Abstract
Bariatric procedures vary in efficacy, but overall are more effective than behavioral and pharmaceutical treatment. Roux-en-Y gastric bypass causes increased secretion of glucagon-like peptide 1 (GLP-1) and reduces body weight (BW) more than adjustable gastric banding (AGB), which does not trigger increased GLP-1 secretion. Since GLP-1–based drugs consistently reduce BW, we hypothesized that GLP-1 receptor (GLP-1R) agonists would augment the effects of AGB. Male Long-Evans rats with diet-induced obesity received AGB implantation or sham surgery. GLP-1R agonism, cannabinoid receptor-1 (CB1-R) antagonism, or vehicle was combined with inflation to evaluate interaction between AGB and pharmacological treatments. GLP1-R agonism reduced BW in both sham and AGB rats (left uninflated) compared with vehicle-treated animals. Subsequent band inflation was ineffective in vehicle-treated rats but enhanced weight loss stimulated by GLP1-R agonism. In contrast, there was no additional BW loss when CB1-R antagonism was given with AGB. We found band inflation to trigger neural activation in areas of the nucleus of the solitary tract known to be targeted by GLP-1R agonism, offering a potential mechanism for the interaction. These data show that GLP-1R agonism, but not CB1-R antagonism, improves weight loss achieved by AGB and suggest an opportunity to optimize bariatric surgery with adjunctive pharmacotherapy.
Recent years have seen an unprecedented rise in the prevalence of obesity driven by the combination of sedentary lifestyle and exposure to energy-dense diets. This dramatic rise, which is associated with numerous serious comorbidities, has led the World Health Organization to describe obesity as the greatest current threat to human health (1). Conventional therapies, in the form of decreased caloric intake and increased physical activity, have proven to be of limited effectiveness at a population level (2). Similarly, current pharmacotherapies have been found to be only mildly efficacious (3). To this point, surgical intervention is the only method that currently achieves sustained reductions of body weight (BW) >15% (4).
Bariatric interventions have proven to be highly efficacious and frequently carry the beneficial side effect of type 2 diabetes (T2D) resolution (5). The most commonly used bariatric procedures are adjustable gastric banding (AGB), vertical sleeve gastrectomy, and Roux-en-Y gastric bypass (RYGB) surgery (6). However, not all of the currently available surgical options are equally effective. AGB results in an average loss of 46% excess BW (5) and a resolution of T2D in 50–70% of individuals (7). Although emerging data may suggest otherwise (8), this procedure is commonly thought of as restrictive in nature. AGB is minimally invasive and reversible but is less effective than RYGB (5); RYGB, a more invasive and irreversible intervention, results in a loss of 60% excess BW on average and is associated with a surprisingly rapid resolution of T2D in ∼80% of patients, but it is also associated with greater mortality (5). We hypothesized that the superior efficacy of RYGB is due to the humoral reprograming observed after RYGB (9) but not AGB (10). Circulating glucagon-like peptide 1 (GLP-1) and therapies based upon it modulate food intake, glucose homeostasis, BW (11), and fat mass (12).
Recent research has focused on identifying the factors responsible for the early benefits of RYGB in the hope that novel, nonsurgical therapies might become possible. Although at least some of the beneficial effects of RYGB on glucose control are secondary to reduced BW, the dramatic changes in gut hormone secretion observed in RYGB but not in AGB may contribute to greater resolution of T2D (13). Studies in humans have identified substantial changes in multiple circulating factors, including GLP-1, following RYGB (14). Levels of GLP-1 are greatly elevated after RYGB but not after AGB, suggesting that it may function as a modulator of both BW and glucose homeostasis (15). Given that several diabetes drugs on the market act as GLP-1 receptor (GLP-1R) agonists, modulation of the GLP-1 system may improve less invasive surgical therapies for weight loss and diabetes.
We hypothesized that treatment with a GLP-1R agonist would augment weight loss with AGB and that this combined benefit is specific for GLP-1. Taken together, our results suggest that less invasive approaches such as AGB may achieve similar results to RYGB when combined with appropriate pharmacotherapies.
RESEARCH DESIGN AND METHODS
Animals.
Male Long-Evans rats (n = 197; 250–300 g) obtained from Harlan Laboratories (Indianapolis, IN) were individually housed and maintained on a 12:12-h light:dark cycle (lights off at 19:00) at 25°C and 50–60% humidity. Rats were provided ad libitum access to water and a high-fat butter diet (HFD; 4.54 kcal/g, 41% fat; Research Diets, New Brunswick, NJ) previously shown to produce diet-induced obesity (DIO) and metabolic impairments. All rats were maintained on HFD for 20 weeks prior to AGB or sham procedure. All procedures for animal use were approved by the University of Cincinnati, Institutional Animal Care and Use Committee.
Adjustable gastric band implantation.
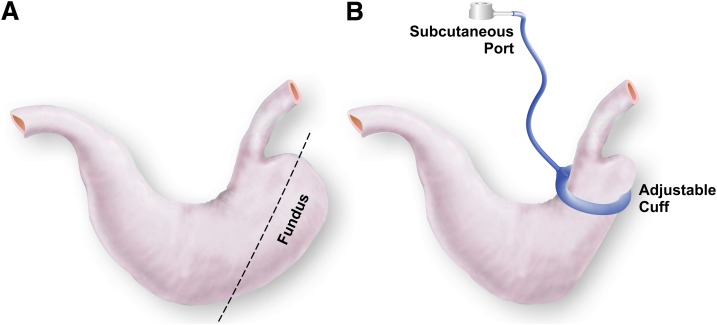
AGB surgery was performed in anesthetized rats (isoflurane) as previously described by Kampe et al. (16) and depicted in Fig. 1. Briefly, rats received laparotomy followed by isolation of the stomach and removal of the forestomach (fundectomy) using an ETS-FLEX 35-mm staple gun (Ethicon Endo-Surgery, Somerville, NJ). Silicone vascular occluders (12 mm lumen diameter; DocXS Biomedical products, Ukiah, CA) were fitted with subcutaneous ports (Braintree Scientific, Braintree, MA) 11.5 cm from the junction of the catheter and the cuff. The occluders were placed between the gastroesophageal junction and the pylorus. Free ends of the occluder (hereafter referred to as gastric bands) were connected using 1–0 Ethilon, nonabsorbable suture (Ethicon Endo-Surgery) such that the band encompassed the greater and lesser curvatures of the stomach. Four additional sutures were used to secure the band in the proximity of the staple line left after removal of the forestomach. The stomach and gastric band were then reintegrated into the peritoneal cavity, and the abdominal wall was closed in layers using a running suture and a running subcuticular suture, respectively. The procedure required ∼45 min of isoflurane-induced anesthetization. For the duration of the implantation and recovery period, all bands were maintained in a deflated state. For the sham surgery, stomachs were exposed and forestomachs removed. The remaining stomach was then returned to peritoneal cavity and the laparotomy closed in layers. Following 3 to 4 weeks of recovery, rats were grouped to match BW and fat mass. Bands were inflated with 10% glycerol solution via a subcutaneous port (Braintree Scientific).
FIG. 1.
Schematic representation of AGB in rats. Location of excision for fundectomy (A) and schematic of band placement (B) as performed in these studies.
Internal band pressure analysis.
Internal band pressure was measured at increasing inflation volumes in 12 rats. Following injection of 10% glycerol solution via a subcutaneous port, a 0–15 psi gauge pressure transducer (PX26–015GV; Omega Engineering, Stamford, Connecticut) was inserted into the port. The transducer was monitored via a 1/8 DIN process meter and controller (DP25B-E-A; Omega Engineering), DAQ hardware interface (12-Bit, 10 kS/s Low-Cost Multifunction, DAQ NI USB-6008; National Instruments Corporation, Austin, TX), and DAQ software interface (LabVIEW; National Instruments Corporation). The change in pressure (from deflated state) was plotted against inflation volume and fitted to a nonlinear, third-order polynomial line (R2 = 0.9991, Y = 12.92 + 0.3862X + 0.002864X2 + 0.000005453X3).
Pre- and postoperative care.
HFD was replaced with Ensure Plus liquid diet (1.41 kcal/g, 29% fat; Abbott Nutrition, Columbus, OH) 24 h prior to surgery. Liquid diet was continued for 48 h postsurgically and then replaced with HFD. Subcutaneous injections of meloxicam (Metacam; Boehringer Ingelheim Vetmedica, Inc; 0.25 mg/100 g BW, once daily for 4 days), gentamicin (0.8 mg/100 g BW on the day of surgery), buprenorphine (Buprenex; Reckitt Benckiser Pharmaceuticals; 0.3 mL twice per day for 5 days), and warm saline (10 and 5 mL twice per day for days 0–3 and 4–5, respectively) were given to all rats postoperatively. A wire grate was used for 3 days postoperatively to prevent rats from eating their bedding.
Body composition measurements.
Whole-body composition (fat and lean mass) was measured using noninvasive NMR technology (EchoMRI, Houston, TX).
Hormone measurements.
Plasma ghrelin was determined using the Meso Scale Discovery Assay System (Meso Scale Discovery, Gaithersburg, MD). Plasma insulin and peptide YY (PYY) were analyzed individually by ELISA assay (#90060; Crystal Chem Inc., Downers Grove, IL; 48-PYYRT-E01.1; ALPCO Diagnostics, Salem, NH). Blood glucose was determined with a TheraSense Freestyle Glucometer (Abbott Diabetes Care, Inc., Alameda, CA).
Brain tissue preparation and immunohistochemistry.
Ninety minutes after inflation, rats were deeply anesthetized with sodium pentobarbital, perfused transcardially with saline, followed by a solution of 4% paraformaldehyde in 0.1 mol/L PBS (pH 7.4) at 4°C. The brains remained in fixative at 4°C overnight, then equilibrated 48 h with 30% sucrose in 0.1 mol/L Tris-buffered saline (TBS; pH 7.2). Serial coronal sections (30 µm) were cut on a cryostat. Immunohistochemistry of c-Fos protein in brain section was processed according to the method we described before (16). Images were then collected, and cell counting was manually performed within a fixed frame outlining the nucleus tractus solitarius (NTS) along its rostral (bregma, 13.30 mm), middle (bregma, 13.68 mm), caudal (bregma, 14.08 mm) axis. Two to six sections per bregma level were counted for each animal (n = 4–6 rats per group).
Peptides.
Exendin-4 (Ex4) was obtained from American Peptide (Sunnyvale, CA). GLP-1 (7-36)amide was obtained from Polypeptide Group (San Diego, CA). Rimonabant (SR141716) was kindly donated by the National Institute of Mental Health chemical synthesis and drug supply program.
Statistical analysis.
All data are represented as mean and SEM. Nonlinear third-order polynomial best-fit, one-way, and two-way ANOVA with Bonferroni multiple comparison posttest or Student t test were performed where appropriate using GraphPad Prism software (GraphPad, San Diego, CA). In all cases, statistical significance was assumed when P < 0.05.
RESULTS
Development and characterization of a rat AGB procedure.
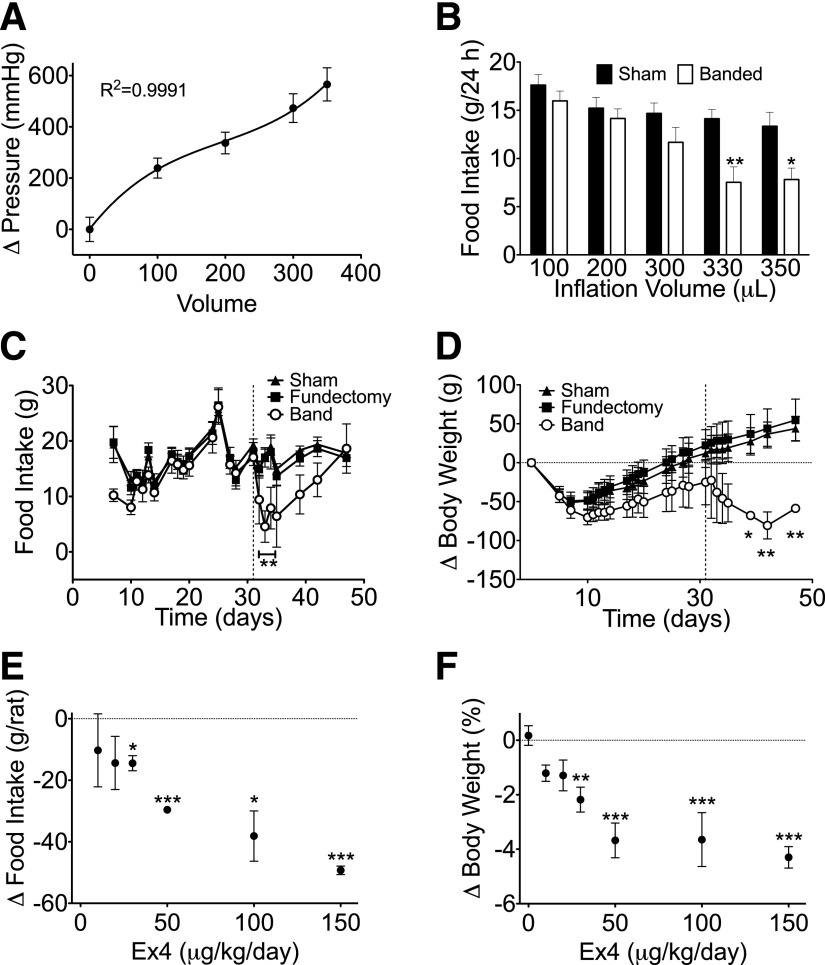
To evaluate the properties of our AGB, we first measured internal band pressure with respect to inflation volume. As expected, increasing inflation volumes of glyerol resulted in increased internal band pressure (Fig. 2A) and was best described by a nonlinear, third-order polynomial best fit (R2 = 0.9991). This model represents a high-pressure/low-volume adjustable band with a very steep linear region of pressure versus inflation (as reviewed in Ref. 17). To assess the optimal pressure/inflation, bands in a cohort of AGB rats were inflated at increasing volumes for 5 days. Twenty-four-hour food intake in these rats was then compared with that of age- and weight-matched sham animals. Consistent with increased restriction, 24-h food intake was reduced in association with the increased band pressure (Fig. 2B). Subsequent long-term studies were conducted to evaluate the effect of chronic AGB inflation. These studies included an additional control group to test any potential effect of the fundectomy on energy balance. Although this procedure involved removal of a considerable portion of the stomach, we identified similar 24-h food intake and BW response in all groups during the 31-day recovery period in which bands were left uninflated (Fig. 2C). Bands were then inflated with increasing volumes on 3 sequential days (days 31–33), resulting in a final volume of 350 μL. Following the final inflation, bands were left in the inflated state, and food and BW was measured. The inflation reduced food intake for 2 days (days 33 and 34) with a return to normal feeding thereafter (Fig. 2C). This rebound is likely attributed to loss of band pressure, as the volume removed from bands 3 days after inflation was often decreased 10–40 μL (data not shown). Although transient, the decrease in food intake stimulated BW loss in AGB rats as compared with rats with either a fundectomy or sham procedure (Fig. 2D).
FIG. 2.
Internal pressure (n = 12 rats) (A) and 24-h food (n = 12–19 rats) intake (B) with respect to inflation volume in DIO Long-Evans rats. Food intake (C) and change in BW (D) following band inflation (day 30) in DIO Long-Evans rats (n = 4 to 5 rats). Suppression of food intake (as compared with vehicle-injected controls [E] and change in BW [F]) in response to Ex4 (0–150 μg/kg/day) in DIO, Long-Evans rats (n = 3 to 4 rats). All data represented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
Administration of a GLP-1R agonist enhances the effect of gastric banding.
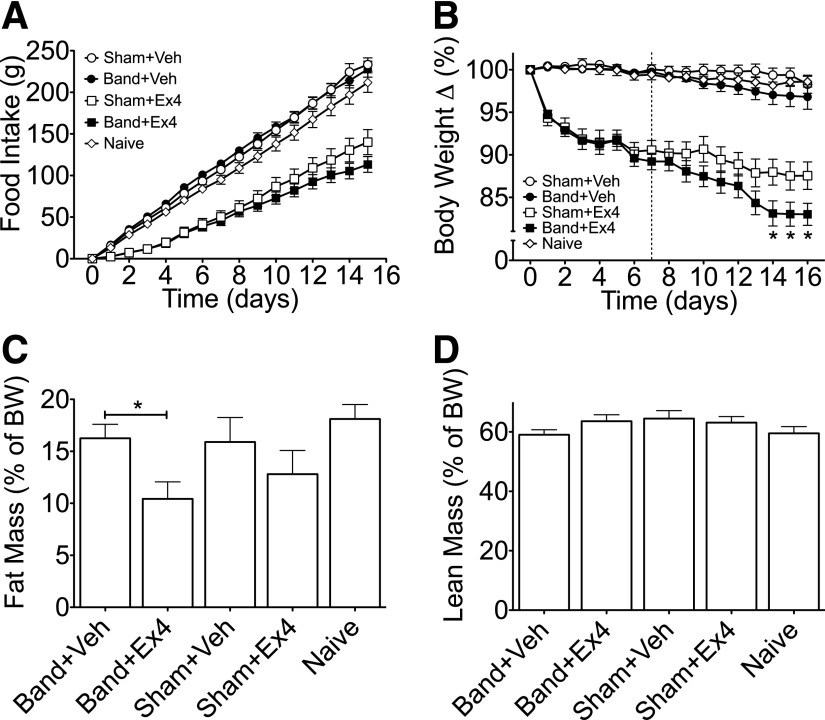
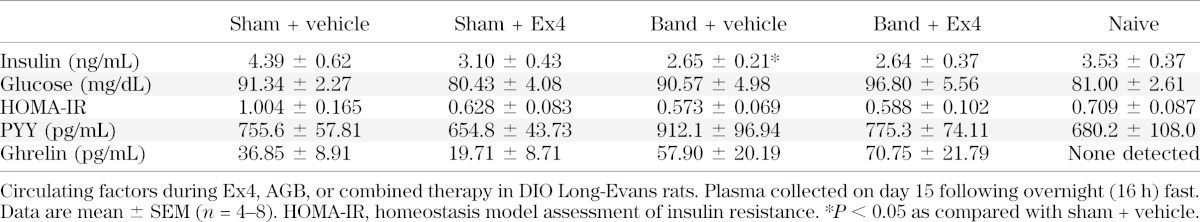
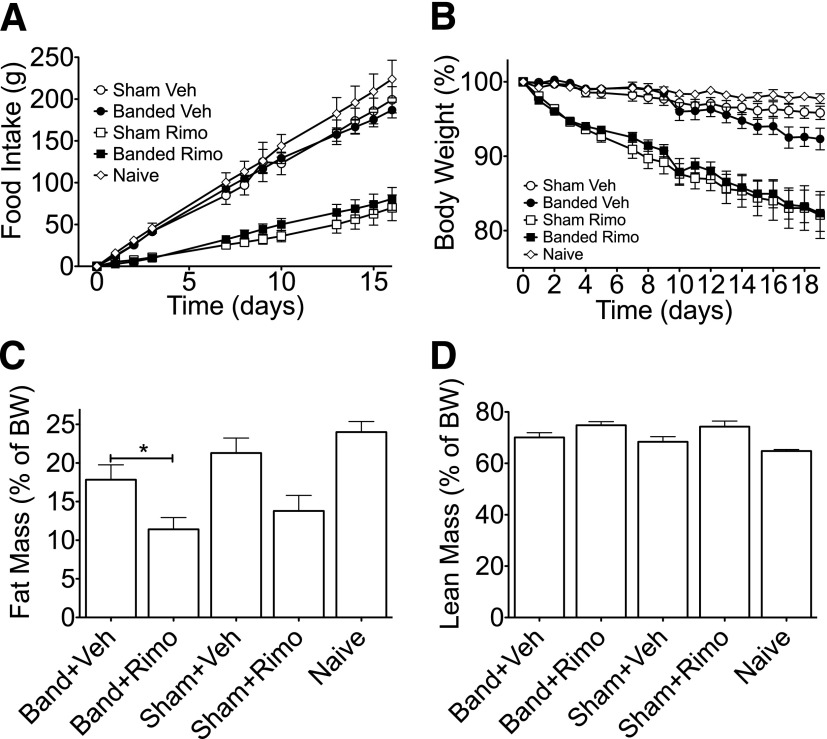
Accumulating data suggest that GLP-1 signaling plays an important role in RYGB-stimulated metabolic improvements. Thus, we hypothesized that administration of a GLP1-R agonist would increase the efficacy of less invasive bariatric interventions. To test this hypothesis, we conducted either AGB surgery or a sham procedure in DIO Long-Evans rats. Following a 3- to 4-week recovery period, rats from both groups were matched for BW and fat mass. In addition, a matched cohort of rats that received no intervention (naive) was included in the study. A pilot 6-day study in DIO rats indicated that with regard to suppression of food intake and BW, maximal efficacy with minimal variability is obtained using Ex4 at a dose of 150 µg/kg/day (Fig. 2E and F). Thus, rats from both surgical groups were then treated with Ex4 (150 µg/kg/day) or vehicle (saline) for 16 days. Ex4 treatment elicited a similar decrease in food intake and BW over the first 7 days, regardless of surgical procedure (Fig. 3A and B, respectively). Beginning on the seventh day of treatment, all rats were anesthetized (5 min isoflurane), and bands in those from the AGB group were filled with 100 µL of a 10% glycerol solution. The inflation was repeated under isoflurane anesthetization over the next 2 days to reach a total volume of 300 µL in AGB rats. Initial studies determined this inflation value to be subthreshold in terms of food intake (Fig. 2B). Thus, we were able to test the interaction between pharmacological weight loss and mild levels of restrictive intervention. To ensure full inflation throughout the study, the bands were emptied and reinflated under isoflurane anesthetization on day 12. Band inflation had no significant effect on food intake or BW in vehicle-treated animals compared with either naive rats or sham-operated rats treated with vehicle (Fig. 3A and B, respectively). However, food intake at day 15 was decreased to a similar degree in both Ex4-sham and Ex4-AGB rats as compared with sham-operated rats (Fig. 3A). Although food intake was similar, a greater loss of BW was observed in Ex4-AGB rats compared with Ex4-treated, sham-operated animals (82.2 vs. 87.5%) (Fig. 3B). This decrease in BW was associated with a decrease in adiposity in rats with combined band and Ex4 therapy as compared with band-vehicle–treated rats (Fig. 3C), with a similar trend observed between Ex4-AGB rats and Ex4-treated, sham-operated animals. In contrast, lean mass was similar between groups (P = 0.34; Fig. 2D). All rats were fasted overnight (18 h) on day 16, and plasma was collected in the fasted state. Analyses of these samples suggest that neither banding nor Ex4 treatment had a significant effect on blood glucose, PYY, ghrelin, or insulin resistance as determined by homeostasis model assessment of insulin resistance (Table 1). However, there was a decrease in fasting insulin in response to banding (sham-vehicle vs. band-vehicle) that is likely due to the decrease in adiposity (Table 1). Taken together, these data suggest that Ex4 enhances AGB-stimulated BW loss in DIO rats, and this BW loss is independent of changes in circulating ghrelin or PYY.
FIG. 3.
Food intake (A), relative BW loss (B), and body composition (relative fat [C] and lean mass [D]) during Ex4 (squares), AGB (closed symbols), or combined therapy (closed squares) in DIO, Long-Evans rats. Ex4 treatment (150 μg/kg/day) administered days 0–15, AGB inflated days 6–8, and reinflated day 12. All data represented as mean ± SEM in n = 8–15 rats. *P < 0.05.
TABLE 1.
Circulating factors in fasted AGB rats
CB1 receptor antagonism does not enhance the effect of gastric banding.
The effect of a GLP-1R agonist on weight loss following subthreshold restriction via AGB highlights a potential role for existing pharmacotherapy for obesity to augment the efficacy of less-invasive surgical interventions. To test the hypothesis that collectively, anorectic signals have synergistic therapeutic potential, we repeated the previous study using rimonabant, a known antagonist of central but not peripheral cannabinoid receptor-1 (CB1-R) (18). Antagonism of the cannabinoid system, and specifically CB1-R, inhibits food intake and reduces BW (19). Rats from sham and AGB groups were matched for BW and fat mass, and animals from both surgical groups were then treated with rimonabant (SR141716; 10 mg/kg/day) or vehicle (10% DMSO and 20% Tween-80 in saline) for 19 days. As with the Ex4 treatment, rimonabant elicited an ∼10% decrease in BW over the first 7 days (Fig. 4B). This weight loss was associated with a substantial inhibition of food intake, and both effects were independent of the surgical procedure (Fig. 4A). Beginning on the seventh day of treatment, all rats were anesthetized (5 min isoflurane), and bands in those from the AGB group were filled with 100 µL of a 10% glycerol solution. The inflation was repeated under isoflurane anesthetization over the next 2 days to reach a total volume of 300 µL in AGB-operated rats. To ensure full inflation throughout the study, the bands were emptied and reinflated under isoflurane anesthetization on day 13. Unlike the effects observed with the combination of Ex4 and AGB (Fig. 3), the addition of mild gastric restriction to rimonabant treatment had no effect on either food intake or BW (Fig. 4A and B). Consistent with its effect on BW, rimonabant treatment stimulated a significant decrease in fat mass (Fig. 4C). AGB also decreased fat mass in vehicle-treated rats during the 16-day study (Fig. 4C). This observation was intriguing, as there was a trend toward loss of BW in these animals that failed to reach significance (Fig. 4B). Furthermore, although each of the interventions stimulated loss of fat mass, the combination provided no additional benefits (Fig. 4C). Consistent with the observations in the combination of Ex4 and AGB, lean mass was similar among all groups following band or rimonabant treatment (Fig. 4D). Together, these observations suggest that some, but not all, anorectic signals can synergistically enhance AGB therapy.
FIG. 4.
Food intake (A), relative BW loss (B), and body composition (relative fat [C] and lean mass [D]) during rimonabant (Rimo; squares), AGB (closed symbols), or combined therapy (closed squares) in DIO, Long-Evans rats. Rimonabant treatment (10 mg/kg/day) administered days 0–18, AGB inflated days 7–9, and reinflated day 13. All data represented as mean ± SEM in n = 7–13 rats. *P < 0.05.
AGB activates neurons in the nucleus of the solitary tract.
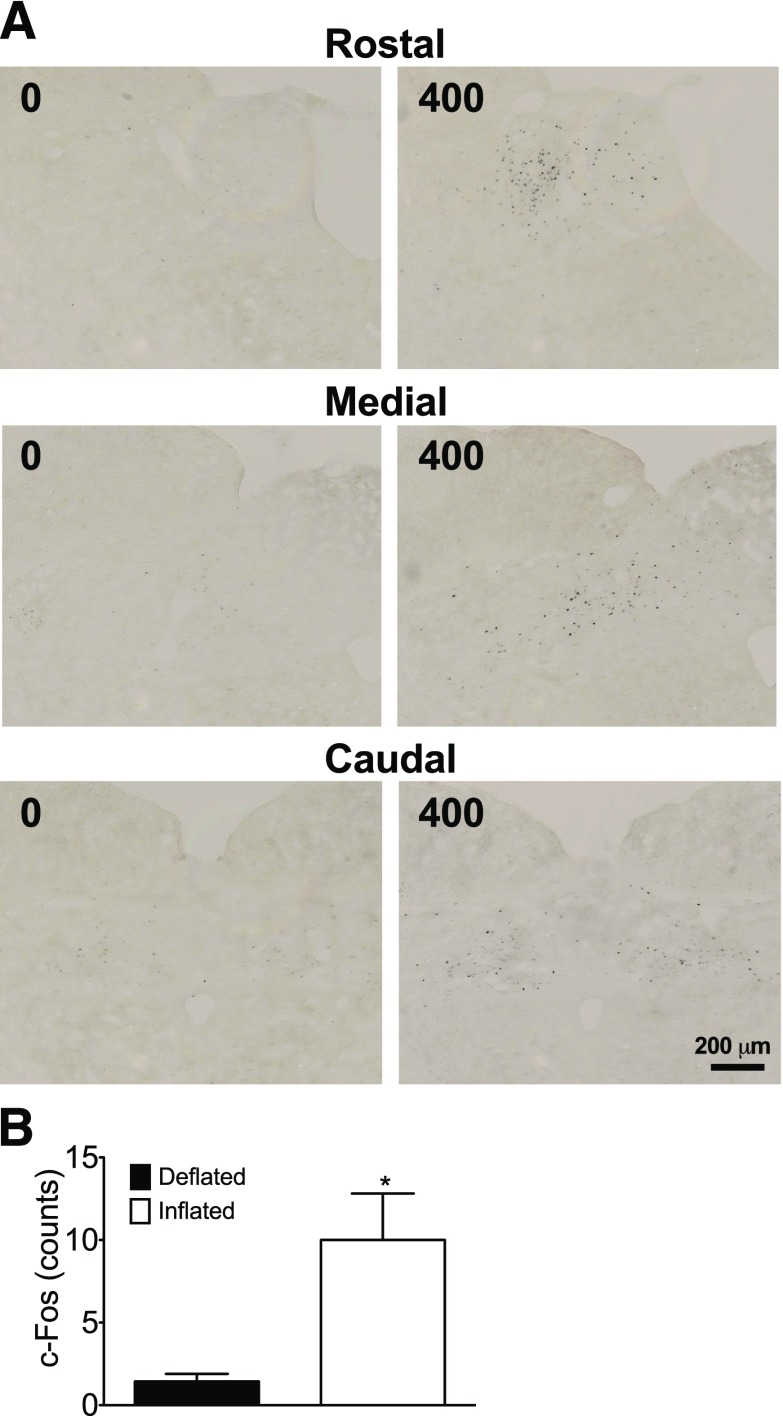
While the physical nature of the AGB imparts direct restriction of gastric volume, it is likely that this manipulation also induces a central nervous system (CNS)-mediated signal. To assess whether AGB-stimulated gastric restriction could be conveyed to the visceral sensory neurons in the NTS, the neuronal activation marker c-Fos was examined in rats with deflated or fully inflated (400 μL) bands. In rats with deflated bands, very few c-Fos–immunoreactive nuclei were observed in any part of the NTS, whereas after 90 min of full inflation, c-Fos–immunoreactive nuclei could be observed in multiple areas of the NTS along the rostrocaudal axis (Fig. 5A). The majority of these nuclei were distributed in the medial, lateral, and central subnuclei, with very few observed in the commissural regions of the NTS (Fig. 5A). Quantification of these nuclei revealed an approximately sevenfold increase in c-Fos signal after inflation (Fig. 5B). These data suggest that acute inflation of the AGB leads to a specific pattern of neuronal activation in visceral-sensing neurons of the NTS reminiscent of the key neuronal circuits controlling energy balance, body adiposity, and glucose metabolism.
FIG. 5.
c-Fos–immunoreactive nuclei of the NTS 90 min after maximal (400 μL) band inflation (A and B: scale bar, 200 μm). All data represented as mean ± SEM in n = 4 to 5 rats. *P < 0.05.
DISCUSSION
This study details the development and characterization of an AGB procedure and, for the first time, its combination with specific pharmacotherapies in a rat model of DIO and insulin resistance. Consistent with its known clinical effects, this model was successfully used to reduce food intake in a pressure-dependent manner. These data also show that treatment with a GLP-1R agonist can be used to enhance the efficacy of AGB, a less invasive but also less effective approach than RYGB. These findings support the use of specific surgical-medical combinations for obesity and raise the possibility that approaches of this kind could be tailored to the needs of individual patients.
Elevated plasma levels of GLP-1 have been described following RYGB (9), vertical sleeve gastrostomy (20), ileal transposition (21), and duodenal-jejunal bypass (22). However, these procedures are all highly invasive and not reversible. Turning a less invasive and reversible bariatric intervention into a comparably beneficial therapeutic approach by adding pharmacotherapy would therefore be a promising additional tool in the fight against obesity and related disorders.
We found that a GLP-1R agonist not only increased the efficacy of AGB to produce weight loss but also reduced the band pressure required to do so. This effect may be specific to GLP-1 agonists, since treatment with the CB1 antagonist rimonabant, which is known to elicit a decrease in food intake, BW, and fat mass in rodents and man (19), failed to enhance AGB. Thus, combining GLP-1R agonists with AGB may represent a unique noninvasive strategy to improve AGB efficacy. Such an approach would provide long-term benefits for patients, as tighter bands can lead to greater food intolerance, vomiting, and greater incidence of stomach erosions, a primary cause of AGB revisions and removals (23).
It should also be noted that this procedure in the rat model involves removal of the proximal gastric region (fundus). Following ingestion of a meal, relaxation of the fundus allows the gastric volume to increase in a process called gastric accommodation (24). Thus, it is not surprising that this procedure can reduce food intake and delay normal growth in rats (25). However, an important difference between our study and this previous report is that our mice were DIO at the time of surgery and maintained on an HFD throughout the studies. It is likely that the caloric density of the diet negated any loss of gastric accommodation. Furthermore, in our procedure, we were careful to maintain all of the glandular stomach, while Axelson et al. (25) intentionally removed acid-producing regions, as well as the nonglandular stomach.
The specific effect of a GLP-1 agonist on AGB efficacy may result from an interaction between CNS circuits regulating appetite and systemic metabolism. Ingestion of food elicits a gastrointestinal response that is conveyed to the brain via both hormonal and afferent vagal activity (26). The lack of differential response in known hormonal components of this regulatory pathway (summarized in Table 1) suggests that this regulation is primarily a vagal-mediated event. The first relay along this pathway is in the brainstem, where, after the central processing and integration of this afferent input, the visceral sensory neurons locate efferent outputs. Somatic and visceral efferent nerves are then used to control ingestive behavior (27). Within the brainstem, the NTS is the first relay area in the central nervous system for sensory stimuli originating from the digestive tract. Emerging evidence confirms that sensory neurons of the gastroesophageal junction project to neurons of the NTS, the arcuate nucleus, and the paraventricular nucleus of the hypothalamus (8). Thus, the NTS could function as an integration point to regulate appetite and systemic metabolism following bariatric surgery and pharmacological intervention.
Consistent with the report of Kampe et al. (16), we observed significant c-Fos induction in the NTS following AGB inflation. This outcome suggests that AGB reduces feeding via two distinct mechanisms. Firstly, it restricts gastric distention and subsequent food volume. Secondly, mechanical pressure from inflation appears to be transmitted to CNS circuits implicated in feeding. While we did not directly assess the c-Fos response in the presence of both a meal and an inflated band, these circuits are also known to respond to GLP-1 activation (28) and provide a logical mechanistic basis for the synergistic effects between AGB and a GLP-1 agonist. Together, these inputs can act at both physiological and behavioral levels to restrict food intake.
Although we were unable to define the underlying mechanism describing the induction of c-Fos and its role in the enhanced therapeutic benefit of GLP-1R agonism in AGB, these observations lead us to several intriguing hypotheses: 1) Elevated c-Fos protein has recently been observed in the NTS following acute or chronic band inflation (8). Interestingly, the NTS is one of the areas of the CNS known to produce GLP-1 (29). Recent studies suggest that these neurons project to the arcuate nucleus (30) and dorsomedial nucleus of the hypothalamus (DMHv) and may be responsible for transmitting satiety signals from the NTS via activation of GLP-1 receptor of the DMHv (31). Thus, Ex4 therapy may enhance actions of the AGB by increasing overall GLP-1 receptor occupancy in the DMHv. 2) GLP-1R populate the NTS (32) and GLP-1R agonism via hindbrain intracerebroventricular (fourth intracerebroventricular) delivery is sufficient to suppress food intake in rats (28). Thus, activation of NTS neurons following AGB inflation may be indicative of increased GLP-1R sensitivity.
In summary, the current study in a rodent model of DIO indicates that Ex4, but not the CB1 antagonist rimonabant, acts in concert with AGB to improve weight loss and metabolic outcomes, possibly via a mechanism involving activation of NTS neurons. Taken together, our results suggest that the GLP-1 system may offer untapped potential for pharmaco-surgical combination therapies for the treatment of T2D and obesity.
ACKNOWLEDGMENTS
This work was supported by grant R01-DK-077975 from the National Institutes of Health, Neuroendocrine Regulation of Adipocyte Metabolism (D.P.-T.), the University of Cincinnati Training Program in Neuroendocrinology of Homeostasis grant T32-DK-059803 (K.M.Ha.), the Netherlands Organization for Scientific Research Rubicon grant (C.-X.Y.), and Ethicon Endo-Surgery. D.P.-T., R.D.D., and M.H.T. have a collaborative association with Roche Research Laboratories pertaining to peptide-based therapeutics in metabolism. M.H.T. receives research funds from and is a consultant for Roche Pharmaceuticals. R.J.S. is a consultant for Ethicon Endo-Surgery, Novo Nordisk, Novartis, Angiochem, Zafgen, Takeda, Zealand Pharma, and Eli Lilly; receives research support from Ethicon Endo-Surgery, Novo Nordisk, Ablaris, Zealand Pharma, and Pfizer; is a paid speaker for Ethicon Endo-Surgery, Novo Nordisk, Merck, and Pfizer; and holds equity in Zafgen. D.A.D. is a consultant for Amylin Pharmaceuticals, Eli Lilly, Novo Nordisk, and Zealand Pharma. No other potential conflicts of interest relevant to this article were reported.
Ethicon Endo-Surgery and its employees had no role in designing or conducting these studies.
K.M.Ha. and M.H.T. were responsible for study conception and design, data analyses and interpretation, and drafting the article. H.K., K.M.He., D.S., N.O., J.H., S.A., C.R., and R.K. acquired data. C.-X.Y. advised on the study concept, acquired data, and critically revised the article. T.D.M., D.P.-T., P.T.P., S.O., R.D.D., D.A.D., and R.J.S. advised on the study concept and critical revision of the article. M.H.T. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011, and at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
REFERENCES
- 1.Kopelman PG. Obesity as a medical problem. Nature 2000;404:635–643 [DOI] [PubMed] [Google Scholar]
- 2.Sarwer DB, von Sydow Green A, Vetter ML, Wadden TA. Behavior therapy for obesity: where are we now? Curr Opin Endocrinol Diabetes Obes 2009;16:347–352 [DOI] [PubMed] [Google Scholar]
- 3.Piya MK, Tahrani AA, Barnett AH. Emerging Treatment Options for Type 2 Diabetes. Br J Clin Pharmacol. Br J Clin Pharmacol. 4 August 2010 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinbrook R. Surgery for severe obesity. N Engl J Med 2004;350:1075–1079 [DOI] [PubMed] [Google Scholar]
- 5.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 2009;122:248–256.e245 [DOI] [PubMed] [Google Scholar]
- 6.Franco JV, Ruiz PA, Palermo M, Gagner M. A review of studies comparing three laparoscopic procedures in bariatric surgery: sleeve gastrectomy, Roux-en-Y gastric bypass and adjustable gastric banding. Obes Surg 2011;21:1458–1468 [DOI] [PubMed] [Google Scholar]
- 7.Dixon JB, Murphy DK, Segel JE, Finkelstein EA. Impact of laparoscopic adjustable gastric banding on type 2 diabetes. Obes Rev 2012;13:57–67 [DOI] [PubMed] [Google Scholar]
- 8.Kampe J, Stefanidis A, Lockie SH, et al. Neural and humoral changes associated with the adjustable gastric band: insights from a rodent model. Int J Obes (Lond) 2012;36:1403–1411 [DOI] [PubMed] [Google Scholar]
- 9.Shin AC, Zheng H, Townsend RL, Sigalet DL, Berthoud HR. Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology 2010;151:1588–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.le Roux CW, Aylwin SJ, Batterham RL, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg 2006;243:108–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blonde L, Klein EJ, Han J, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab 2006;8:436–447 [DOI] [PubMed] [Google Scholar]
- 12.Astrup A, Carraro R, Finer N, et al. NN8022-1807 Investigators . Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond) 2012;36:843–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wickremesekera K, Miller G, Naotunne TD, Knowles G, Stubbs RS. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg 2005;15:474–481 [DOI] [PubMed] [Google Scholar]
- 14.Cummings DE, Overduin J, Foster-Schubert KE. Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab 2004;89:2608–2615 [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Zhou Y, Wang Y, Geng D, Liu J. Roux-en-Y gastric bypass-induced improvement of glucose tolerance and insulin resistance in type 2 diabetic rats are mediated by glucagon-like peptide-1. Obes Surg 2011;21:1424–1431 [DOI] [PubMed] [Google Scholar]
- 16.Kampe J, Brown WA, Stefanidis A, Dixon JB, Oldfield BJ. A rodent model of adjustable gastric band surgery-implications for the understanding of underlying mechanisms. Obes Surg 2009;19:625–631 [DOI] [PubMed] [Google Scholar]
- 17.Fried M. The current science of gastric banding: an overview of pressure-volume theory in band adjustments. Surg Obes Relat Dis 2008;4(Suppl.):S14–S21 [DOI] [PubMed] [Google Scholar]
- 18.Rinaldi-Carmona M, Barth F, Héaulme M, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett 1994;350:240–244 [DOI] [PubMed] [Google Scholar]
- 19.Cota D, Sandoval DA, Olivieri M, et al. Food intake-independent effects of CB1 antagonism on glucose and lipid metabolism. Obesity (Silver Spring) 2009;17:1641–1645 [DOI] [PubMed] [Google Scholar]
- 20.Chambers AP, Stefater MA, Wilson-Perez HE, et al. Similar effects of roux-en-Y gastric bypass and vertical sleeve gastrectomy on glucose regulation in rats. Physiol Behav 2011;105:120–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohli R, Kirby M, Setchell KD, et al. Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities. Am J Physiol Gastrointest Liver Physiol 2010;299:G652–G660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kindel TL, Yoder SM, Seeley RJ, D’Alessio DA, Tso P. Duodenal-jejunal exclusion improves glucose tolerance in the diabetic, Goto-Kakizaki rat by a GLP-1 receptor-mediated mechanism. J Gastrointest Surg 2009;13:1762–1772 [DOI] [PubMed] [Google Scholar]
- 23.Busetto L, Segato G, De Marchi F, et al. Postoperative management of laparoscopic gastric banding. Obes Surg 2003;13:121–127 [DOI] [PubMed] [Google Scholar]
- 24.Azpiroz F, Malagelada JR. Physiological variations in canine gastric tone measured by an electronic barostat. Am J Physiol 1985;248:G229–G237 [DOI] [PubMed] [Google Scholar]
- 25.Axelson J, Persson P, Gagnemo-Persson R, Håkanson R. Importance of the stomach in maintaining calcium homoeostasis in the rat. Gut 1991;32:1298–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 2000;404:661–671 [DOI] [PubMed] [Google Scholar]
- 27.Smith GP. The direct and indirect controls of meal size. Neurosci Biobehav Rev 1996;20:41–46 [DOI] [PubMed] [Google Scholar]
- 28.Hayes MR, Leichner TM, Zhao S, et al. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab 2011;13:320–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 1997;77:257–270 [DOI] [PubMed] [Google Scholar]
- 30.Yi CX, van der Vliet J, Dai J, Yin G, Ru L, Buijs RM. Ventromedial arcuate nucleus communicates peripheral metabolic information to the suprachiasmatic nucleus. Endocrinology 2006;147:283–294 [DOI] [PubMed] [Google Scholar]
- 31.Renner E, Puskás N, Dobolyi A, Palkovits M. Glucagon-like peptide-1 of brainstem origin activates dorsomedial hypothalamic neurons in satiated rats. Peptides 2012;35:14–22 [DOI] [PubMed] [Google Scholar]
- 32.Göke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci 1995;7:2294–2300 [DOI] [PubMed] [Google Scholar]