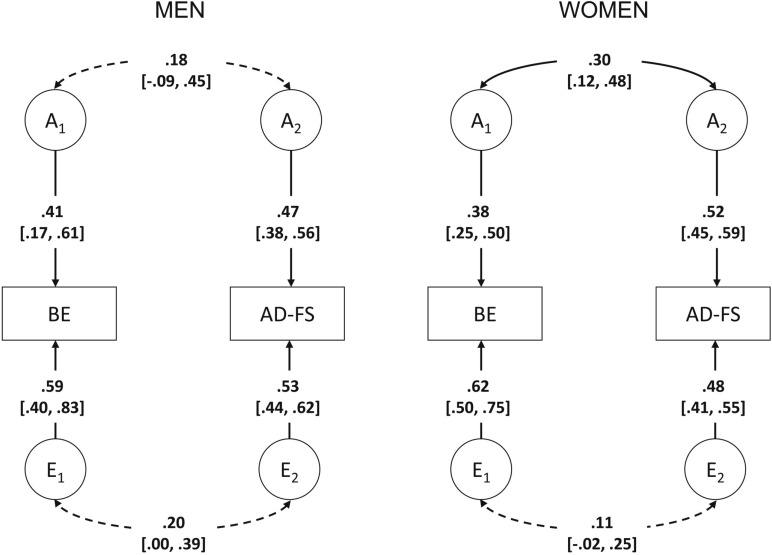
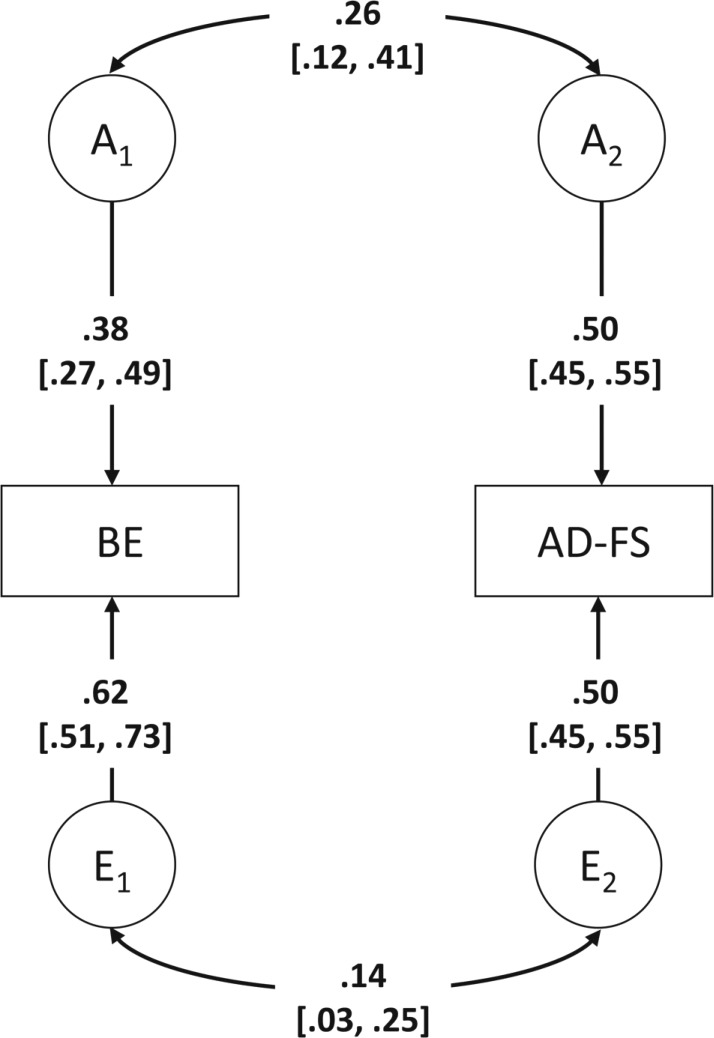
Compared with Model 3, the common effects model provided an adequate fit to the data, Model 4: Δχ2(3) = 1.04, p = .79, whereas the scalar model did not fit the data well, Model 5: Δχ2(7) = 97.93, p < .001 (Table 2). Parameter estimates for men and women under the common effects model are shown in Figure 2. Of the four additional models (Models 4a–4d), a model that equated additive genetic variance and covariance parameters for AD-FS and binge eating for the sexes provided the best fit to the data (Model 4d: AIC = -2608.60, -2LL = 18745.40, df = 10677). Thus, the best-fitting bivariate sex-limitation model was a common effects model that equated all genetic and nonshared environmental parameters for men and women (Figure 3). Additive genetic effects accounted for 38% [27%, 49%] of the variance to binge eating in men and women, with the remaining variance attributable to nonshared environmental effects. Similarly, the variance in AD-FS was also attributable to additive genetic effects (50% [45%, 55%]) in men and women, with the remainder due to nonshared environmental effects. There was significant overlap in both genetic (rg = .26 [.12, .41]) and nonshared environmental (re = .14 [.03, .25]) influences between AD-FS and binge eating, as was indicated by the phenotypic correlation of .19 [.14, .24].
Figure 2.
Bivariate common effects sex-limitation model (Model 4). BE = binge eating; AD-FS = alcohol dependence factor score; A = additive genetic effects; E = nonshared environmental effects. Dotted arrows represent path coefficients/correlations that could be dropped from the model, whereas solid arrows represent path coefficients/correlations that could not be dropped from the model; 95% confidence intervals are presented in brackets.
Figure 3.
Best-fitting bivariate sex-limitation model (Model 4d). BE = binge eating; AD-FS = alcohol dependence factor score; A = additive genetic effects; E = nonshared environmental effects. Dotted arrows represent path coefficients/correlations that could be dropped from the model, whereas solid arrows represent path coefficients/correlations that could not be dropped from the model; 95% confidence intervals are presented in brackets.
Discussion
Our findings indicate that in women, some of the genetic risk factors that influenced vulnerability to AD-FS also influenced vulnerability to both binge eating and compensatory behaviors. The genetic correlations between AD-FS and binge eating and between AD-FS and compensatory behaviors were quite similar (rg = .29 and .32, respectively). The nonshared environmental correlations were not significant for either instance, suggesting that the nonshared environmental effects contributing to AD-FS and bulimia symptoms do not overlap but rather are specific to each behavior. These results partially concur with those from Slane et al. (2012), who found genetic, but not nonshared environmental, correlations between problem alcohol use and both binge eating and compensatory behaviors. The magnitude of the genetic correlation between problem alcohol use and binge eating (rg = .31) in this study was similar, but the genetic correlation between problem alcohol use and compensatory behaviors was higher (rg = .61) than in our study. These differences in results may be attributable to different measures of alcohol consumption and bulimia symptoms or to the ages of the samples (young vs. middle adulthood).
Second, our findings suggest that there are independent as well as shared genetic factors for AD-FS and binge eating in men and women. Our results corroborate prior studies that have found similar heritability estimates of alcohol dependence (Heath et al., 1997; McGue, 1999) and eating disorder symptoms (Klump et al., 2012) in men and women and similar genetic correlations in women (Slane et al., 2012). Importantly, our findings add to the existing literature by investigating genetic and environmental correlations between AD-FS and binge eating in men. Although it was possible to equate all genetic and nonshared environmental parameters in men and women, there could be potential differences between the sexes that we did not have enough power in our sample to detect. It is also possible that the lower endorsement rate of binge eating in men (10.77%), which necessitated that we use a two-level rather than three-level variable for binge eating, reduced our power to detect significant differences in genetic and environmental effects between men and women.
Although this study did not find significant shared environmental influences contributing to the variance in AD-FS, binge eating, or compensatory behaviors, or the covariance between behaviors, it is possible that specific events that are shared by members of the same family were not detected in this study because they interact with genetic factors. When estimated solely from data from twin pairs, these Gene × Environment interactions would be included in estimates of additive genetic effects (Heath and Martin, 1993). For example, it has been shown that in girls, pubertal development is a significant risk factor for both eating disorder symptoms and alcohol use, and alcohol use and onset of bulimic behaviors typically occur during adolescence (Stice et al., 1998; Swendsen et al., 2012). Prior studies have reported that early menarche is a risk factor for many risky behaviors, including bulimic behaviors (Zehr et al., 2007) and alcohol use (Dick et al., 2000). Furthermore, early pubertal onset has been shown to moderate genetic influences on eating disorder symptoms (Klump et al., 2007). Although no such Gene × Environment interaction study has been conducted between pubertal onset and alcohol use, abuse, or dependence, it is possible that early maturation puts one at increased risk for body image issues and increased negative attraction from opposite-sex peers that places one in environments where access to alcohol is common. A combination of pressures to adjust to the changing body at puberty, increased access to alcohol via peer networks, and genetic predispositions for eating disorder symptoms and alcohol problems could result in comorbid alcohol dependence and bulimia symptoms present in adolescence and adulthood.
This study has a number of strengths. First, we were able to examine the association between a factor score of alcohol dependence and bulimia symptoms among both men and women in a large, well-characterized twin sample. Second, we were able to elucidate the relation between alcohol dependence and specific eating disorder symptoms rather than eating disorder diagnoses in women. Prior studies have hypothesized that binge eating and/or compensatory behaviors may specifically be contributing to the association between alcohol dependence and eating disorders. Although we cannot address the issue of causation because these were lifetime assessments of alcohol dependence and eating disorder symptoms, this study begins to address whether binge eating and/or compensatory behaviors are instrumental in the association between alcohol dependence and eating disorders by directly testing shared risk factors between alcohol dependence and binge eating, as well as alcohol dependence and compensatory behaviors. In this sample, the phenotypic correlation between AD-FS and binge eating was similar to that between AD-FS and compensatory behaviors (r = .20 and .22, respectively). Moreover, there was genetic, but not nonshared environmental, overlap between AD-FS and binge eating among women, as well as between AD-FS and compensatory behaviors. These two genetic correlations were also very similar in magnitude (rg = .29 for binge eating and .32 for compensatory behaviors). In addition, the higher heritability estimate for compensatory behaviors compared with binge eating, despite similar genetic correlations between these traits and AD-FS, provides preliminary information that compensatory behaviors may be a more informative eating disorder symptom to explain the genetic association between alcohol dependence and eating disorders. Given that the genetic correlation between binge eating and compensatory behaviors was less than one (which suggests incomplete genetic overlap between both behaviors), both binge eating and compensatory behaviors are important eating disorder behaviors contributing to this association. Future studies that examine alcohol dependence and eating disorders should consider examining both behaviors separately, whenever possible, especially with the change in the DSM-5 for the binge eating and compensatory behaviors frequency criteria (American Psychiatric Association, 2013).
Despite these strengths, there are some limitations. First, because of the older age of the cohort (the mean age was 42.69 years in men and 44.74 years in women), results may not generalize to younger cohorts; however, the results are consistent with those from a prior study investigating genetic and environmental influences on alcohol problems and bulimia symptoms in younger women (Slane et al., 2012). Second, rates of alcohol dependence are higher in this sample of Australian adults compared with estimates from the United States (Teesson et al., 2000, 2006). Further, because we examined lifetime assessments of alcohol dependence criteria, the older age of the individuals means that a larger proportion of the sample would be expected to be through the age of risk compared with younger samples. Still, the heritability estimates and genetic correlations are quite similar to those that were previously reported in studies of U.S. and Australian samples (Heath et al., 1997; McGue, 1999; Slane et al., 2012; Thornton et al., 2011). Third, we operationalized binge eating differently in men and women, such that a two-level variable was used for men and a three-level variable was used for women. In addition, we could not examine loss of control over binge eating in men or women. Even though assessing loss of control for a binge eating episode is important, our sample size was too low to make meaningful comparisons between individuals with and without a sense of loss of control over their binge eating. Thus, the higher prevalence rate of binge eating in our sample compared with others (Hudson et al., 2007) could be because loss of control could not be meaningfully examined in this study. Fourth, we do not know the temporal ordering of the behaviors or the persistence or recovery from alcohol dependence and bulimia symptoms. Finally, we cannot rule out the possibility that other psychopathology comorbid with alcohol dependence and bulimia symptoms, such as depression, may influence this association.
In conclusion, we found independent additive genetic and nonshared environmental influences for AD-FS and binge eating in men and women. Furthermore, there was a moderate proportion of additive genetic influences shared between AD-FS and binge eating for men and women. Among women, there were also independent additive genetic and nonshared environmental effects for compensatory behaviors, as well as common influences between AD-FS and compensatory behaviors. These findings underscore the importance of screening for both alcohol dependence and eating disorder symptoms in samples of men and women with the hope of better detection and treatment, as has recently been advocated by others (Sinha and O’Malley, 2000; Substance Abuse and Mental Health Services Administration, 2010). It will be necessary for future research to elucidate the sources of common genetic and environmental influences so that they can be targets for prevention and intervention.
Footnotes
This work was supported by National Institutes of Health Grants AA11998, AA07728, and AA07580. Arpana Agrawal also received funding from ABMRF/The Foundation for Alcohol Research. The authors do not have any conflicts of interest to report.
References
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd ed., rev. Washington, DC: 1987. Author. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: 2013. Author. [Google Scholar]
- Baker JH, Mitchell KS, Neale MC, Kendler KS. Eating disorder symptomatology and substance use disorders: Prevalence and shared risk in a population based twin sample. International Journal of Eating Disorders. 2010;43:648–658. doi: 10.1002/eat.20856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hessel-brock VM, Nurnberger JI, Jr, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: A report on the reliability of the SSAGA. Journal of Studies on Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Bulik CM. Drug and alcohol abuse by bulimic women and their families. American Journal of Psychiatry. 1987;144:1604–1606. doi: 10.1176/ajp.144.12.1604. [DOI] [PubMed] [Google Scholar]
- Corte C, Stein KF. Eating disorders and substance use: An examination of behavioral associations. Eating Behaviors. 2000;1:173–189. doi: 10.1016/s1471-0153(00)00017-9. [DOI] [PubMed] [Google Scholar]
- Dick DM, Prescott C, McGue M. The genetics of substance use and substance use disorders. In: Kim YK, editor. Handbook of behavior genetics. New York, NY: Springer; 2009. pp. 433–453. [Google Scholar]
- Dick DM, Rose RJ, Viken RJ, Kaprio J. Pubertal timing and substance use: Associations between and within families across late adolescence. Developmental Psychology. 2000;36:180–189. [PubMed] [Google Scholar]
- Gadalla T, Piran N. Co-occurrence of eating disorders and alcohol use disorders in women: A meta analysis. Archives of Women’s Mental Health. 2007;10:133–140. doi: 10.1007/s00737-007-0184-x. [DOI] [PubMed] [Google Scholar]
- Hansell NK, Agrawal A, Whitfield JB, Morley KI, Zhu G, Lind PA, Martin NG. Long-term stability and heritability of telephone interview measures of alcohol consumption and dependence. Twin Research and Human Genetics. 2008;11:287–305. doi: 10.1375/twin.11.3.287. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: Results from the National Epidemio-logic Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PAF, Dinwiddie SH, Slutske WS, Bierut LJ, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: Consistency of findings in women and men. Psychological Medicine. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Heath AC, Madden PA, Slutske WS, Martin NG. Personality and the inheritance of smoking behavior: A genetic perspective. Behavior Genetics. 1995;25:103–117. doi: 10.1007/BF02196921. [DOI] [PubMed] [Google Scholar]
- Heath AC, Martin NG. Genetic models for the natural history of smoking: Evidence for a genetic influence on smoking persistence. Addictive Behaviors. 1993;18:19–34. doi: 10.1016/0306-4603(93)90005-t. [DOI] [PubMed] [Google Scholar]
- Heath AC, Whitfield JB, Martin NG, Pergadia ML, Goate AM, Lind PA, Montgomery GW. A quantitative-trait genome-wide association study of alcoholism risk in the community: findings and implications. Biological Psychiatry. 2011;70:513–518. doi: 10.1016/j.biopsych.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biological Psychiatry. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye WH, Lilenfeld LR, Plotnicov K, Merikangas KR, Nagy L, Strober M, Greeno CG. Bulimia nervosa and substance dependence: Association and family transmission. Alcoholism: Clinical and Experimental Research. 1996;20:878–881. doi: 10.1111/j.1530-0277.1996.tb05266.x. [DOI] [PubMed] [Google Scholar]
- Keel PK, Haedt A, Edler C. Purging disorder: An ominous variant of bulimia nervosa? International Journal of Eating Disorders. 2005;38:191–199. doi: 10.1002/eat.20179. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Walters EE, Neale MC, Kessler RC, Heath AC, Eaves LJ. The structure of the genetic and environmental risk factors for six major psychiatric disorders in women: Phobia, generalized anxiety disorder, panic disorder, bulimia, major depression, and alcoholism. Archives of General Psychiatry. 1995;52:374–383. doi: 10.1001/archpsyc.1995.03950170048007. [DOI] [PubMed] [Google Scholar]
- Klump KL, Culbert KM, Slane JD, Burt SA, Sisk CL, Nigg JT. The effects of puberty on genetic risk for disordered eating: Evidence for a sex difference. Psychological Medicine. 2012;42:627–637. doi: 10.1017/S0033291711001541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Perkins PS, Alexandra Burt S, McGue M, Iacono WG. Puberty moderates genetic influences on disordered eating. Psychological Medicine. 2007;37:627–634. doi: 10.1017/S0033291707000189. [DOI] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Madden PAF, Bucholz KK, Slutske WS, Nelson EC, Martin NG. Genetic effects on alcohol dependence risk: Re-evaluating the importance of psychiatric and other heritable risk factors. Psychological Medicine. 2004;34:1519–1530. doi: 10.1017/s0033291704002922. [DOI] [PubMed] [Google Scholar]
- Krug I, Pinheiro AP, Bulik C, Jiménez-Murcia S, Granero R, Penelo E, Fernández-Arand F. Lifetime substance abuse, family history of alcohol abuse/dependence and novelty seeking in eating disorders: Comparison study of eating disorder subgroups. Psychiatry and Clinical Neurosciences. 2009;63:82–87. doi: 10.1111/j.1440-1819.2008.01908.x. [DOI] [PubMed] [Google Scholar]
- Lilenfeld LR, Kaye WH, Greeno CG, Merikangas KR, Plotnicov K, Pollice C, Nagy L. A controlled family study of anorexia nervosa and bulimia nervosa: Psychiatric disorders in first-degree relatives and effects of proband comorbidity. Archives of General Psychiatry. 1998;55:603–610. doi: 10.1001/archpsyc.55.7.603. [DOI] [PubMed] [Google Scholar]
- Martin NG, Martin PG. The inheritance of scholastic abilities in a sample of twins. I. Ascertainments of the sample and diagnosis of zygosity. Annals of Human Genetics. 1975;39:213–218. doi: 10.1111/j.1469-1809.1975.tb00124.x. [DOI] [PubMed] [Google Scholar]
- Martin NG, Oakeshott JG, Gibson JB, Starmer GA, Perl J, Wilks AV. A twin study of psychomotor and physiological responses to an acute dose of alcohol. Behavior Genetics. 1985a;15:305–347. doi: 10.1007/BF01070893. [DOI] [PubMed] [Google Scholar]
- Martin NG, Perl J, Oakeshott JG, Gibson JB, Starmer GA, Wilks AV. A twin study of ethanol metabolism. Behavior Genetics. 1985b;15:93–109. doi: 10.1007/BF01065891. [DOI] [PubMed] [Google Scholar]
- McGue M. The behavioral genetics of alcoholism. Current Directions in Psychological Science. 1999;8:109–115. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical modeling. 7th ed. Richmond, VA: Department of Psychiatry, VCU Box 900126; 2006. [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Boston, MA: Kluwer Academic; 1992. [Google Scholar]
- Peveler R, Fairburn C. Eating disorders in women who abuse alcohol. British Journal of Addiction. 1990;85:1633–1638. doi: 10.1111/j.1360-0443.1990.tb01653.x. [DOI] [PubMed] [Google Scholar]
- Reichborn-Kjennerud T, Bulik CM, Kendler KS, Røysamb E, Maes H, Tambs K, Harris JR. Gender differences in binge-eating: A population-based twin study. Acta Psychiatrica Scandinavica. 2003;108:196–202. doi: 10.1034/j.1600-0447.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- Root TL, Pisetsky EM, Thornton L, Lichtenstein P, Pedersen NL, Bulik CM. Patterns of co-morbidity of eating disorders and substance use in Swedish females. Psychological Medicine. 2010;40:105–115. doi: 10.1017/S0033291709005662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Tipp JE, Anthenelli RM, Bucholz KK, Hesselbrock VM, Nurnberger JI., Jr Anorexia nervosa and bulimia nervosa in alcohol-dependent men and women and their relatives. American Journal of Psychiatry. 1996;153:74–82. doi: 10.1176/ajp.153.1.74. [DOI] [PubMed] [Google Scholar]
- Sinha R, O’Malley SS. Alcohol and eating disorders: Implications for alcohol treatment and health services research. Alcoholism: Clinical and Experimental Research. 2000;24:1312–1319. [PubMed] [Google Scholar]
- Slane JD, Burt SA, Klump KL. Bulimic behaviors and alcohol use: Shared genetic influences. Behavior Genetics. 2012;42:603–613. doi: 10.1007/s10519-012-9525-2. [DOI] [PubMed] [Google Scholar]
- Stice E, Killen JD, Hayward C, Taylor CB. Age of onset for binge eating and purging during late adolescence: A 4-year survival analysis. Journal of Abnormal Psychology. 1998;107:671–675. doi: 10.1037//0021-843x.107.4.671. [DOI] [PubMed] [Google Scholar]
- Striegel RH, Bedrosian R, Wang C, Schwartz S. Why men should be included in research on binge eating: Results from a comparison of psychosocial impairment in men and women. International Journal of Eating Disorders. 2012;45:233–240. doi: 10.1002/eat.20962. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2009 National Survey on Drug Use and Health: Mental Health Findings (Office of Applied Studies, NSDUH Series H-39, HHS Publication No. SMA 10–4609) Rockville, MD: 2010. Author. Retrieved from http://www.samhsa.gov/data/NSDUH/2k9NSDUH/MH/2K9MHResults.pdf. [Google Scholar]
- Swendsen J, Burstein M, Case B, Conway KP, Dierker L, He J, Merikangas KR. Use and abuse of alcohol and illicit drugs in US adolescents: Results of the National Comorbidity Survey-Adolescent Supplement. Archives of General Psychiatry. 2012;69:390–398. doi: 10.1001/archgenpsychiatry.2011.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teesson M, Baillie A, Lynskey M, Manor B, Degenhardt L. Substance use, dependence and treatment seeking in the United States and Australia: A cross-national comparison. Drug and Alcohol Dependence. 2006;81:149–155. doi: 10.1016/j.drugalcdep.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Teesson M, Hall W, Lynskey M, Degenhardt L. Alcohol- and drug-use disorders in Australia: Implications of the National Survey of Mental Health and Wellbeing. Australian and New Zealand Journal of Psychiatry. 2000;34:206–213. doi: 10.1080/j.1440-1614.2000.00715.x. [DOI] [PubMed] [Google Scholar]
- Thornton LM, Mazzeo SE, Bulik CM. The heritability of eating disorders: Methods and current findings. In: Adan RAH, Kaye WH, editors. Current Topics in Behavioral Neurosciences: Vol. 6. Behavioral neurobiology of eating disorders (pp. 141–156) Berlin, Germany: Springer; 2011. Retrieved from http://link.springer.com/chapter/10.1007/7854_2010_91 p. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Ranson KM, McGue M, Iacono WG. Disordered eating and substance use in an epidemiological sample: II. Associations within families. Psychology of Addictive Behaviors. 2003;17:193–201. doi: 10.1037/0893-164X.17.3.193. [DOI] [PubMed] [Google Scholar]
- Wade T, Heath AC, Abraham S, Treloar SA, Martin NG, Tiggemann M. Assessing the prevalence of eating disorders in an Australian twin population. Australian and New Zealand Journal of Psychiatry. 1996;30:845–851. doi: 10.3109/00048679609065054. [DOI] [PubMed] [Google Scholar]
- Wade TD. A retrospective comparison of purging type disorders: Eating disorder not otherwise specified and bulimia nervosa. International Journal of Eating Disorders. 2007;40:1–6. doi: 10.1002/eat.20314. [DOI] [PubMed] [Google Scholar]
- Wade TD, Bergin JL, Tiggemann M, Bulik CM, Fairburn CG. Prevalence and long-term course of lifetime eating disorders in an adult Australian twin cohort. Australian and New Zealand Journal of Psychiatry. 2006;40:121–128. doi: 10.1080/j.1440-1614.2006.01758.x. [DOI] [PubMed] [Google Scholar]
- Wiederman MW, Pryor T. Substance use among women with eating disorders. International Journal of Eating Disorders. 1996;20:163–168. doi: 10.1002/(SICI)1098-108X(199609)20:2<163::AID-EAT6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Zehr JL, Culbert KM, Sisk CL, Klump KL. An association of early puberty with disordered eating and anxiety in a population of undergraduate women and men. Hormones and Behavior. 2007;52:427–435. doi: 10.1016/j.yhbeh.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]