Abstract
β-Cell function improves in patients with type 2 diabetes in response to an oral glucose stimulus after Roux-en-Y gastric bypass (RYGB) surgery. This has been linked to the exaggerated secretion of glucagon-like peptide 1 (GLP-1), but causality has not been established. The aim of this study was to investigate the role of GLP-1 in improving β-cell function and glucose tolerance and regulating glucagon release after RYGB using exendin(9-39) (Ex-9), a GLP-1 receptor (GLP-1R)–specific antagonist. Nine patients with type 2 diabetes were examined before and 1 week and 3 months after surgery. Each visit consisted of two experimental days, allowing a meal test with randomized infusion of saline or Ex-9. After RYGB, glucose tolerance improved, β-cell glucose sensitivity (β-GS) doubled, the GLP-1 response greatly increased, and glucagon secretion was augmented. GLP-1R blockade did not affect β-cell function or meal-induced glucagon release before the operation but did impair glucose tolerance. After RYGB, β-GS decreased to preoperative levels, glucagon secretion increased, and glucose tolerance was impaired by Ex-9 infusion. Thus, the exaggerated effect of GLP-1 after RYGB is of major importance for the improvement in β-cell function, control of glucagon release, and glucose tolerance in patients with type 2 diabetes.
Hyperglycemia in patients with type 2 diabetes is resolved shortly after Roux-en-Y gastric bypass (RYGB), suggesting that mechanisms independent of weight loss contribute to the improvement in glycemic control (1–4).
Within 1 month and as early as 5 days after RYGB, β-cell function in response to a meal improves in subjects with type 2 diabetes, and this is accompanied by an increased postprandial glucagon-like peptide (GLP)-1 response (3,5,6). In contrast, after intravenous infusion of glucose, which does not elicit the incretin effect, an improvement in β-cell function is absent (5,7,8). Therefore, it could be speculated that the early improvements in β-cell function after RYGB are due to the enhanced GLP-1 secretion related to eating a meal, but causality has not been established (9).
In patients with type 2 diabetes, energy restriction per se is known to result in improved hepatic insulin sensitivity and decreased hepatic glucose production and, as a result, lowered fasting plasma glucose concentrations (10–12). Similar metabolic changes are seen after RYGB, when energy intake is limited (13,14), and this has led to the proposal that caloric restriction with a subsequent reduction in glucotoxicity, rather than an increased effect of GLP-1, is responsible for the improved β-cell function (14,15).
The aim of this study was to investigate the role of GLP-1 in the improved β-cell function and glucose tolerance seen after RYGB in subjects with type 2 diabetes. This was accomplished by pharmacologically blocking the GLP-1 receptor (GLP-1R) during a liquid meal tolerance test before and after surgery using exendin(9-39) (Ex-9; Bachem AG, Bubendorf, Switzerland), a specific GLP-1R antagonist (16).
Previous studies have documented increased meal-related glucagon secretion after RYGB despite improvements in insulin secretion and sensitivity and exaggerated GLP-1 release (3,17,18). This observation is surprising given the glucagonostatic properties of GLP-1 and insulin (19,20). Therefore, a further aim of this study was to evaluate the interaction between GLP-1 and glucagon release after RYGB in both the fasting and postprandial states.
RESEARCH DESIGN AND METHODS
Patients with type 2 diabetes were recruited from the Hvidovre Hospital’s bariatric surgery program (Hvidovre, Denmark), met the criteria for bariatric surgery (age >25 years and BMI >35 kg/m2), and had accomplished a mandatory preoperative, diet-induced loss of 8% of total body wt before inclusion. Patients were excluded if they had uncontrolled hypothyroidism, had been taking antithyroid medication or anorectic agents within 3 months before the experiments, or had a fasting C-peptide level <700 pmol/L. To confirm the diagnosis of type 2 diabetes, an oral glucose tolerance test (OGTT) was performed ≤1 month before the first experiment.
The study was approved by the Municipal Ethical Committee of Copenhagen (reg. nr. H-A-2008-080-31742), was in accordance with the Declaration of Helsinki II, and was registered with clinicaltrials.gov (NCT01579981) and the Danish Data Protection Agency. Written informed consent was obtained from all patients before entering the study. Incretin-based therapies were put on hold for at least 14 days and all other antidiabetic medications for at least 3 days before the first preoperative experiment. Insulin analogs were replaced with NPH insulin at least 2 weeks before the first experiment. RYBG was performed as previously described (18). Patients were examined at 3 visits: before, 1 week after, and 3 months after RYGB. Visits consisted of 2 days where the patients were examined during a liquid meal tolerance test with a concurrent patient-blinded, primed, continuous infusion of Ex-9 or isotonic saline in random order. On each study day, patients met at 0800 h after a 10-h overnight fast. Patients were weighed (Tanita Corp., Tokyo, Japan), a catheter was inserted into the antecubital vein of each arm (one for blood sampling and one for infusion), and three fasting blood samples were drawn (−40 to 30 min). A primed continuous infusion of either saline or Ex-9 was initiated at time −30 min using a precision infusion pump (P2000; IVAC Medical Systems, Hampshire, U.K.). Saline was infused at a rate corresponding to the Ex-9 infusion volumes. After infusion was started, participants maintained a fasting state for 30 min to allow the drug to reach target tissues and drug concentrations to stabilize before the meal. Three further baseline samples were drawn just before ingestion of the meal (−10 to 0 min). At time 0 min, a liquid meal (Fresubin Energy Drink, 200 mL, 300 kcal, carbohydrate [50% of energy], protein [15% of energy], fat [35% of energy]; Fresenius Kabi Deutschland, Bad Homburg, Germany) was provided. To estimate gastric emptying, 1 g of paracetamol (Pamol; Nycomed Danmark, Roskilde, Denmark) was crushed to a powder and added to the meal. To avoid dumping after surgery and to obtain a comparable stimulus before and after RYGB, meal ingestion was supervised to ensure even distribution of meal intake over a 30-min period.
Blood was sampled before and frequently following the meal for a total of 4 h (−40, −35, −30, −10, −5, 0, 30, 45, 60, 90, 120, 180, and 240 min). During each test, patients were placed sitting in a reclined position in a hospital bed, and no strenuous activity was allowed.
Ex-9.
Ex-9 was purchased from Bachem AG. Ex-9 from the same lot was used for all experiments. The peptide was dissolved in sterilized water containing 1% human albumin (Plasma Product Unit PSU; Novo Nordisk A/S, Bagsvaerd, Denmark) and subjected to sterile filtration. The dissolved peptide was dispensed in appropriate volumes and stored frozen (−20°C) under sterile conditions until the day of the experiment. The peptide was demonstrated to be 99.5% pure by high-performance liquid chromatography. The Ex-9 infusion rate of 900 pmol/kg body wt/min was chosen because this had previously been reported to block the GLP-1R by 95% (21,22), and the resulting steady-state concentration of Ex-9 was predicted to be 391 nmol/L using previously published pharmacokinetic parameters (23). Likewise, bolus size was calculated to be 43.000 pmol/kg body wt.
Sample collection and laboratory analyses.
Blood was collected into clot activator tubes for insulin and C-peptide analysis and into prechilled EDTA tubes for analysis of GLP-1, glucose-dependent insulinotropic polypeptide (GIP), glucagon, glucose, paracetamol, and Ex-9.
Clot activator tubes were left to coagulate for 30 min, whereas EDTA tubes were placed on ice until centrifuged at 4°C. Plasma glucose was measured immediately using the glucose oxidase technique (YSI model 2300 STAT Plus; YSI, Yellow Springs, OH). Samples of GLP-1, GIP, glucagon, and Ex-9 were stored at −20°C. All other samples were frozen and stored at −80°C until batch analysis.
Serum insulin and C-peptide concentrations were determined by AutoDELFIA fluoroimmunoassay (Wallac OY, Turku, Finland). Plasma samples were assayed for total GLP-1 immunoreactivity, as described previously (24). Total GIP was measured using a C-terminally directed antiserum 867 with characteristics similar to the previously employed R65 (25,26). Glucagon was measured with the LINCO assay (Millipore, Billerica, MA) because it does not cross-react with Ex-9 (27). Ex-9 was measured using antibody 3145 raised in rabbits immunized with exendin-4, which shows 100% cross-reactivity with Ex-9 but <0.01% cross-reactivity with GLP-1, glucagon, or GIP (27). Paracetamol was measured using a calorimetric assay (Roche Diagnostics GmbH, Mannheim, Germany), and HbA1c was measured using high-performance liquid chromatography with a cation exchange column (Tosoh Bioscience, Tokyo, Japan).
Calculations and statistical analyses.
Fasting glucose and hormone values were calculated as the mean of the time points from the interval −40 to −30 min. Total area under the curve (AUC) was calculated using the trapezoidal model. Baseline values were the mean of the time points from the interval −10 to 0 min. Incremental AUC (I-AUC) was calculated as AUC above baseline.
Insulin resistance was calculated using homeostasis model assessment of insulin resistance (HOMA-IR) using the following equation: Insulinfasting [pmol/L] × Glufasting [mmol/L] / (22.5 × 6.945), where Glu is glucose.
Prehepatic insulin secretion rates (ISRs) were calculated by deconvolution of peripheral C-peptide concentrations and application of population-based parameters for C-peptide kinetics using the ISEC software (28,29). ISR is expressed as picomoles × kilograms−1 × minutes−1. The relationship between glucose concentrations and ISR during the meal, the β-cell glucose sensitivity (β-GS), was characterized as previously described (3). The time to peak paracetamol concentration was used as a marker of gastric emptying.
Data are expressed as means ± SE. Statistical analyses were carried out using Wilcoxon matched pairs signed rank test or Kruskal-Wallis rank sum test, as appropriate. P values <0.05 were considered significant. The analyses were carried out using the R 2.11.1 statistical software package.
RESULTS
Patient characteristics.
Eleven patients with type 2 diabetes were recruited for the study. Two patients were excluded from data analysis: one because intravenous cannulation could not be performed on the second experimental day and the other because RYGB was abandoned because of massive intestinal adherences on the basis of earlier gastrointestinal surgery and peritonitis.
Thus, a total of nine patients with type 2 diabetes (age 50 ± 3 years; three females and six males; BMI 39.2 ± 2.4 kg/m2; diabetes duration 5.7 ± 1.3 years; HbA1c 6.5 ± 0.3% [48 ± 4 mmol/mol]; 2-h OGTT plasma glucose 14.4 ± 1.0 mmol/L) were examined 9 ± 2 days before and 7 ± 1 days (1 week) and 96 ± 2 days (3 months) after RYGB. Preoperatively, one patient was treated with diet alone and eight were treated with metformin either as monotherapy (n = 4) or in combination with glimepiride (1), vildagliptin (1), liraglutide (1), or liraglutide and insulin glargine (1). None of the patients received any antidiabetic medication after RYGB. Further comorbidities were hypertension (6), hypercholesterolemia (2), hypothyroidism (n = 1, well controlled, with thyroid-stimulating hormone within the normal range), and myocardial infarction 4 years earlier (1). None had records or symptoms of gastroparesis, neuropathy, retinopathy, or nephropathy.
The patients entered the bariatric surgery program 337 ± 40 days before RYGB with a BMI of 42.9 ± 2.8 kg/m2. On the day of the OGTT (30 ± 9 days before RYGB), BMI had decreased to 39.5 ± 2.5 kg/m2 (92 ± 0.9% of initial BMI), which was not different from that measured on the first experimental day. One week after RYGB, patients had lost 3.9 ± 0.4% (P < 0.01) of preoperative BMI, and after 3 months they had lost 13.5 ± 1.1% (P < 0.01). HbA1c decreased to 5.7 ± 0.2% (39 ± 2 mmol/mol; P < 0.01) 3 months after surgery.
Ex-9 concentrations.
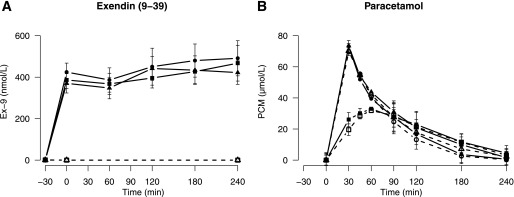
Mean Ex-9 concentrations from time 0 to 240 min were similar before (404 ± 52 nmol/L) and after RYGB (1 week: 444 ± 65 nmol/L; 3 months: 405 ± 51 nmol/L; P = 0.7) and were stable throughout the experiment (Fig. 1).
FIG. 1.
Ex-9 (A) and paracetamol (B) concentrations throughout the experiment. Data are shown as means ± SE. Open symbols, dotted lines: saline infusion; solid symbols, solid line: Ex-9 infusion. Squares: before surgery, circles: 1 week after surgery, triangles: 3 months after surgery.
Gastric emptying.
Paracetamol profiles are shown in Fig. 1. Before the operation, peak paracetamol concentrations were reached 70 ± 10 min after the start of the meal, but after RYGB they peaked at the first postprandial sample point (30 min) (1 week: 33 ± 2 min [P < 0.01]; 3 months: 32 ± 2 min [P < 0.01]). GLP-1R blockade had a minor insignificant effect on time to peak paracetamol concentration before the operation (before Ex-9: 60 ± 10 min [P = 0.49]) but no detectable effect after RYGB (Ex-9 at 1 week: 30 ± 0 min; at 3 months: 30 ± 0 min).
Fasting glucose and hormone concentrations.
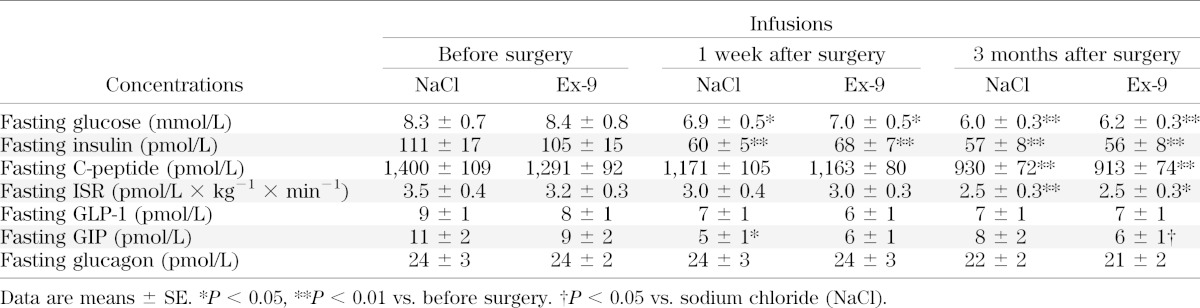
Data regarding fasting glucose and hormone concentrations are listed in Table 1. Following RYGB, fasting glucose, insulin, and C-peptide concentrations decreased, whereas fasting GLP-1, GIP, and glucagon concentrations did not substantially change.
TABLE 1.
Fasting glucose and hormone concentrations (−40, −35, and −30 min) before infusion of saline or Ex-9
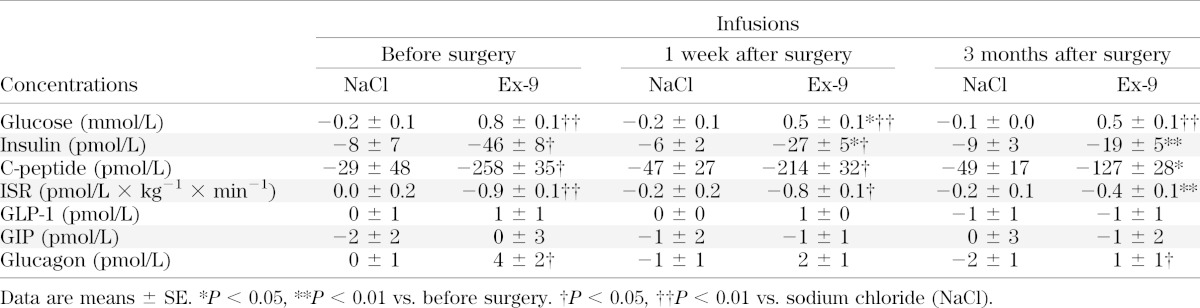
Changes in glucose and hormone concentrations 30 min after start of infusion, that is, before the meal, are reported in Table 2. Ex-9 infusion caused glucose concentrations to increase significantly at all study times, whereas ISR decreased most before and at least 3 months after the operation. Fasting GLP-1 and GIP concentrations were not affected by Ex-9 infusion, but glucagon concentrations increased significantly compared with the changes observed during saline infusion before and 3 months after RYGB.
TABLE 2.
Changes in fasting glucose and hormone concentrations after a primed infusion of saline or Ex-9 but before the meal
Insulin resistance.
Insulin resistance decreased by ∼50% after RYGB (HOMA-IR before RYGB: 5.72 ± 0.74; 1 week after: 2.71 ± 0.41 [P = 0.004]; 3 months after: 2.18 ± 0.30 [P = 0.004]). HOMA-IR did not differ between the days of saline and Ex-9 infusion at any of the three visits (data not shown).
Postprandial glucose concentrations and insulin secretion.
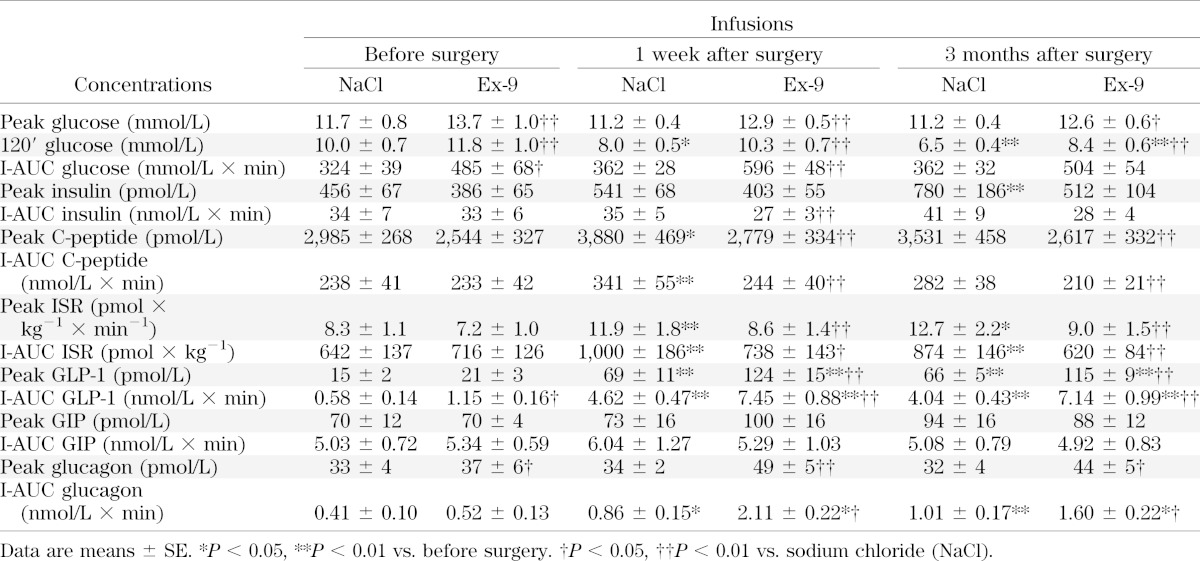
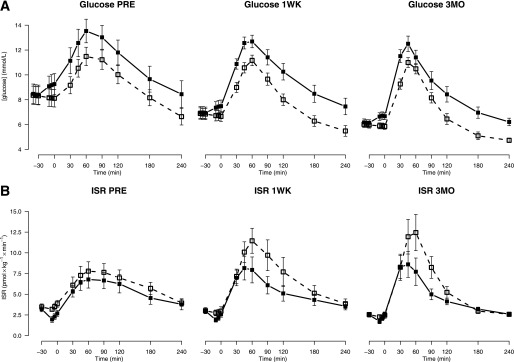
Data for postprandial glucose concentrations and insulin secretion are reported in Table 3, and glucose and ISR profiles are shown in Fig. 2. Following RYGB, 2-h postprandial glucose concentrations were significantly decreased but peak glucose concentrations were unchanged. Blocking the GLP-1R with Ex-9 caused 2-h and peak glucose concentrations to significantly increase compared with saline at all visits.
TABLE 3.
Postprandial glucose and hormone responses with saline or Ex-9 infusion
FIG. 2.
Glucose concentration (A) and ISR (B) profiles before (PRE) and 1 week (1WK) and 3 months (3MO) after RYGB with and without GLP-1R blockade. Data are shown as means ± SE. Open squares, dotted line: saline infusion; solid squares, solid line: Ex-9 infusion.
After the operation, insulin secretion was significantly increased in response to the meal as evidenced by both increased peak ISR and total meal-induced insulin secretion (I-AUC ISR). Ex-9 infusion resulted in significant decreases in peak ISR and I-AUC ISR 1 week and 3 months after RYGB but not before the operation.
β-Cell glucose sensitivity.
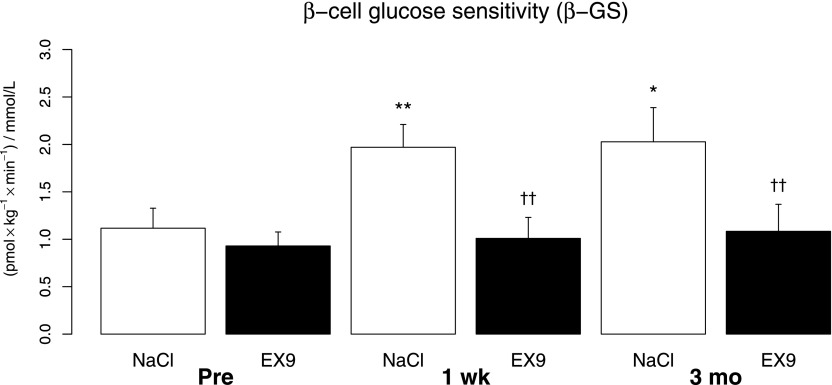
Data for β-GS are shown in Fig. 3. In response to the liquid meal, β-GS increased as a consequence of RYGB. One week after the operation, β-GS had almost doubled (P = 0.008), and it remained elevated at 3 months (P = 0.027). When the GLP-1R was blocked with Ex-9 before the operation, an insignificant decrease in β-GS (P = 0.07) was observed. After RYGB, Ex-9 infusion reduced β-GS to preoperative levels both at 1 week and 3 months after the operation, and these decreases were highly significant (both P = 0.004 vs. saline).
FIG. 3.
β-GS before (Pre) and 1 week (wk) and 3 months (mo) after RYGB with and without GLP-1R block. Data are shown as means ± SE. White bars, saline infusion; black bars, Ex-9 infusion. *P < 0.05 vs. Pre, **P < 0.01 vs. Pre. ††P < 0.01 vs. NaCl.
Postprandial GLP-1, GIP, and glucagon concentrations.
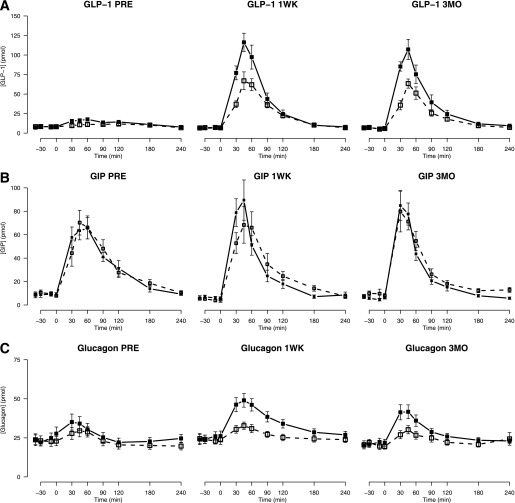
After RYGB, I-AUC GLP-1 was increased eightfold, reflecting a greatly elevated meal-induced GLP-1 response (Fig. 4A). Ex-9 infusion resulted in a further augmentation of GLP-1 release both before and after RYGB. The GIP response (Fig. 4B) was not affected by RYGB or Ex-9 infusion.
FIG. 4.
GLP-1 (A), GIP (B), and glucagon (C) concentration profiles before (PRE) and 1 week (1WK) and 3 months (3MO) after RYGB with and without GLP-1R blockade. Data are shown as means ± SE. Open squares, dotted line: saline infusion; solid squares, solid line: Ex-9 infusion.
Meal-induced glucagon secretion (I-AUC glucagon) (Fig. 4C and Table 3) increased after RYGB. Before the operation, I-AUC glucagon was not affected by GLP-1R blockade (P = 0.3), but after RYGB, Ex-9 infusion resulted in a marked and significant increase in glucagon secretion upon stimulation by the meal compared with the corresponding day of saline infusion.
DISCUSSION
One week and 3 months after RYGB, glucose tolerance and β-GS are improved and the meal-related GLP-1 response and glucagon release are increased in subjects with type 2 diabetes, as we have previously demonstrated (3). The key finding in the current study is that pharmacological blockade of the GLP-1R after RYGB reverses the improvements in β-GS and glucose tolerance and increases postprandial glucagon release. These results strongly suggest that the increased endogenous GLP-1 secretion is of great importance for the acute effects of RYGB on β-cell function and for glucose tolerance in patients with type 2 diabetes. Furthermore, we demonstrate that glucagon secretion is inhibited by GLP-1 after RYGB.
Before RYGB, GLP-1R inhibition impaired glucose tolerance because of an increment in fasting and postprandial glucose concentrations. Fasting insulin secretion decreased but meal-induced secretion was unaltered, and β-GS was not significantly reduced when Ex-9 was infused. Fasting glucagon concentrations increased during GLP-1R blockade, but the meal-induced glucagon response was unaltered. The effects of Ex-9 infusion on insulin and glucagon secretion during fasting were similar to those reported in two recent studies of patients with type 2 diabetes (30,31), but contrary to our results, these studies found a pronounced effect of GLP-1R inhibition on insulin and glucagon secretion when endogenous GLP-1 secretion was stimulated. However, both studies used the hyperglycemic clamp technique to keep plasma glucose concentrations fixed, whereas our study allowed plasma glucose concentrations to vary; as a consequence, they were higher on the day of Ex-9 infusion. This greater glucose stimulus is likely to counteract the effects of GLP-1R antagonism on postprandial insulin and glucagon secretion in our study and may explain these seemingly contradictory results.
One week after RYGB, plasma glucose concentrations had improved in both the fasting and postprandial states. After the operation, insulin secretion increased, β-GS doubled, the GLP-1 response was greatly increased, and meal-induced glucagon release was elevated. These results confirm our previous report and demonstrate that RYGB improves β-cell function soon after surgery (3). GLP-1R blockade caused glucose tolerance to deteriorate and β-GS to decrease to preoperative levels. At the same time, postprandial GLP-1 and glucagon responses increased.
Three months after the operation, glucose tolerance was further improved with enhanced insulin and GLP-1 secretion compared with before surgery, and overall glucose control was improved, as evidenced by a decreased HbA1c, despite patients not receiving antidiabetic medication. GLP-1R antagonism produced results similar to those observed 1 week after the operation, again returning β-GS to preoperative levels and augmenting the meal-related glucagon response, resulting in an impairment of glucose tolerance.
Before the operation, the weight of patients was stable, but after RYGB they experienced rapid weight loss, amounting to ∼4% within a week. It has been suggested that the acute effects of RYGB on β-cell function in patients with type 2 diabetes are strictly related to reduced energy intake (14,15), although reports on the effects of such acute energy restriction are conflicting (12,32). However, while energy restriction has little or no effect on GLP-1 release, postprandial GLP-1 secretion is greatly increased after RYGB (3,5,14,33–35). Previous reports have shown that the incretin effect after RYGB is normalized in patients with type 2 diabetes and that this cannot be explained simply by weight loss (36,37). Furthermore, in patients with type 2 diabetes, β-cell function in response to a meal or an oral glucose load has consistently been reported to improve within the first month after RYGB but not after gastric restrictive surgery where patients undergo the same postoperative dietary regimen or when β-cell function is evaluated by intravenous glucose infusion (3,5–7,33,38,39). The present data illustrate that the exaggerated GLP-1 response in patients with type 2 diabetes seems to be the main explanation for the improvement in β-cell function after RYGB in response to a meal. Reduced glucotoxicity may play a role in the improved β-cell function after surgery, but it seems to be of minor importance compared with the actions of GLP-1 (40,41). Of note, HOMA-IR was not different between saline and Ex-9 infusion at any visit. Therefore, the changes we observed with GLP-1R blockade are unlikely to be a consequence of adaptation of β-cells to changes in insulin resistance (42). It has been suggested that the large GLP-1 response after RYGB is triggered by the accelerated delivery of nutrients to the enteroendocrine cells of the distal ileum (43,44). We recently reported the gastric emptying of liquids to be greatly accelerated in patients who have received RYGB as measured using a scintigraphic method, and this was associated with an increased release of GLP-1 (45). In agreement with this finding, paracetamol absorption in this study was accelerated after RYGB, supporting such a mechanism of action. GLP-1R blockade increased postprandial GLP-1 release by 60–100% before and after RYGB, a finding that may be related to inhibition of GLP-1R on the enteroendocrine cells that work to feed negatively back on GLP-1 secretion (46). Although GLP-1 inhibits gastric emptying (19), paracetamol absorption kinetics were not significantly changed with Ex-9 infusion, making accelerated gastric emptying an unlikely mechanism by which GLP-1R blockade increases GLP-1 secretion in this study. It must be emphasized that estimation of gastric emptying using this method is limited by the blood sampling frequency, especially after RYGB. Previous studies have indicated that GLP-1 tonically suppresses fasting glucagon concentrations in patients with type 2 diabetes, and we were able to reproduce that finding here (31). The glucagonostatic properties of GLP-1 and the exaggerated response after RYGB would predict a reduced release of glucagon as induced by a meal (19); however, the glucagon response increases after the operation. Eliminating the effect of endogenous GLP-1 with Ex-9 further increased glucagon secretion, implying that meal-induced glucagon release continues to be under the inhibitory control of GLP-1. The mechanism underlying the increased meal-induced glucagon response remains to be elucidated.
Salehi et al. (47) reported that Ex-9 infusion resulted in a more pronounced decrease of insulin secretion when a meal was ingested during a hyperglycemic clamp in nondiabetic patients who received RYGB compared with normal controls. Strengths of the current study include the prospective design, the inclusion of patients with type 2 diabetes, and the use of a physiological meal test, allowing us to demonstrate the effects of endogenous GLP-1 signaling before and after RYGB and to evaluate both β-cell function and glucose tolerance. Therefore, our results expand the observations of Salehi et al.
We used a primed infusion of Ex-9 at a rate of 900 pmol × kg−1 × min−1. This dose of Ex-9, which resulted in stable plasma concentrations of ∼400 nmol/L on all experimental days, has previously been reported to result in 95% inhibition of the GLP-1R even in the presence of very high GLP-1 concentrations, such as those expected in the portal vascular bed after RYGB upon stimulation by a meal (21,22). GLP-1R blockade did not influence GIP secretion in this study, and it has previously been shown that Ex-9 infusion does not interfere with GIP signaling on the β-cells (48). In our own studies of the effect of GIP in COS-7 cells transfected with the human GIP receptor, there was no effect of Ex-9 in nanomolar to micromolar concentrations (M. Rosenkilde, unpublished observations). Collectively, this shows that Ex-9 specifically inhibits the actions of GLP-1 with respect to the incretin effect.
A limitation of this study is the small sample size of nine patients, which makes the detection of more subtle changes less likely. Despite this, we were able to demonstrate a highly significant effect of GLP-1R inhibition on insulin secretion and β-cell function after RYGB, underscoring the strength of the association.
In light of the marked inhibition of β-cell function, glucose tolerance was only moderately affected during Ex-9 infusion after the operation. This could reflect an increase in the relative importance of non-insulin-mediated glucose uptake for glucose tolerance (49–51), but the study was not designed to evaluate this.
In addition, we did not account for hepatic glucose production in this study, which could already be decreased 1 week after the operation, perhaps as a result of caloric restriction (9). Further studies are needed to investigate this early aspect of glucose metabolism after RYGB.
In vitro studies of the murine GLP-1R suggest that it is constitutively active and inhibited by Ex-9 even in the absence of GLP-1; i.e., Ex-9 is an inverse agonist (52). It is questionable whether this is a property of the human GLP-1R in vivo (53), but if so our results could be influenced by an increased GLP-1R sensitivity to the inverse agonism of Ex-9 after surgery. However, the effects of Ex-9 on basal insulin and glucagon secretion were greatest before the operation and decreased with time from surgery, which makes it highly unlikely that Ex-9 should more potently inhibit GLP-1R signaling after RYGB.
We conclude that an increased effect of GLP-1 as a consequence of hypersecretion is the main explanation for the improved β-cell function after RYGB in response to a meal in patients with type 2 diabetes. Furthermore, the effects of GLP-1 are important in controlling glucagon release and glucose tolerance in these patients after the operation.
ACKNOWLEDGMENTS
This work was carried out as a part of the program of the UNIK: Food, Fitness & Pharma for Health and Disease (see www.foodfitnesspharma.ku.dk). The UNIK project is supported by the Danish Ministry of Science, Technology and Innovation. Further support was received from the Danish Diabetes Association, The Novo Nordisk Foundation, and The Strategic Research Council for the Capital Area and the Danish Research Agency (Ministry of Science, Technology and Innovation). The purchase of Ex-9 was made possible through a donation from Desirée & Niels Ydes Foundation.
No potential conflicts of interest relevant to this article were reported.
N.B.J. planned and conducted experiments, researched data, and wrote the manuscript. C.D. conducted experiments, contributed to discussion, and reviewed the manuscript. K.N.B.-M., S.H.J., S.M., and J.J.H. contributed to discussion and reviewed the manuscript. D.W., D.L.H., V.B.K., and L.N. reviewed the manuscript. S.M. and J.J.H. planned experiments. N.B.J. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in oral form at the 48th Annual Meeting of the European Association for the Study of Diabetes, Berlin, Germany, 30 September–1 October 2012, and in poster form at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
This work would not have been possible without the technical assistance of Alis Sloth Andersen and Dorthe Baunbjerg Nielsen (Department of Endocrinology, Hvidovre Hospital) and Lene Brus Albæk (Department of Biomedical Sciences, Faculty of Health Sciences, University of Copenhagen).
Footnotes
Clinical trial reg. no. NCT01579981, clinicaltrials.gov.
REFERENCES
- 1.Schauer PR, Burguera B, Ikramuddin S, Cottam D, Gourash W, Hamad G, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg 2003;238:467–484; discussion 84–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 1995;222:339–350; discussion 350–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jørgensen NB, Jacobsen SH, Dirksen C, et al. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with Type 2 diabetes and normal glucose tolerance. Am J Physiol Endocrinol Metab 2012;303:E122–E131 [DOI] [PubMed] [Google Scholar]
- 4.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012;366:1577–1585 [DOI] [PubMed] [Google Scholar]
- 5.Kashyap SR, Daud S, Kelly KR, et al. Acute effects of gastric bypass versus gastric restrictive surgery on beta-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. Int J Obes (Lond) 2010;34:462–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nannipieri M, Mari A, Anselmino M, et al. The role of beta-cell function and insulin sensitivity in the remission of type 2 diabetes after gastric bypass surgery. J Clin Endocrinol Metab 2011;96:E1372–E1379 [DOI] [PubMed] [Google Scholar]
- 7.Morínigo R, Lacy AM, Casamitjana R, Delgado S, Gomis R, Vidal J. GLP-1 and changes in glucose tolerance following gastric bypass surgery in morbidly obese subjects. Obes Surg 2006;16:1594–1601 [DOI] [PubMed] [Google Scholar]
- 8.Camastra S, Gastaldelli A, Mari A, et al. Early and longer term effects of gastric bypass surgery on tissue-specific insulin sensitivity and beta cell function in morbidly obese patients with and without type 2 diabetes. Diabetologia 2011;54:2093–2102 [DOI] [PubMed] [Google Scholar]
- 9.Dirksen C, Jørgensen NB, Bojsen-Møller KN, et al. Mechanisms of improved glycaemic control after Roux-en-Y gastric bypass. Diabetologia 2012;55:1890–1901 [DOI] [PubMed] [Google Scholar]
- 10.Henry RR, Scheaffer L, Olefsky JM. Glycemic effects of intensive caloric restriction and isocaloric refeeding in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1985;61:917–925 [DOI] [PubMed] [Google Scholar]
- 11.Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 2005;54:603–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim EL, Hollingsworth KG, Aribisala BS, Chen MJ, Mathers JC, Taylor R. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011;54:2506–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn JP, Abumrad NN, Breitman I, et al. Hepatic and peripheral insulin sensitivity and diabetes remission at 1 month after Roux-en-Y gastric bypass surgery in patients randomized to omentectomy. Diabetes Care 2012;35:137–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isbell JM, Tamboli RA, Hansen EN, et al. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care 2010;33:1438–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor R. Banting Memorial lecture 2012: reversing the twin cycles of type 2 diabetes. Diabet Med 2013;30:267–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raufman JP, Singh L, Eng J. Exendin-3, a novel peptide from Heloderma horridum venom, interacts with vasoactive intestinal peptide receptors and a newly described receptor on dispersed acini from guinea pig pancreas. Description of exendin-3(9-39) amide, a specific exendin receptor antagonist. J Biol Chem 1991;266:2897–2902 [PubMed] [Google Scholar]
- 17.Falkén Y, Hellström PM, Holst JJ, Näslund E. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J Clin Endocrinol Metab 2011;96:2227–2235 [DOI] [PubMed] [Google Scholar]
- 18.Jacobsen SH, Olesen SC, Dirksen C, et al. Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obes Surg 2012;22:1084–1096 [DOI] [PubMed] [Google Scholar]
- 19.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev 2007;87:1409–1439 [DOI] [PubMed] [Google Scholar]
- 20.Staehr P, Hother-Nielsen O, Levin K, Holst JJ, Beck-Nielsen H. Assessment of hepatic insulin action in obese type 2 diabetic patients. Diabetes 2001;50:1363–1370 [DOI] [PubMed] [Google Scholar]
- 21.Nicolaus M, Brödl J, Linke R, Woerle H-J, Göke B, Schirra J. Endogenous GLP-1 regulates postprandial glycemia in humans: relative contributions of insulin, glucagon, and gastric emptying. J Clin Endocrinol Metab 2011;96:229–236 [DOI] [PubMed] [Google Scholar]
- 22. Morper M, Nicolaus M, Wörle J. B. Göke JS. The efficacy of exendin(9-39)amide as a GLP-1 receptor antagonist in human. Diabetologia 2009;52:803 [Google Scholar]
- 23.Edwards CM, Todd JF, Mahmoudi M, et al. Glucagon-like peptide 1 has a physiological role in the control of postprandial glucose in humans: studies with the antagonist exendin 9-39. Diabetes 1999;48:86–93 [DOI] [PubMed] [Google Scholar]
- 24.Orskov C, Rabenhøj L, Wettergren A, Kofod H, Holst JJ. Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes 1994;43:535–539 [DOI] [PubMed] [Google Scholar]
- 25.Krarup T, Holst JJ. The heterogeneity of gastric inhibitory polypeptide in porcine and human gastrointestinal mucosa evaluated with five different antisera. Regul Pept 1984;9:35–46 [DOI] [PubMed] [Google Scholar]
- 26.Krarup T, Madsbad S, Moody AJ, et al. Diminished immunoreactive gastric inhibitory polypeptide response to a meal in newly diagnosed type I (insulin-dependent) diabetics. J Clin Endocrinol Metab 1983;56:1306–1312 [DOI] [PubMed] [Google Scholar]
- 27.Kielgast U, Holst JJ, Madsbad S. Antidiabetic actions of endogenous and exogenous GLP-1 in type 1 diabetic patients with and without residual β-cell function. Diabetes 2011;60:1599–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 1992;41:368–377 [DOI] [PubMed] [Google Scholar]
- 29.Hovorka R, Soons PA, Young MA. ISEC: a program to calculate insulin secretion. Comput Methods Programs Biomed 1996;50:253–264 [DOI] [PubMed] [Google Scholar]
- 30.Salehi M, Aulinger B, Prigeon RL, D’Alessio DA. Effect of endogenous GLP-1 on insulin secretion in type 2 diabetes. Diabetes 2010;59:1330–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woerle HJ, Carneiro L, Derani A, Göke B, Schirra J. The role of endogenous incretin secretion as amplifier of glucose-stimulated insulin secretion in healthy subjects and patients with type 2 diabetes. Diabetes 2012;61:2349–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malandrucco I, Pasqualetti P, Giordani I, et al. Very-low-calorie diet: a quick therapeutic tool to improve β cell function in morbidly obese patients with type 2 diabetes. Am J Clin Nutr 2012;95:609–613 [DOI] [PubMed] [Google Scholar]
- 33.Umeda LM, Silva EA, Carneiro G, Arasaki CH, Geloneze B, Zanella MT. Early improvement in glycemic control after bariatric surgery and its relationships with insulin, GLP-1, and glucagon secretion in type 2 diabetic patients. Obes Surg 2011;21:896–901 [DOI] [PubMed] [Google Scholar]
- 34.Verdich C, Toubro S, Buemann B, Lysgård Madsen J, Juul Holst J, Astrup A. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety—effect of obesity and weight reduction. Int J Obes Relat Metab Disord 2001;25:1206–1214 [DOI] [PubMed] [Google Scholar]
- 35.Svendsen PF, Jensen FK, Holst JJ, Haugaard SB, Nilas L, Madsbad S. The effect of a very low calorie diet on insulin sensitivity, beta cell function, insulin clearance, incretin hormone secretion, androgen levels and body composition in obese young women. Scand J Clin Lab Invest 2012;72:410–419 [DOI] [PubMed] [Google Scholar]
- 36.Laferrère B, Heshka S, Wang K, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 2007;30:1709–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laferrère B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab 2008;93:2479–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reed MA, Pories WJ, Chapman W, et al. Roux-en-Y gastric bypass corrects hyperinsulinemia implications for the remission of type 2 diabetes. J Clin Endocrinol Metab 2011;96:2525–2531 [DOI] [PubMed] [Google Scholar]
- 39.Lin E, Liang Z, Frediani J, et al. Improvement in β-cell function in patients with normal and hyperglycemia following Roux-en-Y gastric bypass surgery. Am J Physiol Endocrinol Metab 2010;299:E706–E712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Højberg PV, Vilsbøll T, Rabøl R, et al. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia 2009;52:199–207 [DOI] [PubMed] [Google Scholar]
- 41.Meier JJ, Pennartz C, Schenker N, et al. Hyperglycaemia is associated with impaired pulsatile insulin secretion: effect of basal insulin therapy. Diabetes Obes Metab 2013;15:258–263 [DOI] [PubMed] [Google Scholar]
- 42.Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 1993;42:1663–1672 [DOI] [PubMed] [Google Scholar]
- 43.Näslund E, Backman L, Holst JJ, Theodorsson E, Hellström PM. Importance of small bowel peptides for the improved glucose metabolism 20 years after jejunoileal bypass for obesity. Obes Surg 1998;8:253–260 [DOI] [PubMed] [Google Scholar]
- 44.Mason EE. Ileal [correction of ilial] transposition and enteroglucagon/GLP-1 in obesity (and diabetic?) surgery. Obes Surg 1999;9:223–228 [DOI] [PubMed] [Google Scholar]
- 45.Dirksen C, Damgaard M, Bojsen-Møller KN, Jørgensen NB, Kielgast U, Jacobsen SH, et al. Fast pouch emptying, delayed small intestinal transit, and exaggerated gut hormone responses after Roux-en-Y gastric bypass. Neurogastroenterol Motil 2013;25:346–e255 [DOI] [PubMed] [Google Scholar]
- 46.Deacon CF, Wamberg S, Bie P, Hughes TE, Holst JJ. Preservation of active incretin hormones by inhibition of dipeptidyl peptidase IV suppresses meal-induced incretin secretion in dogs. J Endocrinol 2002;172:355–362 [DOI] [PubMed] [Google Scholar]
- 47.Salehi M, Prigeon RL, D’Alessio DA. Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes 2011;60:2308–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schirra J, Sturm K, Leicht P, Arnold R, Göke B, Katschinski M. Exendin(9-39)amide is an antagonist of glucagon-like peptide-1(7-36)amide in humans. J Clin Invest 1998;101:1421–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Best JD, Kahn SE, Ader M, Watanabe RM, Ni TC, Bergman RN. Role of glucose effectiveness in the determination of glucose tolerance. Diabetes Care 1996;19:1018–1030 [DOI] [PubMed] [Google Scholar]
- 50.Yang YJ, Hope I, Ader M, Poulin RA, Bergman RN. Dose-response relationship between lymph insulin and glucose uptake reveals enhanced insulin sensitivity of peripheral tissues. Diabetes 1992;41:241–253 [DOI] [PubMed] [Google Scholar]
- 51.Yang YJ, Hope ID, Ader M, Bergman RN. Insulin transport across capillaries is rate limiting for insulin action in dogs. J Clin Invest 1989;84:1620–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Serre V, Dolci W, Schaerer E, et al. Exendin-(9-39) is an inverse agonist of the murine glucagon-like peptide-1 receptor: implications for basal intracellular cyclic adenosine 3′,5′-monophosphate levels and beta-cell glucose competence. Endocrinology 1998;139:4448–4454 [DOI] [PubMed] [Google Scholar]
- 53.Thorens B, Porret A, Bühler L, Deng SP, Morel P, Widmann C. Cloning and functional expression of the human islet GLP-1 receptor. Demonstration that exendin-4 is an agonist and exendin-(9-39) an antagonist of the receptor. Diabetes 1993;42:1678–1682 [DOI] [PubMed] [Google Scholar]