Abstract
Brain natriuretic peptide (BNP) has an established role in cardiovascular disease (CVD). However, recent animal studies suggest direct metabolic effects of BNP. To determine the association of BNP with the risk of diabetes, we conducted a prospective analysis of participants from the Atherosclerosis Risk in Communities (ARIC) study. We included 7,822 men and women without history of diabetes, CVD, or reduced kidney function at baseline. At baseline, NH2-terminal (NT)-proBNP, a cleavage product of BNP, was inversely associated with adiposity, fasting glucose, insulin, and cholesterol but positively associated with blood pressure and C-reactive protein levels. During a median follow-up of 12 years, 1,740 participants reported a new diagnosis of diabetes or medication use for diabetes. Baseline quartiles of NT-proBNP were inversely associated with diabetes risk, even after multivariable adjustment including fasting glucose. The adjusted HRs for diabetes were 1.0 (reference), 0.84 (95% CI 0.74–0.96), 0.79 (95% CI 0.68–0.90), and 0.75 (95% CI 0.64–0.87) for the 1st, 2nd, 3rd, and 4th quartiles of baseline NT-proBNP, respectively (P for trend <0.001). This inverse association was robust across sex, race, and obesity subgroups. Our results extend animal studies and support a direct and important metabolic role of BNP in humans.
Brain natriuretic peptide (BNP), one of the three natriuretic peptides released by the heart in response to hemodynamic stress, has an established role in cardiovascular (vasodilation) and renal (natriuresis) physiology, in which it confers protection against fluid overload and hypertension (1). It is widely accepted that BNP is closely associated with left ventricular mass and accurately detects heart failure (2). In the general population, elevated levels of BNP are associated with an increased risk of mortality and cardiovascular disease (CVD), especially heart failure (3,4).
More recently, it has been postulated that the effects of natriuretic peptides extend beyond the cardiovascular system and that they may play a role in metabolic regulation, lipolysis, and the development of insulin resistance (5–7). Receptors for natriuretic peptides have been found in cells of tissues other than the classical cardiovascular and renal systems, including adipose tissue (8). In an experiment conducted by Miyashita et al. (6), BNP transgenic mice and wild-type controls were exposed to a high-fat and isocaloric diet; compared with the control mice, the BNP transgenic mice exhibited less weight gain, less ectopic fat accumulation, and less insulin resistance. Further experiments shed some light into some potential mechanisms involved in the protection of these transgenic mice: increased oxygen utilization, increased mitochondrial content in skeletal muscle, and increased gene expression of genes involved in fat oxidation and energy expenditure (peroxisome proliferator–activated receptor-γ coactivator [PGC]-1α). Another animal study, by Bordicchia et al. (9), extended these findings by demonstrating that treating mice with BNP show increased energy expenditure and increased thermogenic activation in brown and white adipose tissue. All together, these studies suggest that BNP exert direct effects on mitochondrial and adipose tissue that can lead to increased glucose utilization and a decrease in adiposity.
Cross-sectional studies have shown low levels of BNP among persons who are overweight or obese (10–13) and, in some studies, lower BNP among adults with diabetes or the metabolic syndrome (11,12). To our knowledge, the independent association between BNP levels and risk of diabetes has been addressed by two small studies in Europe, with inconsistent results (14,15).
The objective of this study was to determine the independent association between BNP levels and risk of diabetes in a community-based population without diabetes or CVD at baseline. We hypothesized that elevated levels of NH2-terminal (NT)-proBNP, a stable cleavage product of BNP, would be associated with decreased risk of diabetes.
RESEARCH DESIGN AND METHODS
The Atherosclerosis Risk in Communities (ARIC) study is an ongoing community-based, predominantly biracial cohort of 15,792 middle-aged adults from four U.S. communities: Forsyth Country, North Carolina; Jackson, Mississippi; Minneapolis, Minnesota; and Washington County, Maryland (16). The first examination of participants took place from 1987 to 1989, with three follow-up visits taking place, each approximately 3 years apart, and a fifth visit currently taking place (2011–2013). The fourth visit (during 1996–1998) was attended by 11,656 and is the baseline for the current study. We excluded participants with race/ethnicity other than black or white (n = 31); persons with a history of diabetes (n = 1,362), coronary heart disease, or heart failure (n = 1,120); persons with reduced kidney function (estimated glomerular filtration rate <60 mL/min/1.73 m2) (n = 623); and persons with missing data (n = 555) or without an 8 h fasting sample (n = 143). The final sample size was 7,822 adults.
All participants signed written informed consent. The institutional review boards at each clinical site approved the study.
Measurement of NT-proBNP.
NT-proBNP was measured in plasma samples collected from participants during visit 4 and stored at −70°C using an electrochemiluminescent immunoassay on an automated Cobas e411 analyzer (Roche Diagnostics) with a lower limit of detection ≤5 pg/mL and coefficient of variation 3.5–4.7%. Studies have demonstrated that NT-proBNP is a good and more stable marker of BNP, with long-term stability (17).
Assessment of diabetes.
Incident diabetes was defined as a self-reported physician diagnosis of diabetes or use of medications for diabetes identified during the annual telephone calls to all participants beginning after visit 4 (1996–1998) with follow-up through April 2011.
Other measurements.
Smoking history, alcohol consumption (never, former, and current drinker and usual ethanol intake [g/week] among current drinkers), medical history, medication use, and family history of diabetes were assessed during a home interview and according to a published protocol (18). We defined history of CVD as self-reported myocardial infarction or stroke before visit 1 or silent myocardial infarction (diagnosed by electrocardiographic changes), adjudicated myocardial infarction, or revascularization (at or before visit 4) and adjudicated hospitalization for congestive heart failure (at or before visit 4). Using standardized methods, height, weight, waist circumference, and blood pressure were measured. Fasting blood samples were obtained, and using standard methods the following assays were performed: serum glucose, insulin, cholesterol (total, LDL, and HDL cholesterol), triglycerides, creatinine, high-sensitivity (hs)–C-reactive protein (hs-CRP), plasma lactate, and hematocrit (19). Cardiac troponin T (cTnT) was measured using a novel high-sensitivity assay, Elecsys Troponin T (Roche Diagnostics, Indianapolis, IN) with a lower limit of detection of 3.0 ng/L.
Statistical methods.
Baseline levels of NT-proBNP were categorized into quartiles. Baseline characteristics of the study population were summarized across quartiles of NT-proBNP. Participants with NT-proBNP below the limit of detection (n = 248 [3.2%]) were assigned a value of 2.5 pg/mL. We used the Kaplan-Meier method to show the differences in the overall risk (cumulative incidence) of diabetes by baseline quartiles of NT-proBNP. Adjusted hazard ratios (HRs) and CIs were estimated using Cox proportional hazards model. We implemented four core models. Model 1 included age, race/center, sex, and education. Model 2 included all variables in model 1 plus BMI (linear spline with a knot at 30 kg/m2). Model 3 included all variables in model 2 plus height (in meters) squared, systolic and diastolic blood pressure, and hs-CRP. Model 4 included all variables in model 3 plus fasting glucose and family history of diabetes. We tested for interactions with race, sex, and obesity status.
We conducted sensitivity analyses excluding 1) persons with elevated hs-cTnT (defined as levels >99th percentile value [14 ng/L] in a healthy subpopulation ages 20–70 years [19]) or 2) participants with impaired fasting glucose or undiagnosed diabetes (fasting glucose ≥100 mg/dL) at baseline. We also conducted competing risk regression analyses to examine the possible effect of competing mortality on our results (20).
To characterize the shape of the association of NT-proBNP with incident diabetes in our adjusted Cox regression model, we implemented a piecewise linear spline model, with knots at the 33rd and 66th percentiles. In this model, we truncated the data at percentile 99th, and the HR at the 10th percentile of NT-proBNP was used as the reference.
RESULTS
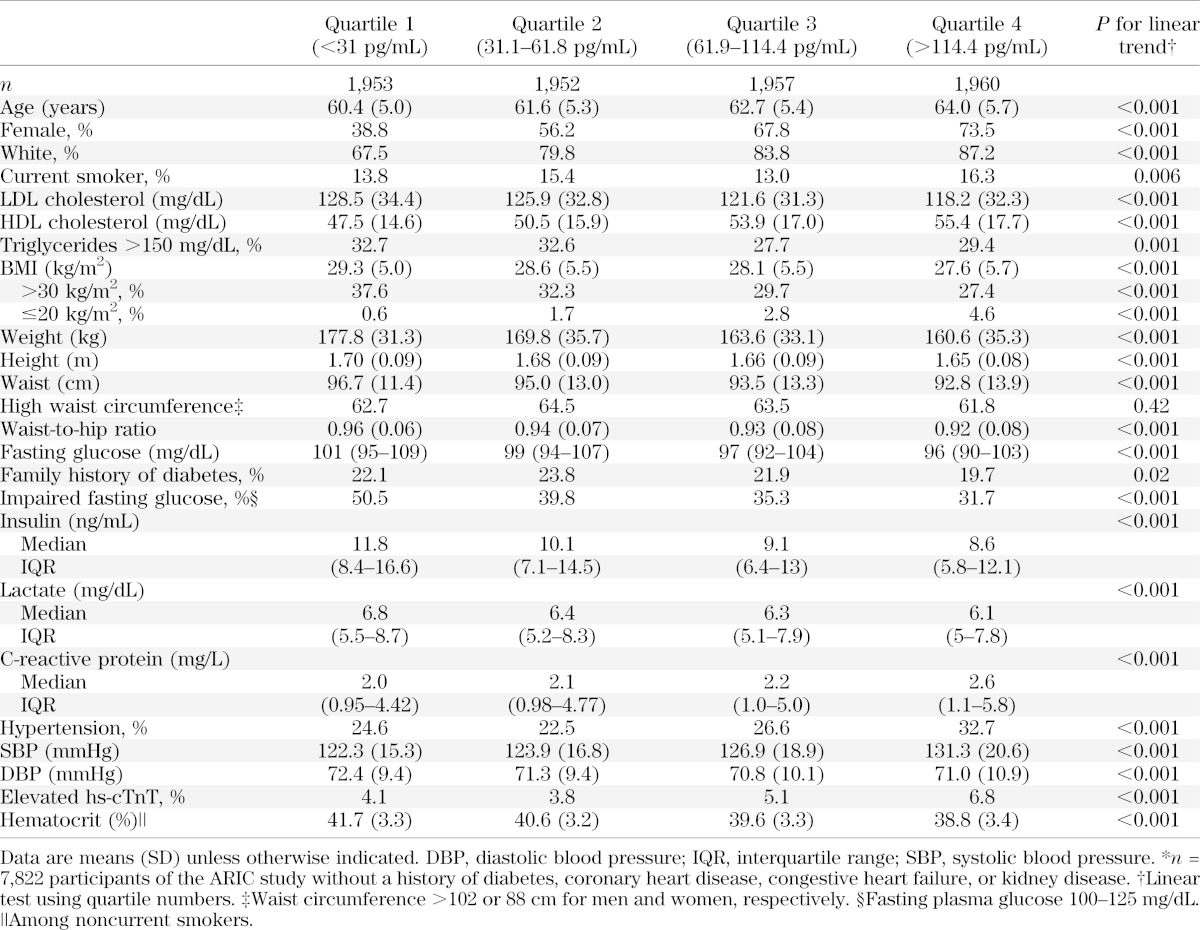
Baseline characteristics of the study population are shown in Table 1. Participants in the lowest quartile of NT-proBNP at baseline were younger; less likely to be white, female, or current smokers; and were more likely to have an adverse lipid profile, higher BMI and waist circumference, and higher levels of fasting glucose, insulin, and lactate compared with participants in the highest quartile of NT-proBNP. In contrast, participants in the lowest quartile of NT-proBNP had lower levels of hs-CRP and systolic blood pressure and lower prevalence of hypertension and lower prevalence of elevated troponin compared with participants in the upper quartile.
TABLE 1.
Characteristics of the study population* according to quartiles of NT-proBNP at baseline
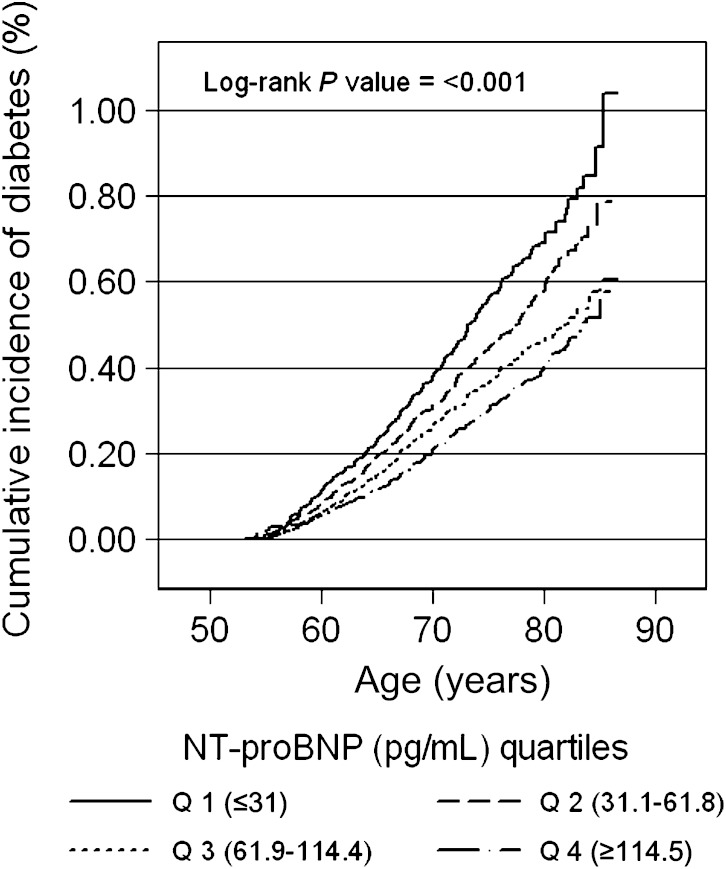
During a median follow-up for 12 years, there were 1,740 new cases of self-reported diabetes among the 7,822 persons in the study population at baseline. The cumulative incidence of diabetes by quartile of baseline NT-proBNP is shown in Fig. 1. The unadjusted incidence rates are shown in Supplementary Table 1.
FIG. 1.
Cumulative incidence (%) of diagnosed diabetes by quartiles (Q) of NT-proBNP at baseline.
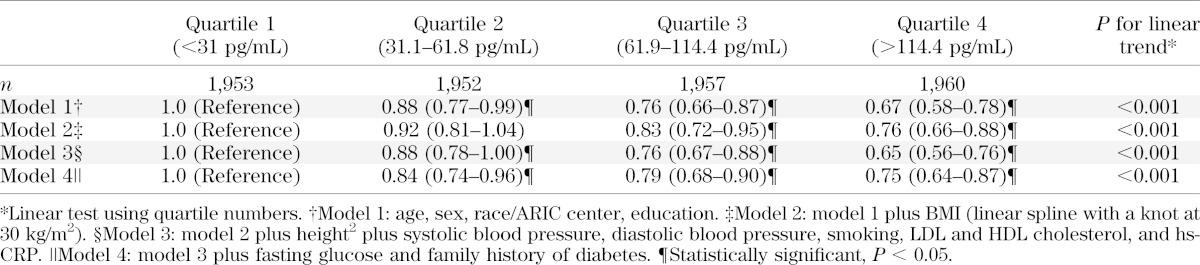
Persons in the lowest quartile of NT-proBNP had a significantly higher risk of diabetes compared with persons in the upper quartiles, even after multivariable adjustment (Table 2). In model 1, the adjusted HRs of diabetes were 1.0 (reference), 0.88 (95% CI 0.77–0.99), 0.76 (95% CI 0.66–0.87), and 0.67 (95% CI 0.58–0.78) for the 1st, 2nd, 3rd, and 4th quartile, respectively (P value for trend < 0.001). After additional adjustment for BMI and cardiovascular risk factors, the associations were attenuated but remained statistically significant. The risk of diabetes remained significantly lower in all three upper quartiles compared with the first quartile of NT-proBNP even after further adjustment for baseline fasting glucose (model 4).
TABLE 2.
Adjusted HRs (95% CI) of incident diagnosed diabetes by quartiles of NT-proBNP at baseline
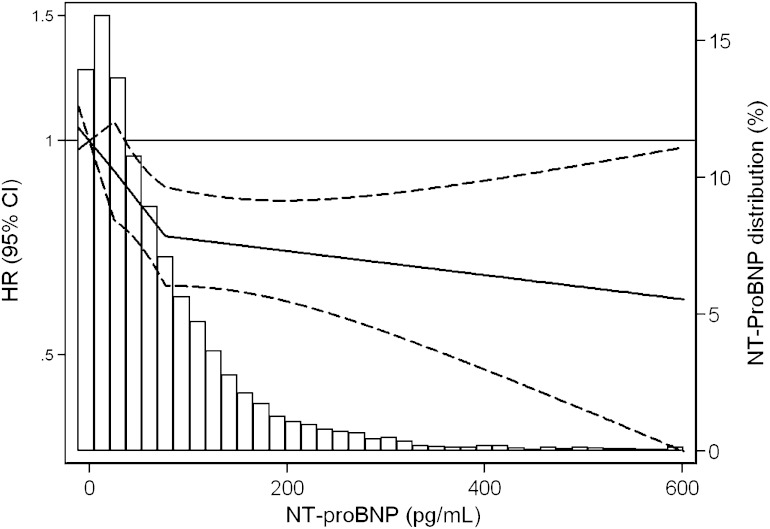
Consistent with the results from the models of NT-proBNP in quartiles, our spline model showed a threshold effect with the steepest and significant decline in diabetes risk at the NT-proBNP levels 14.7–24.9 pg/mL (P value = 0.02), with a shallower and nonsignificant slope at higher levels of NT-proBNP. Indeed, the slope was strongly significant (P value > 0.05) (Fig. 2).
FIG. 2.
Adjusted* HR (95% CI) of incident diagnosed diabetes by baseline NT-proBNP level overlaid on the distribution (frequency histogram) of NT-proBNP in the study population. HRs are presented using a logarithmic scale. Linear spline centered at 14.7 (10th percentile), with knots at the 33rd and 66th percentiles (24.9 and 76.2, respectively), after excluding values ≥99 percentile (NT-proBNP ≥611 pg/mL). *Adjusted for weight, height, age, race/center, sex, systolic blood pressure, diastolic blood pressure, smoking, LDL cholesterol, HDL cholesterol, C-reactive protein, fasting glucose, and family history of diabetes.
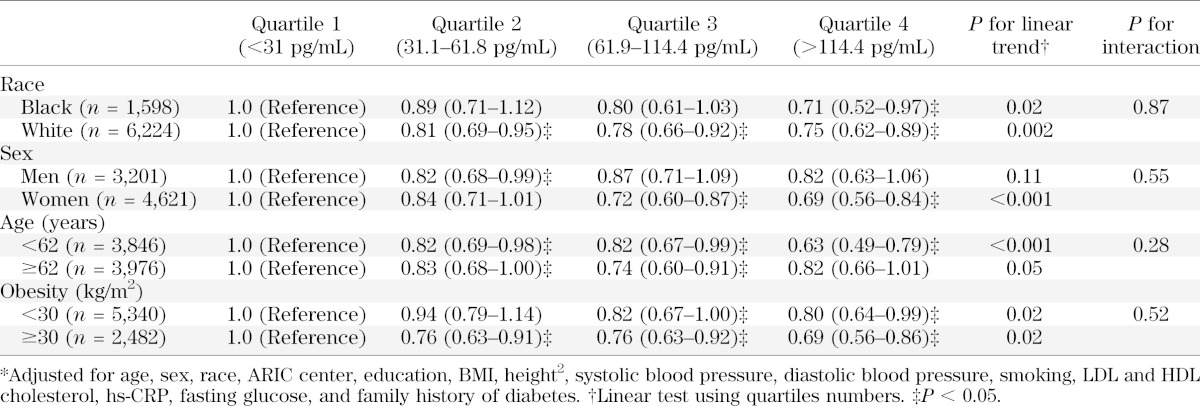
We did not observe any significant interactions by sex, race or obesity (Table 3).
TABLE 3.
Adjusted* HRs (95% CI) of incident diagnosed diabetes by quartiles of NT-proBNP at baseline in population subgroups
In sensitivity analyses, excluding 438 ARIC participants with undiagnosed diabetes or impaired fasting glucose at baseline, the results were very similar or even strengthened, with HRs for diabetes of 1.0 (reference), 0.88 (95% CI 0.76–1.02), 0.80 (95% CI 0.69–0.94), and 0.71 (95% CI 0.60–0.84) for those in the 1st, 2nd, 3rd, and 4th quartile, respectively. Similarly, after exclusion of 386 participants with elevated hs-cTnT, the results were not appreciably altered (Supplementary Table 2). Finally, with use of competing risk models the inverse association of NT-proBNP with diabetes was strengthened. After accounting for competing mortality in model 4 was accounted for, the HRs (95% CIs) for diabetes in the 1st, 2nd, 3rd, and 4th quartile were 1.0 (reference), 0.81 (0.71–0.93), 0.67 (0.57–0.79), and 0.61 (0.52–0.72), respectively.
DISCUSSION
Our findings show that in a community-based population without diabetes, CVD (coronary heart disease or congestive heart failure), or reduced renal function at baseline, higher levels of NT-proBNP were associated with a significantly decreased risk of diabetes, even after adjustment for traditional risk factors and fasting glucose. Our results were consistent across race, sex, and BMI categories. Furthermore, the association remained significant after exclusion of people with impaired fasting glucose at baseline. These results are consistent with limited animal and in vivo studies that suggest circulating levels of BNP levels are metabolically active.
The mechanisms by which BNP and related peptides may influence adipose and glucose metabolism are not well understood. Studies conducted thus far have demonstrated direct effects of BNP on muscle and adipose tissue that could, at least in part, influence the development of insulin resistance. Miyashita et al. (6) showed that BNP transgenic mice with overexpression of BNP had decreased risk of obesity and insulin resistance after receiving a high-fat diet and increased muscle mitochondrial content and fat oxidation. The authors suggest that the cellular effects of BNP emulate the effects observed through catecholamines and include binding to the natriuretic peptide receptor (NPR)-A in adipocytes, activation of the cGMP cascade, and downstream activation of cGMP-dependent protein kinase and p38MAPK, which results in increased mitochondrial biogenesis and uncoupled respiration. More recently, Bordicchia et al. demonstrated in a series of animal and in vivo experiments that natriuretic peptides (BNP and atrial natriuretic peptide [ANP]) activate “brown adipose tissue–like” mechanisms involved with thermogenesis but in white adipose tissue (9,21). In an experimental study in humans, Heinisch et al. (5) intravenously administered BNP or placebo to 20 healthy volunteers and performed an intravenous glucose tolerance test. Their results showed that after infusion of BNP, there were reduced levels of circulating glucose but no effect on insulin secretion or sensitivity.
To our knowledge, little work has been done examining the interrelationship between cardiovascular and noncardiovascular effects of BNP as they pertain to the etiology or development of insulin resistance. In our study, participants in the lowest quartile of NT-proBNP had higher levels of lactate compared with participants in the highest quartile of NT-proBNP, suggesting that blood flow and oxygen delivery may play into the development of insulin resistance. Future studies should be conducted to test this hypothesis.
Most previous epidemiological studies of NT-proBNP and diabetes were cross-sectional and focused on potential mechanisms for the “natriuretic handicap,” which refers to the reduced levels of BNP seen in people with obesity, which in turn could lead to an increased susceptibility for hypertension and heart failure (10,22). It has been postulated that there is increased clearance of BNP by the NPR-C in adipose tissue. However, recent studies have shown that NT-proBNP is cleared by kidney elimination and not by binding to the clearance receptor (NPR-C) (8). Nonetheless, our results were consistent after excluding persons with reduced kidney function and among persons with normal BMI.
To our knowledge, data examining the prospective association between natriuretic peptides and diabetes risk in humans are limited. In Sweden, using data from the Malmo Diet and Cancer study, investigators examined the association between ANP and NT-proBNP and incident diabetes in a sample of 1,828 adults (301 cases of diabetes). The authors found a significant and inverse association between ANP and the risk of diabetes and an inverse but not statistically significant association between NT-proBNP and diabetes risk (odds ratio 0.95 [95% CI 0.84–1.08] comparing the 4th quartile of NT-proBNP to the 1st quartile) (14). In a study using data from the FINRISK97 study (n = 7,827) with 417 cases of incident diabetes, there was a significant inverse association between BNP and diabetes (23). In a case-cohort study with 440 case and 740 control subjects drawn from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk Study, there was a significant inverse association between NT-proBNP and diabetes risk (HR 0.82 [95% CI 0.69–0.97] per 1-SD increase in NT-proBNP) (15). Finally, Pfister et al. (15) combined the results of the EPIC-Norfolk Study with small case-control studies with genotype information and demonstrated that a common genetic variant (rs198389) within the genome region that encodes BNP was associated with a reduced risk of type 2 diabetes.
Our study has some limitations that should be considered in the interpretation of these results. We only had information on new cases of diabetes defined by self-reported diagnosis or diabetes medication use, which is a highly specific definition of diabetes but has relatively low sensitivity (24). We also had only single measurements of NT-proBNP at baseline, and it has been found that all natriuretic peptides have relatively high intraindividual variation (17). This introduces random error to the classification of participants and most likely biased the results toward the null. As with any observational study, the possibility of residual confounding cannot be completely eliminated. Although we had information on fasting glucose and the results were consistent after adjustment for it, we lacked information on oral glucose tolerance test, and therefore it is not possible to test whether NT-proBNP predicts diabetes independently of 2-h oral glucose tolerance test. Our study also has a number of important strengths including the prospective design, long-term follow-up (median of 12 years), large biracial community-based sample with a large number of cases of diabetes occurring during the follow-up (N = 1,740), and rigorous measurements of known risk factors for diabetes using standardized protocols.
In conclusion, our results suggest that persons with very low levels of BNP have a significantly higher risk of developing diabetes compared with persons with moderately high levels of NT-proBNP in a general population. These results provide additional evidence of a novel role for natriuretic peptides in metabolic disorders. Given the availability of recombinant human BNP (25,26), more studies are needed to determine their usefulness in prevention of the development of diabetes.
ACKNOWLEDGMENTS
The Atherosclerosis Risk in Communities study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C. F.L.B. and R.H. received grant support from the National Institutes of Health.
Roche Diagnostics provided reagents and loan of an instrument to conduct the NT-proBNP assays. F.L.B. and R.H. received grant support from Roche Diagnostics. No other potential conflicts of interest relevant to this article were reported.
Roche Diagnostics had no role in the study design, analysis, or presentation preparation.
M.L. developed the research question, analyzed the data, and wrote the manuscript. J.H.Y., F.L.B., J.C., S.W., and C.E.N. reviewed and edited the manuscript. R.H. and C.M.B. collected data and reviewed and edited the manuscript. E.S. refined the research question and study design, collected data, and reviewed and edited the manuscript. M.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
The authors thank the staff and participants of the ARIC study for their important contributions.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db13-0478/-/DC1.
†Deceased.
REFERENCES
- 1.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med 1998;339:321–328 [DOI] [PubMed] [Google Scholar]
- 2.Braunwald E. Biomarkers in heart failure. N Engl J Med 2008;358:2148–2159 [DOI] [PubMed] [Google Scholar]
- 3.de Lemos JA, Lloyd-Jones DM. Multiple biomarker panels for cardiovascular risk assessment. N Engl J Med 2008;358:2172–2174 [DOI] [PubMed] [Google Scholar]
- 4.Kragelund C, Grønning B, Køber L, Hildebrandt P, Steffensen R. N-terminal pro-B-type natriuretic peptide and long-term mortality in stable coronary heart disease. N Engl J Med 2005;352:666–675 [DOI] [PubMed] [Google Scholar]
- 5.Heinisch BB, Vila G, Resl M, et al. B-type natriuretic peptide (BNP) affects the initial response to intravenous glucose: a randomised placebo-controlled cross-over study in healthy men. Diabetologia 2012;55:1400–1405 [DOI] [PubMed] [Google Scholar]
- 6.Miyashita K, Itoh H, Tsujimoto H, et al. Natriuretic peptides/cGMP/cGMP-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes 2009;58:2880–2892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang TJ. The natriuretic peptides and fat metabolism. N Engl J Med 2012;367:377–378 [DOI] [PubMed] [Google Scholar]
- 8.Sarzani R, Dessì-Fulgheri P, Paci VM, Espinosa E, Rappelli A. Expression of natriuretic peptide receptors in human adipose and other tissues. J Endocrinol Invest 1996;19:581–585 [DOI] [PubMed] [Google Scholar]
- 9.Bordicchia M, Liu D, Amri EZ, et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest 2012;122:1022–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das SR, Drazner MH, Dries DL, et al. Impact of body mass and body composition on circulating levels of natriuretic peptides: results from the Dallas Heart Study. Circulation 2005;112:2163–2168 [DOI] [PubMed] [Google Scholar]
- 11.Khan AM, Cheng S, Magnusson M, et al. Cardiac natriuretic peptides, obesity, and insulin resistance: evidence from two community-based studies. J Clin Endocrinol Metab 2011;96:3242–3249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang TJ, Larson MG, Keyes MJ, Levy D, Benjamin EJ, Vasan RS. Association of plasma natriuretic peptide levels with metabolic risk factors in ambulatory individuals. Circulation 2007;115:1345–1353 [DOI] [PubMed]
- 13.Wang TJ, Larson MG, Levy D, et al. Impact of obesity on plasma natriuretic peptide levels. Circulation 2004;109:594–600 [DOI] [PubMed] [Google Scholar]
- 14.Magnusson M, Jujic A, Hedblad B, et al. Low plasma level of atrial natriuretic peptide predicts development of diabetes: the prospective Malmo Diet and Cancer study. J Clin Endocrinol Metab 2012;97:638–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfister R, Sharp S, Luben R, et al. Mendelian randomization study of B-type natriuretic peptide and type 2 diabetes: evidence of causal association from population studies. PLoS Med 2011;8:e1001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol 1989;129:687–702 [PubMed] [Google Scholar]
- 17.Apple FS, Panteghini M, Ravkilde J, et al. Committee on Standardization of Markers of Cardiac Damage of the IFCC . Quality specifications for B-type natriuretic peptide assays. Clin Chem 2005;51:486–493 [DOI] [PubMed] [Google Scholar]
- 18.ARIC Study protocol. Manual 1: General_Description_and_Study_Management Visit 4. Chapel Hill, ARIC, 1997 (Version 3.0)
- 19.ARIC Study protocol. Manual 7: Blood Collection and Processing. Chapel Hill, ARIC, 1993 (Version 3.0)
- 20.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509 [Google Scholar]
- 21.Whittle AJ, Vidal-Puig A. NPs–heart hormones that regulate brown fat? J Clin Invest 2012;122:804–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bayes-Genis A, DeFilippi C, Januzzi JL, Jr. Understanding amino-terminal pro-B-type natriuretic peptide in obesity. Am J Cardiol 2008;101:89–94 [DOI] [PubMed] [Google Scholar]
- 23.Salomaa V, Havulinna A, Saarela O, et al. Thirty-one novel biomarkers as predictors for clinically incident diabetes. PLoS ONE 2010;5:e10100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider AL, Pankow JS, Heiss G, Selvin E. Validity and reliability of self-reported diabetes in the atherosclerosis risk in communities study. Am J Epidemiol 2012;176:738–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cataliotti A, Schirger JA, Martin FL, et al. Oral human brain natriuretic peptide activates cyclic guanosine 3′,5′-monophosphate and decreases mean arterial pressure. Circulation 2005;112:836–840 [DOI] [PubMed] [Google Scholar]
- 26.Rubattu S, Sciarretta S, Valenti V, Stanzione R, Volpe M. Natriuretic peptides: an update on bioactivity, potential therapeutic use, and implication in cardiovascular diseases. Am J Hypertens 2008;21:733–741 [DOI] [PubMed] [Google Scholar]