Abstract
Objective:
Previous research has established a connection between early age at drinking initiation and greater alcohol involvement in adulthood, but it has not yet been established whether this is a causal effect. The current study used a multilevel discordant twin design to examine individual and contextual effects, and an interaction between these effects, of the age at drinking initiation on the frequency and quantity of drinking in adulthood.
Method:
Participants were 4,194 same-sex twins (2,264 monozygotic, 1,924 dizygotic; 2,270 women; Mage = 29.9 years) from the Australian Twin Registry who completed a telephone interview that included assessments of the age at alcohol use initiation and past-year frequency and quantity of alcohol use. Multilevel models were estimated using data from the full sample and using data from only monozygotic twins. Individual (within-twin-pair comparison) and family contextual (between-twin-pair comparison) effects were estimated.
Results:
The age at first drink was related to the past-year frequency (r = -.16) and quantity of drinking (r = -.12) in young adulthood. Individual (causal) and family context effects of age at drinking onset predicted later adult drinking frequency and quantity. There was also a significant cross-level interaction between individual and family contexts for frequency but not quantity of drinking.
Conclusions:
Results of this study indicate a potential causal effect of age at drinking onset on adult alcohol involvement as well as the importance of examining both individual and contextual effects in discordant twin studies.
Studies have demonstrated a link between early age at first drink (e.g., age 14 years or earlier) and later alcohol use disorders (e.g., DeWit et al, 2000; Grant and Dawson, 1997; Guttmannova et al, 2011; Hingson et al., 2006). However, mechanisms behind this association are not completely clear. Some studies document that this relation may be accounted for, at least in part, by other risk factors such as childhood conduct disorder history (e.g., Sartor et al., 2007). Associations with these risk factors indicate that early drinking initiation may not be causally related to heavy drinking in adulthood but rather is a manifestation of a vulnerability to disinhibition (McGue et al., 2001).
A number of studies address the question of whether the association between early onset of drinking and later alcohol use disorder can be explained completely by various sets of correlated risk factors, as this could rule out a causal association. The results of these studies are mixed. For example, King and Chassin (2007) found that after accounting for parental alcoholism and antisocial personality disorder, as well as childhood externalizing behaviors and family conflict, early alcohol initiation and alcohol dependence in young adulthood were not related. In contrast, Buchmann et al. (2009) documented that there was a relation between age at first drink and later alcohol problems, after accounting for childhood externalizing behavior and parental high-risk drinking. Dawson et al. (2008) documented that the association between early drinking and new onsets of alcohol use disorders persisted even after accounting for sociodemographic characteristics and experience of risk factors through the life span.
One difficulty with this approach is that the answer may depend on correlated risk factors that were (or were not) included in the model and their measurement quality. Genetically informative research designs examining genetic and environmental contributions to the association between early-onset drinking and later alcohol use can potentially bypass such difficulties. In a twin design, any latent unmeasured risk factors that are attributable to correlated sets of genetic or shared environmental risk factors can be controlled. Some twin studies found that the relation between early drinking onset and later lifetime alcohol dependence can be primarily attributed to shared genetic risk factors and not to unique environmental influences (Agrawal et al., 2009; Prescott and Kendler, 1999; Sartor et al., 2009). However, other twin studies report modest individual-specific environmental influences shared between early drinking and later problematic use (Fowler et al., 2007; Grant et al., 2006). Taken together, these results suggest that the majority of the association between early age at alcohol use initiation and later alcohol use disorder is attributable to common genetic risk factors, but that this cannot completely explain the relation. A small portion of the relation is likely explained by unique environmental influences, which is consistent with a potential causal influence of early drinking on later alcohol use disorder.
Most research on the prognostic significance of early alcohol use initiation focuses on later disordered drinking. However, studies have also established that early alcohol use initiation is related to drinking involvement in adulthood (e.g., Ellickson et al., 2003; Pitkänen et al., 2005; York et al., 2004). The relation between early drinking initiation and normative adult alcohol involvement has seldom been examined using a genetically informative research design (e.g., Lee et al., 2012). Focusing on alcohol consumption rather than alcohol use disorder is especially important given that most alcohol-related harms occur among the majority of drinkers who are not suffering from an alcohol use disorder (Kreitman, 1986; Spurling and Vinson, 2005). When harm to others is also considered, alcohol use is associated with more harms than any other commonly misused substance (Nutt et al., 2010).
A method particularly well suited for examining the link between age at drinking onset and later alcohol use disorders is the discordant-twin design (e.g., Grant et al., 2006). This design allows researchers to infer causal relationships by examining whether twins discordant for an “exposure” (e.g., early alcohol use initiation) differ on outcomes (e.g., adult alcohol involvement). Such studies have typically focused on the within-twin-pair relationship, comparing the exposed (Twin 1) with the unexposed (Twin 2), and have neglected the between-twin-pair relationship, comparing both twins from Family A with both twins from Family B. The within-twin-pair comparison “controls” for shared familial influences by comparing one twin with his or her co-twin and is often used to help determine causal influence between an exposure and an outcome. After being “controlled for,” between-twin-pair shared familial influences are often ignored in discordant-twin designs. However, focusing solely on the within-twin-pair effect without accounting for higher-level comparisons (i.e., between twin pairs) does not allow for tests of whether there is any additional effect of family context. Statistical techniques such as multilevel mixed modeling allow one to model effects at both the individual (within-twin pairs) and the familial/contextual (between-twin pairs) levels (Snijders and Bosker, 1999).
To our knowledge, there is no research examining potential interactions between individual-level and familial/contextual-level effects of early alcohol initiation age on adult drinking outcomes. It is well established that genes and environments do not operate in isolation. Underlying genetic predispositions can interact with one’s environment (e.g., gene-environment interdependence; Rutter, 2007), and different environments can interact with each other, as well as underlying genetic predispositions (e.g., ecological systems models; Bronfenbrenner and Ceci, 1994). To establish a dynamic understanding of the underlying causes of alcohol use, particularly how early-onset drinking relates to later drinking in adulthood, these interactions must be explored.
There were two goals of this study. First, we isolated the potential unique environmental contribution of early-onset drinking to later alcohol involvement in adulthood by examining the relation within twin pairs discordant for their age at drinking initiation. Second, we examined the extent to which this individual-level effect was moderated by the shared familial context of twin pairs. There were three hypotheses. First, we hypothesized that there would be both within-twin and between-twin effects of onset of drinking on the frequency and quantity of alcohol consumption in adulthood, such that earlier drinking onset would be related to heavier drinking in adulthood. Therefore, twins who drink earlier than their co-twins (within-twin effect) and twins who on average drink earlier than other twins (between-twin effect) will report heavier drinking in adulthood. Second, we hypothesized that there would be a cross-level interaction, such that the unique environmental effect of early drinking onset would be moderated by the shared genetic and environmental context. In particular, we hypothesized that the relationship between the unique environmental effect and adult alcohol involvement would be stronger if there was a stronger familial context of early drinking. Third, we hypothesized that there would be an incremental effect of family context, such that the between-twin-pair effect would be significantly greater than the within-twin-pair effect.
Method
Participants
The sample consisted of adult twins drawn from the Australian Twin Registry. The cohort consisted of 4,268 twins born between 1964 and 1971 (Knopik et al., 2004; Lynskey et al., 2003). Only same-sex monozygotic (MZ) and dizygotic (DZ) twins and only pairs for whom both twins reported on their ages at drinking onset were included in this study. The final sample included 4,194 participants (1,250 MZ female, 1,014 MZ male, 1,020 DZ female, 910 DZ male), with a mean age of 29.9 years (range: 23–39 years).
Procedure
Participants completed a structured psychiatric telephone interview conducted between 1996 and 2000, during which they were administered the Semi-Structured Assessment for the Genetics of Alcoholism–Australian modified version (SSAGA-OZ; Bucholz et al., 1994). See Knopik et al. (2004) and Lynskey et al. (2003) for further information on interview procedures and participant demographics.
Measures
Age at drinking onset.
Age at drinking onset was measured by the question, “How old were you the first time you had more than just a sip of beer, wine, or spirits?” Following from Agrawal et al. (2009), responses were censored such that individuals reporting ages below five were equated to five. Age at onset was treated as a continuous variable (as opposed to a binary variable as in some previous studies [e.g., Grant et al., 2006; Sartor et al., 2009]). This was done to better suit a multilevel modeling framework and to capture nuances within discordance. For example, twins may be assigned to the same dichotomized category of early or late onset but still differ considerably in their ages at drinking onset. Retest data were collected from a subsample of 213 participants about 4 years (SD = 0.4, range: 1.1–4.3 years) after the main interview. The test–retest reliability of age at first drink was very good (r = .78, p < .0001). Potential age-related bias was examined by correlating individuals’ ages at interview with their reported ages at drinking onset. The correlation of .03 suggested minimal bias.
Frequency of alcohol consumption.
The frequency of alcohol consumed in the past year was derived from two questions. First, nonabstainers were asked about their average frequency of drinking during the year they drank the most in their lifetime. They were then asked how old they were when the period began and when it ended. If this period occurred more than 1 year before the interview, participants were asked about their drinking frequency in the past year. Participants who reported that their heaviest period of drinking included the past year were not asked this question. The answers to these questions were combined to yield a measure of past-year drinking frequency. Both questions were measured using a 9-point scale that ranged from every day to less than 3 days per year. The scale was recoded such that the scale midpoint 1 day per week was a score of 1, with every day as a score of 7 and less than 3 days per year as a score of .05 (i.e., 3 ÷ 52, or .06 a week). This was done to facilitate interpretation of the results, such that model estimates represented actual drinking frequencies.
Quantity of alcohol consumption.
The typical quantity of alcohol consumed in the past year was measured using the same procedures as for frequency of alcohol consumption. The answers to two questions were combined to yield a measure of past-year typical drinking quantity. Typical quantity was measured using a 10-point scale ranging from 1–2 drinks to 31 or more drinks. The scale was recoded such that each drink equaled 1 point. Therefore, the response category 1–2 drinks was scored as 1.5, the response category 12–15 drinks was scored as 13.5, and the response category 31 or more drinks was scored as 31. Again, recoding was performed to facilitate interpretation of model estimates as actual quantities of drinking. Skewness of the quantity variable (skewness = 2.78) required log transformation to approximate normality (skewness = 0.78).
Conduct disorder symptoms.
Conduct disorder was evaluated using a 15-item symptom count based on diagnostic criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (American Psychiatric Association, 1994). The mean number of symptoms was 0.74 (SD = 1.34) for the full sample, 1.20 among men (SD = 1.64), and 0.39 among women (SD = 0.89). Skewness of the variable (skewness = 2.63) required a log transformation to approximate normality (skewness = 1.18). The internal consistency reliability (Cronbach’s α) of the symptom count was .63. Four-year test–retest reliability of the conduct disorder symptom count was very good (r =.75, p < .0001). The correlation of -.02 between participant age at interview with reported conduct disorder symptoms suggested minimal bias.
Analytic plan
Two-level models were estimated using SAS version 9.2 (SAS Institute Inc., Cary, NC) PROC GLIMMIX, a statistical procedure used for mixed (or multilevel) models. Multilevel modeling allows for a more informed model that can include both concordant and discordant twins; another benefit is that one is able to examine interactions between individual and contextual effects. In using mixed models for clustered data (i.e., each twin pair = one cluster), the individual twin (within twin pair/Level 1) is nested within the twin pair (between twin pair/Level 2). Both Level 1 and 2 variances are estimated, along with a random intercept. The interpretation of the Level 1 and 2 parameters depends on the method used to center the Level 1 predictor (Enders and Tofighi, 2007). When the Level 1 predictor is group-mean centered (individual twin drinking onset subtracted by the average onset of the twin pair), the Level 1 and Level 2 predictors represent the direct within-twin-pair (comparison against co-twin) and between-twin-pair (comparison against other twin pairs) effects. When the Level 1 predictor is grand-mean centered (individual twin drinking onset subtracted by a constant), the Level 1 predictor represents the direct within-twin-pair effect (as long as the Level 2 predictor is also in the model). The Level 2 predictor will now represent the incremental between-twin-pair effect (i.e., the additional value of the between-twin-pair effect while controlling for the within-twin-pair effect), allowing for a test of significant differences between the within- and between-twin-pair effects. Therefore, the within-twin-pair effect was group-mean centered for the main models and grand-mean centered only when testing for an incremental between-twin-pair effect.
Hypotheses 1 and 2 were tested with a three-step model for each drinking outcome. One set of models predicted the frequency of drinking and the other predicted the typical quantity of alcohol consumed, both in the past year. Each model first tested the main effects of gender, zygosity, age, and Level 1 (individual drinking onset) and Level 2 (twin average drinking onset) effects. Then, cross-level (Level 1 × Level 2) and quadratic Level 2 interactions were added to test for moderation effects. The quadratic between-twin-pair effect is necessary to ensure that moderation is occurring at both levels, that is, to test if the Level 2 effect is also moderated by the Level 1 effect. Finally, the third step added Level 1 and 2 conduct disorder effects. Level 1 and 2 conduct disorder variables were both used to account for the effect of conduct disorder when examining both levels of age at drinking onset. Thus, Level 1 conduct disorder controlled for Level 1 drinking initiation, and Level 2 conduct disorder controlled for Level 2 drinking initiation.
Potential differences by zygosity and gender were estimated for each predictor by examining interactions with the drinking onset variables (both Level 1 and Level 2). There was one significant interaction between gender and the main effect of within-twin-pair age at first drink for the model predicting quantity. This interaction was included in the final model for quantity. In one set of analyses, MZ and DZ twins were combined to examine overall twin effects. Another set of analyses was restricted to MZ twin data to allow for the most stringent tests of unique environmental causality.
Finally, the third hypothesis was tested by using a grand-mean centered Level 1 variable, which was centered at age 16 years (the overall sample mean). Both the grand-mean centered Level 1 variable and the Level 2 variable were entered in a model along with gender, zygosity, and age in order to test if between-twin-pair differences in the age at drinking initiation had an incremental effect on drinking frequency and quantity in adulthood.
Results
Descriptive analyses
The mean past-year frequency of drinking was 1.60 (i.e., on average, individuals drank 2–3 days a week), and the typical quantity of alcohol consumed was 3.27 drinks (i.e., on average, during a typical drinking episode, individuals drank 3–4 drinks) in adulthood. Frequency and quantity of drinking had a modest positive association for men (r = .05, p = .02) and for women (r = .05, p = .01).
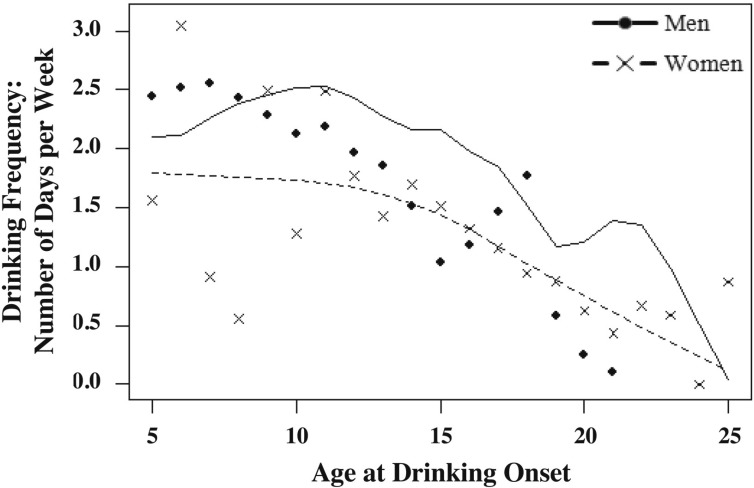
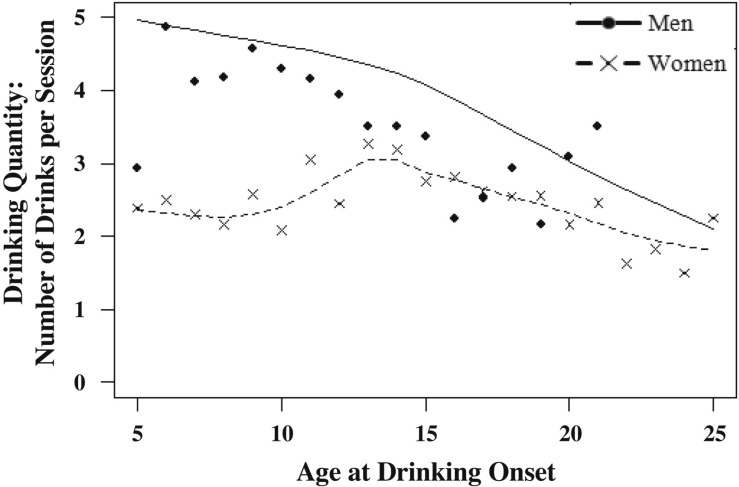
The average age at first drink for the sample was 15.74 years (SD = 2.86). Most twin pairs (75%) were discordant for their age at first drink. The average discordance was 1.90 (SD = 2.32) years. The age at drinking onset was negatively associated with both the frequency of drinking (men: r = -.13, p = .01; women: r = -.14, p = .01) and the typical quantity of alcohol consumed (men: r = -.12, p = .01; women: r = .04, p = .02 for). Figures 1 and 2 depict smoothing spline plots that represent the mean frequency and quantity of alcohol consumption in adulthood as a function of the age at drinking initiation (separately for men and women). As seen in Figure 1, although individuals who initiated drinking at early ages (e.g., before age 14 years) drank more frequently as adults than those who initiated drinking at later ages, there appears to be additional reduction in the frequency of drinking in adulthood with each additional year of the age at first drink. As shown in Figure 2, there was not as strong a decline in the typical quantity of alcohol consumed as a function of the age at first drink. Conduct disorder was also inversely associated with the age at drinking onset (men: r = -.19, p = .01; women: r = -.18, p = .01) and positively associated with quantity of drinking in adulthood (men: r = .11, p = .01; women: r = -.14, p = .01) but only positively associated with frequency of drinking in adulthood for men (men: r = .05, p = .01; women: r = .03, p = .06).
Figure 1.
Smoothing spline plot representing drinking frequency (number of drinking days per week) in adulthood as a function of age at onset of drinking for men and women. Line represents predicted data points as indicated by the smoothing spline parameter. Data points (dots and crosses) represent observed data points for men and women. Smoothing splines provide the best-fitting function by considering its average smoothness in conjunction with its goodness-of-fit. Goodness-of-fit is measured by residual sum of squares, whereas average smoothness is measured by the integral of the function’s second derivative. The smoothing parameter controls the influence of smoothness on the overall best-fitting function. A smoothing spline was fit through the data in Figures 1 and 2 to help visualize trend in the age at drinking onset. The smoothing parameter was chosen using the generalized cross-validation approach. The spline was weighted by number of men and women at each age at drinking onset.
Figure 2.
Smoothing spline plot representing drinking quantity (number of drinks per drinking session) in adulthood as a function of age at onset of drinking for men and women. Line represents predicted data points as indicated by the smoothing spline parameter. Data points (dots and crosses) represent observed data points for men and women.
Mixed models
Drinking frequency.
The three steps of the two-level models predicting drinking frequency in adulthood from age at first drink for both MZ and DZ twins are presented in Table 1. The within-twin-pair main effect (Level 1) was significant (β = -4.68, p = .01), indicating that for every year earlier that Twin 1 initiated drinking compared with Twin 2, Twin 1 increased his or her drinking frequency by approximately 3 days per year (b = -0.06, p = .01). In other words, the main effect of the unique environmental experience of drinking earlier than one’s twin significantly influenced an individual’s frequency of drinking in adulthood. The between-twin-pair main effect (Level 2) was also significant (β = -14.89, p = .00), indicating that for every year the average age at drinking initiation of the twin pair (i.e., familial context) was lower compared with other twin pairs, drinking frequency increased by approximately 5 days per year (b = -0.12, p = .01).
Table 1.
Standardized and unstandardized estimates of past-year drinking frequency as predicted by age at drinking onset and conduct disorder for the full sample and the monozygotic (MZ) twin samples
| Total sample |
MZ twins |
|||||
| Variable | Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 |
| Zygosity | 1.03 (0.04) | 0.67 (0.02) | 0.73 (0.02) | – | – | – |
| Gender | 17.94** (0.61) | 17.64** (0.60) | 18.56** (0.61) | 12.02** (0.55) | 11.85** (0.54) | 12.29** (0.56) |
| Age | 7.92** (0.05) | 8.09** (0.06) | 8.04** (0.06) | 5.49* (0.05) | 5.41** (0.05) | 5.33** (0.05) |
| Within-twin drinking onseta | -4.68** (-0.06) | -6.59** (-0.08) | -6.24** (-0.08) | -3.44** (-0.06) | -4.13** (-0.07) | -4.14** (-0.07) |
| Between-twin drinking onsetb | -14.89** (-0.12) | 18.30** (-0.15) | -18.56** (-0.15) | -11.66** (-0.12) | -14.21** (-0.15) | -.14.62** (-0.15) |
| Within Drinking Onset × Between Drinking Onset | -3.67* (-0.01) | -3.67* (-0.01) | -1.22 (-0.01) | -1.21 (-0.01) | ||
| Between Drinking Onset × Between Drinking Onset | -6.51** (-0.01) | -6.45** (-0.01) | -4.92* (-0.01) | -4.87* (-0.01) | ||
| Within-twin conduct disordera | 2.13 (0.12) | -0.06 (-0.005) | ||||
| Between-twin conduct disorderb | -1.19 (-0.04) | -1.53 (-0.08) | ||||
Notes: Unstandardized estimates in parentheses.
Level 1 (unique environment effect) variable;
Level 2 (familial context effect) variable.
p < .05;
p < .01.
Both cross-level and quadratic interactions were also significant. The significant interaction between within-twin-pair and between-twin-pair drinking onset indicated that the effect of Twin 1 drinking earlier than Twin 2 on adult alcohol consumption became more negative (β = 3.67 p = .01; Table 1, Model 3) as the twin pair’s average age at drinking onset decreased. Therefore, being in a shared genetic and/or environmental context that promotes early drinking behavior will additionally increase the individual effect of drinking earlier relative to one’s twin. However, at very high levels of familial risk, the shared genetic/environmental context may be much more predictive of drinking frequency than individual drinking onset, as evidenced by the significant quadratic between-twin-pair effect.
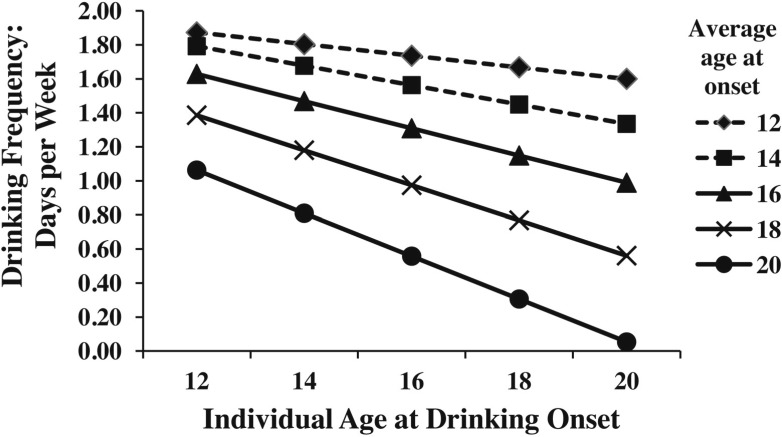
The quadratic effect of Level 2 (between-twin-pair) drinking onset indicated that after controlling for individual drinking onset, the between-twin-pair effect became more negative (β = -6.45, p = .01; Table 1, Model 3) as the average age at drinking onset increased. Figure 3 displays the cross-level interaction (accounting for quadratic Level 2 interaction) for twins with individual ages at onset of 12–20 years and twin-pair average ages at onset of 12–20 years. As seen in Figure 3, frequency of drinking, as predicted by individual age at onset, decreases as the average twin-pair onset increases. Thus, the frequency of alcohol consumption for a twin who started at age 16 years is higher if she is from a twin pair with an average age at onset of 14 years, as the between-twin effect makes the relationship between individual age at onset and drinking frequency more negative compared with a twin who started at age 16 years but has an average twin-pair onset of 18 years. However, because of the significant quadratic between-twin-pair effect, differences between twins with individual onsets of 14 and 18 years and average onsets of 14 years is smaller than twins with individual onsets of 14 and 18 years and an average onset of 18 years. Finally, conduct disorder was not a significant predictor as a within-twin-pair or between-twin-pair effect.
Figure 3.
Cross-level and quadratic Level 2 drinking onset predicting drinking frequency (number of drinking days per week) in adulthood for MZ and DZ twins. Y-axis represents the past-year frequency of drinking (days per week); X-axis represents individual ages at onset of drinking. Lines represent different average ages at onset of drinking for twin pairs.
Results from models using MZ pairs were similar to models using the full sample, although there was no significant cross-level interaction. However, given that there was no interaction between zygosity and cross-level interaction (b = -0.008, p = .36), failure to find these effects among MZ twins might be attributable to a reduction in statistical power when using the smaller subsample. This indicates that while controlling for 100% of shared environment and genetics, the unique environmental effect of starting to drink earlier than one’s twin has a causal effect on drinking frequency later in adulthood. Failure to find a significant effect of conduct disorder at either Level 1 or 2 indicates that this cannot be explained by the earlier-drinking twin also having more symptoms of conduct disorder than the later-drinking co-twin.
Drinking quantity.
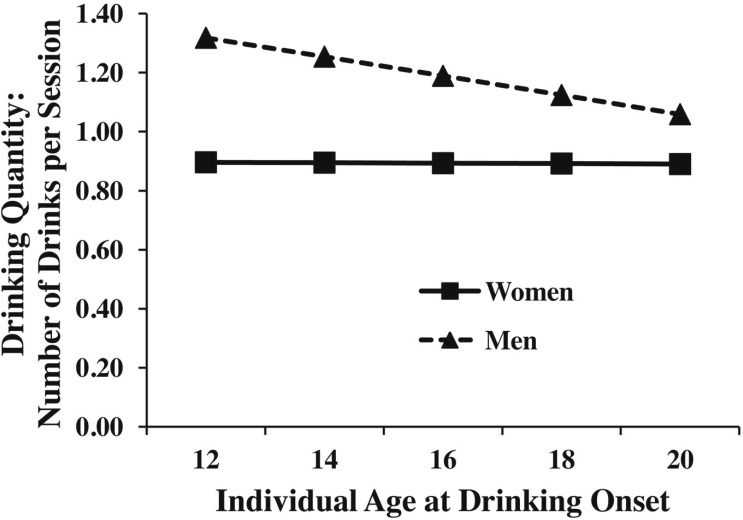
Table 2 displays the results for drinking quantity from models using the full sample and only MZ twins. The pattern of main effects was similar to that found for drinking frequency: both the Level 1 within-twin-pair main effect (β = -1.11, p = .01) and Level 2 between-twin-pair effect (β = -3.00, p = .01) were significant. However, the cross-level interaction was not (β= -0.77, p = .14; Table 2, Model 2). There was a significant within-twin-pair Level 1 effect of conduct disorder. This means that (while controlling for the between-twin-pair effect of conduct disorder) a greater number of conduct disorder symptoms of a twin relative to the co-twin was associated with larger quantities of alcohol consumed in adulthood. Finally, as seen in Figure 4, there was an interaction between the within-twin-pair effect and gender, such that individual age at drinking onset had a stronger effect on men’s compared with women’s adult drinking quantity.
Table 2.
Standardized and unstandardized estimates of past-year drinking quantity as predicted by age at drinking onset and conduct disorder for the full sample and the monozygotic (MZ) twin samples
| Total sample |
MZ twins |
|||||
| Variable | Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 |
| Zygosity | 0.01 (0.0003) | -0.09 (-0.003) | -0.22 (-0.007) | – | – | – |
| Gender | 9.76** (0.33) | 9.67** (0.33) | 8.68** (0.30) | 6.79** (0.31) | 6.77** (0.31) | 6.04** (0.28) |
| Age | -3.28** (-0.02) | -3.23** (-0.02) | -3.20** (-0.02) | -2.49** (-0.02) | -2.50** (-0.02) | -2.37** (-0.02) |
| Within-twin drinking onseta | -1.11* (-0.01) | -1.53* (-0.02) | -0.06 (-0.001) | -0.23 (-0.004) | -0.41 (-0.01) | -0.16 (-0.003) |
| Between-twin drinking onsetb | -3.00** (-0.02) | -4.02** (-0.03) | -3.25** (-0.03) | -2.20** (-0.02) | -2.64** (-0.03) | -1.95* (-0.02) |
| Within Drinking Onset × Between Drinking Onset | -0.77 (-0.002) | -0.89 (-0.002) | -0.41 (-0.002) | -0.54 (-0.003) | ||
| Between Drinking Onset × Between Drinking Onset | -1.98* (-0.003) | -2.06* (-0.003) | -0.84 (-0.002) | -0.91 (-0.002) | ||
| Within-twin drinking onset × Gender | -1.80** (-0.03) | -0.93 (-0.02) | ||||
| Within-twin conduct disordera | 1.48** (0.09) | 0.37 (0.03) | ||||
| Between-twin conduct disorderb | 3.03** (0.11) | 2.53** (0.12) | ||||
Notes: Unstandardized estimates are in parentheses.
Level 1 (unique environment effect) variable;
Level 2 (familial context effect) variable.
p < .05;
p < .01.
Figure 4.
Within-twin-pair drinking onset predicting quantity of drinking in adulthood (number of drinks per drinking session) moderated by gender for twins with twin pair average ages at drinking onset of 14 and 18 years.
The model using only MZ twins did not replicate the model using the full sample. Only the between-twin main effect of drinking onset and the between-twin-pair effect of conduct disorder were significant (Table 2). The within-twin main effect and the interaction between gender and within-twin-pair effect were not significant. However, because there were no significant interactions with these predictors and zygosity for the quantity outcome, failure to find these effects among MZ twins might be because of a reduction in statistical power when using the smaller subsample.
Finally, grand-mean centered models were estimated to test the third hypothesis that the between-twin-pair (family context) variable would incrementally contribute to the models. For the frequency outcome, the between-twin-pair effect was significant using a grand-mean centered Level 1 predictor, for both the full sample (β = -7.82, p < .05) and the MZ-only sample (β = -5.89, p < .05). Therefore, the between-twin-pair effect (e.g., genetics or shared family environment) incrementally contributed to the model for the frequency outcome, indicating that the between-twin-pair effect was significantly larger than the within-twin-pair effect. However, for the quantity outcome, the between-twin-pair effect did not contribute incrementally to the models for the full sample (β = -1.33, p = .19) or the MZ-only sample (β = -1.81, p = .10). This indicates that, when controlling for the within-twin-pair effect, there is no additional between-twin-pair effect, and as such, the unique environment and family context effects are of similar magnitude.
Kendler et al. (2010) demonstrated that current alcohol involvement measures may not account for underlying genetic predispositions to alcohol use disorder or alcohol involvement during the lifetime period of heaviest drinking. Therefore, we reran all models using the quantity and frequency of drinking during the lifetime heaviest drinking period rather than the past year to examine whether this yielded different results. The results of the models were similar to models predicting current alcohol involvement; the same effects were significant in models with either set of outcomes.
Discussion
The purpose of this study was to examine a causal model of age at drinking initiation on later alcohol involvement in adulthood using a multilevel discordant twin design. In particular, we were interested in examining within-twin-pair effects, between-twin-pair effects, and potential interactions. We found support for the hypothesis that there would be both within-twin-pair and between-twin-pair effects for each outcome. As seen in previous research (e.g., Ellickson et al., 2003; Pitkänen et al., 2005; York et al., 2004), there was a relationship between age at drinking onset and adult alcohol involvement as measured by frequency and quantity of drinking. As hypothesized, the within-twin-pair effect (i.e., unique environmental effect) of age at onset was negatively related to both drinking frequency and quantity (except in the MZ-only model predicting quantity). There was also a significant between-twin-pair (i.e., familial context) effect of drinking onset on both drinking frequency and quantity. Previous studies have documented that the relationship between age at onset and lifetime alcohol use disorder diagnoses can be attributed mainly to genetic and shared environmental influences rather than unique environmental influences (e.g., Agrawal et al., 2009; Fowler et al., 2007; Sartor et al., 2009). By contrast, both unique environmental and familial context effects seem to be predictors of adult alcohol involvement.
As predicted, there was a significant cross-level interaction for within-twin-pair and between-twin-pair effects, but only for the frequency outcome, such that a riskier familial drinking context strengthened the relationship between individual context and adult drinking frequency. Finally, the third hypothesis was supported for only the frequency outcome. The between-twin-pair incremental effect was also significant in the model predicting frequency, indicating that the between-twin-pair effect was significantly stronger than the within-twin-pair effect. However, the nonsignificant incremental effect for the model predicting quantity indicated that the between-twin-pair and within-twin-pair effects were of equal magnitude.
This study provides evidence for drinking onset age as a possible causal effect regarding quantity and frequency of alcohol use in adulthood, as indicated by a significant within-twin-pair effect on later drinking. In this study we were unable to address potential mechanisms of action for this relationship. However, other research provides insight into ways in which drinking initiation may influence later alcohol involvement. These include both biological/genetic changes because of specific behaviors (e.g., changes in neurological functioning because of early drinking behavior) and classic environmental changes (e.g., social interactions) incorporating diathesis–stress effects or gene–environment interdependence (Rutter, 2007). For example, early drinking onset in adolescence affects neurological development, particularly in sensitive periods of adolescent brain development (Windle et al., 2008; Witt, 2010), leading to increased tolerance and craving behavior. Therefore, earlier drinking may change neurological responses to alcohol, which facilitates higher levels of alcohol involvement. Research also indicates that early-onset drinking can be socially reinforced through peers. For example, Knecht et al. (2011) documented that as adolescents drink more over time, they gain more friends who drink similarly. Simons-Morton and Chen (2006) documented that this relationship works the other way as well: as an adolescent gains more friends who drink, the adolescent will start to drink more alcohol. Therefore, adolescents who drink earlier may gravitate toward other individuals who drink. These individuals consequently reinforce the adolescents’ drinking behavior.
Taking these studies together, an adolescent’s neurological and social environment may be influenced by early age at first drink, and this experience could subsequently influence and reinforce drinking behavior. Unlike genetic or shared environmental contexts, these factors would be more related to the individual’s unique experience of drinking initiation. Longitudinal studies of adolescent drinking involvement that examine potential mechanisms such as these foster improved understanding of how age at drinking onset could have a causal influence on later alcohol use.
It is worth noting that these results indicate that the unique effect of early age at drinking onset is not as important as underlying genetic predisposition or family environmental influence when predicting frequency of drinking in adulthood. The third hypothesis, that the between-twin-pair effect would have an incremental contextual effect, was supported such that the between-twin-pair effect was significantly stronger than the within-twin effect. This finding indicates that the unique environment plays a smaller role in predicting adult drinking frequency than family context (genes and/or shared environment). This supports previous studies (e.g., Agrawal et al., 2009; Sartor et al., 2009) documenting that family context effects are the main predictors for alcohol disorders. This hypothesis was not supported for drinking quantity, however, indicating that unique environment and family context effects were of similar magnitude in predicting adult alcohol quantity.
The second hypothesis, that there would be significant interactions between within-twin-pair and between-twin-pair effects, was supported for only the frequency outcome. The cross-level interaction indicates a strong individual–contextual interdependence; high-risk familial drinking contexts can increase drinking frequency for individuals who have later ages at drinking initiation. Therefore, individuals with a family context that predisposes individuals to drink (e.g., genetic liability or socialization of drinking), as evidenced by an early-onset co-twin, will drink more frequently and in higher quantities than individuals who initiated drinking at the same age who do not have the same level of familial risk. However, the quadratic between-twin effect indicates that high familial risk can be a much more powerful predictor than individual drinking age. Therefore, for individuals who have high familial risk, an earlier drinking age may result in only a small increase in adult drinking frequency. These results document the importance of examining both contextual and individual-level effects in discordant twin studies and reinforce the importance of a gene–environment interdependence framework.
Limitations and implications
This study has limitations. Reports of age at alcohol use initiation and conduct disorder symptoms were retrospective, and the sample was Australian young adults studied in the late 1990s. These results may not generalize to other countries, age groups, or historical periods. Another limitation is the implicit assumption of equivalence of “exposures” (similarity in ages at drinking onset) within and between twin pairs. Limited contextual information about drinking initiation does not allow us to test this assumption. Finally, current findings provided evidence for a potential causal influence of age at first drink on later alcohol involvement; however, it is possible that other unmeasured unique environmental effects on drinking initiation and alcohol consumption could explain this relationship. This study also has implications for future research. Although we document that age at initiation can possibly be a causal mechanism for later adult alcohol involvement, there are many specific processes by which this may happen (e.g., neurological development, socialization). Using prospective studies to examine multiple contexts of how alcohol use and its influences change over time may be well suited for such research. These studies could examine individuals from the time at which they initiate drinking and assess relationships between the individual’s own alcohol use and the contextual variables that influence drinking. Furthermore, these results highlight the importance of modeling both within-twin and between-twin effects in discordant twin designs, as it allows for modeling potential cross-level interactions. This framework might also provide insights into a range of adolescent risk behaviors.
Acknowledgments
The authors thank Lesa Hoffman for help with the statistical analyses for this study; Dixie Statham for coordinating the data collection for the twins; and David Smyth, Olivia Zheng, and Harry Beeby for data management of the Australian Twin Registry. We thank the Australian Twin Registry twins for their continued participation.
Footnotes
This work was supported by National Institute on Alcohol Abuse and Alcoholism Grant AA07728.
References
- Agrawal A, Sartor CE, Lynskey MT, Grant JD, Pergadia ML, Grucza R, Heath AC. Evidence for an interaction between age at first drink and genetic influences on DSM-IV alcohol dependence symptoms. Alcoholism: Clinical and Experimental Research. 2009;33:2047–2056. doi: 10.1111/j.1530-0277.2009.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. 4th ed. Washington, DC: Author; 1994. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- Bronfenbrenner U, Ceci SJ. Nature-nurture reconceptualized in developmental perspective: A bioecological model. Psychological Review. 1994;101:568–586. doi: 10.1037/0033-295x.101.4.568. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hessel-brock VM, Nurnberger JI, Jr, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: A report on the reliability of the SSAGA. Journal of Studies on Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Buchmann AF, Schmid B, Blomeyer D, Becker K, Treutlein J, Zimmermann US, Laucht M. Impact of age at first drink on vulnerability to alcohol-related problems: Testing the marker hypothesis on a prospective study of young adults. Journal of Psychiatric Research. 2009;43:1205–1212. doi: 10.1016/j.jpsychires.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Chou SP, Ruan WJ, Grant BF. Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcoholism: Clinical and Experimental Research. 2008;32:2149–2160. doi: 10.1111/j.1530-0277.2008.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: A risk factor for the development of alcohol disorders. American Journal of Psychiatry. 2000;157:745–750. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- Ellickson PL, Tucker JS, Klein DJ. Ten-year prospective study of public health problems associated with early drinking. Pediatrics. 2003;111:949–955. doi: 10.1542/peds.111.5.949. [DOI] [PubMed] [Google Scholar]
- Enders CK, Tofighi D. Centering predictor variables in crosssectional multilevel models: A new look at an old issue. Psychological Methods. 2007;12:121–138. doi: 10.1037/1082-989X.12.2.121. [DOI] [PubMed] [Google Scholar]
- Fowler T, Lifford K, Shelton K, Rice F, Thapar A, Neale MC, Van Den Breex MBM. Exploring the relationship between genetic and environmental influences on initiation and progression of substance use. Addiction. 2007;102:413–422. doi: 10.1111/j.1360-0443.2006.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: Results from the National Longitudinal Alcohol Epidemiologic Survey. Journal of Substance Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Grant JD, Scherrer JF, Lynskey MT, Lyons MJ, Eisen SA, Tsuang MT, Bucholz KK. Adolescent alcohol use is a risk factor for adult alcohol and drug dependence: Evidence from a twin design. Psychological Medicine. 2006;36:109–118. doi: 10.1017/S0033291705006045. [DOI] [PubMed] [Google Scholar]
- Guttmannova K, Bailey JA, Hill KG, Lee JO, Hawkins JD, Woods ML, Catalano RF. Sensitive periods for adolescent alcohol use initiation: Predicting the lifetime occurrence and chronicity of alcohol problems in adulthood. Journal of Studies on Alcohol and Drugs. 2011;72:221–231. doi: 10.15288/jsad.2011.72.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: Age at onset, duration, and severity. Archives of Pediatrics & Adolescent Medicine. 2006;160:739–746. doi: 10.1001/archpedi.160.7.739. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Myers J, Dick D, Prescott CA. The relationship between genetic influences on alcohol dependence and on patterns of alcohol consumption. Alcoholism: Clinical and Experimental Research. 2010;34:1058–1065. doi: 10.1111/j.1530-0277.2010.01181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King KM, Chassin L. A prospective study of the effects of age of initiation of alcohol and drug use on young adult substance dependence. Journal of Studies on Alcohol and Drugs. 2007;68:256–265. doi: 10.15288/jsad.2007.68.256. [DOI] [PubMed] [Google Scholar]
- Knecht AB, Burk WJ, Weesie J, Steglich C. Friendship and alcohol use in early adolescence: A multilevel social network approach. Journal of Research on Adolescence. 2011;21:475–487. [Google Scholar]
- Knopik VS, Heath AC, Madden PAF, Bucholz KK, Slutske WS, Nelson EC, Martin NG. Genetic effects on alcohol dependence risk: Re-evaluating the importance of psychiatric and other heritable risk factors. Psychological Medicine. 2004;34:1519–1530. doi: 10.1017/s0033291704002922. [DOI] [PubMed] [Google Scholar]
- Kreitman N. Alcohol consumption and the preventive paradox. British Journal of Addiction. 1986;81:353–363. doi: 10.1111/j.1360-0443.1986.tb00342.x. [DOI] [PubMed] [Google Scholar]
- Lee LO, Young-Wolff KC, Kendler KS, Prescott CA. The effects of age at drinking onset and stressful life events on alcohol use in adulthood: A replication and extension using a population-based twin sample. Alcoholism: Clinical and Experimental Research. 2012;36:693–704. doi: 10.1111/j.1530-0277.2011.01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynskey MT, Heath AC, Bucholz KK, Slutske WS, Madden PAF, Nelson EC, Martin NG. Escalation of drug use in early-onset cannabis users vs co-twin controls. Journal of the American Medical Association. 2003;289:427–433. doi: 10.1001/jama.289.4.427. [DOI] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Malone S, Elkins I. Origins and consequences of age at first drink. I. Associations with substance-use disorders, disinhibitory behavior and psychopathology, and P3 amplitude. Alcoholism: Clinical and Experimental Research. 2001;25:1156–1165. [PubMed] [Google Scholar]
- Nutt DJ, King LA, Phillips LD. & the Independent Scientific Committee on Drugs. Drug harms in the UK: A multicriteria decision analysis. The Lancet. 2010;376:1558–1565. doi: 10.1016/S0140-6736(10)61462-6. [DOI] [PubMed] [Google Scholar]
- Pitkänen T, Lyyra AL, Pulkkinen L. Age of onset of drinking and the use of alcohol in adulthood: A follow-up study from age 8-42 for females and males. Addiction. 2005;100:652–661. doi: 10.1111/j.1360-0443.2005.01053.x. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Age at first drink and risk for alcoholism: A noncausal association. Alcoholism: Clinical and Experimental Research. 1999;23:101–107. [PubMed] [Google Scholar]
- Rutter M. Gene-environment interdependence. Developmental Science. 2007;10:12–18. doi: 10.1111/j.1467-7687.2007.00557.x. [DOI] [PubMed] [Google Scholar]
- Sartor CE, Lynskey MT, Bucholz KK, Madden PAF, Martin NG, Heath AC. Timing of first alcohol use and alcohol dependence: Evidence of common genetic influences. Addiction. 2009;104:1512–1518. doi: 10.1111/j.1360-0443.2009.02648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor CE, Lynskey MT, Heath AC, Jacob T, True W. The role of childhood risk factors in initiation of alcohol use and progression to alcohol dependence. Addiction. 2007;102:216–225. doi: 10.1111/j.1360-0443.2006.01661.x. [DOI] [PubMed] [Google Scholar]
- Simons-Morton B, Chen RS. Over time relationships between early adolescent and peer substance use. Addictive Behaviors. 2006;31:1211–1223. doi: 10.1016/j.addbeh.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Snijders TAB, Bosker R. London, England: Sage; 1999. Multilevel analysis: An introduction to basic and advanced multilevel modeling (pp. 52–56) [Google Scholar]
- Spurling MC, Vinson DC. Alcohol-related injuries: Evidence for the prevention paradox. Annals of Family Medicine. 2005;3:47–52. doi: 10.1370/afm.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle M, Spear LP, Fuligni AJ, Angold A, Brown JD, Pine D, Dahl RE. Transitions into underage and problem drinking: Developmental processes and mechanisms between 10 and 15 years of age. Pediatrics, 121, Supplement. 2008;4:S273–S289. doi: 10.1542/peds.2007-2243C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt ED. Research on alcohol and adolescent brain development: Opportunities and future directions. Alcohol. 2010;44:119–124. doi: 10.1016/j.alcohol.2009.08.011. [DOI] [PubMed] [Google Scholar]
- York JL, Welte J, Hirsch J, Hoffman JH, Barnes G. Association of age at first drink with current alcohol drinking variables in a national general population sample. Alcoholism: Clinical and Experimental Research. 2004;28:1379–1387. doi: 10.1097/01.alc.0000139812.98173.a4. [DOI] [PubMed] [Google Scholar]