Abstract
Delayed diabetic wound healing is, in part, the result of inadequate endothelial progenitor cell (EPC) proliferation, mobilization, and trafficking. Recently, we developed a serum-free functional culture system called the quality and quantity culture (QQc) system that enhances the number and vasculogenic potential of EPCs. We hypothesize that QQc restoration of diabetic EPC function will improve wound closure. To test this hypothesis, we measured diabetic c-kit+Sca-1+lin− (KSL) cell activity in vitro as well as the effect of KSL cell–adoptive transfer on the rate of euglycemic wound closure before and after QQc. KSL cells were magnetically sorted from control and streptozotocin-induced type I diabetic C57BL6J bone marrow. Freshly isolated control and diabetic KSL cells were cultured in QQc for 7 days and pre-QQc and post-QQc KSL function testing. The number of KSL cells significantly increased after QQc for both diabetic subjects and controls, and diabetic KSL increased vasculogenic potential above the fresh control KSL level. Similarly, fresh diabetic cells form fewer tubules, but QQc increases diabetic tubule formation to levels greater than that of fresh control cells (P < 0.05). Adoptive transfer of post-QQc diabetic KSL cells significantly enhances wound closure compared with fresh diabetic KSL cells and equaled wound closure of post-QQc control KSL cells. Post-QQc diabetic KSL enhancement of wound closure is mediated, in part, via a vasculogenic mechanism. This study demonstrates that QQc can reverse diabetic EPC dysfunction and achieve control levels of EPC function. Finally, post-QQc diabetic EPC therapy effectively improved euglycemic wound closure and may improve diabetic wound healing.
Although blood supply is essential for tissue viability, new blood vessel formation is critical for tissue recovery, regeneration, and repair. Postnatal new blood vessel formation was long thought to be restricted to angiogenesis, the sprouting of new blood vessels from existing vascular structures. However, in 1997, we demonstrated that the de novo formation of new blood vessel derived from bone marrow (BM)-derived cells (i.e., vasculogenesis) is an important part of postnatal healing (1–3). The BM-derived endothelial progenitor cells (EPCs) are precursors of endothelial cells (ECs) and are characterized by their surface expression of KDR, CD133, and CD34 for humans and of lineage-negative c-kit+Sca-1+ (KSL) cells for murine BM cells (4–6).
After injury, locally derived circulating factors mobilize EPCs from their endosteal BM niche. Circulating BM-derived EPCs traffic to the site of injury, experience diapedesis, cluster, tubulize, and canalize to form nascent vessels that inosculate with the existing vasculature (7,8). EPCs have been shown to revascularize numerous ischemic tissues, including myocardium (i.e., myocardial infarction), brain (i.e., cerebral infarction), and skin (i.e., cutaneous wounding) (9,10). Whereas BM-derived EPCs contribute to only 25% of newly formed endothelium in healing tissues, when EPC function is impaired there are marked deficits in tissue repair mechanisms (11,12).
Compared with nondiabetic patients, diabetic EPCs have impaired proliferation, adhesion, migration, and differentiation (13–15). Although the pathogenesis of impaired diabetic wound healing is multifactorial, EPC dysfunction plays a central role (16,17). These intrinsic diabetic EPC vasculogenic impairments may result in >83,000 amputations each year and a postamputation 3-year mortality rate of 75.9% (18). In preclinical studies, the administration of exogenous EPCs has improved ventricular function after myocardial ischemia (19,20), enhanced neuronal recovery after cerebral vascular occlusion, and accelerated restoration of blood flow to ischemic limbs (13,16,17,21–23). Based on these exciting results, we have conducted a phase 3 clinical trial of autologous granulocyte colony-stimulating factor–mobilized peripheral blood EPC therapy for nonhealing diabetic foot patients (24). The results demonstrated that more successful therapeutic results were seen in patients receiving high-vasculogenic EPCs. From these results, we hypothesize that successful autologous diabetic EPC therapy relies on the vasculogenic function of transplanted EPCs and speculate that the intrinsic diabetic EPC dysfunction will limit the efficacy of the therapeutic strategy (25,26).
Recently, our group established a serum-free quality and quantity culture (QQc) system (containing stem cell factor, thrombopoietin, vascular endothelial growth factor, interleukin-6, and Flt-3 ligand) that enhances the vasculogenic potential of EPCs (27). We hypothesize that QQc can reverse the detrimental effects of diabeties-induced EPC dysfunction and can supply a sufficient number of functional EPCs for adoptive autologous cell–based therapy for diabetic patients. In the current study, we tested this hypothesis.
RESEARCH DESIGN AND METHODS
Diabetic mouse model.
C57BL/6J male mice aged 8–10 weeks and weighing 20–25 g were purchased from Crea Japan (Kawasaki, Japan) and The Jackson Laboratory (Bar Harbor, ME). Obliteration of pancreatic β-cells was achieved with intraperitoneal injections of 50 mg/kg streptozotocin (STZ; Sigma-Aldrich, St. Louis, MO) in 50 mmol/L sodium citrate buffer (pH 4.5) for 5 consecutive days (28). Ten days after the initial injection, mice with a blood glucose level >300 mg/dL were deemed diabetic, whereas those with a level <300 mg/dL received an additional 3 days of STZ injections (50 mg/kg). Mice were considered diabetic if they maintained glucose levels >300 mg/dL for at least 4 weeks before the date of wounding. Control mice received intraperitoneal injections at the same time points with an equal volume of 50 mmol/L sodium citrate buffer. A total of 200 mice were used in this experiment (n = 100 per group). All procedures were conducted in accordance with the guidelines set forth by the committee of Ethical Animal Care and Use at Tokai University School of Medicine and the Institutional Animal Care and Use Committee at New York University Medical Center.
BM progenitor cell isolation.
BM cells were harvested from diabetic and control mouse femurs and tibias as previously described (7). Mononuclear cells were washed with PBS-EDTA, and erythrocytes were removed by ammonium chloride hemolyzation and stained with a lineage-positive antibody cocktail containing CD45R/B220, TER119, CD3e, CD11b, Ly-6G, and Ly6C (Gr-1) for 20 min at 4°C (all antibodies obtained from BD PharMingen, San Diego, CA). After labeling the lineage-positive antibodies with biotin-labeled magnetic beads, cells underwent a negative selection process with a magnetic cell sorting system. Lineage-negative cells were counted and then incubated with rat fluorescein isothiocyanate antimouse Ly-6A/E (Sca-1; BD PharMingen) and rat phycoerythrin CD117 (c-kit; BD PharMingen) for 20 min at 4°C, washed three times, and resuspended in 20% Iscove modified Dulbecco medium (Gibco, Carlsbad, CA). Fluorescein isothiocyanate–conjugated Sca-1 and phycoerythrin-conjugated c-kit double-positive cells (KSL cells) then were obtained by fluorescent-activated cell sorting.
Serum-free QQc.
Diabetic and control BM KSL cells were isolated as described, and 1 × 103 cells were placed in each well of a 24-well plate (BD Falcon, Bedford, MA) and cultured in QQc for 7 days as previously described (27). Briefly, QQc is an optimized growth factor/cytokine combination (20 ng/mL thyroid peroxidase, 20 ng/mL interleukin-6, 100 ng/mL SCF, 100 ng/mL Flt-3 ligand, and 50 ng/mL vascular endothelial growth factor; all from Peprotech, Rocky Hills, NJ) serum-free stem span (Stem Cell Technologies) media. After 7 days of QQc, control KSL cells were termed CQQc and diabetic KSL cells were termed DMQQc. Growth in QQc has been shown to dramatically expand and enhance the vasculogenic potential of EPCs.
EPC colony-forming assay of KSL population.
The vasculogenic potential of diabetic and control BM KSL cells was assessed using the EPC colony-forming assay as previously described (6,29,30). EPC colony-forming assay is designed to distinguish total EPC colony-forming units (tEPC-CFUs) into two different types of EPC-CFUs: primitive (small cell) and definitive (large cell). The primitive EPC-CFU (pEPC-CFUs) is a predominantly proliferative population of cells and the definitive EPCs-CFU (dEPC-CFU) is a predominantly vasculogenic population with greater adhesion, migration, and differentiation and tabularization potential. Briefly, a total of 500 BM KSL cells per dish were seeded into a 35-mm hydrophilic tissue culture dish. Seven days later, tEPC-CFUs, pEPC-CFUs, and dEPC-CFUs were counted by two investigators who were blinded to the experimental conditions. Experiments were performed in triplicate.
Tube formation assay.
Tubule formation assay was performed by adding Biocoat Matrigel (Becton Dickinson; Franklin Lakes, NJ) into 24-well plates and incubating in a CO2-free incubator at 37°C for 30 min. The same lot of Matrigel was used for all experiments. The gels were then overlaid with 3 × 103 fresh and expanded diabetic and control cells cocultured with 1 × 104 endothelial cells suspended in culture medium and incubated at 37°C in an atmosphere of 5% CO2. The well cultured with endothelial cells only was used as a control. After 12 h of incubation, gels were examined by using a phase-contrast microscope equipped with a digital camera (Nikon eclipse TE2000-U; Nikon, Melville, NY). A blinded observer measured the total number of tube-like structures per high-power field (HPF) in five random fields.
Quantitative real-time PCR.
Total RNA from 2 × 104 diabetic and control KSL cells was extracted using the RNeasy Micro kit (Qiagen, Basel, Switzerland) based on the manufacturer’s protocol, and reverse-transcription was performed using high-capacity cDNA reverse-transcription kit (Applied Biosystems, Foster City, CA). The transcription reaction was performed at 37°C for 2 h. The obtained cDNA was amplified using the reaction mixture of TaqMan FAST Universal PCR Master Mix (Applied Biosystems). The following TaqMan probes (Applied Biosystems) were used: 18S rRNA (ribosomal RNA control reagents 4308329), endothelial growth factor (Mm00438696_m1; Egf), hepatocyte growth factor (Mm01135193_m1; Hgf), fibroblast growth factor-2 (Mm00433287_m1; Fgf2), fibroblast growth factor-7 (Mm00433291_m1; Fgf7), von Willebrand factor (Mm00550376_m1; vWF), CD29 (Mm0125320_m1; Itgb1), and Flk-1 (Mm0122419_m1; Kdr). The PCR mixtures were preincubated at 95°C for 20 s, followed by 40 cycles of 95°C for 3 s and 62°C for 30 s by ABI 7500 FAST (Applied Biosystems). The real-time data were analyzed by change (Δ) in threshold cycle (Ct) method. The ΔCt was calculated as (gene of target Ct) − (18S rRNA Ct). The relative quantity of mRNA of the target gene was determined by the ΔCt calculation 2−ΔCt.
Wound model and KSL adoptive cellular therapy.
To reduce the confounding variables that would affect KSL function in a diabetic wound, we first used 8- to 10-week-old euglycemic C57BL/6J male mice (n = 45; 3 per experimental group in triplicates) as recipients for diabetic and control KSL therapy (31). To verify the efficacy of QQc diabetic cells in diabetic wound healing, a diabetic STZ mouse wound model was similarly prepared. Briefly, each mouse was anesthetized and depilated, and one set of bilateral 6-mm punch biopsy specimens was excised on the dorsum. Excisions were full-thickness, including the hypodermis and panniculus carnosum. India ink was applied intradermally at the margins to permanently mark the wound edge. A silicone stent (Grace Bio-Laboratories, Bend, OR) with an 8-mm inner diameter was sutured with 5–0 nylon (Ethicon, Somerville, NJ) around each wound to minimize skin contracture and to ensure healing by secondary intention. On postoperative day 3, a 1-mL syringe with a 30-gauge needle was used to inject 25 μL saline, 2 × 104 freshly isolated control KSL, 2 × 104 freshly isolated diabetic KSL, 2 × 104 post-QQc control KSL, or 2 × 104 post-QQc diabetic KSL (QD) into the center of the muscle at the base of the wound. The wounds were covered with Tegaderm to prevent the cells from leaking and drying.
Wound photographs were acquired with a 7-megapixel digital camera (Canon USA, Lake Success, NY) from a distance of 6.5 cm, with the lens oriented parallel to the wound. Wound area was measured digitally (Photoshop CS3; Adobe Systems, San Jose, CA) and calibrated against the internal diameter of the silicon stent to correct for magnification, perspective, or parallax effects. Percent wound closure {1 − [(wound area)/(original wound area)]} was measured photogrammetrically on days 0, 3, 7, 10, 14, 18, and 21.
Wound harvest.
Wounds were harvested from killed animals at postoperative days 7, 14, and 21 (n = 4 per group at each time point). A full-thickness excision including 3 mm beyond the margin of the original wound edge (demarcated with India ink) was performed. Each wound was bisected, and one-half of the wound was frozen in optimal cutting temperature compound for cryosectioning. The other half was fixed in 100% methanol and embedded in paraffin. Sections were cut from the central region of the wound at a thickness of 5 µm. Before staining, paraffin sections were deparaffinized and rehydrated by successive passages through xylene and decreasing concentrations of ethanol.
Van Gieson stain for wound maturity.
Wound maturity can be quantified with Van Gieson staining protocol, which simultaneously stains mature collagen deep red and stains immature collagen pink (32). Horizontal sections were cut from each specimen at each time point. Paraffin sections were processed with staining solution as described previously (33). Sections were imaged and digitized in their entirety at 200× resolution with an Aperio ScanScope GL scanning optical microscope (Aperio Technologies, Vista, CA). Images were then analyzed with Adobe Photoshop CS3 (Adobe Systems). Percentage mature collagen was quantified by measuring the total pixel area of the wound and the percentage of pixels therein that were consistent in color with mature collagen. Lateral wound margins were identified at the border of the panniculus carnosum layer.
CD31 staining for vascularity and proliferating cell nuclear antigen staining for cellular proliferation.
Paraffin sections were incubated in either CD31 (an endothelial marker) or proliferating cell nuclear antigen (PCNA; a nuclear marker for proliferation) (both from Cell Signaling Technology, Danvers, MA) antibodies, washed, and stained with DAB (Vector Laboratories, Burlingame, CA). Slides were examined under 200× magnification and captured as digital images (Olympus BX51 microscope and DP12 camera). In CD31-labeled sections, patent vessels were tallied, and numeral density was reported as vessels per 200× field. Cross-sectional area of each vessel was obtained with Adobe Photoshop CS3 and reported as total cross-sectional area normalized to wound area as well as average cross-sectional area per vessel. In PCNA-labeled sections, nuclei exhibiting positive PCNA staining were tallied and reported as cells per 200× field.
Green fluoroscent protein and vWF costaining.
To follow the adoptively transferred cell trafficking, BM-KSL cells from diabetic and controls were isolated from 8- to 10-week-old green fluorescent protein (GFP)-expressing C57BL6 mice (CLEA Japan) as described. The KSL cells were cultured in QQc medium for 1 week as described; 2 × 104 GFP-KSL cells were injected into 8- to 10-week-old euglycemic C57BL/6J male mice as described. Wounds were harvested from killed animals at postoperative days 7, 14, and 21 (n = 4 per group at each time point) as described. Tissue sections were fixed in 4% paraformaldehyde overnight at 4°C, processed through 100% ethanol and xylenes, and embedded in paraffin. To enhance GFP expression, samples were incubated with a 1:300 dilution of anti-GFP mouse polyclonal antibody (Invitrogen) for 1 h at room temperature, washed, and stained with DAB (DOTITE). For vWF staining, the sections were further treated with 1:300 dilution anti-vWF rabbit polyclonal antibody (DAKO) for 4°C overnight and washed and blocked in 5% normal sheep serum for 5 min followed by anti-rabbit IgG alkaline phosphatase-streptavidin complex (NICHIREI). The double-stained images with vWF images were obtained with the same equipment as described. Dual-filter images were superimposed to illustrate GFP trafficking and wound vascular architecture.
Statistical analysis.
All data are presented as the mean ± SD. A Kruskal-Wallis one-way ANOVA with Tukey-Kramer post hoc analysis was performed when comparisons involved more than two groups. Mann-Whitney test was used for pairwise comparisons. Significance was considered at P < 0.05. The number of animals in each group was determined with an a priori power analysis using a standard for adequacy of 80% to reject the null hypothesis of zero correlation using G*Power.
RESULTS
QQc restores growth and vasculogenic potential of diabetic EPCs.
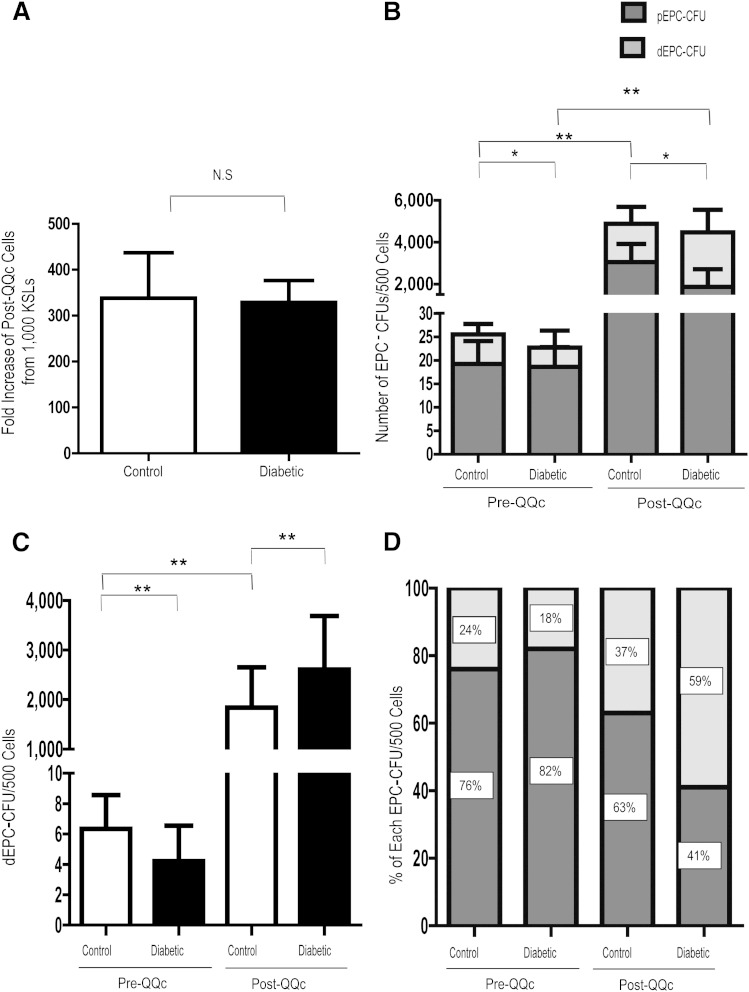
Because ex vivo expansion is an important step in adoptive cellular therapy, we measured the effects of QQc on KSL proliferation. After 7 days of QQc, the CQQc population increased 338.2- ± 260.7-fold (Fig. 1A). Similarly, after 7 days of QQc, the DMQQc population increased 329.0- ± 125.7-fold (Fig. 1A). There was no significant difference (P = 0.6) in the QQc cellular expansion of diabetic and control KSL cells.
FIG. 1.
QQc restores growth and vasculogenic potential of diabetic progenitor cells to more than the level of controls. A: Fold increase of control and diabetic post-QQc cells. B: The frequency of EPC-CFU production from pre-QQc and post-QQc control and diabetic KSL cells. C: The frequency of DEPC-CFU production from pre-QQc and post-QQc control and diabetic KSL cells. D: The percent of pEPC-CFUs and dEPC-CFUs from total EPC-CFUs. *P < 0.05; **P < 0.01. n = 3 dishes/group for 4 trials. NS, not significant.
Because QQc restored ex vivo diabetic KSL expansion to control rates, we assessed the effects of QQc on the vasculogenic potential of diabetic KSL cells using EPC colony-forming assay. Before expansion, diabetic KSL cells had similar numbers of pCFUs (19.2 ± 4.9 vs. 18.6 ± 04.2; P = 0.5) but significantly fewer tCFUs (22.7 ± 5.2 vs. 25.0 ± 3.8; P < 0.01) and dEPC-CFUs (4.2 ± 2.3 vs. 6.3 ± 2.2; P < 0.01) compared with control KSL cells. After QQc, the number of diabetic tEPC-CFUs (4,469 ± 1,593; P < 0.01), pEPC-CFUs (1,862 ± 842; P < 0.01), and dEPC-CFUs (2,607 ± 1,084; P < 0.01) increased significantly from pre-QQc levels, as did the number of diabetic tEPC-CFUs (4,469 ± 1,593 vs. 4,884 ± 1,495; P = 0.4), and dEPC-CFUs (2,607 ± 1,084 vs. 1,839 ± 813; P = 0.06) were restored to control levels (Fig. 1B). Importantly, QQc increased the percentage of diabetic dEPC-CFUs (the EPC population that most readily forms new vessels) more than three-fold (17.8 ± 8.8 vs. 58.2 ± 12.7%; P < 0.01) (Fig. 1C and D).
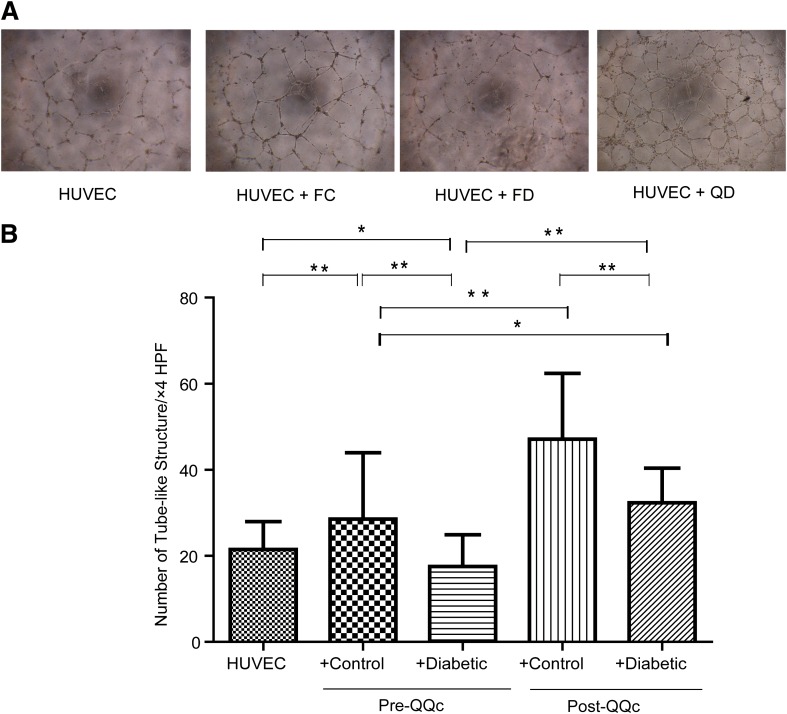
QQc restores tubular formation of diabetic progenitor cells.
Organization of endothelial cells in a three-dimensional network of tubes is the final step of angiogenesis. Because QQc increased the rate of diabetic KSL proliferation as well as differentially increased the proportion of diabetic dCFUs, we tested the effects of QQc on diabetic KSL tubule formation in vitro. Before QQc, on matrigel, diabetic KSL cells had significantly fewer tubules per HPF than controls (17.55 ± 7.4 vs. 28.53 ± 15.4; P < 0.01) (Fig. 2). Moreover, the tube formation with diabetic KSL cells demonstrated significantly less tubules per HPF compared with human umbilical vein endothelial cells with no cell group, suggesting that diabetic KSL may have a negative effect on augmenting angiogenesis (21.5 ± 6.5 vs. 17.55 ± 7.4; P < 0.05). Although the number of tubules per HPF with DMQQc KSL cells was still significantly lower (33.3 ± 8. vs. 47.1 ± 15.3; P < 0.05) than CQQc KSL cells tubules per HPF, diabetic KSL tube formation significantly increased (17.55 ± 7.4 vs. 33.3 ± 8.0; P < 0.01) after QQc, and it significantly increased compared with pre-QQc control KSL cells (33.3 ± 8.0 vs. 28.53 ± 15.4; P < 0.05)
FIG. 2.
QQc restores tubular formation of diabetic progenitor cells. A: Representative features of tube formation assay of human umbilical vein endothelial cells (HUVECs) by coculturing with presence or absence of pre-QQc and post-QQc control and diabetic cells (4× magnification). The ratio of HUVEC:KSL cells is 1 × 104:3 × 103 (10:3). B: Graph of the numbers of tubules formed in each group. n = 10 wells/group. *P < 0.05; **P < 0.01. FC, freshly isolated control cells; FD, freshly isolated diabetic cells.
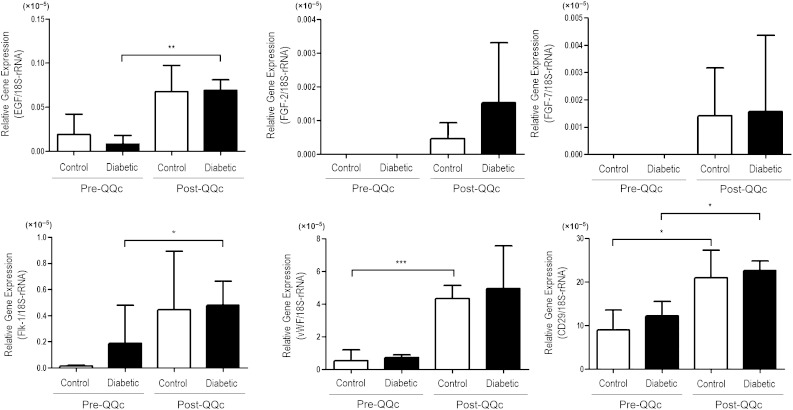
QQc enhances expression of vasculogenic and wound healing factors.
Because QQc increased the rate of diabetic KSL proliferation, differentially increased the proportion of diabetic dEPC-CFUs, and increased the tubule-forming potential of diabetic KSL cells, we tested the effects of QQc on diabetic KSL gene expression. After QQc, diabetic KSL cells increased their expression of wound healing–related growth factor genes endothelial growth factor, fibroblast growth factor-2, and fibroblast growth factor-7 and vasculogenesis-related genes vWF, CD29, and Flk-1 (Fig. 3). Although all key wound healing–related factors increased after QQc, endothelial growth factor production increased 3.5-fold in control KSL cells and 8.3-fold in diabetic KSL. Among the vasculogenesis-related genes, the expression of vWF increased by 8.3-fold and 6.7-fold in control and diabetic KSL, respectively, post-QQc. The expression of Flk-1 in CQQc KSL remarkably increased (32.7-fold), and in DMQQc KSL cells were increased 2.5-fold.
FIG. 3.
QQc enhances expansion of vasculogenic and wound healing factors. The total RNA were prepared from pre-QQc and post-QQc cells from control and diabetic mice KSL cells. The gene expression levels of proangiogenic growth factors were estimated by real-time PCR, and the data were shown as the relative gene expression of the target genes vs. 18S rRNA. The target genes of quantitative PCR were EGF, FGF-2, FGF-7, vWF, CD29, and Flk-1. The data are shown as means ± SD. n = 4. *P < 0.05; **P < 0.01; ***P < 0.001. Flk-1, VEGFR-2 (KDR/Flk-1).
In addition to the upregulation of key wound healing and vasculogenic genes, KSLs also increase their expression of CD29/integrin β-1, an integrin unit associated with the angioblastic growth cone during vasculogenesis. After QQc, CD29 expression in both CQQc and DMQQc KSL cells increased significantly (2.3-fold for control and 1.9-fold for diabetic; P < 0.05). Moreover, the expression of CD29 in DMQQc KSL cells was not significantly different from CD29 expression in CQQc KSL cells.
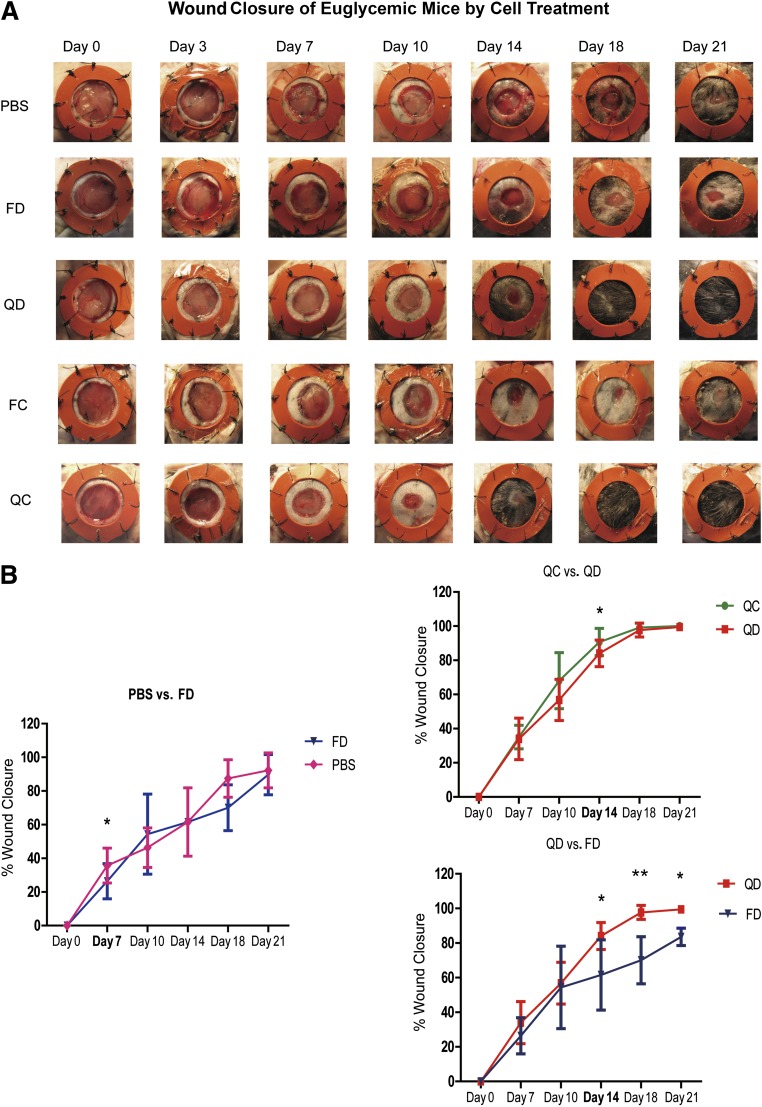
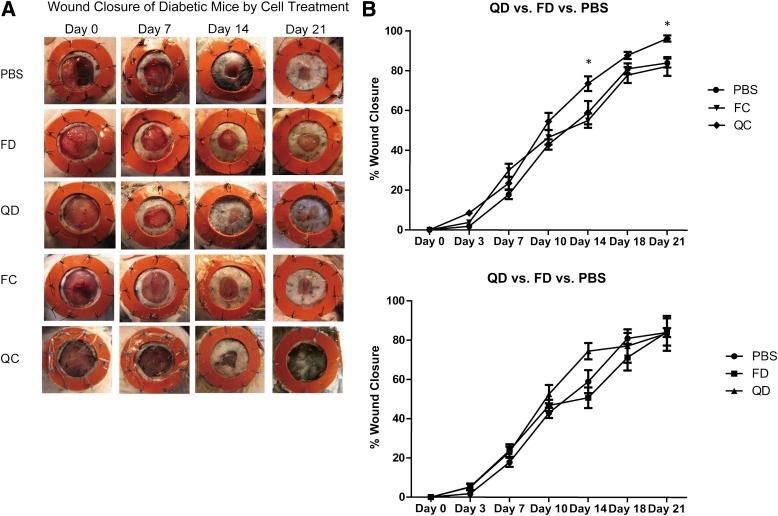
Post-QQc diabetic progenitor cell therapy accelerates wound closure.
To reduce the number of confounding variables that might affect EPC function during wound closure, we tested pre-QQc and post-QQc diabetic and control KSL-adoptive cellular therapy in wounded euglycemic mice. Adoptive cellular therapy with pre-QQc diabetic KSL cells had little impact on wound healing compared with PBS treatment on day 7 (26.1 ± 3.0 vs. 30.7 ± 1.9%; P < 0.05), day 14 (61.5 ± 5.6 vs. 70.1 ± 3.5%; P = 0.50), day 18 (70.0 ± 13.6 vs. 87.4 ± 11.1%; P = 0.6), or day 21 (83.5 ± 5.0 vs. 89.7 ± 5.5%; P = 0.05) (Fig. 4A and B). In marked contrast, adoptive transfer of DMQQc KSL cells accelerated the percent wound closure compared with pre-QQc diabetic treatment on day 14 (81.3 ± 8.7 vs. 61.5 ± 20.3%; P < 0.05), day 18 (70.0 ± 13.6 vs. 97.7 ± 4.0%; P < 0.01), or day 21 (83.5 ± 5.0 vs. 89.7 ± 5.5%; P < 0.05) (Fig. 4A and B). Moreover, the percent wound closure achieved with adoptive transfer of DMQQc KSL cells was not significantly different than the percent wound closure achieved with adoptive transfer of CQQc KSL on day 14 (81.3 ± 7.2 vs. 89.98 ± 7.7%; P < 0.05), but it was not significantly different on day 18 (97.7 ± 4.0 vs. 99.1 ± 1.5%; P = 0.9) or day 21 (94.4 ± 1.6 vs. 100 ± 0%; P = 0.3) (Fig. 4A and B).
FIG. 4.
Post-QQc adoptive diabetic progenitor cell therapy accelerates wound healing. A: Representative images show wound healing in euglycemic mice treated with PBS, pre-QQc, and post-QQc control and diabetic cells. Wounds were photographed at the times indicated from day 0 to day 21. B: The graphs show the comparison of percent wound closure between PBS and freshly isolated diabetic cell (FD)-treated group (left), post-QQc control cell (QC)-treated group, QD-treated group (top right), and QD-treated and FD-treated group (bottom right). *P < 0.05; **P < 0.01. FC, freshly isolated control cell.
Post-QQc adoptive diabetic progenitor therapy enhances wound vascularization and collagen maturation.
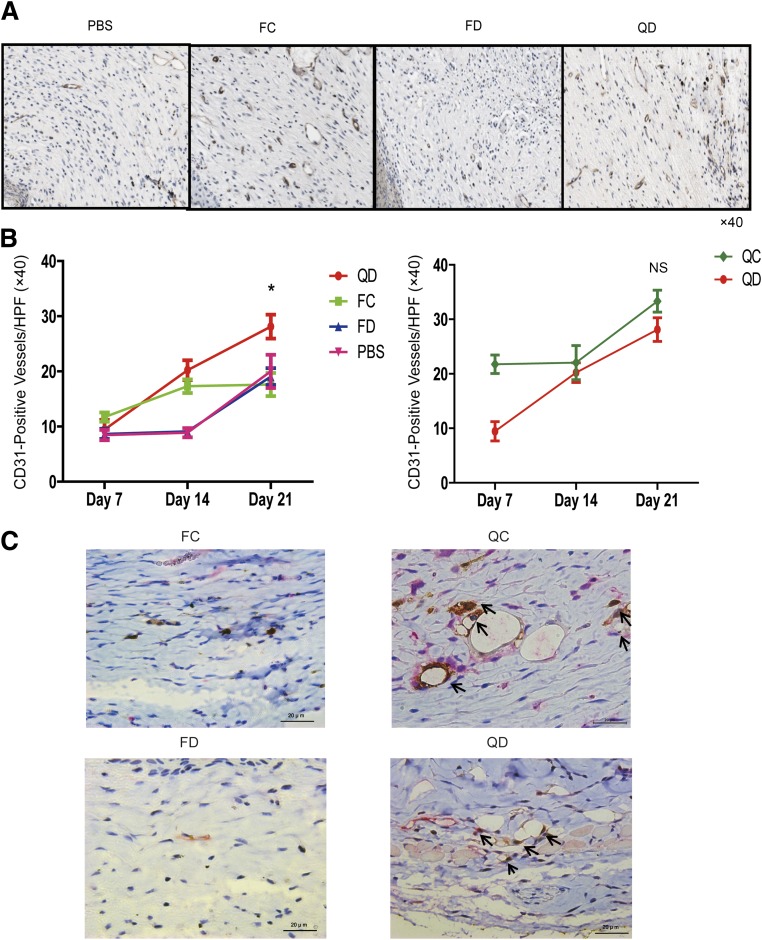
To understand how QQc improved diabetic KSL-mediated wound closure, we measured wound vascularization and collagen maturation. Interestingly, wounds injected with QD KSL cells showed significantly higher CD31 counts compared with the freshly isolated diabetic KSL treatment group and PBS (13.8 ± 1.8 vs. 8.6 ± 0.9 vs. 7.2 ± 0.8; P < 0.01) starting at day 7. On day 21, the vascularity in the post-QQc diabetic KSL treatment groups significantly increased compared with freshly isolated control and diabetic KSL treatment groups, as well as compared with the PBS control group (25.5 ± 1.7 vs. 15.0 ± 1.8 vs. 18.0 ± 1.2 vs. 11.5 ± 1.0; P < 0.05). Interestingly, post-QQc KSL treatment groups showed a rapid increase in vascularity after injection, as compared with the relatively delayed response in the freshly isolated KSL treatment group (Fig. 5A and B).
FIG. 5.
Post-QQc diabetic progenitor cell therapy enhances wound vascularization. A: Representative immunohistochemistry staining of CD31 to evaluate vascular density in the wounds of PBS-treated, freshly isolated control cell (FC)-treated, freshly isolated diabetic cell (FD)-treated, and QD-treated groups at day 21 (×40). B: The graphs show the CD31-positive vessels per HPF at days 7, 14, and 21. Left graph shows the comparison between QD-treated and FC-treated, FD-treated, and PBS-treated groups. Right graph shows the comparison between post-QQc control cell (QC)-treated and QD-treated groups. *P < 0.05. C: Representative immunohistochemistry double staining of GFP (brown) and vWF (red). The arrows point to the vessels with positive double staining. ×60 scale bar = 20 μm. The staining demonstrates that GFP/vWF double-stained vessels are only observed in the post-QQc control- and diabetic cell-treated wounds. GFP-positive cells observed more in the QC treated group. NS, not significant.
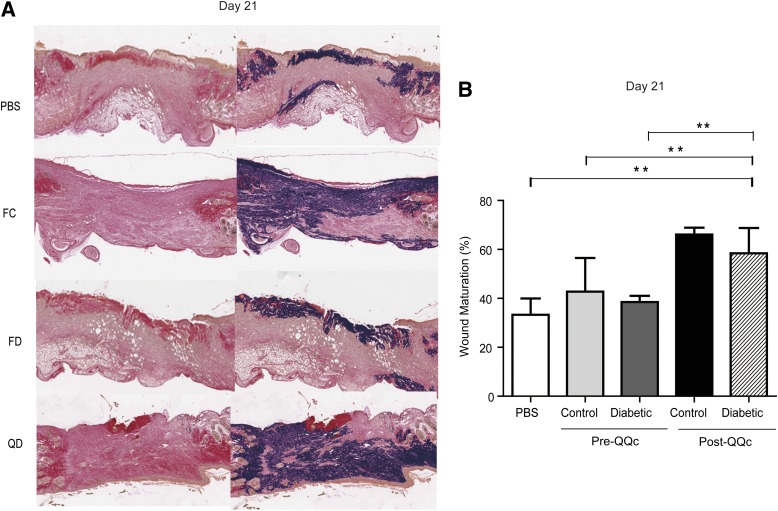
On day 21, the percentage of mature collagen as assessed by Van Gieson staining in the wounds treated with post-QQc diabetic KSL cells (58 ± 11%) was greater compared with wounds treated with freshly isolated control (43 ± 14%) and diabetic KSL cells (38 ± 3%) and PBS (33 ± 7%; P < 0.01 and P < 0.01, respectively) (Fig. 6A and B).
FIG. 6.
Post-QQc diabetic progenitor cell therapy enhances the percentage of mature collagen in the wound. A: Representative Van Gieson staining demonstrating mature collagen staining in PBS-treated, freshly isolated control cell (FC)-treated, freshly isolated diabetic cell (FD)-treated, and QD-treated groups on day 21 (×10). B: The graph shows the percentage of mature collagen in wounds treated with PBS, FC, FD, and QD cells on day 21. **P < 0.01.
Post-QQc diabetic progenitor cells have high potential for direct vasculogenesis.
To identify whether the increased vascularity is attributable to differentiation of injected post-QQc KSL cells or is attributable to increased numbers of resident endothelial cells, we injected pre-QQc and post-QQc GFP control and diabetic KSL cells in the wound and identified the GFP and vWF costaining cells. As a result, GFP-positive cells costained with vWF were only identified in the post-QQc control and the diabetic cell-treated groups at day 21. Comparing post-QQc control and diabetic cell-treated groups, the post-QQc control cell-treated group showed higher numbers of GFP cells incorporated into the vasculature, suggesting higher vasculogenesis of post-QQc control KSL cells (Fig. 5C).
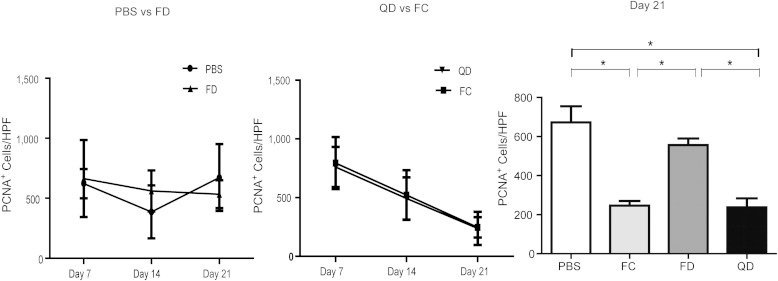
Post-QQc diabetic progenitor cell therapy increases cellular proliferation in the wound.
To study the effects of QQc-expanded diabetic KSL cells on native cells in the wound, we measure fibroblastic proliferation in situ. After adoptive QD KSL treatment, wound fibroblastic proliferation peaked early and declined significantly from day 7 to day 21 in the groups treated with freshly isolated control KSL cells (795 ± 221 vs. 247 ± 86 cells/field; P = 0.044) and post-QD KSL cells (761 ± 171 vs. 238 ± 141 cells/field; P = 0.011). Contrarily, there was no increase in cellular proliferation in wounds treated with freshly isolated diabetic KSL cells (664 ± 321 vs. 534 ± 116 cells/field; P = 0.41) or PBS (621 ± 122 vs. 672 ± 278 cells/field; P = 0.31) (Fig. 7).
FIG. 7.
Post-QQc diabetic progenitor cell therapy increases cellular proliferation in the wound. The left and middle graphs show the number of PCNA-positive cells in the wound per HPF on day 7, day 14, and day 21 in the following treated groups: PBS vs. freshly isolated diabetic cells (FD; left), QD vs. FC (middle). The right bar graph shows the number of PCNA-positive cells in the wound per HPF on day 21. *P < 0.05.
Function of post-QQc diabetic progenitor cells is deteriorated in glycemic diabetic wounds.
The in vivo efficacy of post-QQc diabetic KSL cells also was tested in STZ-induced glycemic murine diabetic wounds. As a result, post-QQc KSL cells indicated significant percent wound closure compared with pre-QQc KSL cells and PBS on day 14 (73.60 ± 3.69 vs. 55.02 ± 3.61 vs. 58.98 ± 5.86%; P < 0.05) and day 21 (96.34 ± 1.52 vs. 82.29 ± 4.72 vs. 84.01 ±2.28%; P < 0.05) (Fig. 8). However, there was no significant difference between the wound closures between pre-CQQc KSL, pre-DMQQc and post-DMQQc KSL, and PBS at all times. Although post-DMQQc KSL can accelerate wound healing and possess the restored vasculogenic potential in euglycemic in vivo conditions, these data suggest functional limitation of post-DMQQc KSL function in glycemic diabetic condition.
FIG. 8.
Efficacy of post-QQc diabetic progenitor cell therapy is deteriorated by diabetic condition. A: Representative images show wound healing in STZ-induced diabetic mice treated with PBS, before and after control, and treated with diabetic QQc cells. Wounds were photographed at the times indicated, from day 0 to day 21. B: The graph shows the comparison of percent wound closure between post-QQc control cell (QC)-treated vs. freshly isolated control cell (FC)-treated vs. PBS-treated groups. QC indicated significant percent wound closure compared with FC and PBS on day 14 (QC: 73.60 ± 3.69 vs. FC: 55.02 ± 3.61 vs. PBS: 58.98 ± 5.86%; P < 0.05) and day 21 (QC: 96.34 ± 1.52 vs. FC: 82.29 ± 4.72 vs. PBS: 84.01 ± 2.28%; P < 0.05). The wound closure between FC and PBS was similar without any significance. C: The graph shows the comparison of percent wound closure between QD-treated vs. freshly isolated diabetic cell (FD)-treated vs. PBS-treated groups. There was no significant difference between the three compared groups at all time points. *P < 0.05.
DISCUSSION
Current diabetic wound treatment hinges on patient education, prevention, and early diagnosis. Once a wound has developed, however, invasive therapies are costly and noninvasive therapies are less effective. Ultimately, because current treatments do not correct the underlying pathophysiology, many patients experience untoward complications and require amputations. Although investigators have long focused on the detrimental effects of elevated blood glucose on diabetic wound healing, recent data suggest that diabetic impairment of EPC function has a secondary effect on diabetic wound healing (5,34). This latter point is highlighted in the results of our recent clinical trial (24). By injecting autologous granulocyte colony-stimulating factor–mobilized peripheral blood EPCs into the nonhealing wounds of diabetic patients, we found that successful wound healing correlated with the vasculogenic function of transplanted EPCs. Moreover, we discovered that autologous EPC therapy has two inherent limitations: low EPC cell number and low vasculogenic function.
In an effort to overcome the limitations of autologous EPC therapy in diabetic patients, we studied the effect of QQc diabetic EPC ex vivo expansion on wound healing. In our study, mouse BM KSL cells were used as an EPC-enriched population based on a recent study reported by Kwon et al. (6) QQc is a serum-free culture system recently developed and reported by our group (27). QQc is a functional culture system that not only increases the number of EPCs but also increases the population of differentiated colony-forming EPCs (i.e., vasculogenic EPCs). Our in vitro experiments demonstrate that QQc significantly increases diabetic EPC cell number, definitive colony formation, and tubulization. Because QQc not only increased the number of diabetic EPCs but also restored their function to the level of control EPCs, we tested the effects of QQc diabetic EPCs ex vivo–expanded cells on wound closure. We used a stented wound closure model to minimize the effects of wound contracture (35). To focus our investigation on the function and efficacy of post-QQc diabetic EPCs compared with fresh healthy allogeneic EPCs, we selected a euglycemic wound closure mice model. We hypothesized that the use of a euglycemic recipient would eliminate the effect of confounding variables present in a diabetic recipient model.
Because new blood vessel formation is crucial for successful wound healing, we hypothesize that DMQQc therapy leads to accelerated wound closure by enhancing vasculogenesis (36). CD31 staining demonstrated that post-QQc diabetic EPC treatment increased wound vascularity compared with freshly isolated diabetic EPC treatment and control groups at all time points. Moreover, as demonstrated previously by Masuda et al. (27,29), because QQc increases the number of dEPC-CFUs (i.e., vasculogenic EPCs) and dEPCs more readily form new vessels (compared with pEPCs), we hypothesize that they are the vitally important EPC fraction mediating the therapeutic vasculogenesis observed in our in vivo experiments. Collectively, our findings suggest a potential mechanism by which DMQQc EPCs accelerate wound closure; transplanted post-QQc EPCs accelerate wound closure by forming tubules and inosculating with existing vasculature. This idea is further supported by the finding that GFP-labeled KSL cells incorporated into the native vascular network.
Enhanced new blood vessel formation may accelerate wound closure in a number of different ways. We found that DMQQc therapy significantly enhanced the percentage of mature collagen in the wound. Interestingly, post-QQc diabetic EPCs exhibit significantly higher CD29mRNA expression compared with fresh diabetic EPCs. Recently, it was reported that CD29 directly influences growth factor signaling and promotes fibroblast migration (37). Together with the PCNA data, we hypothesize that DMQQc stimulates fibroblast migration to the wound and accelerates wound closure.
To confirm the efficacy of QQc therapy in diabetic mice, we injected pre-QQc and post-QQc control and diabetic KSL cells to a full-thickness wound in STZ-induced glycemic diabetic mice. The results indicated that accelerated wound healing was seen only in post-QQc control KSL cell–treated group. The pre-diabetic and post-diabetic QQc KSL cells group, pre-QQc control KSL cells group, and the PBS-treated group demonstrated the same percent wound closure at all times from day 3 to day 21. In other words, the healing of post-QQc diabetic KSL cells in eugylcemic wounds was not seen in a diabetic condition. We assume from this result that hyperglycemic conditions of diabetic mice may have deteriorated the function of post-QQc diabetic KSL cells. Because post-QQc diabetic KSL cells have a highly therapeutic effect in euglycemic conditions, we believe that QQc therapy may be effective in euglycemic diabetic patients, i.e., patients with controlled blood glucose levels (blood glucose <8 mmol/L or <140 mg/dL) according to the practical guidelines on the management and prevention of diabetic foot (38). Our data are similar, and we daily treat diabetic patients with chronic wounds and high blood glucose. Many previous reports have shown that one of the standards of care for diabetic wounds involves systemic glucose control, and effective wound healing cannot be expected for uncontrolled diabetes even with highly effective therapy. Our results for EPC therapy for uncontrolled diabetic mice show that the condition of the host has a great impact on deterioration of the cells being administered, and we believe that “metabolic memory” and epigenetic modification by hyperglycemia are possibilities for why this is. In euglycemic diabetic patients the efficacy of EPC therapy for wound healing is limited because of autologous EPC vasculogenic dysfunction, as shown in our previous report (24). We believe that present EPC therapy with application of autologous dysfunctional EPC may not be effective even for diabetic patients with controlled glucose. The application of QQc in these patients may be the key for highly therapeutic autologous diabetic EPC therapy. To test our hypothesis, we have tried to establish a stented wound healing model of insulin-treated STZ diabetic mice with controlled glucose levels and treated these mice with diabetic pre-QQc and post-QQc KSL cells. Unfortunately, the model was difficult to establish because of the many interventions on the mice. Therefore, this hypothesis remains to be proven.
Another limitation of our study includes not knowing the exact mechanism of how QQc restores the vasculogenic dysfunction of diabetic EPCs. We recently have looked into the effect of QQc on oxidative stress of control and diabetic BM KSL cells and found that QQc relieves oxidative stress on both control and diabetic BM KSL cells (data not shown). However, this was not the specific mechanism for restoring diabetic BM KSL dysfunction. We plan to investigate further in a future study.
In summary, we have demonstrated that QQc not only restores diabetic EPC function but also achieves supraphysiologic EPC vasculogenic function in vitro and in vivo. Because QQc is serum-free and rapidly expands the number of diabetic EPCs, this system may facilitate cell-based therapies for diabetic patients. Although this study has limitations regarding future clinical applications for diabetic patients, this study can be considered the first step in establishing an ideal cell-based therapy for diabetic patients. Moreover, the rapidly expanded post-QQc EPC population could be aliquoted, cryopreserved, and used again for metachronous wounds or other ischemic conditions (e.g., myocardial ischemia).
Conclusions.
Here, we demonstrate that a novel serum-free QQc system expands the number of cells and enhances the vasculogenic and therapeutic potential of diabetic EPCs. We hypothesize that adoptive post-QQc diabetic EPC therapy may be an effective cell-based therapy for nonhealing diabetic wounds.
ACKNOWLEDGMENTS
This work was supported by a Health and Labor Sciences research grant from the Japanese Ministry of Health, Labor, and Welfare (20890227, 22791737), a funding program for Next Generation World Leading Researchers LS113, a Tokai University research aid grant, and Plastic Surgery Research Award 2007 (awarded to R.T.).
No potential conflicts of interest relevant to this article were reported.
R.T. conceived and designed the study, obtained financial support, wrote the manuscript, provided the study material, collected data, analyzed data, and interpreted data. M.V. collected data, analyzed data, interpreted data, and wrote the manuscript. H.M. conceived and designed the study and wrote the manuscript. R.I. collected and assembled data, analyzed data, and interpreted data. M.K. collected data, assembled data, analyzed data, and interpreted data. M.M. provided administrative support. H.M. wrote the manuscript. S.M.W. obtained financial support, provided administrative support, wrote the manuscript, and approved the final manuscript. T.A. obtained financial support, provided administrative support, wrote the manuscript, and approved the final manuscript. T.A. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors acknowledge the contributions of Christopher C. Chang, Dan Bassiri, Clarence Lin, Oren M. Tepper, and Anthony L. Rios (all from Institute of Reconstructive Plastic Surgery Laboratories, Department of Plastic Surgery, New York University Medical Center, New York, New York), Mika Wada (from Tokai University School of Medicine, Department of Regenerative Medicine), and Satoshi Fujimura, Kayo Okada, and Kayoko Arita (from Juntendo University School of Medicine, Department of Plastic and Reconstructive Surgery) for their kind technical assistance. The authors also thank Dr. Yoshinori Okada, Dr. Hiroshi Kamiguchi, and Yoko Kameyama (from Tokai University School of Medicine) for their outstanding technical support regarding flow cytometry, real-time PCR, and immunohistochemistry.
REFERENCES
- 1.Drake CJ. Embryonic and adult vasculogenesis. Birth Defects Res C Embryo Today 2003;69:73–82 [DOI] [PubMed] [Google Scholar]
- 2.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964–967 [DOI] [PubMed] [Google Scholar]
- 3.Shi Q, Rafii S, Wu MH, et al. Evidence for circulating bone marrow-derived endothelial cells. Blood 1998;92:362–367 [PubMed] [Google Scholar]
- 4.Hristov M, Erl W, Weber PC. Endothelial progenitor cells: isolation and characterization. Trends Cardiovasc Med 2003;13:201–206 [DOI] [PubMed] [Google Scholar]
- 5.Tanaka R, Wada M, Kwon SM, et al. The effects of flap ischemia on normal and diabetic progenitor cell function. Plast Reconstr Surg 2008;121:1929–1942 [DOI] [PubMed] [Google Scholar]
- 6.Kwon SM, Lee YK, Yokoyama A, et al. Differential activity of bone marrow hematopoietic stem cell subpopulations for EPC development and ischemic neovascularization. J Mol Cell Cardiol 2011;51:308–317 [DOI] [PubMed] [Google Scholar]
- 7.Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 1999;85:221–228 [DOI] [PubMed] [Google Scholar]
- 8.Tepper OM, Capla JM, Galiano RD, et al. Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow-derived cells. Blood 2005;105:1068–1077 [DOI] [PubMed] [Google Scholar]
- 9.Ii M, Nishimura H, Iwakura A, et al. Endothelial progenitor cells are rapidly recruited to myocardium and mediate protective effect of ischemic preconditioning via “imported” nitric oxide synthase activity. Circulation 2005;111:1114–1120 [DOI] [PubMed] [Google Scholar]
- 10.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 2004;10:858–864 [DOI] [PubMed] [Google Scholar]
- 11.Murayama T, Tepper OM, Silver M, et al. Determination of bone marrow-derived endothelial progenitor cell significance in angiogenic growth factor-induced neovascularization in vivo. Exp Hematol 2002;30:967–972 [DOI] [PubMed] [Google Scholar]
- 12.Tepper OM, Carr J, Allen RJ, Jr, et al. Decreased circulating progenitor cell number and failed mechanisms of stromal cell-derived factor-1alpha mediated bone marrow mobilization impair diabetic tissue repair. Diabetes 2010;59:1974–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schatteman GC, Hanlon HD, Jiao C, Dodds SG, Christy BA. Blood-derived angioblasts accelerate blood-flow restoration in diabetic mice. J Clin Invest 2000;106:571–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loomans CJ, de Koning EJ, Staal FJ, et al. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes 2004;53:195–199 [DOI] [PubMed] [Google Scholar]
- 15.Li Calzi S, Neu MB, Shaw LC, Grant MB. Endothelial progenitor dysfunction in the pathogenesis of diabetic retinopathy: treatment concept to correct diabetes-associated deficits. EPMA J 2010;1:88-100 [DOI] [PMC free article] [PubMed]
- 16.Albiero M, Menegazzo L, Boscaro E, Agostini C, Avogaro A, Fadini GP. Defective recruitment, survival and proliferation of bone marrow-derived progenitor cells at sites of delayed diabetic wound healing in mice. Diabetologia 2011;54:945–953 [DOI] [PubMed] [Google Scholar]
- 17.Fadini GP, Sartore S, Albiero M, et al. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler Thromb Vasc Biol 2006;26:2140–2146 [DOI] [PubMed] [Google Scholar]
- 18.Neukomm LJ, Frei AP, Cabello J, et al. Loss of the RhoGAP SRGP-1 promotes the clearance of dead and injured cells in Caenorhabditis elegans. Nat Cell Biol 2011;13:79–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawamoto A, Gwon HC, Iwaguro H, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation 2001;103:634–637 [DOI] [PubMed] [Google Scholar]
- 20.Kocher AA, Schuster MD, Szabolcs MJ, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 2001;7:430–436 [DOI] [PubMed] [Google Scholar]
- 21.Taguchi A, Soma T, Tanaka H, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest 2004;114:330–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin CD, Allori AC, Macklin JE, et al. Topical lineage-negative progenitor-cell therapy for diabetic wounds. Plast Reconstr Surg 2008;122:1341–1351 [DOI] [PubMed] [Google Scholar]
- 23.Sivan-Loukianova E, Awad OA, Stepanovic V, Bickenbach J, Schatteman GC. CD34+ blood cells accelerate vascularization and healing of diabetic mouse skin wounds. J Vasc Res 2003;40:368–377 [DOI] [PubMed] [Google Scholar]
- 24.Tanaka R, Masuda H, Kato S, et al. Autologous G-CSF mobilized peripheral blood CD34(+) cell therapy for diabetic patients with chronic non-healing ulcer. Cell Transplant. 25 October 2012 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Jarajapu YP, Grant MB. The promise of cell-based therapies for diabetic complications: challenges and solutions. Circ Res 2010;106:854–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan Q, Qiu L, Li G, et al. Transplantation of healthy but not diabetic outgrowth endothelial cells could rescue ischemic myocardium in diabetic rabbits. Scand J Clin Lab Invest 2010;70:313–321 [DOI] [PubMed] [Google Scholar]
- 27.Masuda H, Iwasaki H, Kawamoto A, et al. Development of serum-free quality and quantity control culture of colony-forming endothelial progenitor cell for vasculogenesis. Stem Cells Transl Med 2012;1:160–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Westermann D, Rutschow S, Van Linthout S, et al. Inhibition of p38 mitogen-activated protein kinase attenuates left ventricular dysfunction by mediating pro-inflammatory cardiac cytokine levels in a mouse model of diabetes mellitus. Diabetologia 2006;49:2507–2513 [DOI] [PubMed] [Google Scholar]
- 29.Masuda H, Alev C, Akimaru H, et al. Methodological development of a clonogenic assay to determine endothelial progenitor cell potential. Circ Res 2011;109:20–37 [DOI] [PubMed] [Google Scholar]
- 30.Tanaka R, Wada M, Kwon S, et al. The effects of flap ischemia on normal and diabetic progenitor cell function. Plast Reconstr Surg 2008;121:1929–1942 [DOI] [PubMed] [Google Scholar]
- 31.Park S, Tepper OM, Galiano RD, et al. Selective recruitment of endothelial progenitor cells to ischemic tissues with increased neovascularization. Plast Reconstr Surg 2004;113:284–293 [DOI] [PubMed] [Google Scholar]
- 32.Pugazhenthi K, Kapoor M, Clarkson AN, Hall I, Appleton I. Melatonin accelerates the process of wound repair in full-thickness incisional wounds. J Pineal Res 2008;44:387–396 [DOI] [PubMed] [Google Scholar]
- 33.Carson FL. Histotechnology: A Self-Instructional Text. Chicago, ASCP Press, 1997 [Google Scholar]
- 34.Fadini GP, Avogaro A. Potential manipulation of endothelial progenitor cells in diabetes and its complications. Diabetes Obes Metab 2010;12:570–583 [DOI] [PubMed] [Google Scholar]
- 35.Michaels J, Churgin SS, Blechman KM, Greives MR, Aarabi S, Galiano RD, Gurtner GC. db/db mice exhibit severe wound-healing impairments compared with other murine diabetic strains in a silicone-splinted excisional wound model. Wound Repair Regen 2007;15:665–670 [DOI] [PubMed]
- 36.Glotzbach JP, Levi B, Wong VW, Longaker MT, Gurtner GC. The basic science of vascular biology: implications for the practicing surgeon. Plast Reconstr Surg 2010;126:1528–1538 [DOI] [PubMed] [Google Scholar]
- 37.King SJ, Worth DC, Scales TM, Monypenny J, Jones GE, Parsons M. β1 integrins regulate fibroblast chemotaxis through control of N-WASP stability. EMBO J 2011;30:1705–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bakker K, Apelqvist J, Schaper NC, International Working Group on Diabetic Foot Editorial Board . Practical guidelines on the management and prevention of the diabetic foot 2011. Diabetes Metab Res Rev 2012;28(Suppl. 1):225–231 [DOI] [PubMed] [Google Scholar]