Abstract
The objective of this study was to describe longitudinal relations of serum total free fatty acids (FFAs) to insulin resistance (IR) and cardiovascular (CV) risk factors from adolescence into adulthood. The cohort included participants in a longitudinal study of obesity and IR with complete data, including anthropometric measures, FFAs, IR measured by euglycemic clamp, blood pressure, fasting serum lipids, and insulin at mean 15 and 22 years of age (n = 207) and their parents (n = 272). FFAs and IR were not significantly related at mean 15 years of age but were significantly related at mean age 22 years. FFA did not relate to BMI at either age. FFA at 15 years of age estimated IR at 22 years of age. In parents (mean age 51 years), FFA was significantly correlated with BMI, percent body fat, systolic blood pressure, LDL, and IR. Associations with all risk factors except IR in parents were attenuated by adjustment for BMI. Most 22 years of age correlations with parents were higher than corresponding 15 years of age correlations. This study finds that FFA is associated with IR starting in young adulthood. The relation between FFA and CV risk factors does not become significant until later adulthood. The results support a significant impact of early metabolic dysfunction on later CV risk.
Free fatty acids (FFAs) are elevated in obese individuals, primarily as a result of release from increased fat mass. This release is enhanced by the resistance of obese adipose tissue to the antilipolytic effect of insulin and inability of obese adipocytes to effectively recycle FFAs via re-esterification (1,2). Elevated FFAs compete with glucose as an energy source in both muscle and fat and are associated with decreased glucose oxidation and increased insulin resistance (IR) in muscle tissue and liver (3–5). Further, increased FFAs in adults have been associated with endothelial dysfunction, blood pressure, and pancreatic β-cell dysfunction (6–9).
Lipid infusion in lean, obese, and type 2 diabetic adults, a model of short-term elevation of FFA, results in peripheral IR and decreased peripheral glucose disposal (10–15). The FFA effect on peripheral IR is dose dependent and occurs at the level of insulin-stimulated glucose uptake, adversely affecting insulin signaling pathways (6,16,17). FFA production is altered in individuals with known genetic risk for type 2 diabetes mellitus, regardless of weight. Glucose-tolerant Mexican American adult children of two parents with type 2 diabetes mellitus were found to have impaired glucose tolerance by insulin clamp and reduced insulin-mediated suppression of lipid oxidation and plasma FFA concentration compared with age-matched, sex-matched, and weight-matched control subjects with nondiabetic parents (7,18).
Information is limited for FFA in childhood and for the natural history of changes in FFA during transition from childhood to adulthood. The goal of the current study was to determine relations of FFA with adiposity, IR, and cardiovascular (CV) risk factors during late childhood/early adolescence and early adulthood. We examined cross-sectional relations of these factors at mean ages 15 and 22 years and longitudinal relations of factors between these age-groups. As an older adult comparison, relations between factors were examined in parents of subjects.
RESEARCH DESIGN AND METHODS
Subjects.
This study was approved by the University of Minnesota Committee for the Use of Human Subjects in Research. At each time point, the study was explained to participants and parents; parents and participants 18 years of age and older signed informed consent, and children younger than 18 years of age gave informed oral assent.
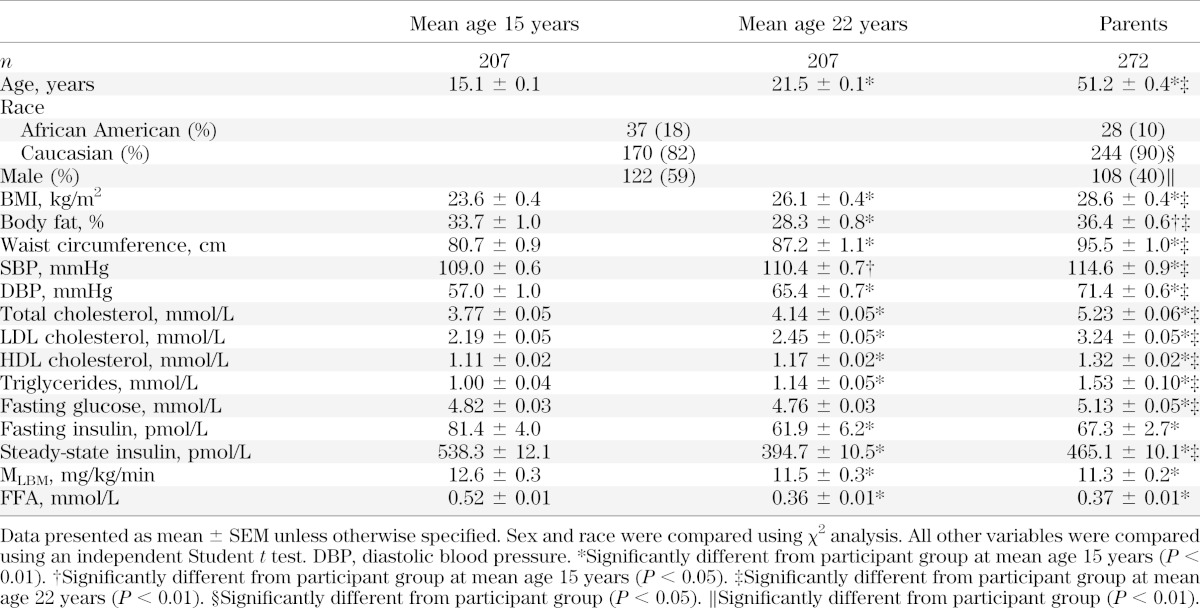
This longitudinal study of the influence of obesity and IR in childhood on development of adult CV risk has been described previously (19). Initial studies were conducted at mean 13 years of age, with additional cohort studies at mean 15, 19, and 22 years of age. The present report consists of data from the mean 15 years of age (range 12–18) and 22 years of age (range 18–25) visits, the times during which samples were obtained and analyzed for FFA. Of 329 individuals seen at mean age 15 years and 280 individuals seen at mean age 22 years, inclusion in this study was limited to those with complete datasets at both time points (n = 207). Of these, 187 had Tanner stage at mean 15 years of age recorded. There were no significant differences in clinical factors between those included and excluded at either age, except for slightly higher fasting glucose in the included group at mean 15 years of age (4.82 vs. 4.71 mmol/L; P = 0.024) and lower steady-state insulin during euglycemic-hyperinsulinemic clamp at age 22 years (394.7 vs. 463.8 pmol/L; P = 0.044). Among the subjects with complete datasets, there was no difference in any measure between those with or without Tanner stage recorded at mean 15 years of age.
Parents of participants were recruited throughout the study (n = 379) and, at either 13 or 15 years of age visits of their children, underwent many of the same measurements as their offspring. Analysis was limited to parents with complete datasets (n = 272). There were differences between those included and excluded in BMI (28.6 vs. 30.3 kg/m2; P < 0.05), waist circumference (95.5 vs. 100.2 cm; P < 0.05), systolic blood pressure (SBP) (114.6 vs. 119.2 mmHg; P < 0.01), fasting glucose (5.13 vs. 5.58 mmol/L; P < 0.001), and steady-state insulin during euglycemic-hyperinsulinemic clamp (465.1 vs. 685.8; P < 0.01).
Clinical measurements.
Height, weight, and blood pressure were measured as previously described (19). Waist circumference was measured in duplicate midway between anterior superior iliac spine and lower rib margin. Percentage of body fat and lean body mass (LBM) were determined by the skinfold method of Slaughter et al. (20) at mean 15 years of age and by dual-energy X-ray absorptiometry at mean 22 years of age and in the parent group. LBM values at mean 15 years of age were adjusted to dual-energy X-ray absorptiometry values according to previously derived equations (21).
Euglycemic-hyperinsulinemic clamps were conducted at the University of Minnesota Clinical Research Center after a 10-h overnight fast as previously described (19,22). Insulin sensitivity was determined from the amount of glucose administered over the final 40 min of euglycemic-hyperinsulinemic clamp and expressed as milligrams of glucose utilization per kilogram of lean LBM per minute (MLBM). A lower MLBM indicates greater IR.
Serum insulin samples were collected on ice, centrifuged within 20 min, and analyzed by radioimmunoassay using a double-antibody method (Equate RIA; Binax, Portland, ME). Blood samples for serum lipids were analyzed in the University of Minnesota laboratory with a Cobas Fara. Cholesterol was determined by a standard enzymatic cholesterol oxidase-based method; HDL cholesterol was determined after precipitation of non-HDL lipoproteins with magnesium/dextran precipitating reagent. Triglycerides were determined with a standard glycerol-blanked, enzymatic triglyceride method. LDL cholesterol was estimated by the Friedewald equation. Total mmol/L FFA in serum was determined at mean 22 years of age and in the parent group by quantitative spectrophotometry. At mean 15 years of age, individual FFA levels were determined by tandem mass spectrometry and converted from microgram of each fatty acid per milliliter (μg/mL) sample to mmol/L of total FFA using the molar mass of each FFA identified. Supplementary Tables 1 and 2 show a comparison of mean total and percentage of FFA before and after conversion to mmol/L.
Statistical analysis.
All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC). Participant characteristics were expressed as means ± SEM for continuous variables and as frequencies for categorical variables. Comparisons were made using Student t test and χ2 analysis, respectively. Pearson partial correlation coefficients, adjusting for age, sex, and race, with or without baseline BMI, evaluated within-person tracking of clinical measures between ages 15 and 22 years. Unadjusted Pearson correlations were used to evaluate relations between clinical measures in subjects at mean 15 and 22 years of age and their parents. Of 272 individuals in the parent group, only 196 were parents of included participants. Of 139 child-parent sets, 25 included fathers, 57 included mothers, and 57 included both parents, for whom midparental value was used. Pearson partial correlation coefficients, adjusted for sex, race, and baseline age, were used for cross-sectional analyses of FFA with other variables at mean 15 and 22 years of age and those of the parents. Multiple regression analysis evaluated relations of insulin sensitivity (MLBM) with FFA level, adjusting for age, sex, and BMI for adolescents (mean 15 years of age), young adults (mean 22 years of age), and their parents (mean 51 years of age). Additionally, multiple regression analysis was used to estimate MLBM at mean 22 years of age from FFA level at mean 15 years of age, adjusting for age, sex, race, and BMI at initial visit. Multiple regression analyses also were performed with the interaction term of sex and FFA; in no case was this term significant. Natural log transformation of the skewed variable, triglycerides, was evaluated and presented when different from the untransformed variable. Three multivariable models were developed adjusting for baseline age, sex, and race (Caucasian vs. African American) as follows: model 1, baseline FFA and BMI as independent variables; model 2, baseline FFA and change in FFA, baseline BMI, and change in BMI; and model 3, the same as model 2 with the addition of baseline level of the outcome variable. Changes in BMI or FFA were calculated as mean 22 years of age value minus mean 15 years of age value. Analyses at mean 15 years of age also were adjusted with and without Tanner stage for each analysis and were not different, unless noted otherwise. P < 0.05 was considered to be statistically significant.
RESULTS
Participant characteristics.
Of 207 individuals studied at both average 15 and 22 years of age, 59% were male and 82% were Caucasian. Most of the 15-year-olds were in the late pubertal stage (Tanner 1, none; Tanner 2, 2%; Tanner 3, 5%; and Tanner 4–5, 93%). BMI increased from 24 to 26 kg/m2 between mean 15 and 22 years of age, but percent body fat decreased from 34 to 28%. Both SBP and diastolic blood pressure increased significantly from mean 15 to mean 22 years of age. Lipids, including total cholesterol, LDL, HDL, and triglycerides, were significantly higher at mean 22 years of age than at mean 15 years of age. No difference in fasting glucose was observed between mean 15 and 22 years of age. Fasting insulin decreased significantly from mean age 15 to mean 22 years of age, coincident with a significant increase in IR (MLBM). FFAs were significantly lower at mean 22 years of age.
The parent group (n = 272, mean age 51 years) had a significantly smaller percentage of males and a higher percentage of Caucasians (Table 1). All anthropometric, blood pressure, lipid, and fasting glucose measures were significantly higher in parents. MLBM, fasting insulin, and FFA levels in parents were significantly lower than those in participants at 15 years of age (P < 0.01) but were not significantly different from those in participants at 22 years of age.
TABLE 1.
Characteristics of repeated measures in study participants at mean ages 15 and 22 years and of a single measure of their parents
Relations of clinical measures between 15 and 22 years of age.
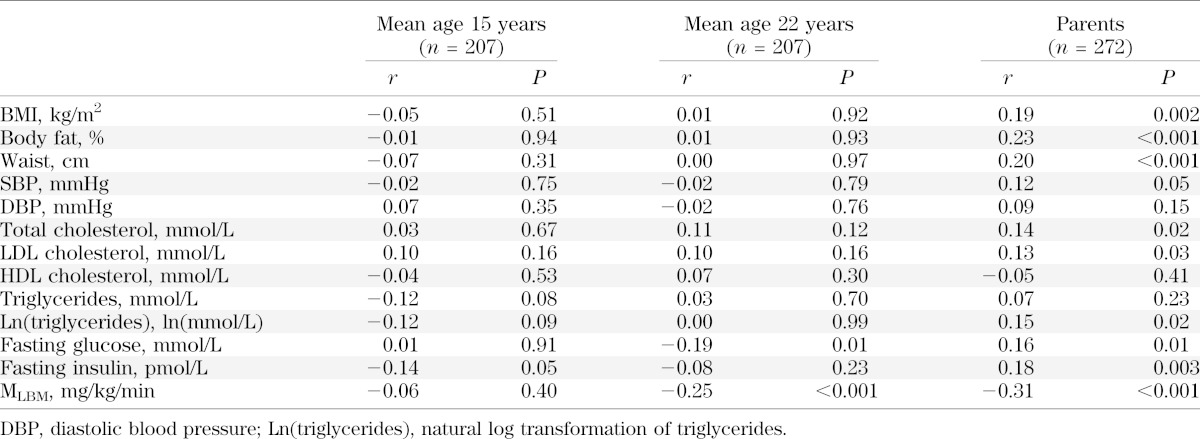
All clinical and laboratory measures were correlated significantly between the two ages, except for FFA (Table 2). When partial correlations were additionally adjusted for Tanner stage at mean 15 years of age, these relations were unchanged. When partial correlations were additionally adjusted for BMI at mean 15 years of age, relations did not change with the exception of insulin, which was greatly attenuated (r = 0.07; P = 0.30). Relationships in males and females separately were similar to those computed in the entire group, with the exception of FFAs, which were significantly correlated between mean 15 and 22 years of age in females only (r = 0.27; P = 0.01).
TABLE 2.
Tracking correlations of CV disease risk factors between mean ages 15 and 22 years
Relations of clinical measures between 15 and 22 years of age and midparental measure.
For the 139 subjects with parental data, clinical measures were compared between subjects and parents (Table 3). At 15 and 22 years of age, there was a significant correlation between offspring and parents for BMI, percent body fat, waist circumference, diastolic blood pressure, and natural log transformation of triglycerides. Correlations between offspring and parents became stronger for virtually all CV risk factors at 22 years of age, and SBP, total cholesterol, HDL, fasting insulin, and MLBM were significantly related between parents and their children only at mean 22 years of age. When adjusted for parental contribution (i.e., mother, father, or both), these correlations were not significantly different.
TABLE 3.
Correlations of CV risk factors between subjects at mean ages 15 and 22 years and midparental measures
Relations of FFA to other measures.
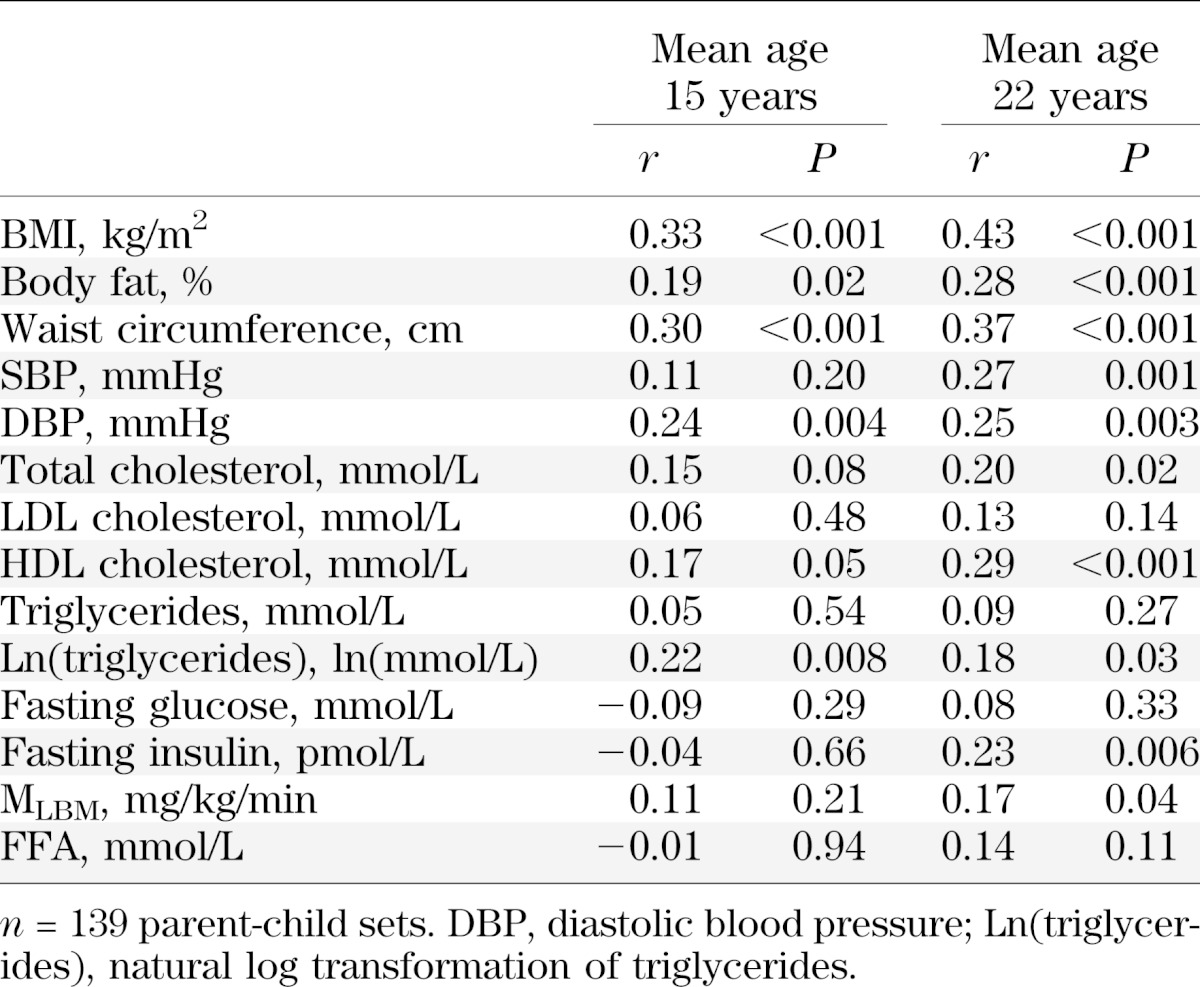
As shown in Table 4, FFAs at mean 15 years of age, adjusted for age, sex, and race, were not significantly correlated with blood pressure, measures of adiposity, lipids, fasting glucose, fasting insulin, FFA, or IR (MLBM), with similar results when analyzed separately for males and females. When additionally adjusted for Tanner stage (n = 183), only the relation with fasting insulin and natural log transformation of triglycerides became significant (r = −0.17 and P = 0.02 and r = −0.16 and P = 0.03, respectively). At mean 22 years of age, FFAs were not significantly correlated with blood pressure, measures of adiposity, serum lipids, or fasting insulin. There was a significant inverse relation with fasting glucose and MLBM. Adjustment for BMI did not appreciably affect these relations at either age (data not shown). In separate analyses by sex, results were unchanged, except correlations in females between FFAs and both fasting glucose and insulin were not significant.
TABLE 4.
Cross-sectional correlation of serum FFA with measures of adiposity and CV risk factors, adjusted for age, sex, and race, at participant mean ages 15 and 22 years and in their parents
In contrast to 15- and 22-year-olds, FFAs in parents were significantly correlated with measures of adiposity, SBP, fasting glucose, fasting insulin, MLBM, total cholesterol, natural log transformation of triglycerides, and LDL. After adjustment for BMI in the parent group, there was no longer a significant relation with waist circumference (r = 0.09; P = 0.16), SBP (r = 0.091; P = 0.14), fasting glucose (r = 0.09; P = 0.14), natural log transformation of triglycerides (r = 0.09; P = 0.14), or insulin (r = 0.09; P = 0.13), but relations with percent body fat, blood lipids, and IR continued to be significant. Separate analyses by sex showed some differences between mothers and fathers. Significant correlations between FFA and BMI, waist circumference, and fasting insulin were found only in mothers; a significant relation between FFA and SBP was seen only in fathers.
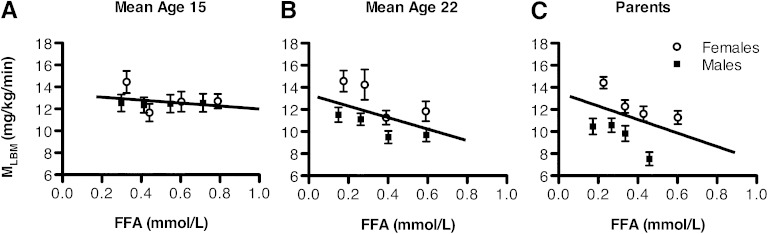
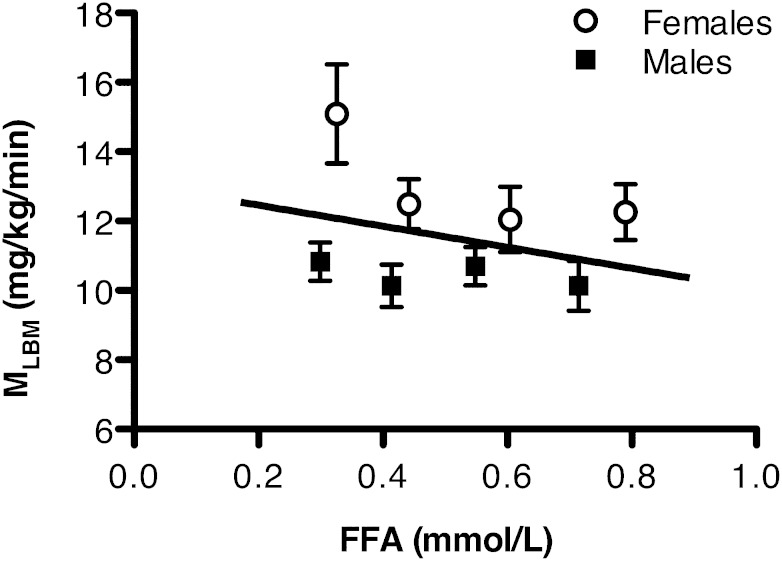
The relation between FFA and IR (MLBM) by age and sex, adjusted for BMI and race, is shown in Fig. 1. Linear regression of MLBM on FFA with age, sex, race, and BMI in the model showed a nonsignificant inverse trend at mean age 15 years (β = −1.35; P = 0.37), a significant inverse relation at mean 22 years of age (β = −5.22; P < 0.001), and the strongest inverse relation in parents (β = −6.12; P < 0.0001). Further adjustment of IR at 15 years of age for Tanner stage did not change results (data not shown).
FIG. 1.
Cross-sectional multiple regression of IR (MLBM) on FFA level, adjusted for age, race, BMI, and sex in adolescents (mean 15 years of age), young adults (mean 22 years of age), and their parents (mean 51 years of age). At mean 15 years of age, β = −1.35 mg/kg/min per mmol/L (P = 0.37); at mean 22 years of age, β = −5.22 mg/kg/min per mmol/L (P < 0.001); and in parents, β = −6.12 mg/kg/min per mmol/L (P < 0.0001). Sex-specific mean MLBM quartiles are included for goodness-of-fit assessment. ○, females; ■, males.
Estimation of CV risk factors and IR at mean 22 years of age from mean 15 years of age, FFAs, and changes in FFAs from mean 15 to 22 years of age.
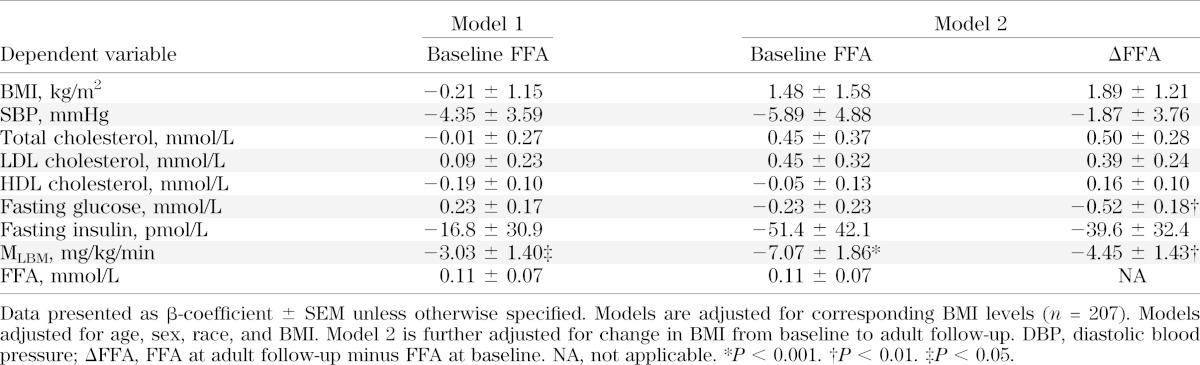
In multivariable model 1, with baseline (mean 15 years of age) FFAs and BMI as independent variables and age, sex, and race as covariates, baseline FFA was associated only with MLBM (P = 0.03) and not with later FFA (Table 5 and Fig. 1). In model 2, which included change in both FFA and BMI from mean 15 years of age to mean 22 years of age in addition to baseline FFA and BMI, fasting glucose was estimated by change in FFA (P = 0.004) and MLBM was estimated by baseline FFA (P = 0.0002) and change in FFA (P = 0.002). In model 3 (data not shown), in which the baseline CV risk factor under consideration was included in addition to the independent variables in model 2, there was little change from model 2; MLBM was again estimated by baseline FFA (P = 0.0003) and change in FFA (P = 0.002), and fasting glucose was estimated by change in FFA (P = 0.002); in addition, change in FFA now estimated LDL (P = 0.02). Adjustment for Tanner stage at mean 15 years of age did not change these results (data not shown).
TABLE 5.
Results of multivariable models using baseline FFA at mean age 15 years to estimate CV risk factor levels at mean age 22 years
Linear regression analyses were used to assess the relation between FFAs at mean 15 years of age and MLBM at mean 22 years of age, adjusting for sex, baseline age, and BMI (Fig. 2). FFAs at mean 15 years of age was a significant estimator of MLBM at mean 22 years of age (β = −3.03; P = 0.03). After adjustment for Tanner stage at 15 years of age, this relationship persisted (β = −3.29; P = 0.03).
FIG. 2.
Multiple regression of IR (MLBM) at mean 22 years of age on FFA level at mean 15 years of age, adjusted for age, race, BMI, and sex (β = −3.03 mg/kg/min per mmol/L; P = 0.03). Sex-specific mean MLBM quartiles are included for goodness-of-fit assessment. ○, females; ■, males.
DISCUSSION
This study provides new information on relations between FFA and both CV risk factors and IR in children and adults by showing longitudinal developmental changes during the transition from adolescence to young adulthood, the increasing strength of relations between FFA and IR with aging, and the increasing strength of the relations between parents and children from mean 15 years of age to mean 22 years of age. At mean 15 years of age, FFA levels were not associated with any of the measures of adiposity, CV risk factors, or IR. By mean 22 years of age, FFA levels began to show a significant relation to IR but not to adiposity or CV risk factors. Only in the older adult group (parents; mean 51 years of age) was there a significant relation of FFA to a broad group of cardiometabolic risk factors, including adiposity, systolic blood pressure, lipids, fasting insulin, and glucose, and in particular, there was a more pronounced significant relation to IR.
Although there were significant correlations between parents and children for a number of CV risk factors, relation with FFA was not significant. To our knowledge, this is the first time FFA and IR by euglycemic-hyperinsulinemic clamp have been compared in a large parent-child cohort. Similar to previous studies, we also found significant relations between parents and children for BMI, blood pressure, and lipids, with the exception of LDL (23). Although LDL also was significantly related between parents and children in the Bogalusa Heart Study, the correlation was similarly fairly weak and that cohort was significantly larger, with greater power to detect such associations (23).
Previous studies have reported relations between FFA and both adiposity and IR; however, studies in the pediatric population are limited. In contrast to adults, fasting FFAs in prepubertal children were not associated with IR as measured by the euglycemic-hyperinsulinemic clamp (24). Although an inverse relation between FFAs and fasting insulin has been reported in prepubertal children, other studies showed no relation of FFAs with fasting insulin in both prepubertal and pubertal children (24–28). Lipid infusion, which results in elevated FFA levels, increased IR in pubertal but not in prepubertal children (29). There was no difference in FFA-mediated changes in IR after lipid infusion in African American adolescents when compared with Caucasian adolescents (30). It is unclear whether increased basal levels of FFAs seen in obese adolescents relate to increased fat mass or impaired antilipolytic effect of insulin (31,32). Whereas a definite pattern cannot be established from those studies, they suggest a potential age-dependent or development-dependent change in the relation between FFAs and both fasting insulin and IR.
Longitudinal studies in adults have shown higher FFA levels were associated with increased risk of impaired glucose tolerance or type 2 diabetes (33); in Pima Indians with normal glucose tolerance, there is an association of baseline FFA levels with later IR by euglycemic clamp (34). In contrast, another study showed no association of baseline FFAs with fasting insulin and glucose on follow-up (35). The current study expands on previous studies of children or adults by exploring longitudinal changes between adolescence and adulthood and relating FFA levels between adolescents and their adult parents.
The relation between FFA levels and IR at mean 22 years of age is independent of degree of adiposity, existing before any association with adiposity or FFA levels develops, as shown in the older adults. Together with the observation that higher FFA levels at mean 15 years of age are associated with higher IR at mean 22 years of age, this suggests that FFA-related metabolic abnormalities develop with increasing age, exhibiting a causal relation in the development of IR. Association of FFA levels with other CV risk factors does not appear until later adulthood, suggesting a progressive effect of FFAs on the pathogenesis of CV disease. This is supported by studies in a rat model system, which showed an increased FFA-induced inflammatory response and IR with aging (36). Together, these findings support the hypothesis that fat metabolism and distribution are early pathogenic factors in development of IR.
The relations between FFAs and IR did not completely parallel relations of FFAs with fasting insulin, probably, in part, because of developmental changes associated with transition from adolescence to young adulthood. Both fasting insulin and insulin sensitivity decreased from 15 to 22 years of age. Previous studies of this normal cohort, with a wide range of BMI and IR, have shown during adolescence a poor correlation between fasting insulin and IR (37), a significant increase in IR without a significant change in fasting insulin during puberty (19), and separate patterns of change in fasting insulin and IR from 11 to 19 years of age (19). Thus, these studies provide evidence to support the complex relation between fasting insulin and IR, particularly during the second decade of life. The finding that levels of both were similar at 22 and 51 years of age (parents) suggests that the relations become more stable during adulthood.
FFA levels were overall higher, independent of BMI, at mean 15 years of age compared with later measures in young adulthood and in their parents. This observation may relate to differences in FFA assays; however, it is consistent with a previous study in which higher FFA levels in children were associated with higher rates of basal lipolysis than in adults (38). Moreover, increased lipolytic activity was correlated with IGF-I levels, which are substantially higher in children and in adolescents than during adulthood (38,39).
FFA levels in circulation reflect the balance between lipolysis, formation of triglyceride droplets in adipocytes, and utilization of fatty acids by muscle. Lipolysis is regulated hormonally, stimulated by catecholamines, and inhibited by insulin. In the insulin-sensitive state, triglyceride storage is promoted via the peroxisome proliferator–activated receptor-γ signaling pathway, and peroxisome proliferator–activated receptor-γ agonists decrease plasma FFA levels (40). In obesity, lipolysis is driven, in part, by increased adipocyte size but also results from adipocyte resistance to the antilipolytic effect of insulin. The latter appears to be related to local adipose tissue inflammation, which is associated with macrophage infiltration and increased local production of tumor necrosis factor-α (40). Tumor necrosis factor-α promotes lipolysis via inhibition of the insulin signaling pathway, modulation of G-protein signaling, and downregulation of perilipin, a lipid droplet-associated protein that plays a critical role in lipolysis (41). Beyond inflammatory changes in adipose tissue, there is some evidence that dysfunction of other adipocyte processes may play a role in lipolysis. Oxidative stress in the adipocyte has been shown to result in protein carbonylation, a covalent modification of proteins by oxidized lipids. Protein carbonylation has been linked to mitochondrial dysfunction and increased lipolysis (42). It is also increased in human obesity and positively associated with serum FFA (43).
Reducing FFA levels early in life may be protective against development of adult CV disease. FFAs are among a group of circulating lipids shown to induce chronic inflammation and cellular dysfunction in nonadipose tissues, a process termed lipotoxicity (44). Macrophages and other tissues take up excess FFA, resulting in steatotic liver injury, IR, atherosclerosis, and chronic kidney disease (44). Lipid infusion causes increased oxidative stress and IR in both liver and muscle and is associated with increased serum levels of malondialdehyde, a product of adipose oxidative stress (45,46). Lipid infusion also impairs microvascular recruitment in skeletal and cardiac muscle, suggesting a potential role for FFAs in CV disease (47).
Among the limitations of this study, the predominately Caucasian cohort, reflecting the local population, reduces the generalizability to more diverse populations. Participants in the original cohort without complete datasets at 15 or 22 years of age were excluded; however, there were no significant differences between included versus excluded participants, except for slightly higher fasting glucose at mean 15 years of age and higher steady-state insulin during euglycemic-hyperinsulinemic clamp at mean age 22 years. Differences in laboratory techniques for measuring FFAs between visits required results from mean 15 years of age to be converted from concentration by mass (μg/mL) into concentration by molarity (mmol/L). The sensitivity of this conversion is not known, and we were unable to find literature directly comparing the two methods. This study did not include data regarding diet and physical activity. Either of these factors could affect FFA levels, as shown previously (48–50). Finally, the parental group was limited by availability of only one parent in some cases, potentially affecting relation of midparental measures to offspring. The sex of the parent or availability of both parents was not significant when added to the analysis. Whereas comparison of parents and children allows some evaluation of genetic contribution, this is confounded by environment, which also may be shared. Measures in which genetics and environment have opposite effects may make these relations more difficult to detect.
This study also had some specific strengths. It is unique in its analysis of relations between FFAs and both CV risk factors and IR in a longitudinal cohort, using the gold standard euglycemic-hyperinsulinemic clamp to measure IR. The large size of the cohort is a further strength. This is the first time FFA levels during childhood have been related to later IR in adulthood. Finally, inclusion of the parental group allows for studying developmental changes in FFA relations, suggesting a gradually increasing association with CV risk factors and IR from adolescence to young adulthood.
In summary, this study shows that the relation between FFAs and IR is not significant in early adolescence; however, FFA estimates future IR, the relation becomes significant during the transition to young adulthood, and it continues to increase, along with development of significant relations to adiposity and CV risk factors, during aging. The observation that FFA in the parental group was significantly associated with BMI and most of the CV risk factors, unlike at younger ages, may suggest that elevated FFA and adipose dysfunction have cumulative effects on metabolic disease that are not fully expressed until later adulthood. Better understanding of mechanisms underlying serum FFA levels may help define development of IR and interactions with adiposity.
ACKNOWLEDGMENTS
The work presented in this article was supported by National Institutes of Health grants HL-52851 and M01-RR-00400 (General Clinic Research Centers).
No potential conflicts of interest relevant to this article were reported.
B.I.F. researched and analyzed data and wrote the manuscript. D.R.J. researched and analyzed data and reviewed and edited the manuscript. J.S., A.M., L.M.S., and A.R.S. researched data and reviewed and edited the manuscript. A.R.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the individuals who participated in the study and study coordinator Lauri Schafer, Department of Pediatrics, University of Minnesota.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db12-1122/-/DC1.
REFERENCES
- 1.Jensen MD, Haymond MW, Rizza RA, Cryer PE, Miles JM. Influence of body fat distribution on free fatty acid metabolism in obesity. J Clin Invest 1989;83:1168–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arner P. Insulin resistance in type 2 diabetes: role of fatty acids. Diabetes Metab Res Rev 2002;18(Suppl. 2):S5–S9 [DOI] [PubMed] [Google Scholar]
- 3.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963;1:785–789 [DOI] [PubMed] [Google Scholar]
- 4.Lillioja S, Bogardus C, Mott DM, Kennedy AL, Knowler WC, Howard BV. Relationship between insulin-mediated glucose disposal and lipid metabolism in man. J Clin Invest 1985;75:1106–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boden G, She P, Mozzoli M, et al. Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-kappaB pathway in rat liver. Diabetes 2005;54:3458–3465 [DOI] [PubMed] [Google Scholar]
- 6.Kashyap SR, Belfort R, Cersosimo E, Lee S, Cusi K. Chronic low-dose lipid infusion in healthy patients induces markers of endothelial activation independent of its metabolic effects. J Cardiometab Syndr 2008;3:141–146 [DOI] [PubMed] [Google Scholar]
- 7.Kashyap SR, Belfort R, Berria R, et al. Discordant effects of a chronic physiological increase in plasma FFA on insulin signaling in healthy subjects with or without a family history of type 2 diabetes. Am J Physiol Endocrinol Metab 2004;287:E537–E546 [DOI] [PubMed] [Google Scholar]
- 8.Florian JP, Pawelczyk JA. Non-esterified fatty acids increase arterial pressure via central sympathetic activation in humans. Clin Sci (Lond) 2010;118:61–69 [DOI] [PubMed] [Google Scholar]
- 9.Carpentier A, Mittelman SD, Bergman RN, Giacca A, Lewis GF. Prolonged elevation of plasma free fatty acids impairs pancreatic beta-cell function in obese nondiabetic humans but not in individuals with type 2 diabetes. Diabetes 2000;49:399–408 [DOI] [PubMed] [Google Scholar]
- 10.Boden G, Chen X, Rosner J, Barton M. Effects of a 48-h fat infusion on insulin secretion and glucose utilization. Diabetes 1995;44:1239–1242 [DOI] [PubMed] [Google Scholar]
- 11.Zhang H, Dellsperger KC, Zhang C. The link between metabolic abnormalities and endothelial dysfunction in type 2 diabetes: an update. Basic Res Cardiol 2012;107:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Randle PJ, Priestman DA, Mistry SC, Halsall A. Glucose fatty acid interactions and the regulation of glucose disposal. J Cell Biochem 1994;55(Suppl.):1–11 [DOI] [PubMed] [Google Scholar]
- 13.Baldeweg SE, Golay A, Natali A, Balkau B, Del Prato S, Coppack SW, European Group for the Study of Insulin Resistance (EGIR) . Insulin resistance, lipid and fatty acid concentrations in 867 healthy Europeans. Eur J Clin Invest 2000;30:45–52 [DOI] [PubMed] [Google Scholar]
- 14.Boden G, Laakso M. Lipids and glucose in type 2 diabetes: what is the cause and effect? Diabetes Care 2004;27:2253–2259 [DOI] [PubMed] [Google Scholar]
- 15.Golay A, Felber JP, Jallut D, Munger R, Ruiz J, Jéquier E. Effect of lipid oxidation on the regulation of glucose utilization in obese patients. Acta Diabetol 1995;32:44–48 [DOI] [PubMed] [Google Scholar]
- 16.Belfort R, Mandarino L, Kashyap S, et al. Dose-response effect of elevated plasma free fatty acid on insulin signaling. Diabetes 2005;54:1640–1648 [DOI] [PubMed] [Google Scholar]
- 17.Roden M, Price TB, Perseghin G, et al. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest 1996;97:2859–2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gulli G, Ferrannini E, Stern M, Haffner S, DeFronzo RA. The metabolic profile of NIDDM is fully established in glucose-tolerant offspring of two Mexican-American NIDDM parents. Diabetes 1992;41:1575–1586 [DOI] [PubMed] [Google Scholar]
- 19.Moran A, Jacobs DR, Jr, Steinberger J, et al. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes 1999;48:2039–2044 [DOI] [PubMed] [Google Scholar]
- 20.Slaughter MH, Lohman TG, Boileau RA, et al. Skinfold equations for estimation of body fatness in children and youth. Hum Biol 1988;60:709–723 [PubMed] [Google Scholar]
- 21.Steinberger J, Jacobs DR, Raatz S, Moran A, Hong C-P, Sinaiko AR. Comparison of body fatness measurements by BMI and skinfolds vs dual energy X-ray absorptiometry and their relation to cardiovascular risk factors in adolescents. Int J Obes (Lond) 2005;29:1346–1352 [DOI] [PubMed] [Google Scholar]
- 22.Sinaiko AR, Jacobs DR, Jr, Steinberger J, et al. Insulin resistance syndrome in childhood: associations of the euglycemic insulin clamp and fasting insulin with fatness and other risk factors. J Pediatr 2001;139:700–707 [DOI] [PubMed] [Google Scholar]
- 23.Chen W, Srinivasan SR, Bao W, Berenson GS. The magnitude of familial associations of cardiovascular risk factor variables between parents and offspring are influenced by age: the Bogalusa Heart Study. Ann Epidemiol 2001;11:522–528 [DOI] [PubMed] [Google Scholar]
- 24.Ijzerman RG, Stehouwer CDA, Serné EH, et al. Incorporation of the fasting free fatty acid concentration into quantitative insulin sensitivity check index improves its association with insulin sensitivity in adults, but not in children. Eur J Endocrinol 2009;160:59–64 [DOI] [PubMed] [Google Scholar]
- 25.Garcés C, Cano B, Granizo JJ, et al. Insulin and HOMA in Spanish prepubertal children: relationship with lipid profile. Clin Biochem 2005;38:920–924 [DOI] [PubMed] [Google Scholar]
- 26.Chu N-F, Chang J-B, Shieh S-M. Plasma leptin, fatty acids, and tumor necrosis factor-receptor and insulin resistance in children. Obes Res 2003;11:532–540 [DOI] [PubMed] [Google Scholar]
- 27.Sabin MA, De Hora M, Holly JMP, et al. Fasting nonesterified fatty acid profiles in childhood and their relationship with adiposity, insulin sensitivity, and lipid levels. Pediatrics 2007;120:e1426–e1433 [DOI] [PubMed] [Google Scholar]
- 28.Allard P, Delvin EE, Paradis G, et al. Distribution of fasting plasma insulin, free fatty acids, and glucose concentrations and of homeostasis model assessment of insulin resistance in a representative sample of Quebec children and adolescents. Clin Chem 2003;49:644–649 [DOI] [PubMed] [Google Scholar]
- 29.Arslanian S, Suprasongsin C. Glucose-fatty acid interactions in prepubertal and pubertal children: effects of lipid infusion. Am J Physiol 1997;272:E523–E529 [DOI] [PubMed] [Google Scholar]
- 30.Burns SF, Kelsey SF, Arslanian SA. Effects of an intravenous lipid challenge and free fatty acid elevation on in vivo insulin sensitivity in African American versus Caucasian adolescents. Diabetes Care 2009;32:355–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson C, Tamborlane WV, Maggs DG, et al. Effect of insulin on glycerol production in obese adolescents. Am J Physiol 1998;274:E737–E743 [DOI] [PubMed] [Google Scholar]
- 32.Caprio S, Hyman LD, Limb C, et al. Central adiposity and its metabolic correlates in obese adolescent girls. Am J Physiol 1995;269:E118–E126 [DOI] [PubMed] [Google Scholar]
- 33.Salgin B, Ong KK, Thankamony A, Emmett P, Wareham NJ, Dunger DB. Higher fasting plasma free fatty acid levels are associated with lower insulin secretion in children and adults and a higher incidence of type 2 diabetes. J Clin Endocrinol Metab 2012;97:3302–3309 [DOI] [PubMed] [Google Scholar]
- 34.Stefan N, Stumvoll M, Bogardus C, Tataranni PA. Elevated plasma nonesterified fatty acids are associated with deterioration of acute insulin response in IGT but not NGT. Am J Physiol Endocrinol Metab 2003;284:E1156–E1161 [DOI] [PubMed] [Google Scholar]
- 35.Byrne CD, Maison P, Halsall D, Martensz N, Hales CN, Wareham NJ. Cross-sectional but not longitudinal associations between non-esterified fatty acid levels and glucose intolerance and other features of the metabolic syndrome. Diabet Med 1999;16:1007–1015 [DOI] [PubMed] [Google Scholar]
- 36.Einstein FH, Huffman DM, Fishman S, et al. Aging per se increases the susceptibility to free fatty acid-induced insulin resistance. J Gerontol A Biol Sci Med Sci 2010;65:800–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz B, Jacobs DR, Moran A, Steinberger J, Hong C-P, Sinaiko AR. Measurement of insulin sensitivity in children: Comparison between the euglycemic hyperinsulinemic clamp and surrogate measures. Diabetes Care 2008;31:783–788 [DOI] [PubMed]
- 38.Arslanian SA, Kalhan SC. Correlations between fatty acid and glucose metabolism. Potential explanation of insulin resistance of puberty. Diabetes 1994;43:908–914 [DOI] [PubMed] [Google Scholar]
- 39.Moran A, Jacobs DR, Jr, Steinberger J, et al. Association between the insulin resistance of puberty and the insulin-like growth factor-I/growth hormone axis. J Clin Endocrinol Metab 2002;87:4817–4820 [DOI] [PubMed] [Google Scholar]
- 40.Larsen TM, Toubro S, Astrup A. PPARgamma agonists in the treatment of type II diabetes: is increased fatness commensurate with long-term efficacy? Int J Obes Relat Metab Disord 2003;27:147–161 [DOI] [PubMed] [Google Scholar]
- 41.Langin D, Arner P. Importance of TNFalpha and neutral lipases in human adipose tissue lipolysis. Trends Endocrinol Metab 2006;17:314–320 [DOI] [PubMed] [Google Scholar]
- 42.Curtis JM, Grimsrud PA, Wright WS, et al. Downregulation of adipose glutathione S-transferase A4 leads to increased protein carbonylation, oxidative stress, and mitochondrial dysfunction. Diabetes 2010;59:1132–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frohnert BI, Sinaiko AR, Serrot FJ, et al. Increased adipose protein carbonylation in human obesity. Obesity (Silver Spring) 2011;19:1735–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schaffer JE. Lipotoxicity: when tissues overeat. Curr Opin Lipidol 2003;14:281–287 [DOI] [PubMed] [Google Scholar]
- 45.He B, Zhao S, Zhang W, Li Y, Han P. Effect of sodium salicylate on oxidative stress and insulin resistance induced by free fatty acids. Hepatobiliary Pancreat Dis Int 2010;9:49–53 [PubMed] [Google Scholar]
- 46.Park E, Wong V, Guan X, Oprescu AI, Giacca A. Salicylate prevents hepatic insulin resistance caused by short-term elevation of free fatty acids in vivo. J Endocrinol 2007;195:323–331 [DOI] [PubMed] [Google Scholar]
- 47.Chai W, Liu J, Jahn LA, Fowler DE, Barrett EJ, Liu Z. Salsalate attenuates free fatty acid-induced microvascular and metabolic insulin resistance in humans. Diabetes Care 2011;34:1634–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee M-G, Sedlock DA, Flynn MG, Kamimori GH. Resting metabolic rate after endurance exercise training. Med Sci Sports Exerc 2009;41:1444–1451 [DOI] [PubMed] [Google Scholar]
- 49.Chen S-Y, Chen S-M, Chang W-H, et al. Effect of 2-month detraining on body composition and insulin sensitivity in young female dancers. Int J Obes (Lond) 2006;30:40–44 [DOI] [PubMed] [Google Scholar]
- 50.Steffen LM, Vessby B, Jacobs DR, Jr, et al. Serum phospholipid and cholesteryl ester fatty acids and estimated desaturase activities are related to overweight and cardiovascular risk factors in adolescents. Int J Obes (Lond) 2008;32:1297–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]