Abstract
Angiotensin receptor blockers are renoprotective in hypertensive azotemic patients with type 2 diabetes, but their efficacy in early diabetic kidney disease is uncertain. We performed a 6-year randomized clinical trial in 169 American Indians with type 2 diabetes and normoalbuminuria (albumin/creatinine ratio [ACR] <30 mg/g; n = 91) or microalbuminuria (ACR 30–299 mg/g; n = 78) at baseline. The primary outcome was decline in glomerular filtration rate (GFR) to ≤60 mL/min or to half the baseline value in subjects who entered with GFR <120 mL/min. Another outcome was differences in glomerular structure at end of treatment. Subjects received 100 mg losartan or placebo daily. GFR was measured annually; 111 subjects underwent kidney biopsies. Only nine subjects reached the GFR outcome, and the unadjusted hazard ratio (losartan vs. placebo) was 0.50 (95% CI, 0.12–1.99). Differences in mesangial fractional volume were not estimated in the combined albuminuria groups because of an interaction with treatment assignment. In separate analyses, mesangial fractional volume was lower in subjects treated with losartan in the microalbuminuria group (18.8 vs. 25.6%; P = 0.02), but not in the normoalbuminuria group (19.6 vs. 17.8%; P = 0.86). Treatment with losartan may preserve some features of kidney structure in American Indians with type 2 diabetes and microalbuminuria.
Angiotensin receptor blockers (ARBs) reduce the rate of diabetic kidney disease progression in hypertensive azotemic patients with type 2 diabetes (1,2). Their efficacy in slowing progression of early kidney disease, however, is less certain, and surrogate end points, such as albuminuria progression, complicate interpretation of most studies (3). In the Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria (IRMA 2) study (4), frequency of progression to macroalbuminuria was reduced by the study drug, but decline in glomerular filtration rate (GFR), as estimated by creatinine clearance, was not. Similarly, in the Steno-2 study (5), intensive multifactorial intervention in patients with type 2 diabetes and microalbuminuria significantly reduced progression to proteinuria but did not alter the rate of GFR decline.
Efficacy of ARBs in the primary prevention of diabetic kidney disease is less certain. The Renin Angiotensin System Study (RASS) (6), a clinical trial of losartan or enalapril versus placebo in patients with type 1 diabetes and normoalbuminuria, found no benefit of treatment over 5 years in either change in GFR or change in mesangial fractional volume of serial kidney biopsies. Moreover, the 5-year cumulative incidence of microalbuminuria was higher in those receiving losartan than in either of the other groups, suggesting the potential for harm.
We performed a randomized, placebo-controlled, clinical trial to test whether the ARB losartan offered renoprotection to persons with type 2 diabetes who had either normal urinary albumin excretion or microalbuminuria at baseline. Clinical outcomes included changes in GFR from baseline and differences in glomerular structure on kidney biopsies performed at the end of the study period. We also examined the effect of losartan on urinary albumin excretion.
RESEARCH DESIGN AND METHODS
Study design.
A planned 6-year, single-center, randomized, double-blind, placebo-controlled, clinical trial compared the effect of losartan (Cozaar; Merck) versus placebo on development and progression of diabetic nephropathy in type 2 diabetes. Subjects were stratified at baseline by albumin excretion category (normoalbuminuria: albumin/creatinine ratio [ACR] <30 mg/g; microalbuminuria: ACR 30–299 mg/g) based on the geometric mean of three screening measurements and were allocated randomly to receive either losartan or placebo within each category. The prespecified primary study outcome was a decline in GFR to ≤60 mL/min or to half the baseline value in subjects who entered the study with GFR <120 mL/min. Another study outcome was the difference between treatment groups in predefined glomerular structural variables measured on kidney biopsy samples obtained at the end of treatment. Subjects were enrolled between 1996 and 2001, the last biopsy was performed in 2007, and morphometric evaluation was completed in 2012. Progression to macroalbuminuria (ACR ≥300 mg/g) also was examined as an outcome.
Treatment group was assigned by computer-generated random blocks of <10 subjects stratified by albuminuria category. Treatment with losartan began at 50 mg daily, with the dose increasing to 100 mg daily after 1 week if symptomatic hypotension did not develop. A placebo corresponding to each dose of losartan was supplied. Compliance was assessed by pill counts, and subjects were considered compliant if ≥50% of study drug was used. Clinical care was provided outside the study in accordance with existing clinical care guidelines while avoiding the use of ACE inhibitors or ARBs for management of hypertension. In 2000, changes in standards of care and new evidence of cardiovascular protection by treatment with ACE inhibitors in similar patients threatened continuation of the study, because treatment with either an ACE inhibitor or an ARB was recommended for patients with diabetes and microalbuminuria (7,8). Accordingly, the protocol was modified to suggest that primary care providers consider adding inhibitors of the renin-angiotensin-aldosterone system (RAAS) to the masked treatment regimen of study subjects.
Women were tested quarterly for pregnancy. Each woman with childbearing potential was instructed to stop the study drug if she thought she might be pregnant and to notify the study team for confirmatory testing. If a woman became pregnant while using treatment, then the treatment was withheld. If requested by the woman or her health care provider, then the treatment code was broken and the subject was informed whether she was receiving active drug or placebo. GFR was not measured in women who were pregnant. In those who remained blinded to treatment category, both treatment and GFR measurements were resumed 3 months postpartum or after completion of breastfeeding, whichever was later. In the one patient who was unblinded, unmasked treatment with losartan was reinitiated.
Losartan and placebo were provided by Merck. The study was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases and was overseen by a safety monitoring board. Each subject gave informed consent at each renal clearance study and before kidney biopsy.
Study subjects.
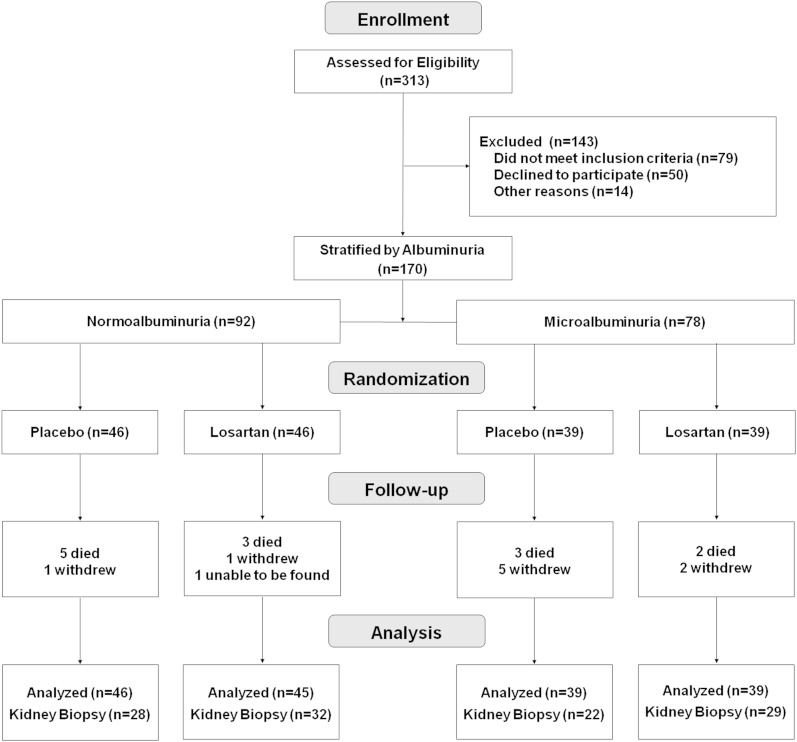
Between 1965 and 2007, American Indians from the Gila River Indian Community participated in a longitudinal study of diabetes and its complications. Participants in the present trial were selected from this cohort. Eligible participants included those between 18 and 65 years old who had type 2 diabetes for at least 5 years, serum creatinine concentration <120 μmol/L (<1.4 mg/dL), serum potassium concentration ≤5.5 mEq/L, and ACR <300 mg/g on at least two of three occasions at least 1 week apart within 3 months of enrollment. Exclusion criteria included uncontrolled hypertension, pregnancy, nondiabetic kidney disease, bleeding disorders that would prevent performance of a kidney biopsy, and BMI ≥45 kg/m2. All potentially eligible members of the community were identified from the longitudinal study database and their medical records were reviewed to confirm eligibility. Of 313 patients with type 2 diabetes who were screened, 79 were ineligible, 50 declined to participate, and 14 had other reasons that prevented participation despite meeting eligibility requirements. The remaining 170 subjects were stratified into two groups, 92 with normal urinary albumin excretion and 78 with microalbuminuria. Within each stratum, subjects were assigned randomly to one of the two treatment groups (Fig. 1).
FIG. 1.
Enrollment, randomization, and follow-up of the study subjects. All subjects were included in intention-to-treat analyses, except one person in the normoalbuminuria group who was randomized to losartan but could not be found after the baseline examination.
Other measures.
BMI was defined as weight divided by the square of height (kg/m2). Mean arterial pressure (MAP) was calculated as MAP = two-thirds diastolic blood pressure + one-third systolic blood pressure. Hypertension was defined as systolic blood pressure ≥130 mmHg, diastolic blood pressure ≥80 mmHg, or treatment with antihypertensive medicine. Urinary albumin was measured by nephelometric immunoassay (9) and urinary creatinine by a modified Jaffé reaction (10). Urinary albumin concentrations below the threshold detected by the assay (≤6.8 mg/L) were set to 6.8 mg/L in the analyses. HbA1c was measured by high-performance liquid chromatography. A high-performance liquid chromatography system also was used to measure iothalamate concentrations for GFR determination (11). Urine and serum samples were stored at −80°C until day of assay. Pill counts and measures of blood pressure and ACR were obtained 1 week after initiation of treatment and then quarterly. GFR was measured in the morning after an overnight fast at baseline, 1 month after initiation of treatment, and then annually by urinary clearance of iothalamate (11). ACR was measured at each of these clearance studies, and results from these ACR collections are reported.
Morphometric methods.
A subset of subjects underwent percutaneous kidney biopsy at the end of the treatment period to determine whether treatment with losartan was associated with structural differences. Baseline kidney biopsies were not performed because of safety concerns. Tissue was processed and embedded in epoxy resin (Epon 812) and prepared for microscopy as described previously (12). Light and electron microscopy were performed either in the Beckman Center for Electron Microscopy at Stanford University or in the Division of Nephrology at the University of Minnesota. Digital light and electron micrographs were used to make measurements using formal stereologic methods to account for two-dimensional sampling of three-dimensional objects (13). Predefined morphometric variables included glomerular volume, percent globally sclerotic glomeruli, fractional interstitial area, mesangial fractional volume, filtration surface area density, glomerular basement membrane width, number of endothelial cells, mesangial cells and podocytes per glomerulus, filtration slit frequency, and foot process width (12,14–16).
Statistical analysis.
Clinical features at baseline were compared between groups by ANOVA for normally distributed continuous variables, by the Kruskal-Wallis test for those with non-normal distributions, and by Fisher exact test for categorical variables. Compliance with pill usage by treatment group, the proportion of participants with hypertension by treatment group at baseline, and the proportion of miscarriages by treatment group also were assessed by Fisher exact test. Change in GFR 1 month after initiating treatment was examined by paired t tests. Mean GFR and blood pressure throughout the study were compared between treatment groups using mixed models to account for serial correlations over time. Times to outcomes according to treatment group were compared by product-limit survival curves and the log-rank test. Hazard ratios were computed using Cox proportional hazards regression. The exact Wilcoxon two-sample test was used to compare morphometric variables by treatment assignment; nominal P values were reported without adjusting for multiple comparisons. Effect of treatment assignment on mesangial fractional volume was further examined by linear regression after adjusting for duration of diabetes. Interaction between treatment assignment and albuminuria group in the morphometric analysis was tested using generalized additive models. All analyses were based on the intention-to-treat principle.
RESULTS
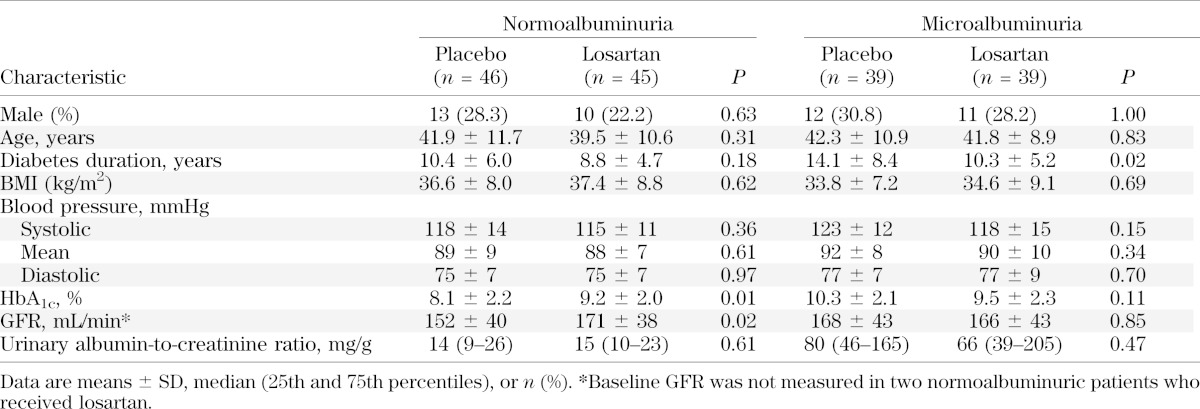
Of 170 subjects randomized, one had no follow-up measurements and was excluded from further analysis. The remaining 169 subjects had follow-up measurements of GFR to assess the primary GFR outcome and 111 had morphometric information from kidney biopsy to assess the structural outcome (Fig. 1). Nine subjects withdrew before their target completion dates. Baseline characteristics of the groups were similar, except for higher GFR (P = 0.02) and HbA1c (P = 0.01) in subjects with normoalbuminuria who received losartan, and longer duration of diabetes (P = 0.02) in subjects with microalbuminuria who received placebo (Table 1). Hypertension was present at baseline in 5 (5.5%) subjects with normoalbuminuria (2 of 45 in the losartan group, 3 of 46 in the placebo group; P = 1.00) and in 16 (20.5%) with microalbuminuria (9 of 39 in the losartan group, 7 of 39 in the placebo group; P = 0.78).
TABLE 1.
Baseline characteristics of the 169 subjects by albuminuria group and treatment group assignment
GFR was measured at randomization and again after 1 month to examine the acute effect of treatment. Eighty-two subjects in the placebo group (96.5%) and 77 in the losartan group (91.7%) completed both measurements. At one month, mean percent change (±SD) in GFR among subjects receiving losartan was −3.16 ± 16.48% vs. 0.59 ± 16.00% in the placebo-treated group (P = 0.15).
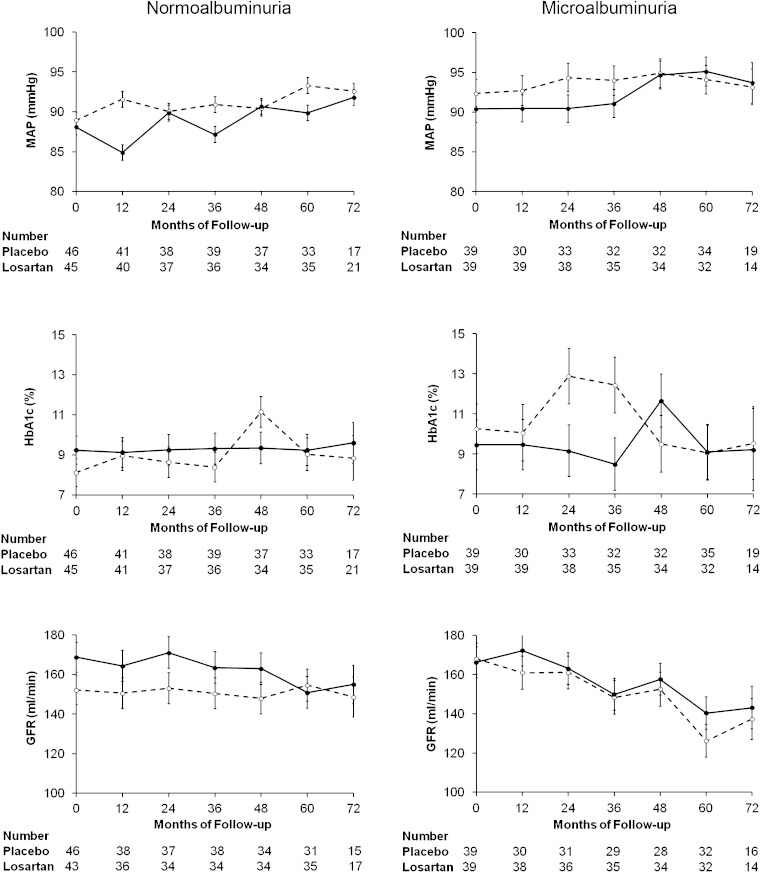
Patients were followed-up for a median of 5.9 years (interquartile range, 5.0–6.0 years). Mean MAP, HbA1c, and GFR throughout treatment are shown in Fig. 2. Mean MAP was similar throughout the study in subjects receiving either losartan or placebo in the normoalbuminuria (P = 0.21) and microalbuminuria (P = 0.73) groups, and increased modestly in both treatment groups as the study progressed. Mean HbA1c was higher at baseline (P = 0.01) in the normoalbuminuria group receiving losartan and generally converged during the study so that overall differences in HbA1c between the losartan and placebo groups throughout the study were not statistically significant (P = 0.48); mean HbA1c was similar at baseline (P = 0.11), and throughout the study (P = 0.43) in subjects receiving either losartan or placebo in the microalbuminuria group. Mean GFR was higher at baseline in subjects with normoalbuminuria who received losartan than in those who received placebo (P = 0.02) and was equivalent at baseline in both treatment groups in those with microalbuminuria (P = 0.85). Differences in mean GFR throughout the study according to treatment assignment were not statistically significant in either the normoalbuminuria (P = 0.11) or the microalbuminuria (P = 0.42) groups.
FIG. 2.
Annual mean ± standard error MAP (upper panels), HbA1c (middle panels), and GFR (lower panels) in subjects with normoalbuminuria (left panels) or microalbuminuria (right panels) at baseline by treatment group (dashed line = placebo; solid line = losartan). There were no significant differences in any of these measures by treatment group throughout the treatment period (for MAP, P = 0.21 in the normoalbuminuria group and P = 0.73 in the microalbuminuria group; for HbA1c, P = 0.48 in the normoalbuminuria group and P = 0.43 in the microalbuminuria group; and for GFR, P = 0.11 in the normoalbuminuria group and P = 0.42 in the microalbuminuria group).
Adherence was satisfactory; 68.2% of subjects in the losartan group and 72.6% in the placebo group used at least half of their study medicines. Adherence by treatment with losartan or placebo was similar in both the normoalbuminuria (32 of 45 in the losartan group, 29 of 46 in the placebo group; P = 0.51) and microalbuminuria (29 of 39 in the losartan group, 29 of 39 in the placebo group; P = 1.00) groups. Although 112 (66.3%) subjects also reported using other RAAS inhibitors during the study, overall exposure was limited because it occurred in only 19.6% of the total person-time under treatment; 52 of these subjects received dual blockade during a portion of the study because they were receiving losartan as the study drug.
Primary study outcome.
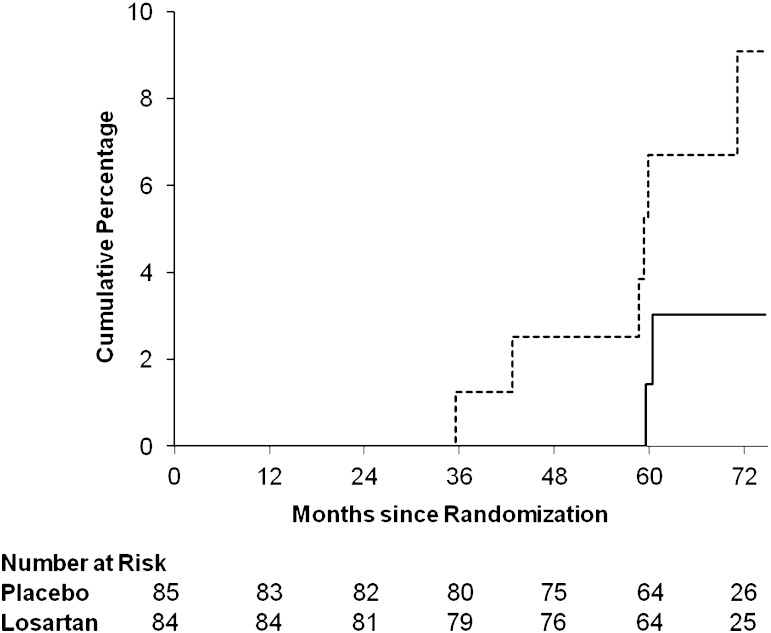
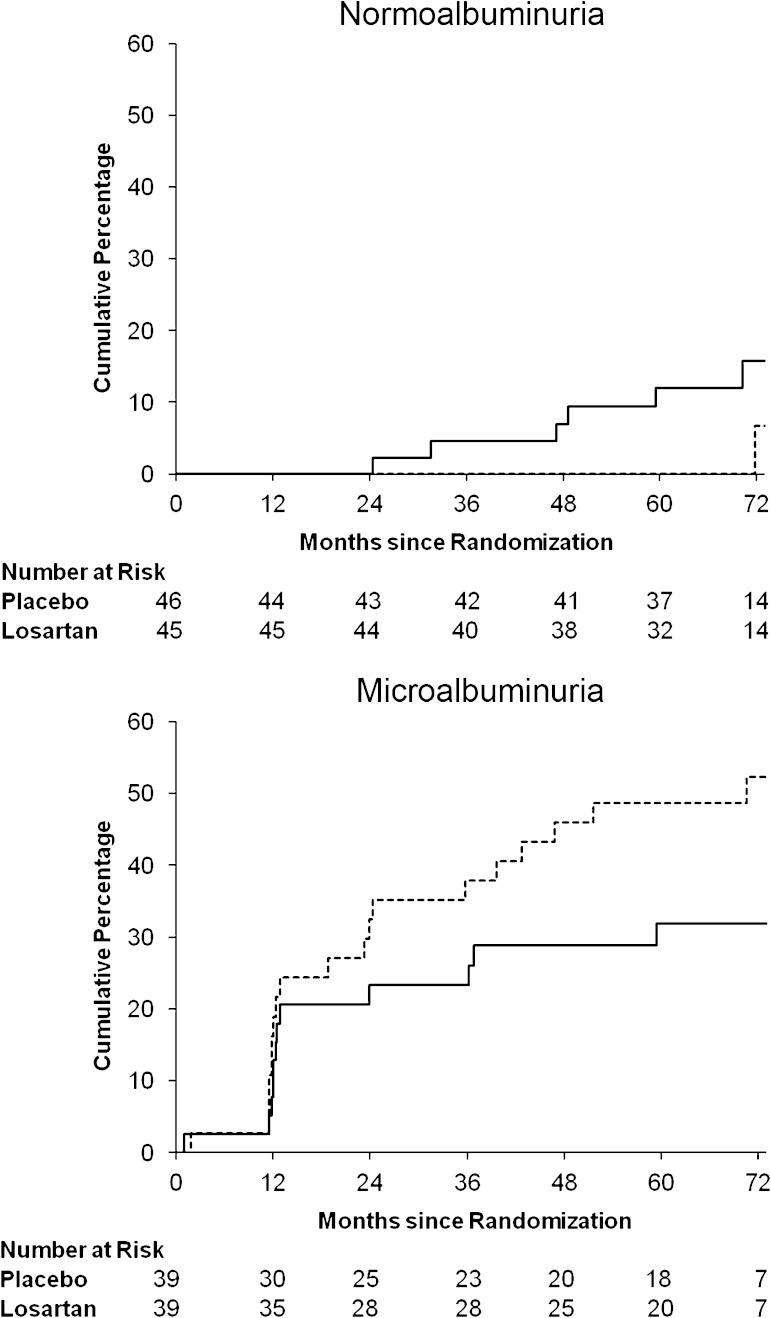
Only nine subjects (5.3%) reached the GFR outcome; two (4.3%) were randomized to placebo and two (4.4%) were randomized to losartan in the normoalbuminuria group, and four (10.3%) were randomized to placebo and one (2.6%) was randomized to losartan in the microalbuminuria group. Because no interaction was found between treatment assignment and albuminuria group (P = 0.35), the overall treatment effect was estimated and the unadjusted hazard ratio for reaching the primary outcome in those receiving losartan versus placebo stratified by albuminuria group was 0.50 (95% CI, 0.12–1.99). Cumulative percentage of first occurrence of the GFR outcome by treatment assignment is shown in Fig. 3.
FIG. 3.
Cumulative percentage of the first occurrence of the GFR outcome by treatment group (dashed line = placebo; solid line = losartan). Log-rank tests for the GFR outcome yielded P = 0.31.
Other study outcomes.
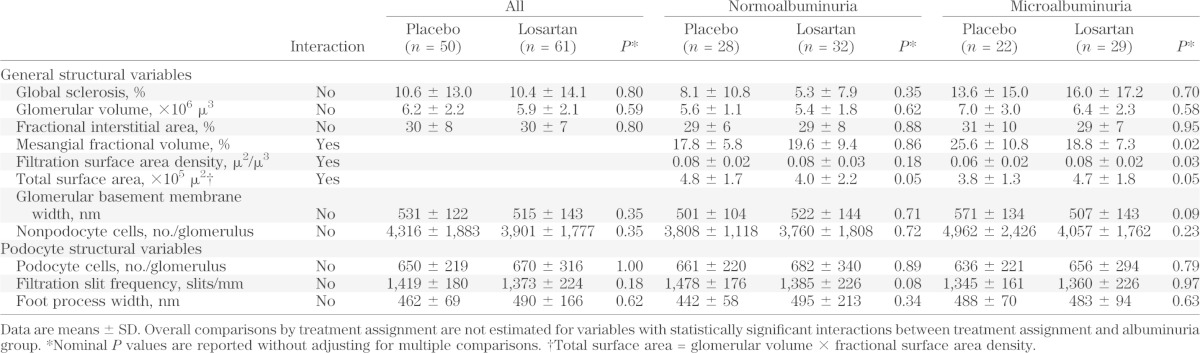
Differences in morphometric variables between treatment groups at end of follow-up are shown in Table 2. Differences in mesangial fractional volume, filtration surface area density, and total surface area were not estimated in the combined albuminuria groups because of a statistically significant interaction between treatment assignment and albuminuria group. Mesangial fractional volume was significantly lower among subjects with microalbuminuria who received losartan than in those who received placebo (18.8 vs. 25.6%; P = 0.02), whereas no difference in mesangial fractional volume was observed by treatment group in subjects with normoalbuminuria at baseline (19.6 vs. 17.8%; P = 0.86). Similar results were obtained after adjusting for the duration of diabetes (Fig. 4). Filtration surface area density was also significantly higher (0.08 μ2/μ3 vs. 0.06 μ2/μ3; P = 0.03) and total surface area (4.7 × 105 μ2 vs. 3.8 × 105 μ2; P = 0.05) was marginally higher in subjects with microalbuminuria who received losartan than in those who received placebo, whereas the total surface area was marginally lower compared with placebo in subjects with normoalbuminuria (4.0 × 105 μ2 vs. 4.8 × 105 μ2; P = 0.05).
TABLE 2.
Morphometric variables by baseline albuminuria level and treatment group assignment
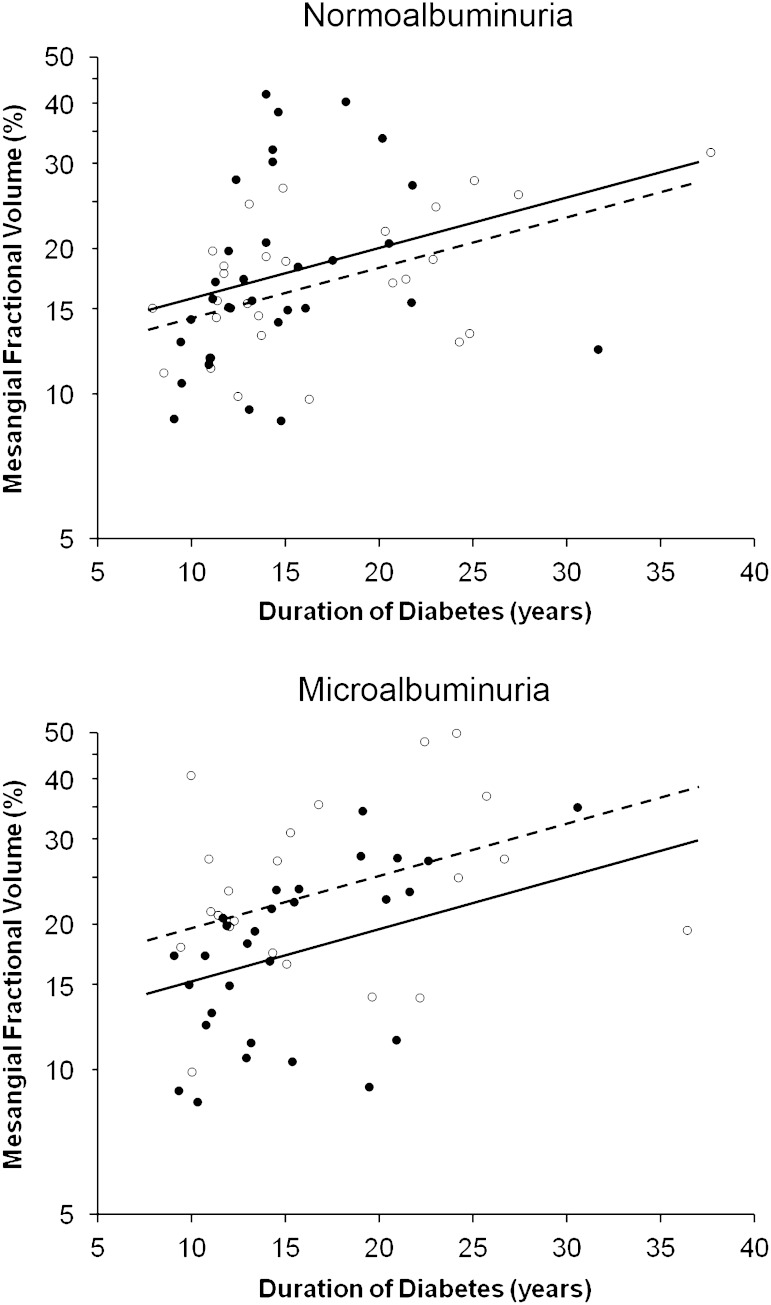
FIG. 4.
Effect of treatment with losartan or placebo on mesangial fractional volume (logarithmic scale) according to duration of diabetes in subjects with normoalbuminuria or microalbuminuria at baseline (open circles [○] = treatment with placebo; filled circles [●] = treatment with losartan). Regression lines are shown by treatment group (dashed line = placebo; solid line = losartan). After adjusting for duration of diabetes, mesangial fractional volume was 22.9% (95% CI, 4.6–37.7%) lower in subjects with microalbuminuria who received losartan than in those who received placebo. Mesangial fractional volume was not statistically significantly higher (10.1% [−8.9 to 33.0%]) in subjects with normoalbuminuria who received losartan than in those who received placebo.
Forty-one subjects (24.3%) progressed to macroalbuminuria during the trial (Fig. 5); 1 (2.2%) was randomized to placebo and 8 (17.8%) were randomized to losartan in the normoalbuminuria group (P = 0.02), and 20 (51.3%) were randomized to placebo and 12 (30.8%) were randomized to losartan in the microalbuminuria group (P = 0.08). Because of a statistically significant interaction between treatment assignment and albuminuria group (P = 0.016), the overall treatment effect was not estimated and the hazard ratio for reaching this outcome in those receiving losartan versus placebo was examined separately by albuminuria group; the hazard ratio was 8.12 (95% CI, 1.02–64.98) in the normoalbuminuria group and 0.54 (0.26–1.10) in the microalbuminuria group. The hazard ratio for the first appearance of elevated albuminuria (ACR ≥30 mg/g) was 0.65 (0.23–1.82) in the normoalbuminuria group. Because randomization tended to equalize covariates between treatment groups, further adjustment for baseline covariates did not modify the effect of treatment.
FIG. 5.
Cumulative percentage of the first occurrence of the macroalbuminuria outcome in subjects with normoalbuminuria or microalbuminuria at baseline by treatment group (dashed line = placebo; solid line = losartan). Log-rank tests for the macroalbuminuria outcome yielded P = 0.02 in the normoalbuminuria group and P = 0.08 in the microalbuminuria group.
Adverse events.
Side effects potentially related to treatment were few, mild, and reported only soon after initiation of treatment. In the losartan group, one subject reported skin rash and another reported stomach cramps; in the placebo group, one reported skin rash and another reported nausea. Each symptom resolved spontaneously and did not require changing the dose of the study medicine. There were no cases of hyperkalemia. One subject developed urethral obstruction after kidney biopsy because of blood clots in the bladder and required bladder catheterization and irrigation for 24 h after biopsy but did not require transfusion. Nine deaths occurred during the study: six in the placebo group (one death from coronary artery disease, one from leukemia, one from hepatocellular carcinoma, one from alcoholic cirrhosis, and two from automobile accidents) and three in the losartan group (one death from coronary artery disease, one from multiple myeloma, and one from a bleeding gastric ulcer).
There were 32 pregnancies: 21 in 12 women who received losartan and 11 in 8 women who received placebo. Twenty-six of the pregnancies produced healthy live births; of the six miscarriages, five occurred in the losartan group and one occurred in the placebo group (P = 0.64). No malformations were seen among the live births.
DISCUSSION
Among American Indians with type 2 diabetes and microalbuminuria, treatment with losartan for nearly 6 years reduced some indices of progression of diabetic nephropathy compared with placebo treatment. Expansion of the mesangial fractional volume, a structural hallmark of diabetic nephropathy that is strongly associated with GFR decline (14), was significantly attenuated in the group that received losartan. This phenomenon was accompanied by higher filtration surface density and area compared with placebo, a finding that would enhance glomerular filtration capacity. Although none of the morphometric comparisons were significant when adjusted for multiple comparisons using a Bonferroni correction, we reported nominal P values because we were interested in the individual hypotheses associated with each of the measured morphometric variables and we were concerned about increasing the risk of type 2 error by applying such a correction. We also observed a 51% reduction in risk of GFR decline in losartan-treated subjects compared with placebo, and a 46% reduction in risk of progression to macroalbuminuria in those with microalbuminuria at baseline. Given lower than expected rates of progression, however, our study was underpowered, and neither of these differences in functional outcomes was statistically significant. Nevertheless, our findings suggest that early treatment with losartan in those with microalbuminuria may preserve glomerular architecture, possibly providing a structural basis for the renoprotective effect of angiotensin receptor blockade seen previously (1,2,4).
Treatment with losartan in the absence of elevated albuminuria at initiation of therapy was associated with a significant eight-fold increased risk, albeit with wide CIs, of developing macroalbuminuria over 6 years compared with placebo, and with a modestly lower total filtration surface area at the end of treatment, suggesting that treatment with losartan to prevent diabetic nephropathy offers no benefit. Although the first appearance of elevated albuminuria (ACR ≥30 mg/g) was similar in those treated with losartan or placebo, further progression to macroalbuminuria (ACR ≥300 mg/g) among those who developed microalbuminuria was higher in the losartan group. The extent to which baseline differences in HbA1c and GFR between treatment groups influenced these findings cannot be determined, but these differences did not persist throughout the study. The RASS also reported increased incidence of elevated albuminuria in normotensive normoalbuminuric patients with type 1 diabetes who were treated with losartan as compared with either enalapril or placebo. It further demonstrated that RAAS blockade did not prevent the increase in mesangial fractional volume in these patients (6). Other studies also have found that RAAS blockade does not prevent development of microalbuminuria in normoalbuminuric normotensive patients with either type 1 (17,18) or type 2 diabetes (17).
Mean rate of GFR decline in the microalbuminuria group was virtually identical throughout the study in subjects treated with either losartan or placebo (Fig. 2). Previous renoprotective clinical trials in patients with microalbuminuria also found that GFR declined similarly in drug and placebo groups in early diabetic kidney disease despite reduced progression of proteinuria in those receiving ACE inhibitors or ARBs (4,5). Compensatory hypertrophy and adaptive hyperfiltration of remaining glomeruli in response to sclerosis of individual glomeruli may complicate interpretation of GFR changes. In the current study, both the proportion of globally sclerotic glomeruli (15.0 ± 16.2%) and mean glomerular volume (6.7 ± 2.6 ×106 μ3) in the microalbuminuria group were significantly higher (P < 0.01 and P = 0.02, respectively) at the end of follow-up than in the normoalbuminuria group (6.6 ± 9.4% and 5.5 ± 1.5 ×106 μ3).
Although a decline in GFR may indicate progressive kidney disease, it is not sufficiently sensitive to be a useful surrogate end point in clinical trials of early diabetic kidney disease. We reported previously that decline in GFR in early diabetic kidney disease did not predict progression to ESRD unless it was accompanied by progression to macroalbuminuria (19). These findings may support superiority of structural over functional outcomes in clinical trials involving patients with early diabetic kidney disease.
Three small clinical trials previously examined effect of RAAS inhibitors on structural end points in patients with type 1 (20,21) or type 2 (22) diabetes who already had elevated albuminuria or proteinuria at baseline. In each study, kidney biopsies were performed at the beginning and end of the treatment period, and RAAS inhibition was associated with stabilization of mesangial fractional volume, whereas treatment with placebo was associated with an increase in this volume. Stabilization of mesangial fractional volume was not statistically significant in one trial (22), but a significant increase in capillary luminal volume fraction (an indication of expanded glomerular filtration surface) was noted in the treatment group compared with placebo. Results of these clinical trials are consistent with the current study suggesting that inhibition of the RAAS is associated with renal structural preservation in persons with diabetes who already have clinical evidence of diabetic nephropathy. We did not observe an effect of treatment on podocyte number despite previously finding a decline in podocyte number after progression to macroalbuminuria (16,23). Progression rates to macroalbuminuria in the current study were similar between the losartan and placebo groups among subjects with microalbuminuria and were low among those with normoalbuminuria at baseline, which may explain the absence of an association.
Many of the women participants had childbearing potential, and 32 pregnancies occurred during the study. No congenital anomalies were reported in 26 live births but there were six miscarriages, one (9%) in the placebo group and five (24%) in the losartan group. Although the association between treatment with losartan and miscarriage was not statistically significant, women of childbearing age who are using these medicines should be advised of the potential risk of miscarriage and encouraged to stop the medicines once they plan to or become pregnant.
Strengths of the current study include long duration of treatment and use of quantitative structural outcomes in patients treated early in the course of their kidney disease. The study also included members of a minority population with a high frequency of type 2 diabetes and diabetic kidney disease (24,25) who are not represented in most clinical trials and who are younger, on average, than subjects in similar clinical trials (1,2,4).
Limitations of this study include low power to evaluate the primary outcome, which may have been adversely affected by the decision midway through the study to suggest that those who managed these patients consider using other RAAS inhibitors in their treatment regimens. Standard of care for people with diabetic kidney disease was evolving, and this modification was required by the ethics committee overseeing the study. Although the use of these additional agents was low (20% of person-time), the study ultimately examined efficacy of losartan versus standard care, which may have included treatment with an ACE inhibitor, an ARB, or an aldosterone blocker.
Previous studies that examined the impact of RAAS inhibition on glomerular structure used comparisons of pretherapy and posttherapy kidney biopsy samples to gauge the efficacy of the study drug. Previous morphometric work suggests that structural changes of diabetic kidney disease precede development of albuminuria (15), and that structural alterations typically found in normoalbuminuric subjects do not differ significantly from those in subjects with microalbuminuria (16). These findings were confirmed in a subset of participants from the current study (12). Therefore, to avoid the additional risk to study participants generated by undergoing two kidney biopsies, we chose to examine effects of treatment on differences in glomerular structure only at the end of treatment.
Conclusions.
In conclusion, treatment with losartan was associated with a possible structural benefit in early diabetic nephropathy. This potential benefit did not extend to those with normoalbuminuria at baseline. Treatment with losartan in the normoalbuminuria group was associated with a substantially increased incidence of macroalbuminuria, which also was reported previously in type 1 diabetes (6). Whether or not increased risk of macroalbuminuria is accompanied by increased risk of adverse clinical outcomes is unknown, but these findings support the current recommendation that inhibitors of the RAAS should not be used for primary prevention of diabetic nephropathy in normotensive normoalbuminuric persons with diabetes (26). However, these medicines seem to mitigate progression of diabetic kidney disease when used after the onset of microalbuminuria.
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, the American Diabetes Association (Clinical Science Award 1-08-CR-42), and by Merck, which provided the study drug and placebo tablets. No other potential conflicts of interest relevant to this article were reported.
E.J.W. researched data and wrote the manuscript. G.F. researched data and reviewed and edited the manuscript. L.I.J. researched data and reviewed and edited the manuscript. T.L. researched data and reviewed and edited the manuscript. K.V.L. researched data, reviewed and edited the manuscript, and contributed to the discussion. R.L.H. researched data and reviewed and edited the manuscript. W.C.K. researched data, reviewed and edited the manuscript, and contributed to the discussion. P.H.B. researched data, reviewed and edited the manuscript, and contributed to the discussion. B.Y. researched data and reviewed and edited the manuscript. B.D.M. researched data, reviewed and edited the manuscript, and contributed to the discussion. R.G.N. researched data and wrote the manuscript. R.G.N. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 26th Annual Meeting of the European Diabetic Nephropathy Study Group, Castelldefels, Spain, 24–25 May 2013, and at the 9th Conference on Kidney Disease in Disadvantaged Populations, a Satellite Symposium of the 2013 World Congress of Nephrology, Hong Kong, 4–5 June 2013.
The authors are indebted to the participants in this investigation.
Footnotes
Clinical trial reg. no. NCT00340678, clinicaltrials.gov.
See accompanying commentary, p. 3014.
REFERENCES
- 1.Brenner BM, Cooper ME, de Zeeuw D, et al. RENAAL Study Investigators . Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345:861–869 [DOI] [PubMed] [Google Scholar]
- 2.Lewis EJ, Hunsicker LG, Clarke WR, et al. Collaborative Study Group . Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001;345:851–860 [DOI] [PubMed] [Google Scholar]
- 3.Levey AS, Cattran D, Friedman A, et al. Proteinuria as a surrogate outcome in CKD: report of a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis 2009;54:205–226 [DOI] [PubMed] [Google Scholar]
- 4.Parving HH, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S, Arner P, Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group . The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 2001;345:870–878 [DOI] [PubMed] [Google Scholar]
- 5.Gaede P, Vedel P, Larsen N, Jensen GVH, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003;348:383–393 [DOI] [PubMed] [Google Scholar]
- 6.Mauer M, Zinman B, Gardiner R, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med 2009;361:40–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Diabetes Association . Standards of medical care for patients with diabetes mellitus. Diabetes Care 2000;23(Suppl. 1):S32–S42 [PubMed] [Google Scholar]
- 8.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G, The Heart Outcomes Prevention Evaluation Study Investigators . Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med 2000;342:145–153 [DOI] [PubMed] [Google Scholar]
- 9.Vasquez B, Flock EV, Savage PJ, et al. Sustained reduction of proteinuria in type 2 (non-insulin-dependent) diabetes following diet-induced reduction of hyperglycaemia. Diabetologia 1984;26:127–133 [DOI] [PubMed] [Google Scholar]
- 10.Chasson AL, Grady HJ, Stanley MA. Determination of creatinine by means of automatic chemical analysis. Tech Bull Regist Med Technol 1960;30:207–212 [PubMed] [Google Scholar]
- 11.Myers BD, Nelson RG, Tan M, et al. Progression of overt nephropathy in non-insulin-dependent diabetes. Kidney Int 1995;47:1781–1789 [DOI] [PubMed] [Google Scholar]
- 12.Weil EJ, Lemley KV, Mason CC, et al. Podocyte detachment and reduced glomerular capillary endothelial fenestration promote kidney disease in type 2 diabetic nephropathy. Kidney Int 2012;82:1010–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weibel ER. Practical Methods for Biological Morphometry. New York, Academic Press, 1979 [Google Scholar]
- 14.Mauer SM, Steffes MW, Ellis EN, Sutherland DE, Brown DM, Goetz FC. Structural-functional relationships in diabetic nephropathy. J Clin Invest 1984;74:1143–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fioretto P, Steffes MW, Mauer M. Glomerular structure in nonproteinuric IDDM patients with various levels of albuminuria. Diabetes 1994;43:1358–1364 [DOI] [PubMed] [Google Scholar]
- 16.Pagtalunan ME, Miller PL, Jumping-Eagle S, et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 1997;99:342–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bilous R, Chaturvedi N, Sjølie AK, et al. Effect of candesartan on microalbuminuria and albumin excretion rate in diabetes: three randomized trials. Ann Intern Med 2009;151:11–20, W3-4 [DOI] [PubMed] [Google Scholar]
- 18.The EUCLID Study Group . Randomised placebo-controlled trial of lisinopril in normotensive patients with insulin-dependent diabetes and normoalbuminuria or microalbuminuria. Lancet 1997;349:1787–1792 [PubMed] [Google Scholar]
- 19.Pavkov ME, Knowler WC, Lemley KV, Mason CC, Myers BD, Nelson RG. Early renal function decline in type 2 diabetes. Clin J Am Soc Nephrol 2012;7:78–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudberg S, Osterby R, Bangstad HJ, Dahlquist G, Persson B. Effect of angiotensin converting enzyme inhibitor or beta blocker on glomerular structural changes in young microalbuminuric patients with type I (insulin-dependent) diabetes mellitus. Diabetologia 1999;42:589–595 [DOI] [PubMed] [Google Scholar]
- 21.Ahmad J, Shafique S, Abidi SM, Parwez I. Effect of 5-year enalapril therapy on progression of microalbuminuria and glomerular structural changes in type 1 diabetic subjects. Diabetes Res Clin Pract 2003;60:131–138 [DOI] [PubMed] [Google Scholar]
- 22.White KE, Pinel N, Cordonnier DJ, Bilous RW, Diabiopsies Group . Does ACE inhibition slow progression of glomerulopathy in patients with type 2 diabetes mellitus? Diabet Med 2001;18:933–936 [DOI] [PubMed] [Google Scholar]
- 23.Lemley KV, Abdullah I, Myers BD, et al. Evolution of incipient nephropathy in type 2 diabetes mellitus. Kidney Int 2000;58:1228–1237 [DOI] [PubMed] [Google Scholar]
- 24.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–1053 [DOI] [PubMed] [Google Scholar]
- 25.Pavkov ME, Bennett PH, Knowler WC, Krakoff J, Sievers ML, Nelson RG. Effect of youth-onset type 2 diabetes mellitus on incidence of end-stage renal disease and mortality in young and middle-aged Pima Indians. JAMA 2006;296:421–426 [DOI] [PubMed] [Google Scholar]
- 26.National Kidney Foundation . KDOQI Clinical Practice Guidelines for Diabetes and CKD: 2012 Update. Am J Kidney Dis 2012;60:850–886 [DOI] [PubMed] [Google Scholar]