Abstract
Atypical antipsychotic (AAP) medications that have revolutionized the treatment of mental illness have become stigmatized by metabolic side effects, including obesity and diabetes. It remains controversial whether the defects are treatment induced or disease related. Although the mechanisms underlying these metabolic defects are not understood, it is assumed that the initiating pathophysiology is weight gain, secondary to centrally mediated increases in appetite. To determine if the AAPs have detrimental metabolic effects independent of weight gain or psychiatric disease, we administered olanzapine, aripiprazole, or placebo for 9 days to healthy subjects (n = 10, each group) under controlled in-patient conditions while maintaining activity levels. Prior to and after the interventions, we conducted a meal challenge and a euglycemic-hyperinsulinemic clamp to evaluate insulin sensitivity and glucose disposal. We found that olanzapine, an AAP highly associated with weight gain, causes significant elevations in postprandial insulin, glucagon-like peptide 1 (GLP-1), and glucagon coincident with insulin resistance compared with placebo. Aripiprazole, an AAP considered metabolically sparing, induces insulin resistance but has no effect on postprandial hormones. Importantly, the metabolic changes occur in the absence of weight gain, increases in food intake and hunger, or psychiatric disease, suggesting that AAPs exert direct effects on tissues independent of mechanisms regulating eating behavior.
Over the past decade, there has been increasing recognition that some of the second-generation antipsychotic medications, termed the atypical antipsychotics (AAPs), are associated with an increased incidence of obesity (1), type 2 diabetes (2,3), and cardiovascular disease (4,5). The implications for public health are tremendous (6) due to the large number of adult patients treated with these agents and the increasing use of off-label prescriptions to children (7) and the elderly (8). Prospective studies have provided evidence of drug-specific effects within the broad category of AAPs (9,10). Olanzapine, a well-tolerated and highly effective agent, is associated with some of the most severe metabolic consequences, including weight gain and increases in fasting glucose, insulin (11,12), and lipids (13,14). Aripiprazole tends to cause less weight gain than olanzapine (15) and is often considered metabolically neutral (16–18). Despite accumulating evidence of AAP-induced metabolic impairments, there remain unresolved issues as to whether metabolic disease is part of the natural history of schizophrenia and bipolar illness or if the metabolic impairments are only secondary to weight gain.
By administering the drugs to healthy volunteers, one can determine whether metabolic effects are independent of disease. A handful of studies have used this approach, reporting either no effect (19–21) or decreases in insulin sensitivity in the presence of modest weight gain after short-term administration (10–15 days) of olanzapine compared with other AAPs or placebo (22). Weight-independent effects in control subjects have only been reported in two studies, with olanzapine decreasing insulin sensitivity (23) and increasing fasting glucose and leptin after an oral glucose tolerance test (24). The effects of the AAPs on hormonal responses to the “real-world” stimulus of a mixed-nutrient meal challenge have not been thoroughly investigated, and no study has been conducted in an in-patient setting in which activity was controlled.
We hypothesized that meal ingestion that elicits both neural and incretin-mediated hormonal responses would be more likely to reveal olanzapine-induced changes in meal-related metabolism compared with the traditional measurements used to assess insulin secretion and sensitivity, which involve intravenous glucose administration and bypass activation of the brain-gut-pancreas axis. We also expected that detrimental effects on metabolism would be specific to olanzapine and that a comparator AAP such as aripiprazole would not be different from placebo. To address these hypotheses, we administered olanzapine, aripiprazole, or placebo for 9 days to healthy subjects under controlled in-patient conditions while maintaining their activity levels. Prior to and after the interventions, we conducted a meal challenge to replicate the physiological stimuli that patients would typically experience in daily life as well as a euglycemic-hyperinsulinemic clamp to evaluate insulin sensitivity and glucose disposal.
RESEARCH DESIGN AND METHODS
Selection and description of participants.
The study was approved by the institutional review board of the University of Pennsylvania, and all participants gave their written informed consent. Subjects underwent screening at the Clinical and Translational Research Center (CTRC) of the Hospital of the University of Pennsylvania after an overnight fast. All subjects were given a structured neuropsychiatric interview, the Mini International Neuropsychiatric Interview (25). Inclusion criteria included no past or present psychiatric history, weight stable, minimal exercise routine that only included walking, BMI = 19–24.5 kg/m2, systolic blood pressure <130 mmHg, diastolic blood pressure <85 mmHg, and women taking oral contraceptives with constant dosing regimens. Exclusion criteria included prescription medications, hemoglobin <11 g/dL, drug or alcohol dependence, homelessness or inability to give informed consent, currently on a weight loss diet, and moderate to significant exercise regimen. If subjects met the inclusion/exclusion criteria, they were instructed in pedometer usage. Subjects wore the pedometer for 5 days: 3 weekdays and 2 weekend days. The average number of steps over the 5 days was used as the target level of activity during their inpatient stay.
Study design and experimental procedures.
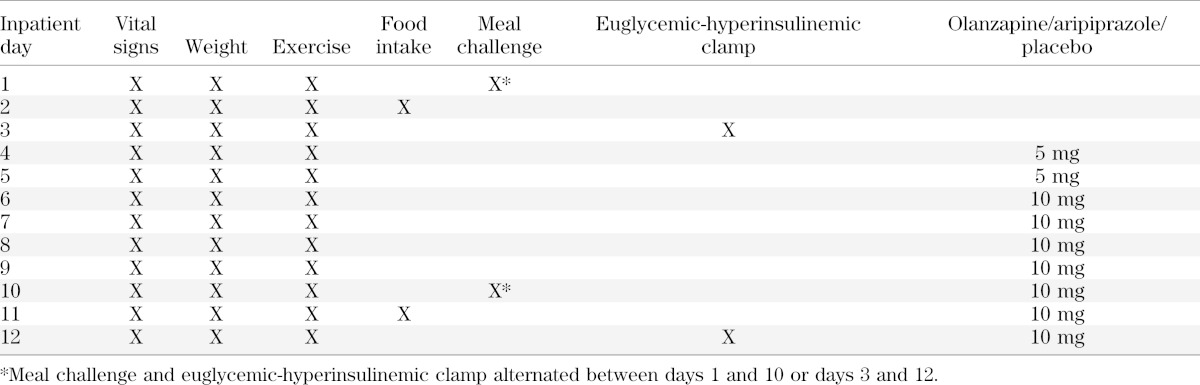
Subjects were admitted into CTRC for 12 nights and randomly assigned into one of three arms. Subjects and study personnel were blinded as to the assignment. As illustrated in Table 1, vital signs and weight were measured daily prior to breakfast, and subjects were supervised on a daily walk to maintain activity levels. Subjects ate ad libitum throughout the inpatient stay. Visual analog scales for ratings of hunger and satiety were given daily prior to and after each meal and snack. Subjects were given daily symptom questionnaires to evaluate side effects of drug administration. On days 1 and 3, after administration of a standardized meal the evening before an overnight fast, subjects underwent either a meal challenge or a euglycemic-hyperinsulinemic clamp administered in a randomized order. On day 2, unknown to the subjects, food intake was monitored. Food items selected from the menu of the metabolic kitchen were given in excess and were weighed prior to and after each meal. On days 4 and 5, subjects were given 5 mg of drug or placebo in the morning to determine drug tolerability. On days 6–12, subjects were administered 10 mg of olanzapine, aripiprazole, or placebo each evening until the end of the study. On days 10 and 12, the metabolic challenges were repeated, and food intake was monitored on day 11. Upon discharge, subjects were given 5 mg of drug to take the next two evenings and then received follow-up calls to ensure no adverse effects.
TABLE 1.
Study design
Hyperinsulinemic-euglycemic clamp.
At 6:30 a.m. after an overnight fast, two intravenous catheters were inserted: one into an anticubital vein for infusions and one retrograde into a contralateral hand vein warmed by a heated hand box or heating pad to obtain arterialized venous blood. Prior to initiation of isotope infusion, a baseline blood sample (1 mL) was taken to measure baseline concentrations of stable isotopes. At 7:00 a.m., a priming dose of 5 mg/kg of [6,6,2H2]glucose (99% enriched; Cambridge Isotopes Laboratories, Andover, MA) was administered over a 5-min period, followed by a continuous infusion (0.05 mg/kg/min) until the end of the clamp. After baseline blood samples (5 mL) at t = −30, −15, and −1 min, at 9:00 a.m. (t = 0 min) at steady-state enrichment, a primed (1.6 mU/kg ⋅ min for 10 min) continuous (0.8 mU/kg ⋅ min for 240 min) infusion of insulin was administered. A variable infusion rate of 20% glucose was initiated to maintain plasma glucose at 90 mg/dL. To reduce changes in plasma enrichment of [6,6,2H2]glucose during the clamp, the glucose infusate was enriched to ∼2.0% with [6,6,2H2]glucose. All infusions were administered by a volumetric pump (Gemini PC-2TX; Alaris Medical Systems, San Diego, CA). Blood samples (0.8 mL) were taken every 5 min, centrifuged, and measured at bedside with an automated glucose analyzer (YSI 2300; Yellow Springs Instruments, Yellow Springs, OH) to adjust the glucose infusion rate (GIR) and achieve the desired plasma glucose concentration over the 4.0-h period. Additional blood samples at t = 30, 60, 90, 120, 150, 180, 210, 230, and 240 min were obtained for biochemical analysis.
Mixed-meal challenge.
A mixed-nutrient meal was prepared with a caloric content of 10 kcal/kg of body weight per subject. Each meal contained 45% carbohydrate, 15% protein, and 40% fat and was composed of scrambled eggs, bacon, 100 mL of water, and a gelatin dessert containing [1-13C]glucose. Jello was prepared using 1.2 g/kg body weight of dextrose dissolved in 200 mL of water. Once cooled to room temperature, [1-13C]glucose was added to obtain an enrichment of 4% (26). An aliquot was removed for subsequent measurement of stable isotope. The morning of the meal challenge, two intravenous catheters were inserted as described above. At 6:30 a.m. after an overnight fast, a baseline blood sample (1 mL) was taken to measure baseline concentrations of stable isotopes. At 7:00 a.m., 2 h prior to meal ingestion, the [6,6,2H2]glucose was infused as described for the clamp above. After the 2-h infusion of stable isotope, two blood samples were taken at −15 and −1 min. At t = 0, subjects ingested the mixed-nutrient meal with the stable isotope over 15 min. Blood (6 mL) was collected at 2, 4, 6, 8, 10, 12, 15, 20, 25, 30, 45, 60, 75, 90, 105, 120, 135, 150, 165, 180, 210, 230, 270, 300, and 330 min.
Analysis of blood samples.
Plasma glucose was measured in duplicate by the glucose oxidase method using an automated glucose analyzer (YSI2300) at the Monell Chemical Senses Center. Analysis of hormones was performed by the Diabetes Research Center of the University of Pennsylvania. Plasma immunoreactive insulin, C-peptide, glucagon, and leptin were measured in duplicate by double-antibody radioimmunoassay (Millipore, Billerica, MA). Glucagon-like peptide 1 (GLP-1) and ghrelin were measured by fluorescent ELISA from Millipore. Plasma free fatty acids (FFAs) and triglyceride levels were measured at the Monell Chemical Senses Center using the WAKO chemical assay. Plasma enrichment of [1-13C]glucose and [6,6,2H2]glucose was measured using gas chromatography–mass spectrometry at Metabolic Solutions (Nashua, NH) to simultaneously monitor the C1–2 and C3–6 fragments as well as the labeled glucose.
Calculations.
Hormonal and metabolic responses to the meal challenge meal were determined by calculating the integrated area over baseline (area under the curve [AUC]). AUCs were calculated using Origin Graphing Software (7.0; Northampton, MA). For postprandial glucagon and triglycerides, which exhibit both increases and decreases relative to baseline, AUCs were calculated from zero as opposed to baseline. The acute insulin response (AIR) to the meal was calculated for the first 10-min period starting from the onset of food ingestion.
During the euglycemic-hyperinsulinemic clamp, basal levels of glucose and insulin were calculated from samples taken at the end of the tracer equilibration period prior to t = 0. Basal rates of endogenous glucose production (EGP) were calculated using the Steele steady-state equation. The rate of appearance of glucose (Ra) was calculated using the modified Steele equation for nonsteady states: Ra = [F/E(t)] − {V × [(C2 + C1)/2]/[1 + E(t)] × [E1 + E2/2]/[T2 − T1]}/[E(t)], where F is the rate of tracer infusion (accounting for the percent mole fraction in the basal infusate and the percent of glucose enrichment added to the 20% glucose infused during the clamp), E(t) is the average of the plasma enrichment of two adjacent samples, V is the volume of distribution (40 mL/kg), C2 + C1 are the glucose concentrations at time 2 and time 1, respectively, in mg/mL, and E1 and E2 represent the isotopic enrichment at the respective time points. During the clamps, EGP was calculated by subtracting the rate of exogenous glucose infusion from Ra. The rate of glucose disposal (Rd) was determined as follows: Rd = Ra − V[(C2 + C1)/(t2 − t1)]. Peripheral insulin sensitivity (SI) was calculated by adjusting for differences in steady state (SS) insulin and glucose concentrations at the end of clamp by using the following equation: SI = (Rdss − Rdbasal)/[(INSss − INSbasal) × GLUss)] (27). We estimated the disposition index (DI) by multiplying the SI by the AIR to the meal.
Statistics.
This was a two-factor experimental design with one repeated measure (pre- and postintervention) and one nonrepeated measure (olanzapine, aripiprazole, and placebo). Subject characteristics and data are presented as mean ± SE in graphs and mean ± SD in tables and text. One-way ANOVAs were used to compare fasting baseline values or preintervention AUCs. Post hoc analysis was conducted using Tukey t test. To determine if olanzapine or aripiprazole induced significant changes from baseline, ΔAUCs (post-AUC − preintervention AUC) for each drug were compared with the change in placebo using independent Student t tests. Statistical significance was considered at the two-tailed P < 0.05. Statistical analysis was conducted using Statistica Software 10 (StatSoft Inc., Tulsa, OK).
RESULTS
Effect of AAP administration on body weight, food intake, and hunger.
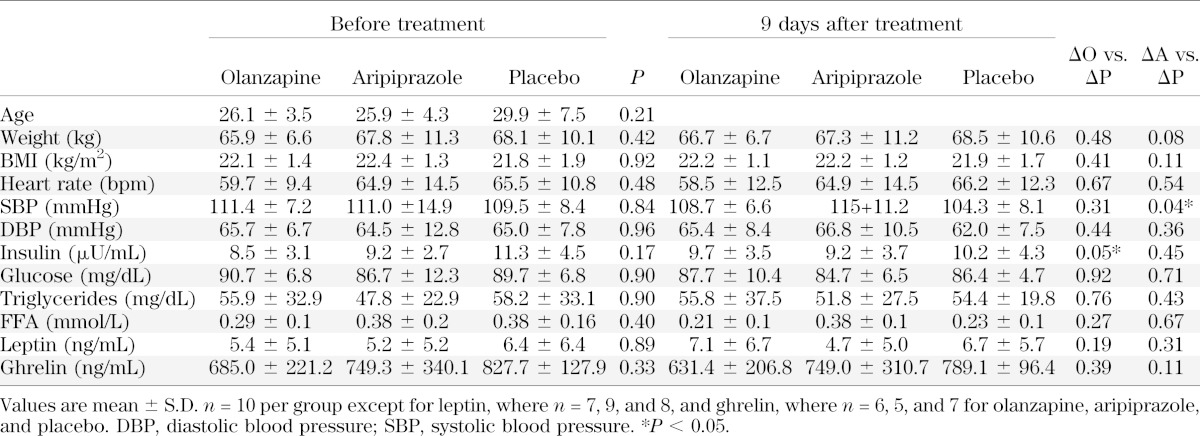
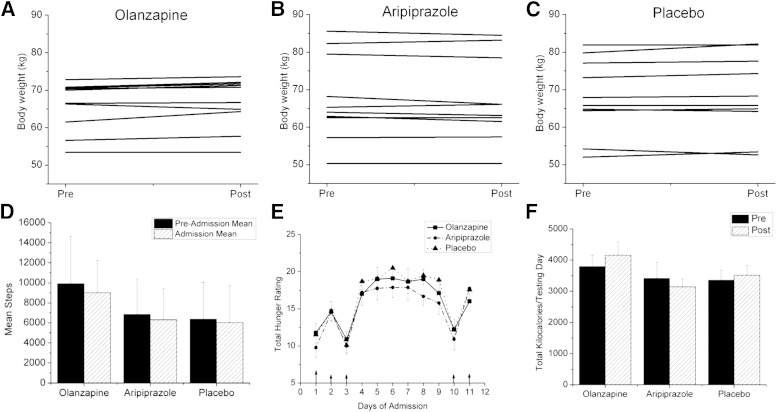
The final number of participants was 30 subjects, with 7 men and 3 women in each experimental condition. None of the subjects dropped out of the study due to study-related adverse events. The primary side effect of olanzapine was drowsiness. Baseline characteristics were not significantly different among the three groups of subjects prior to the interventions (Table 2). After olanzapine, no significant changes in any of the variables were observed except for an increase in fasting plasma insulin (P < 0.05). Aripiprazole had no effect on any of the variables, with the exception of an increase in systolic blood pressure relative to placebo. No significant change in weight was observed after olanzapine (Fig. 1A) or aripiprazole (Fig. 1B) compared with placebo (Fig. 1C); although a trend toward a decrease in weight was evident in the aripiprazole group (P < 0.08) (Table 2), resulting in a difference in the change in weight compared with the change in olanzapine (P < 0.05). Figure 1D illustrates the mean number of steps taken over 5 days prior to hospital admission compared with the mean number of steps during the 12-day in-patient period. No differences were found, suggesting that our goal to maintain activity levels while in the study was met. Figure 1E shows the cumulative daily score of the hunger ratings (four per day except for the metabolic testing days) over the course of the study. No significant differences in hunger ratings were found among the interventions. Ad libitum food intake (Fig. 1F) was monitored on days 2 and 11 when metabolic tests were not conducted. Total kilocalorie intake on the test days was similar among treatments. For the group as a whole, total kilocalorie intake on day 11 was highly correlated with change in body weight over the 12-day period (R = 0.63, P < 0.01), indicating that this acute measurement of kilocalorie intake is a reliable indicator of overall intake.
TABLE 2.
Baseline subject characteristics prior to and after 9 days of olanzapine, aripiprazole, or placebo
FIG. 1.
Body weight, activity levels, hunger, and food intake after short-term administration of olanzapine, aripiprazole, or placebo. Body weight of subjects on the first (pre) and last day of the study (post) after 9 days of either olanzapine (A), aripiprazole (B) or placebo (C). D: Activity level of each group measured by the average number of steps taken during 5 days of monitoring in the free living state (solid bar) and during the 12 inpatient days (hatched bar). E: Sum of the numerical score of the hunger ratings taken over the course of each day throughout the inpatient period for each intervention arm: olanzapine (solid line, solid square), aripiprazole (dashed line, solid circle), or placebo (dotted line, solid triangle). Number of hunger ratings administered was lower on test days (days 1–3 and 10–12), hence lower mean scores. F: Mean kilocalorie intake as measured ad libitum on day 2 prior to administration of drug (solid bar) and on day 11, the 8th day of drug administration (hatched bar). No significant differences were found on any of the variables illustrated in these figures. Values are means ± SEM, n = 10 each study arm.
Effect of AAPs on postprandial glucose and hormone concentrations.
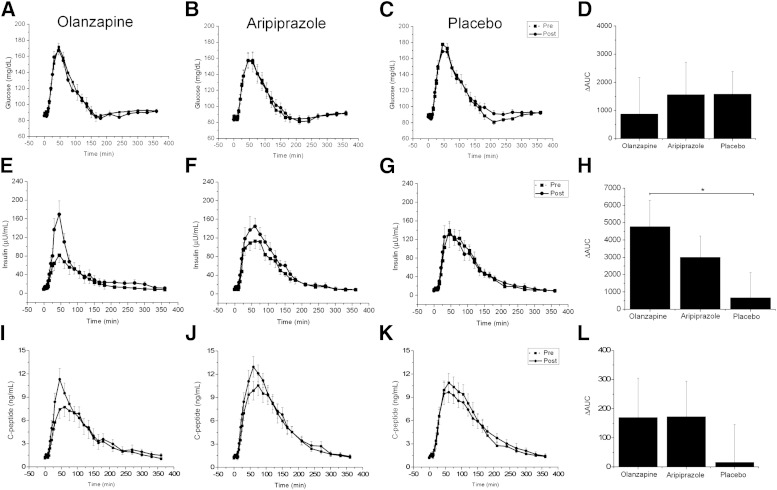
Preintervention, postprandial glucose levels were not significantly different across treatments (F(2,27) = 0.2, P = 0.78) (Fig. 2A–C). No significant differences were found postintervention when the Δglucose AUCs for olanzapine (P = 0.64) or aripiprazole (P = 0.98) were each compared with the change in placebo (Fig. 2D). ANOVA revealed that postprandial insulin AUCs prior to the interventions were significantly lower in the olanzapine group compared with the placebo (F(2,27) = 5.0, P = 0.01) (Fig. 2E–G) despite comparable glucose concentrations across groups. The lower insulin levels were most likely due to trending toward lower weight, higher activity, and insulin sensitivity. When Δinsulin AUCs for olanzapine or aripiprazole were each compared with placebo, only the change in olanzapine was found to be significantly different from the change in placebo (P < 0.05) (Fig. 2H). Olanzapine increased postprandial insulin AUC by 73%, whereas postintervention aripiprazole and placebo AUCs were only increased by 24 and 5%, respectively. Preintervention, postprandial C-peptide concentrations were similar across the three groups (F(1,27) = 2.1, P = 0.14) (Fig. 2I–K). The ΔC-peptide AUCs for olanzapine (170.0 ± 427.6 pg/mL/360 min, P < 0.42) and aripiprazole (173.1 ± 387.7 pg/mL/360 min, P = 0.39) were not different from placebo (16.3 ± 407.8 pg/mL/360 min) despite large differences in the means (Fig. 2L).
FIG. 2.
Postprandial plasma glucose, insulin, and C-peptide concentrations after short-term administration of olanzapine, aripiprazole, or placebo. Postprandial plasma glucose concentrations prior to (dashed line, solid square) and after administration (solid line, solid circles) of olanzapine (A), aripiprazole (B), or placebo (C). D: ΔAUC calculated from the postintervention glucose AUC minus preintervention glucose AUC. No significant differences were found. Postprandial plasma insulin concentrations prior to (dashed line, solid square) and after administration (solid line, solid circles) of olanzapine (E), aripiprazole (F), or placebo (G). ΔInsulin AUC for olanzapine was significantly greater compared with placebo (H). Postprandial plasma C-peptide concentrations prior to (dashed line, solid square) and after administration (solid line, solid circles) of olanzapine (I), aripiprazole (J), or placebo (K). ΔC-peptide AUC (L). No significant differences were found. Values are mean ± SEM. *P < 0.05.
Preintervention, the ratio of C-peptide/insulin AUCs was not different across the treatments (F(2,27) = 0.91, P = 0.41), but differences were found within each treatment arm postintervention. Olanzapine administration decreased the C-peptide/insulin ratio (0.18 ± 0.11 vs. 0.10 ± 0.04, P < 0.05) as did aripiprazole (0.13 ± 0.05 vs. 0.10 ± 0.03, P < 0.03), whereas no difference in placebo was observed (0.12 ± 0.10 vs. 0.10 ± 0.05, P = 0.29). Since changes in the ratio of C-peptide to insulin are an indirect index of hepatic insulin clearance (28,29), the observed decrease in the ratio after AAPs may be due to decreases in hepatic insulin clearance compensatory to prevailing insulin resistance. Changes in hepatic insulin clearance are increasingly being recognized as an important mechanism contributing to glucose homeostasis in insulin resistance (30).
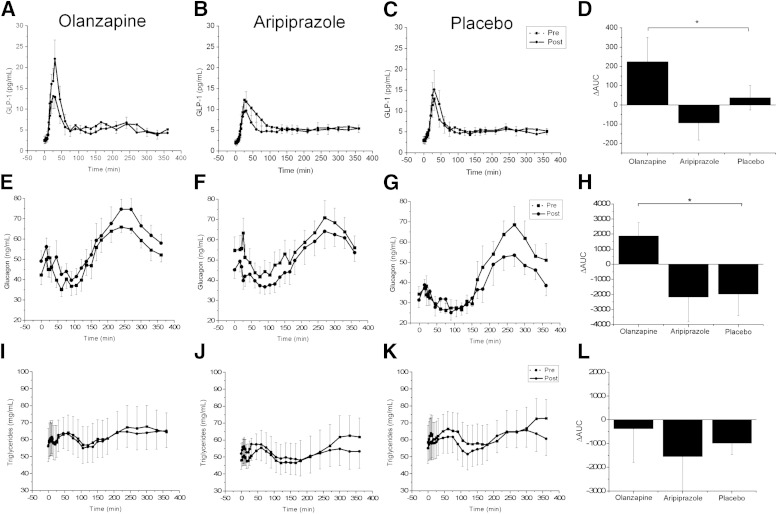
Postprandial GLP-1 AUC concentrations were not different across the groups prior to the intervention (F(2,27) = 0.27, P = 0.76). Olanzapine administration induced a rapid increase in postprandial GLP-1 concentrations (Fig. 3A) compared with placebo (P < 0.05) (Fig. 3C) and aripiprazole (P < 0.05) (Fig. 3B). Preintervention, there were no differences in postprandial glucagon AUC across treatments (F(2,26) = 1.52, P = 0.23). After olanzapine administration, an increase in postprandial glucagon AUC was observed (P < 0.05), but the Δglucagon AUC after aripiprazole was similar to placebo (Fig. 3H). Postprandial triglyceride concentrations were not significantly different across groups prior to invention (Fig. 3L) and were unaffected by AAP administration. Similarly, no differences in ΔFFA AUC after olanzapine (7.9 ± 43.0 mmol/L, P = 0.24) or aripiprazole (−23.9 ± 80.9 mmol/L, P = 0.07) compared with placebo (31.5 ± 44.1 mmol/L) were found (data not shown).
FIG. 3.
Postprandial plasma GLP-1, glucagon, and triglyceride concentrations after short-term administration of olanzapine, aripiprazole, or placebo. Postprandial plasma GLP-1 concentrations prior to (dashed line, solid square) and after administration (solid line, solid circles) of olanzapine (A), aripiprazole (B), or placebo (C). D: ΔGLP-1 AUC. Olanzapine significantly increased GLP-1 compared with placebo. No significant differences were found after aripiprazole or placebo administration. Postprandial plasma glucagon concentrations prior to (dashed line, solid square) and after administration (solid line, solid circles) of olanzapine (E), aripiprazole (F), or placebo (G). ΔGlucagon AUC for olanzapine was significantly greater compared with placebo (H). Triglyceride concentrations prior to (dashed line, solid square) and after administration (solid line, solid circles) of olanzapine (I), aripiprazole (J), or placebo (K). L: ΔTriglyceride AUC. No significant differences were found. Values are means ± SEM. *P < 0.05.
Effect of AAPs on glucose metabolism and insulin resistance.
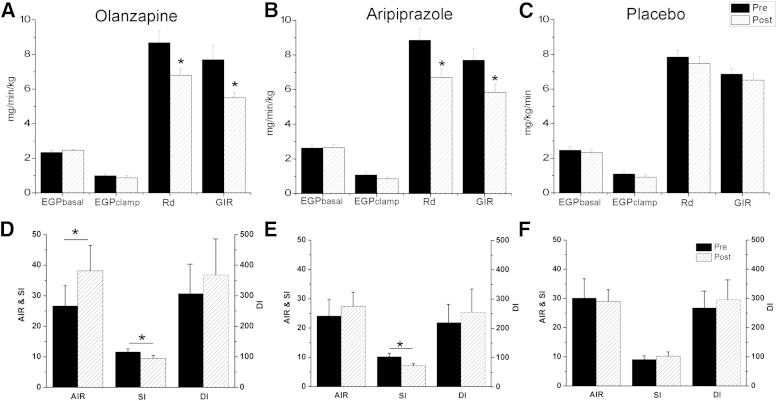
During the euglycemic-hyperinsulinemic clamp, steady-state conditions of euglycemia-hyperinsulinemia and C-peptide were achieved prior to and after administration of olanzapine (Supplementary Fig. 1A, D, and G), aripiprazole (Supplementary Fig. 1B, E, and H), and placebo (Supplementary Fig. 1C, F, and I). The rates of basal EGP were similar across all three groups prior to the interventions (F(2,27) = 0.59, P = 0.56) (Fig. 4A–C). Basal rates of EGP were not influenced by administration of olanzapine, aripiprazole, or placebo. During steady-state clamp conditions, EGP was suppressed by the hyperinsulinemia to the same extent as observed prior to the intervention for all treatment arms, indicating no change in hepatic insulin sensitivity. The rates of glucose disposal (Rd) at steady state were similar across all three groups prior to the interventions (Rd, F(2,27) = 0.89, P = 0.42). Both olanzapine (Fig. 4A) and aripiprazole (Fig. 4B) decreased the rate of glucose disposal (26 and 28%, olanzapine and aripiprazole, respectively; P < 0.05 for both) and the GIRs (21 and 23%, olanzapine and aripiprazole, respectively; P < 0.05, for both). Correcting Rd for circulating insulin concentrations at steady state, the change in Rd was significantly decreased after olanzapine (−0.025 ± 0.026 mg/kg/min) and aripiprazole (−0.028 ± 0.019 mg/kg/min) compared with placebo (−0.007 ± 0.013 mg/kg/min; P < 0.01 for both). Insulin sensitivity (SI) was also reduced by both AAPs (olanzapine, 11.7 ± 3.8 to 9.1 ± 3.6 mg/kg/min, P < 0.05; aripiprazole, 9.2 ± 1.6 to 7.0 ± 2.3 mg/kg/min, P < 0.05) (Fig. 4D and E). No significant differences were observed after placebo administration (Fig. 4F). Supplementary Fig. 2 illustrates EGP, Ra, and Rd from the meal labeled with [1-13C]glucose. No differences were found in Δolanzapine (Supplementary Fig. 2A, D, and E) or aripiprazole (Supplementary Fig. 2B, E, and H) compared with placebo (Supplementary Fig. 2C, F, and I), respectively. To estimate the DI (Fig. 4D–F), we multiplied the AIR from the meal challenge and SI during the clamps. Compared with placebo, olanzapine administration resulted in a 46% compensatory increase in AIR (26.0 ± 21.8 to 38.2 ± 26.4 μU/mL/10 min) due to the prevailing insulin resistance. In contrast, after aripiprazole administration, AIR did not increase (23.8 ± 15.3 to 27.4 ± 18.3 μU/mL/10 min). The resulting DI was not altered after either AAP.
FIG. 4.
Indices of glucose metabolism, disposal, and insulin sensitivity prior to and after short-term administration of olanzapine, aripiprazole, or placebo. Olanzapine administration had no effect on EGP during the basal period or during steady state but significantly reduced glucose disposal (Rd) (P < 0.05) and the GIR (P < 0.05) (A). Aripiprazole similarly reduced glucose disposal and the GIR (P < 0.05) (B) compared with placebo (C). The AIR to the meal was calculated as the first 10 min of insulin release. Olanzapine (D) but not aripiprazole (E) significantly increased AIRmeal compared with placebo (F). Both olanzapine and aripiprazole decreased insulin sensitivity (P < 0.05) but the DI was not significantly different after the intervention, demonstrating adequate compensation for the prevailing insulin resistance. SI was calculated as the change in glucose disposal and dividing by the change in insulin and multiplying by glucose at steady state. DI was estimated by multiplying the SI by the AIR to the meal. Values are mean ± SEM. *P < 0.05.
DISCUSSION
Here we show that short-term administration of the AAPs olanzapine and aripiprazole induces insulin resistance in healthy subjects, but only olanzapine results in significant changes in postprandial metabolism after a mixed-meal challenge. Olanzapine was found to elicit hyperinsulinemia as well as acute increases in postprandial GLP-1 and small elevations in glucagon concentrations. The rapidly induced metabolic dysregulation occurred in the absence of weight gain and psychiatric disease, independent of changes in hunger or food intake, as indicated by our behavioral and metabolic data. These results confirm that olanzapine exerts direct effects on insulin-sensitive tissues and suggest that the mechanisms regulating the increase in food intake may be distinct from those mediating the metabolic abnormalities.
Unique to the current study is the use of the mixed-nutrient meal challenge to unveil olanzapine-induced changes in postprandial responses. One of the most notable findings is the magnitude and consistency of the postprandial hyperinsulinemia. Nine out of ten subjects exhibited an increase in postprandial insulin AUC compared with baseline, with 6 out of 10 subjects doubling their insulin response. Olanzapine-induced increases in postprandial insulin have not previously been documented, but investigation has been limited to studies that had insufficient postmeal sampling frequency (20) or did not use a mixed-nutrient stimulus (24).
The olanzapine-induced postprandial hyperinsulinemia was associated with a decrease in insulin sensitivity as measured by euglycemic-hyperinsulinemic clamp. In contrast, aripiprazole administration, which also induced insulin resistance, was not accompanied by significant increases in postprandial insulin concentrations. The effects of aripiprazole on glucose metabolism and insulin sensitivity using standardized methodologies for assessment of insulin sensitivity have not previously been investigated. The clamp technique facilitated our ability to document the decrease in insulin sensitivity even in the absence of significant changes in postprandial insulin. Insulin resistance is typically characterized by elevations in EGP and circulating FFAs (31,32), but we did not find changes in either variable after AAP administration. These data agree with the report that modest decreases in glucose disposal independent of increases in EGP or lipolysis occurred after olanzapine administration to healthy control subjects (24).
We had hypothesized that meal ingestion, which elicits incretin responses (33,34) and neurally mediated insulin release (35,36), would be more likely to unveil the effects of a centrally mediated psychiatric agent than glucose methodologies that bypass activation of the brain-gut-pancreas axis. Our findings of increases in postprandial insulin and the incretin hormone GLP-1 support this initial hypothesis. However, elevations in GLP-1 coincident with insulin resistance and increases in glucagon are surprising since GLP-1 inhibits glucagon release (37) and is attenuated in type 2 diabetes (38). The olanzapine-induced insulin resistance observed in this study does not parallel the normal etiology of insulin resistance associated with increased body adiposity, hyperlipidemia, and attenuated GLP-1 concentrations. The lack of consistency suggests that other unknown factors may be mediating the increases in postprandial insulin and GLP-1, which were correlated (R = 0.44, P < 0.05). Glucose-dependent insulinotropic peptide, an important physiological incretin, also stimulates insulin release (37) and could be playing a role in the increased insulin concentrations. We speculate that meal ingestion activates a central nervous system mechanism, perhaps vagal, contributing to the hyperinsulinemia and the increases in GLP-1 as well as glucagon, which is also sensitive to vagal mediation (36). The observed postprandial hyperinsulinemia may then be a consequence of peripheral insulin resistance and increased neural activation of gut and pancreatic hormone release. Elevated postprandial GLP-1 has not previously been observed after olanzapine administration; although there is one negative report (39).
Olanzapine has been shown to be a high-affinity muscarinic receptor antagonist (40) and in vitro can block acetylcholine binding to muscarinic receptors on the pancreatic islet, thereby inhibiting insulin release (41). Based on these data and data from our own laboratory showing that vagally mediated, early phase insulin can be inhibited by muscarinic blockade (36), we (42) and others (40,43,44) speculated that olanzapine induces metabolic impairments by attenuating insulin release through muscarinic blockade. Surprisingly, we found significant increases in both early phase and postprandial insulin release after olanzapine. As vagally mediated insulin secretion can be induced by changes in peripheral metabolism (45), and olanzapine has been shown to exert procholinergic effects independent of muscarinic antagonism (46), we now speculate that antagonism of peripheral muscarinic receptors may result in a compensatory centrally mediated increase in vagal efferent activity, thereby enhancing insulin release.
The study has a number of limitations. It is possible that small changes in body fat may have mitigated the reported decrease in Rd and SI so we have limited the interpretation of findings to be independent of weight gain, rather than body adiposity. Increases in body adiposity seem less likely for aripiprazole, which trends toward decreasing body weight. Since dual-energy X-ray absorptiometry measurements were not conducted, the reported values for EGP and changes in Rd were not expressed as a function of lean body mass. However, within the short timeframe of this study, it is unlikely that small increases in visceral or hepatic fat would have been detected by dual-energy X-ray absorptiometry. Due to limited plasma volume, we did not measure certain metabolic variables that could be mediating the observed outcomes. Two important variables that will be measured in future studies are glucose-dependent insulinotropic peptide, known to enhance insulin secretion (47), and cortisol, which can induce insulin resistance (48). Finally, although the study was powered to detect significant differences in olanzapine-induced changes in postprandial insulin release based on pilot data from our own laboratory, it was not powered to detect aripiprazole-induced changes in postprandial metabolism.
In summary, we have demonstrated that olanzapine induces insulin resistance and postprandial metabolic dysregulation in response to the real-life stimulus of meal ingestion. Postprandial hyperinsulinemia may be one of the early precipitating factors in the pathophysiology of olanzapine administration contributing to fat deposition. We have also shown that aripiprazole, an AAP considered metabolically sparing, has modest effects on insulin sensitivity. These data suggest direct and differential effects of the AAPs on insulin-sensitive tissues in the absence of psychiatric disease, weight gain, or increases in hunger. The rapidly induced metabolic impairments are likely mediated by mechanisms separate from those regulating food intake as we did not observe increases in food intake, hunger, or the hunger-related hormone ghrelin. With longer olanzapine administration, AAP-induced central nervous system effects would likely mediate the increased food intake necessary for the known weight gain and this would then exacerbate the metabolic effects reported here. Our findings suggest that interventions inhibiting weight gain in AAP-treated patients may be only partially effective in preventing metabolic disease since the drugs are exerting direct effects on tissue function. Developing AAPs without the debilitating metabolic side effects will depend on the individual contribution of the different neurotransmitters and also on the complex interaction between the peripheral and central nervous system and their effects on behavior and metabolism.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health (NIH) Grant DK-084383 (K.L.T.). Core services were provided by the Diabetes Research Center at the University of Pennsylvania from a grant sponsored by NIH DK-19525 and CTRC facilities from NIH UL1-RR-024134 from the National Center for Research Resources.
No potential conflicts of interest relevant to this article were reported.
K.L.T. designed, analyzed, and interpreted the study and wrote the manuscript. M.R.R. contributed to the design, data interpretation, and clinical oversight and edited the manuscript. J.G. contributed to the design and initiation of the study. C.F. conducted the euglycemic-hyperinsulinemic clamps and preparation of the isotope. H.-L.N. conducted the glucose, FFA, and triglyceride analyses. K.R. advised on the design, drug administration, and safety and provided clinical oversight. K.L.T. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, Illinois, 21–25 June 2013.
The authors thank Erica Alshehabi (Monell Chemical Senses Center) for her excellent coordinator support, Dr. Heather Collins (Penn Diabetes Research Center Bioassay Core) for her technical expertise, and the nurses at the CTRC of the University of Pennsylvania for their dedicated attention to detail and nursing care. The authors also thank Dr. Mitchell Lazar, University of Pennsylvania, Philadelphia, Pennsylvania, for his comments on an earlier version of the manuscript.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db13-0430/-/DC1.
See accompanying commentary, p. 3022.
REFERENCES
- 1.Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999;156:1686–1696 [DOI] [PubMed] [Google Scholar]
- 2.Gianfrancesco F, Pesa J, Wang RH, Nasrallah H. Assessment of antipsychotic-related risk of diabetes mellitus in a Medicaid psychosis population: sensitivity to study design. Am J Health Syst Pharm 2006;63:431–441 [DOI] [PubMed] [Google Scholar]
- 3.Basu A, Meltzer HY. Differential trends in prevalence of diabetes and unrelated general medical illness for schizophrenia patients before and after the atypical antipsychotic era. Schizophr Res 2006;86:99–109 [DOI] [PubMed] [Google Scholar]
- 4.Michelsen JW, Meyer JM. Cardiovascular effects of antipsychotics. Expert Rev Neurother 2007;7:829–839 [DOI] [PubMed] [Google Scholar]
- 5.Daumit GL, Goff DC, Meyer JM, et al. Antipsychotic effects on estimated 10-year coronary heart disease risk in the CATIE schizophrenia study. Schizophr Res 2008;105:175–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newcomer JW. Metabolic considerations in the use of antipsychotic medications: a review of recent evidence. J Clin Psychiatry 2007;68(Suppl. 1):20–27 [PubMed] [Google Scholar]
- 7.Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA 2009;302:1765–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenheck R, Leslie D, Sernyak M. From clinical trials to real-world practice: use of atypical antipsychotic medication nationally in the Department of Veterans Affairs. Med Care 2001;39:302–308 [DOI] [PubMed] [Google Scholar]
- 9.Patel JK, Buckley PF, Woolson S, et al. CAFE Investigators . Metabolic profiles of second-generation antipsychotics in early psychosis: findings from the CAFE study. Schizophr Res 2009;111:9–16 [DOI] [PubMed] [Google Scholar]
- 10.Strassnig M, Miewald J, Keshavan M, Ganguli R. Weight gain in newly diagnosed first-episode psychosis patients and healthy comparisons: one-year analysis. Schizophr Res 2007;93:90–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henderson DC, Copeland PM, Borba CP, et al. Glucose metabolism in patients with schizophrenia treated with olanzapine or quetiapine: a frequently sampled intravenous glucose tolerance test and minimal model analysis. J Clin Psychiatry 2006;67:789–797 [DOI] [PubMed] [Google Scholar]
- 12.Newcomer JW. Abnormalities of glucose metabolism associated with atypical antipsychotic drugs. J Clin Psychiatry 2004;65(Suppl. 18):36–46 [PubMed] [Google Scholar]
- 13.Haupt DW, Fahnestock PA, Flavin KA, et al. Adiposity and insulin sensitivity derived from intravenous glucose tolerance tests in antipsychotic-treated patients. Neuropsychopharmacology 2007;32:2561–2569 [DOI] [PubMed] [Google Scholar]
- 14.Meyer JM, Davis VG, McEvoy JP, et al. Impact of antipsychotic treatment on nonfasting triglycerides in the CATIE Schizophrenia Trial phase 1. Schizophr Res 2008;103:104–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res 2005;80:19–32 [DOI] [PubMed] [Google Scholar]
- 16.Ganguli R, Brar JS, Garbut R, Chang CC, Basu R. Changes in weight and other metabolic indicators in persons with schizophrenia following a switch to aripiprazole. Clin Schizophr Relat Psychoses 2011;5:75–79 [PubMed] [Google Scholar]
- 17.Wang LJ, Ree SC, Huang YS, Hsiao CC, Chen CK. Adjunctive effects of aripiprazole on metabolic profiles: comparison of patients treated with olanzapine to patients treated with other atypical antipsychotic drugs. Prog Neuropsychopharmacol Biol Psychiatry 2013;40:260–266 [DOI] [PubMed] [Google Scholar]
- 18.Nielsen J, Skadhede S, Correll CU. Antipsychotics associated with the development of type 2 diabetes in antipsychotic-naïve schizophrenia patients. Neuropsychopharmacology 2010;35:1997–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sowell MO, Mukhopadhyay N, Cavazzoni P, et al. Hyperglycemic clamp assessment of insulin secretory responses in normal subjects treated with olanzapine, risperidone, or placebo. J Clin Endocrinol Metab 2002;87:2918–2923 [DOI] [PubMed] [Google Scholar]
- 20.Sowell M, Mukhopadhyay N, Cavazzoni P, et al. Evaluation of insulin sensitivity in healthy volunteers treated with olanzapine, risperidone, or placebo: a prospective, randomized study using the two-step hyperinsulinemic, euglycemic clamp. J Clin Endocrinol Metab 2003;88:5875–5880 [DOI] [PubMed] [Google Scholar]
- 21.Fountaine RJ, Taylor AE, Mancuso JP, et al. Increased food intake and energy expenditure following administration of olanzapine to healthy men. Obesity (Silver Spring) 2010;18:1646–1651 [DOI] [PubMed] [Google Scholar]
- 22.Sacher J, Mossaheb N, Spindelegger C, et al. Effects of olanzapine and ziprasidone on glucose tolerance in healthy volunteers. Neuropsychopharmacology 2008;33:1633–1641 [DOI] [PubMed] [Google Scholar]
- 23.Vidarsdottir S, de Leeuw van Weenen JE, Frölich M, Roelfsema F, Romijn JA, Pijl H. Effects of olanzapine and haloperidol on the metabolic status of healthy men. J Clin Endocrinol Metab 2010;95:118–125 [DOI] [PubMed] [Google Scholar]
- 24.Albaugh VL, Singareddy R, Mauger D, Lynch CJ. A double blind, placebo-controlled, randomized crossover study of the acute metabolic effects of olanzapine in healthy volunteers. PLoS ONE 2011;6:e22662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59(Suppl. 20):22–33; quiz 34–57 [PubMed] [Google Scholar]
- 26.Toffolo G, Basu R, Dalla Man C, Rizza R, Cobelli C. Assessment of postprandial glucose metabolism: conventional dual- vs. triple-tracer method. Am J Physiol Endocrinol Metab 2006;291:E800–E806 [DOI] [PubMed] [Google Scholar]
- 27.Bergman RN, Finegood DT, Ader M. Assessment of insulin sensitivity in vivo. Endocr Rev 1985;6:45–86 [DOI] [PubMed] [Google Scholar]
- 28.Polonsky KS, Rubenstein AH. C-peptide as a measure of the secretion and hepatic extraction of insulin. Pitfalls and limitations. Diabetes 1984;33:486–494 [DOI] [PubMed] [Google Scholar]
- 29.Polonsky K, Jaspan J, Pugh W, et al. Metabolism of C-peptide in the dog. In vivo demonstration of the absence of hepatic extraction. J Clin Invest 1983;72:1114–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim SP, Ellmerer M, Kirkman EL, Bergman RN. Beta-cell “rest” accompanies reduced first-pass hepatic insulin extraction in the insulin-resistant, fat-fed canine model. Am J Physiol Endocrinol Metab 2007;292:E1581–E1589 [DOI] [PubMed] [Google Scholar]
- 31.Basu R, Chandramouli V, Dicke B, Landau B, Rizza R. Obesity and type 2 diabetes impair insulin-induced suppression of glycogenolysis as well as gluconeogenesis. Diabetes 2005;54:1942–1948 [DOI] [PubMed] [Google Scholar]
- 32.Bergman RN, Ader M. Free fatty acids and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol Metab 2000;11:351–356 [DOI] [PubMed] [Google Scholar]
- 33.Holst JJ. Glucagonlike peptide 1: a newly discovered gastrointestinal hormone. Gastroenterology 1994;107:1848–1855 [DOI] [PubMed] [Google Scholar]
- 34.Ahrén B, Holst JJ, Mari A. Characterization of GLP-1 effects on beta-cell function after meal ingestion in humans. Diabetes Care 2003;26:2860–2864 [DOI] [PubMed] [Google Scholar]
- 35.Teff KL, Engelman K. Oral sensory stimulation improves glucose tolerance in humans:effects on insulin, C-peptide, and glucagon. Am J Physiol 1996;270:R1371–R1379 [DOI] [PubMed] [Google Scholar]
- 36.Teff KL, Townsend RR. Early phase insulin infusion and muscarinic blockade in obese and lean subjects. Am J Physiol 1999;277:R198–R208 [DOI] [PubMed] [Google Scholar]
- 37.Drucker DJ. The biology of incretin hormones. Cell Metab 2006;3:153–165 [DOI] [PubMed] [Google Scholar]
- 38.Vilsbøll T, Krarup T, Deacon CF, Madsbad S, Holst JJ. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes 2001;50:609–613 [DOI] [PubMed] [Google Scholar]
- 39.Vidarsdottir S, Roelfsema F, Streefland T, Holst JJ, Rehfeld JF, Pijl H. Short-term treatment with olanzapine does not modulate gut hormone secretion: olanzapine disintegrating versus standard tablets. Eur J Endocrinol 2010;162:75–83 [DOI] [PubMed] [Google Scholar]
- 40.Nasrallah HA. Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol Psychiatry 2008;13:27–35 [DOI] [PubMed] [Google Scholar]
- 41.Johnson DE, Yamazaki H, Ward KM, et al. Inhibitory effects of antipsychotics on carbachol-enhanced insulin secretion from perifused rat islets: role of muscarinic antagonism in antipsychotic-induced diabetes and hyperglycemia. Diabetes 2005;54:1552–1558 [DOI] [PubMed] [Google Scholar]
- 42.Teff KL, Kim SF. Atypical antipsychotics and the neural regulation of food intake and peripheral metabolism. Physiol Behav 2011;104:590–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stahl SM, Mignon L, Meyer JM. Which comes first: atypical antipsychotic treatment or cardiometabolic risk? Acta Psychiatr Scand 2009;119:171–179 [DOI] [PubMed] [Google Scholar]
- 44.Hahn M, Chintoh A, Giacca A, et al. Atypical antipsychotics and effects of muscarinic, serotonergic, dopaminergic and histaminergic receptor binding on insulin secretion in vivo: an animal model. Schizophr Res 2011;131:90–95 [DOI] [PubMed] [Google Scholar]
- 45.Teff KL, Townsend RR. Prolonged mild hyperglycemia induces vagally mediated compensatory increase in C-peptide secretion in humans. J Clin Endo Metab 2004;89:5606–5613 [DOI] [PubMed] [Google Scholar]
- 46.Tzavara ET, Bymaster FP, Nomikos GG. The procholinergic effects of the atypical antipsychotic olanzapine are independent of muscarinic autoreceptor inhibition. Mol Psychiatry 2006;11:619–621 [DOI] [PubMed] [Google Scholar]
- 47.Nauck MA. Unraveling the science of incretin biology. Eur J Intern Med 2009;20(Suppl. 2):S303–S308 [DOI] [PubMed] [Google Scholar]
- 48.Andrews RC, Walker BR. Glucocorticoids and insulin resistance: old hormones, new targets. Clin Sci (Lond) 1999;96:513–523 [DOI] [PubMed] [Google Scholar]