Abstract
The importance of predation as a mortality factor in adult mosquitoes has received only limited attention in the scientific literature. Despite the lack of consensus among researchers as to whether bats are important predators of mosquitoes, there have been no attempts to directly document the effect of bats on mosquito populations or behavior. We conducted an enclosure experiment to test the hypothesis that bats reduce the local abundance of ovipositing female mosquitoes by examining whether the northern long-eared bat (Myotis septentrionalis Trouessart) had an effect on Culex spp. (Diptera: Culicidae) oviposition, using naturally occurring mosquitoes, either through direct predation or trait mediated effects on mosquito behavior. We found a signiÞcant, 32% reduction in egg-laying activity associated with bat predation. Artificial oviposition habitats directly outside bat enclosures experienced no reduction in oviposition; we attributed the observed reduction in egg-laying activity to direct predation on ovipositing females by bats and not changes in mosquito behavior. In addition, we noted a decrease in the number of larval mosquitoes in enclosures exposed to bat predation. These results suggest the impact of aerial predators on pathogen transmission may be large, and warrants further scientific investigation.
Keywords: predation, mosquitoes, predator effects, enclosure experiments, vector borne disease
There have been few studies examining the effects of predation on adult mosquitoes, despite the broad distribution and public health importance of culicids. Most studies of aerial predation on adult mosquitoes have been anecdotal or descriptive (Lamborn 1890, Rydell et al. 2002) or have focused on male mosquitoes (Yuval and Bouskila 1993), which do not transmit disease. Other studies have focused on sit-and-wait terrestrial predators, including arachnid, anuran, or reptilian predators (Dabrowska-Prot et al. 1968, Takagi et al. 1996, Canyon and Hii 1997, Strickman et al. 1997, Fox 1998, Roitberg et al. 2003, Jackson et al. 2005). In general, these studies have been conducted in small terraria and show predation rates from less than one mosquito per day per spider (Strickman et al. 1997) to >100 per day per gecko (Canyon and Hii 1997). Thus far, a lack of focused inquiry into realistic encounters between adult mosquitoes and their predators has precluded any true understanding of the role predators play in regulating mosquito populations.
Predators that are likely to have a large impact on ovipositing mosquitoes must be voracious, forage at the times that mosquitoes are seeking oviposition sites, and should be generalists, so they can persist on other prey when mosquitoes are less abundant. One group of potential predators that may impact mosquito populations is bats that forage at the same time of night Culex mosquitoes (Diptera: Culicidae) are seeking oviposition sites (Reddy et al. 2007, MacDonald et al. 1981, Lee and McCracken 2004); that are voracious feeders; and that are known to depredate mosquitoes, as evidenced by analyses of gut contents and fecal material (Buchler 1976, Whitaker and Lawhead 1992). However, few studies have explicitly examined bats feeding on mosquitoes. Rydell et al. (2002) discovered a unique study site in a barn in Alaska that allowed the direct observation of little brown bats (Myotis lucifugus) foraging on mosquitoes of an unknown species, and presumably representing males, host-seeking and possibly gravid females. Another study has noted high feeding rates of M. lucifugus and Myotis subulatus leibii bats (up to 10 mosquitoes per min) under laboratory settings with very high densities of released mosquitoes (Griffin et al. 1960). Although neither study of mosquito feeding observed changes in insect behavior, other studies of bat predation on insects have, including changes in the behavior of water striders (Hetereoptera: Gerridae) (Svensson et al. 2002) and many moths species, if they can detect ultrasound (Fenton and Fullard 1979, Acharya and McNeil 1998, Fullard 2001, Fullard and Napoleone 2001, Schoeman and Jacobs 2003, Ratcliffe and Fullard 2005).
There have been no studies of bat predation on mosquitoes that have attempted to quantify the impact on natural mosquito populations, instead relying primarily on gut or fecal analysis (Buchler 1976; Whitaker and Lawhead 1992). One reason for this lack of empirical data is that assessing the impacts of aerial predators on insect prey populations is logistically difficult using direct observations. One approach is to estimate the effects of bats on mosquitoes using artificial predator enclosures, which have been used in many systems to assess the effects of predators on individual prey populations (reviewed in Sih et al. 1985). Many studies have successfully used predator enclosures to study the behavioral interactions between bats and their prey. (Ratcliffe and Dawson 2003, Siemers and Ivanova 2004, Ratcliffe and Fullard 2005, Wund 2005). However, the use of aviaries or enclosures to quantify the impact of bats on natural populations of insects has not, to our knowledge, been published.
Although the overall importance of predation on adult mosquitoes is unknown, it may be an especially important source of mortality for recently blood-fed and gravid females that are encumbered by additional weight (Roitberg et al. 2003). Culex spp. mosquitoes, implicated in the majority of West Nile virus transmission in North America (Apperson et al. 2002), usually lay a single clutch of eggs between bloodmeals (Clements 1999). With the exception of vertically transmitted pathogens, mosquitoes that have recently oviposited are more likely to be carrying a pathogen, having fed at least once on a possibly infected host. Therefore, the increased predation risk on gravid females has implications for disease transmission above the overall impact of predation on mosquito populations. However, this fundamental hypothesis of how predation on vectors may affect disease transmission has not been examined empirically or in theoretical models.
Our objective was to demonstrate that bats may impact ovipositing Culex mosquitoes. Using predator enclosures in a field setting that would contain foraging bats but allow mosquitoes access to attractive oviposition habitats, we hypothesized that bats reduce the local abundance of ovipositing Culex mosquitoes. If bats were eating ovipositing mosquitoes, we predicted that enclosures containing northern long-eared bats (Myotis septentrionalis) would have fewer Culex egg rafts than enclosures without bats. Furthermore, if the presence of bats leads mosquitoes to avoid an area, we predicted that artificial oviposition habitats near bat-containing enclosures (but not accessible to the bats) would likewise show lower rates of oviposition, relative to habitats near enclosures not containing bats.
Materials and Methods
Study Site
This study was conducted at the E.S. George Reserve, located outside of Pickney, MI. The E.S. George Reserve is a 110-hectare protected reserve consisting of a mosaic of oak– hickory, maple– beech, and old field vegetation types. There are many semi-permanent and seasonal bodies of water in the site. Three sites were chosen based upon their common distance to wetland areas (40–60 m), openness of the local understory, distance from other sites, and utility in erecting enclosures. All sites were under closed canopy, and two (one and 3) were on abandoned two-track roads.
Bat Care and Handling
All bats were caught at the University of Michigan Biological Station located in Pellston, MI with standard harp trapping and mist-netting techniques. All bats used were identified using morphological characters as the northern long-eared bat. In total, nine bats were used throughout the study, six males and three females. Four bats escaped during the study. Animals were collected under Michigan DNR permit SC1139 (to M.A.W.) and cared for in accordance with the University of Michigan University Committee on the Use and Care of Animals (approval 8086).
Captive housing protocols followed the recommendations of Lollar and French (1998). Bats were housed in 0.108-m3 wooden cages with a screened window on the door. The cage interiors were lined in plastic and soft screening for ease of cleaning and to allow the bats to crawl around the cage comfortably. Fresh water was provided ad libitum. The bats were trained to take mealworms (Coleoptera: Tenebrionidae) from a dish and were fed twice daily, unless used in an evenings experiment, in which case they were only fed in the morning. Mealworms were raised on vitamin-enriched medium to provide adequate nutrition for the bats. On nights in which they were not used in the experiment, bats were allowed to βy in a 4- by 2.5- by 2-m screen-walled room all evening, while having full access to their cages. They would be returned to their roosting cages the following morning. A window with closed blinds provided a low level of natural lighting to the room. Ambient temperature was 24°C, and a heat lamp was placed 0.5 m from the back of each cage to provide a thermal gradient within the cages. After the experiment was completed, the bats were released at the capture site on 24 August 2004.
Bats were transported to the study site in a ventilated soft food container lined with screening and cloth. Although it is unclear how much stress the bats experienced during transport, they were generally calm and entered torpor during each trip. Before release, bats were fed two to three meal worms to initiate a gustatory response, and given water. Bats were released between 7 and 9 p.m., and retrieved the following morning between 9 and 11a.m. The individual identity of each bat was recorded before being released.
Enclosures
At each site, two 3-m3 enclosures were built. The frames of the enclosures were made of 1.905-cm (0.75-in.) internal diameter polyvinyl chloride pipe. The enclosures were screened on all sides using 0.635-cm (0.25-in.) mesh landscape netting (Landware Corporation, Reston, VA). To prevent escapes by bats, seams were sealed with hot glue and expanding, spray insulating foam (Great Stuff, Dow Chemical Co., Midland, MI). Access to each enclosure was via a 1-m2 zippered door made of heavy tarp material. The two enclosures at each site were positioned 50 m apart. The mesh size used in the enclosures had been shown to allow mosquitoes (Culex spp.) and other small insects access to the enclosure while preventing little brown bats, a similar sized-species to M. septentrionalis, from escaping (M.H.R. and M.A.W., unpublished data). Each enclosure also contained a small, shallow pan of fresh water for the bats to drink. The enclosure that contained bats also had a small wooden “bat box”to provide a comfortable roost. The bats used these roosts habitually, which allowed for ease in recapture by hand after each trial.
For both trials, artificial oviposition habitats (AOHs) were used to sample the ovipositing mosquito population (Reiskind and Wilson 2004). Each AOH was a 64–l plastic container (Rubbermaid Co., Columbus, OH). In each AOH we placed 10 liters of well water and 100 g of hay, wrapped in nylon netting and weighted with silica pebbles. The AOHs had screening stapled to the insides, to facilitate bats climbing out should they fall into the habitat.
Experimental Design
Two experiments were conducted during June and July 2004. At the beginning of each experiment, one of two enclosures at each site was randomly chosen to contain bats. This enclosure was the “bat enclosure” for the duration of the experiment, the other enclosure serving as a control in a paired design. This allowed us to examine both the effects of the bats on oviposition of mosquitoes (by comparing daily egg-clutch counts) and the number of larvae in the AOHs at the end of each experiment. During both months, bat calls were recorded using a D-100 Hetereodyne Detector (Pettersson Elektronik AB, Uppsala, Sweden), attached to a sound-activated cassette recorder. The regular production of “feeding buzzes,” characteristic noises produced by bats when they attack aerial prey (Griffin et al. 1960) qualitatively demonstrated that bats foraged within the enclosures on most nights. In addition, bats were regularly observed hunting in their enclosures while ambient light was available (M.A.W. and M.H.R., unpublished data). Although attacks were obvious, the identity of prey taken was not determined.
June Experiment
During the first experiment, five AOHs were placed in each study site: one was abutting the control enclosure, one inside the control enclosure, one equidistant (25 m) from each enclosure, one inside the bat enclosure, and one abutting the bat enclosure. Two bats were placed in one enclosure of each pair for nine nights with at least one night between “bat nights,” from 2 June to 25 June 2004. Bats were not released on nights rain was predicted. Every day for the duration of the experiment, Culex mosquito egg clutches were removed and counted from all AOHs. Egg clutches were not returned to the AOHs. Although every effort was made to collect all egg clutches before hatching, some eggs may have hatched before removal or partial egg clutches left behind. All egg clutches from this experiment were identified as Culex restuans (Theobald) by using characters from neonate larvae. On 25 June, the experiment was terminated, and all AOHs were harvested. Harvesting was conducted in an identical manner for all AOHs: a 400-ml water sample was taken with eight dips of a 50-ml tube attached to a wooden dowel. The remainder of the water was filtered through a 0.1 mm gauge net, and all of the unfiltered material was put with the 400-ml water sample. These samples were taken back to the laboratory, and all macroinvertebrates were counted and identified to higher taxa following Merritt and Cummins (1996), and then to morphospecies (data not shown). Mosquito larvae were identified to species using Darsie and Ward (1981).
July Experiment
During the second experiment, only three AOHs were placed in each study site: one in each enclosure and one equidistant between the two enclosures. Between 7 July and 28 July 2004, a single bat was released in the bat enclosure for nine nights, with at least one night between bat nights. Culex spp. egg clutches were counted and removed daily. Almost all egg clutches (94%) were identified as Cx. restuans, with the remaining 6% identified as Culex pipiens (L.). On 28 July 2004, all AOHs were harvested as in the June experiment.
Statistical Analysis
All statistical analyses were conducted in SAS 9.0 (SAS Institute, Cary, NC). Oviposition data could not be transformed to approximate a normal distribution. In all analyses, goodness-of-fit measures (assessed by dividing measures of deviance by degrees of freedom and comparing which distribution resulted in a df/dev closest to 1) suggested a better fit to the negative binomial than a Poisson or normal probability distribution. Consequently, to compare oviposition activity among AOHs on nights bats were released, we used a repeated measures negative binomial regression with an exchangeable correlation structure to model the correlations between AOHs within each site and night (PROC GENMOD). Based upon previous studies showing that on any given night, when multiple AOHs are present, egg-clutch abundance is aggregated but idiosyncratic for each night (Reiskind and Wilson 2004), each site by night combination was considered an independent observation. The repeated measures regression was used to capture the paired nature of the experimental design (bat cage versus nonbat cage within each site). Unadjusted means are presented in the results to demonstrate the effect sizes observed.
Comparisons between nights when bats were released and nights when bats were not released were made with the same analytical approach (PROC GEN-MOD), with site alone as a clustering factor. The use of this repeated measures methodology necessitates the comparison of parameters from generalized estimating equations (GEEs) to compare main effects, along with Wald statistics for type 3 analysis (comparable to a type III sum of squares analysis in analysis of variance [ANOVA]) (Candy 2000, Diggle et al. 2002). To further strengthen the robustness of our results, as well as to present a more familiar statistical analysis, a paired t-test was performed on the sums of all egg clutches laid for each month for each site, comparing cages with bats and cages without bats, both on nights bats were released and on nights bats were not released. Because there were few macroin-vertebrates in the AOHs other than Culex spp. larvae, only the total abundance of mosquito larvae were compared for each month using a linear mixed-model ANOVA to account for the paired design of the experiment, with site as a random effect.
Results
Effects of Bat Predation on Mosquito Oviposition
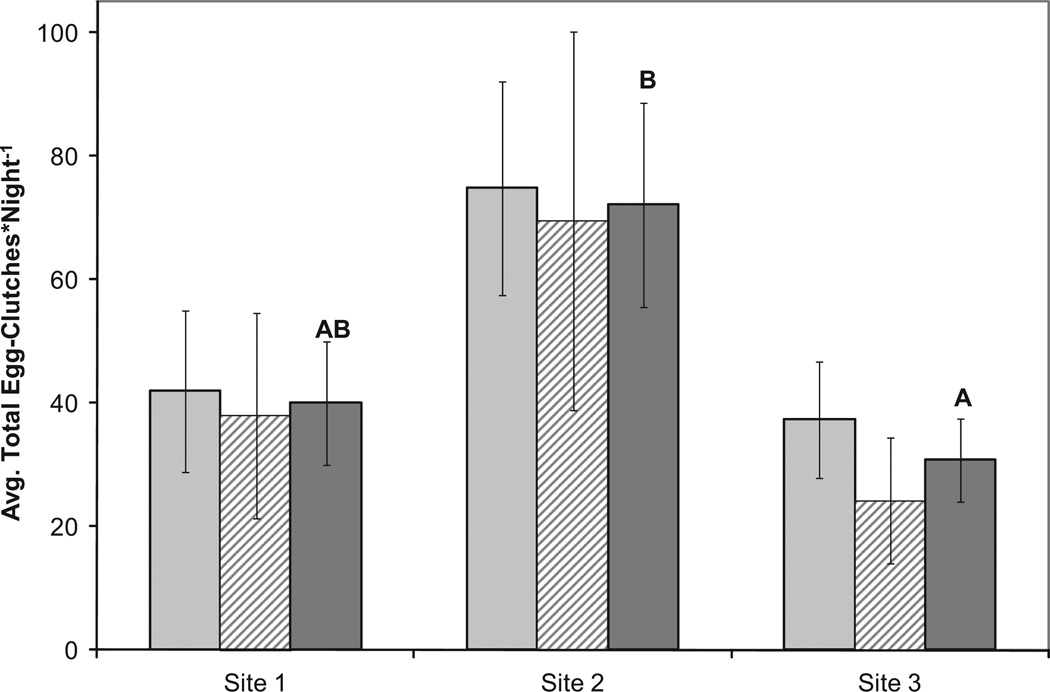
When total egg clutches (sum of all containers) per site per day are compared for the two experiments (June and July), there is a significant effect of site, but no significant effect of month nor interaction between month and site (type 3 effect of site: χ2df = 2 = 7.86, P = 0.0196) (Fig. 1; Table 1). Examining each month separately yields a similar pattern of oviposition activity in each of the three sites, suggesting the consistency of total mosquito activity for each site (Fig. 1). Therefore, we combined the data from the 2 mo and compared the number of egg clutches laid per night in the AOH in enclosures with bats, the control AOH (enclosed but not exposed to bats), and the unenclosed AOH within each site by night combination. This comparison demonstrated a significant effect of bats on oviposition activity, reducing oviposition in bat-containing enclosures by 32% relative to enclosures without bats (bat present: 8.44 egg clutches per night; bat absent: 12.42 egg clutches per night; Z1 = 3.26, P = 0.0011) (Table 2). Post hoc tests show significantly fewer egg clutches laid in the bat enclosure relative to both the unenclosed and control habitats, and no difference between the enclosed without bat AOH and the unenclosed AOH (χ2 df = 1 = 2.62, P = 0.1055).
Fig. 1.
Average egg clutches laid per night in each site in June and July. Light gray bars are for June (n= 22), hatched bars are for July (n= 21) and dark gray bars for both months combined (n= 45). Error bars are ± 1 SEM. Letters denote significant differences by post hoc tests from a negative binomial regression (see Table 1).
Table 1.
Initial parameter estimates of negative binomial regression of the total egg clutches laid per site, per night (22 nights in June, 21 nights in July)
| Parameter | Categorya | df | Estimate | SE | 95% CL | χ2 | P | |
|---|---|---|---|---|---|---|---|---|
| Intercept | 1 | 3.6411 | 0.2007 | 3.2477 | 4.0344 | 329.11 | < 0.0001 | |
| Site | 1 | 1 | 0.2912 | 0.2363 | −0.1720 | 0.7543 | 1.52 | 0.2179 |
| Site | 2 | 1 | 0.6604 | 0.2362 | 0.1975 | 1.1233 | 7.82 | 0.0052 |
| Site | 3 | 0 | 0 | 0 | 0 | 0 | ||
| Month | June | 1 | −0.1085 | 0.1931 | −0.4870 | 0.2699 | 0.32 | 0.5740 |
| Month | July | 0 | 0 | 0 | 0 | 0 | ||
| Dispersion | 1 | 1.2552 | 0.1448 | 0.9713 | 1.5390 | |||
Site 3 and July are used as reference categories.
Table 2.
GEE parameter estimates from the negative binomial regression of numbers of egg clutches laid per night in bat enclosures, no-bat enclosures, and unenclosed AOHs on nights bats were released
| Parameter | Categorya | Estimate | SE | 95% CL | Z-score | P | |
|---|---|---|---|---|---|---|---|
| Intercept | 2.1335 | 0.2048 | 1.7320 | 2.5350 | 10.42 | < 0.0001 | |
| Treatment | Unenclosed | 0.5795 | 0.1502 | 0.2851 | 0.8768 | 3.86 | 0.0001 |
| Treatment | No-bat | 0.3863 | 0.1185 | 0.1541 | 0.6185 | 3.26 | 0.0011 |
| Treatment | Bat | 0 | 0 | 0 | 0 | ||
The bat treatment is used as a reference category.
We also were able to compare the oviposition activity on nights bats were released to nights bats were not released, in the same AOH. This comparison demonstrates a significant effect of the presence of bats in lowering the number of egg clutches laid, as opposed to some unmeasured factor specific to the bat enclosures. Comparing nights with bats in the bat enclosure to nights without bats in the same enclosure, there was an overall 34% reduction in egg clutches laid, very similar to the reduction observed between bat and no bat enclosures within each site on any given night (bats present: 8.44 egg clutches per night or bats absent: 12.72 egg clutches per night; Z1 = 3.45 P = 0.0006) (Table 3A). The other AOHs (unenclosed and control enclosure) did not show any significant differences between nights bats were released and nights bats were not released (Table 3B and C). Furthermore, comparing bat enclosures to control enclosures on nights bats were not released, showed no significant difference in number of egg-clutches laid (least square means (backtransformed from log means) (±1 SE): 13.72 (±1.86) versus 11.48 (±1.62) egg-clutches per night (χ21 = 3.73, P = 0.0534; negative binomial regression).
Table 3.
GEE parameter estimates of the negative binomial regression comparing number of egg clutches laid on nights bats were released to nights bats were not released in AOHs in bat enclosure (A), no-bat enclosure (B), and unenclosed (C) treatments
| Parametera | Estimate | SE | 95% CL | Z-score | P | ||
|---|---|---|---|---|---|---|---|
| A. Bat enclosure | Intercept | 2.5809 | 0.2399 | 2.1108 | 3.0510 | 10.76 | < 0.0001 |
| Bats absent | 0.1948 | 0.0564 | 0.0843 | 0.3054 | 3.45 | 0.0006 | |
| Bats present | 0 | 0 | 0 | 0 | |||
| June | −0.9224 | 0.1637 | −1.2432 | −0.6015 | −5.63 | < 0.0001 | |
| July | 0 | 0 | 0 | 0 | . | . | |
| B. No-bat enclosure | Intercept | 2.8967 | 0.2078 | 2.4893 | 3.3040 | 13.94 | < 0.0001 |
| Bat absent | 0.0131 | 0.1154 | −0.2131 | 0.2393 | 0.11 | 0.9097 | |
| Bat present | 0 | 0 | 0 | 0 | |||
| June | −0.7541 | 0.2174 | −1.1803 | −0.3279 | −3.47 | < 0.0005 | |
| July | 0 | 0 | 0 | 0 | |||
| C. Unenclosed | Intercept | 3.0829 | 0.2794 | 2.5353 | 3.6304 | 13.94 | < 0.0001 |
| Bat absent | 0.0105 | 0.914 | −0.1687 | 0.1897 | 0.11 | 0.9087 | |
| Bat present | 0 | 0 | 0 | 0 | |||
| June | −0.7905 | 0.0944 | −0.9755 | −0.6056 | −8.38 | < 0.0001 | |
| July | 0 | 0 | 0 | 0 | |||
Nights bats were released (bats present) and July are used as reference categories.
Using a paired t-test, we compared the total number of egg clutches laid over a month on nights bats were released and nights bats were not released in habitats that contained a bat versus control habitats. We found significantly fewer egg clutches in habitats that contained a bat compared with control habitats on nights bats were released (t5 = 2.71, P < 0.05), but not on nights bats were not released (t5 = 1.46, P < 0.25).
Effects of Bat Predation on Mosquito Behavior
The impact of trait-mediated effects on mosquito oviposition was examined in the June trial only, by comparing AOHs immediately outside enclosures containing bats. There were no significant differences between containers immediately outside a bat-containing enclosure and those outside a nonbat enclosure in the number of egg clutches laid (data not shown). There were significantly fewer egg clutches laid in AOHs in a bat containing enclosure relative to the AOH immediately outside the same enclosure (χ21 = 9.00, P = 0.003; negative binomial regression).
Effects of Enclosures on Mosquito Oviposition
By comparing AOHs within the bat-free enclosure to the AOHs between the two enclosures, we were able to assess the effects of the enclosure on oviposition activity. Considering all nights, there was no significant difference between the control AOH and the unenclosed AOH (χ21 = 3.03, P = 0.082; negative binomial regression).
Effects of Bat Predation on Larval Mosquitoes
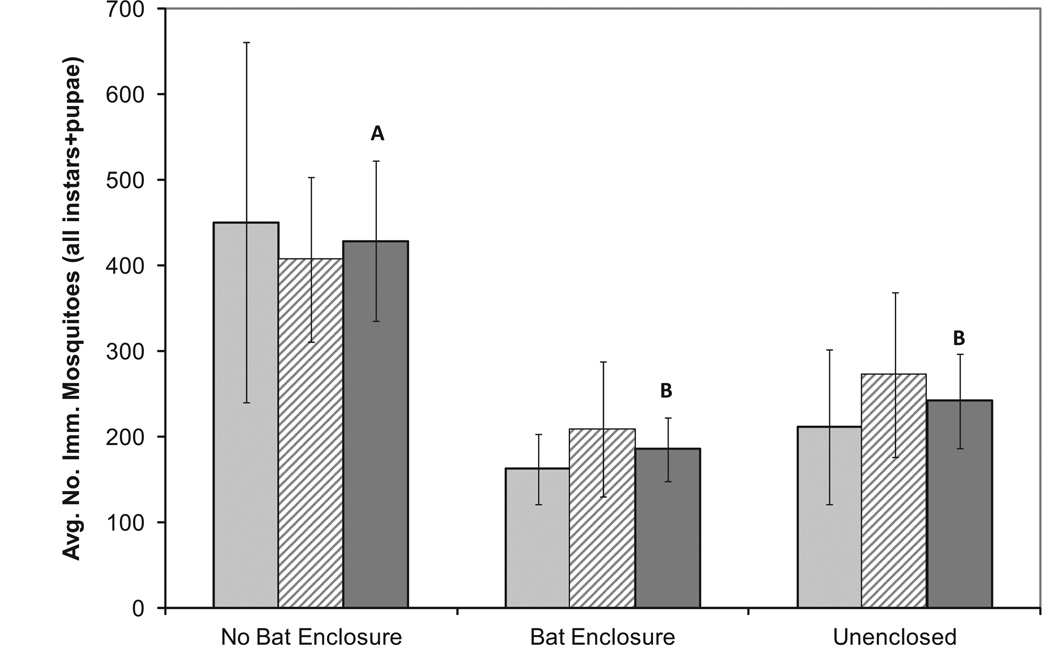
Both months of observation showed similar patterns in number of immature mosquitoes (Fig. 2). Combining both months of observations, the total number of immature Culex mosquitoes was significantly reduced in bat enclosures (Fig. 2, linear mixed model ANOVA, F2, 6 = 4.89, P = 0.026).
Fig. 2.
Average number of immature Culex (larvae and pupae) spp. collected at the end of each month in each artificial oviposition habitat location. Light gray bars are for June (n= 3), hatched bars are for July (n= 3) and dark gray bars for both months combined (n= 6). Error bars are ± 1 SEM. Letters denote significant differences by post hoc tests with an adjusted α(α = 0.0183) from the linear mixed model regression.
Discussion
The observed 32% reduction in egg clutches laid in habitats placed within enclosures containing one or two M. septentrionalis, relative to habitats placed in control enclosures that did not contain bats, indicates that bats reduce mosquito oviposition under these experimental conditions. A similar effect size (34% reduction) of eggs laid in the bat enclosures on nights a bat was present relative to nights we did not put a bat in the enclosure demonstrates that the reduction in egg masses in bat enclosures only occurred when bats were present. On nights bats were not released, there was no significant difference between the designated bat enclosures and control enclosures, further strengthening the conclusion that the presence of bats is the only factor affecting oviposition activity in this study. The similarity in effect size between control and bat enclosures, and between nights with bats and nights without bats in the designated bat enclosures suggests the robustness of the effect of bats on oviposition activity. Although we consider the most likely explanation to be predation on adult, ovipositing (or oviposition seeking) females, we cannot reject the alternative hypothesis that bats are eating egg rafts directly, although to our knowledge, bats, including M. septentrionalis, have never been observed feeding on egg rafts, nor have rafts been found in previous studies of gut or fecal material of bats.
We found no support for the hypothesis that mosquitoes change their oviposition behavior in response to foraging bats, because there was no difference in the number of egg rafts laid in AOHs immediately outside bat containing enclosures and AOHs immediately outside control enclosures in the June experiment. Because there was no evidence for behavioral changes in ovipositing mosquitoes, it is likely this effect on mosquitoes is entirely density mediated. It is possible that mosquitoes do respond to the presence of bats by avoiding large areas where bats are flying, but we observed no evidence for this based upon identical oviposition rates on nights bats were released to nights bats were not released in AOHs not exposed to bats. Rydell et al. (2002) also did not note any behavioral modification by mosquitoes in response to bats, although these mosquitoes were likely host or mate seeking, not ovipositing. Other researchers have noted behavioral changes in bat prey (Fenton and Fullard 1979, Svensson et al. 2002), and the lack of behavioral response to bat presence in mosquitoes may suggest that bats are not an important source of adult mortality in these mosquitoes.
This study is the first documented measurement of the effects of bats on female mosquitoes. All previous studies of the presence of mosquitoes in bats’ diets have suggested that mosquitoes may make up a small proportion of bats’ diets, but they cannot estimate any impact of bats on mosquito populations, or even suggest a number of mosquitoes eaten (Whitaker and Lawhead 1992, Brack and Whitaker 2001, Carter et al. 2003, Whitaker 2004). In addition to the significant differences in oviposition in the enclosures, we have qualitative evidence of bats foraging in our enclosures, based upon ultrasound tape recordings and observations of hunting behavior (M.A.W. and M.H.R., unpublished data) made during the experiment. In spite of the fact that our effective bat densities are orders of magnitude higher than natural densities of 10 –17 bats per hectare for related species Myotis sodalis, Myotis myotis, and Myotis blythii (Humphrey et al. 1977, Arlettaz 1996), M. septentrionalis does forage in restricted, understory spaces and may forage intensely in an area (Kurta 1995, Caceres and Barclay 2000, Ratcliffe and Dawson 2003; Broders et al. 2004; M.A.W., personal observation). We can conclude, under the right circumstances, M. septentrionalis can substantially reduce oviposition even when other prey are available. The question remains as to whether our results translate to more natural conditions. Based on our findings, we suggest that more naturalistic investigations of this question should focus on measuring the intensity of bat predation where and when ovipositing females are locally abundant.
There were significantly lower numbers of immature mosquitoes in habitats in bat enclosures, relative to habitats in no bat enclosures. There are three possible explanations for this pattern. First, collecting egg clutches is difficult to do in such a manner as to prevent the colonization of habitats by Culex mosquitoes. Often small pieces of a clutch will break away and remain in the container. In addition, some eggs may hatch before being collected. Assuming a consistent rate of collection failure, we could interpret this pattern as reflecting the differences in egg input. Second, because both the bat enclosure and the unenclosed habitat had some degree of exposure to aerial predation, the nonbat cage could have acted as a predator exclosure, protecting the AOH from exposure to predators foraging, but not staying, in the AOHs. Therefore, although we did not measure natural levels of predation, it is possible that this pattern is due to predation directly on the aquatic mosquito larvae. Finally, bats, or other predators in the case of the unenclosed habitat, may be directly foraging on larval mosquitoes. Taking larval, aquatic insects has not been observed in this species of bat, but congeners of M. septentrionalis are known to take insects off surfaces by scooping them within their tail membranes, including insects alighting on water (Siemers 2001). Although we do not have observations of this behavior in bats, it may be worth investigating.
In this study, we have found evidence for bats depredating ovipositing mosquitoes. Although the experimental set up is artificial, our data suggest M. septentrionalis will forage on ovipositing mosquitoes, given the appropriate conditions. The weight burden of blood and eggs may increase susceptibility to predation of blood fed or gravid females (Roitberg et al. 2003, Jackson et al. 2005), although the resting behavior of recently blood fed mosquitoes may decrease the opportunity for contact between recently blood-fed mosquitoes and vertebrate predators. Eventually, gravid females need to oviposit and will aggregate in large numbers around attractive oviposition sites (Reiskind and Wilson 2004), possibly providing an opportunity for bats and gravid mosquitoes to come into contact. Studies have shown bat foraging activity to be determined by the abundance of emergent, aquatic insects, both spatially and temporally (Fukui et al. 2006). Together, these points suggest bat predation on mosquitoes could help regulate disease vectors. We were not able to test all of these hypotheses in our study, however, we have shown, experimentally, that a single M. septentrionalis, can exert a major reduction in local abundance of potentially disease carrying Culex mosquitoes. These results suggest the impact of aerial predators on pathogen transmission may be large and warrants further scientific investigation.
Acknowledgments
We thank Graham Newman, Lyssa Sperlich, and Thomas Chappel for assistance in the field. We thank Todd Livdahl, Mark Wilson, L. Phil Lounibos, Stacy Philpott, Steve Yanoviak, and Kristi Judd for useful comments and discussion on this research. We also thank two anonymous reviewers, whose comments greatly improved this manuscript. This work was funded by the E.S. George Reserve Scholarship (to M.H.R.), The University of Michigan Department of Ecology and Evolutionary Biology Block grant (to M.H.R. and M.A.W.), The American Museum of Natural History Theodore Roosevelt Memorial Fund (to M.A.W.), and an Animal Behavior Society student research grant (to M.A.W.). M.H.R. is supported by the Oklahoma Agricultural Experiment Station under Hatch Project 2702 and MSS NE507 “Mosquitoes and Public Health” Project 2712.
References Cited
- Acharya L, McNeil J. Predation risk and mating behavior: the responses of moths to bat-like ultrasound. Behav. Ecol. 1998;9:552–558. [Google Scholar]
- Apperson C, Harrison B, Unnasch T, Hassan H, Irby W, Savage H, Aspen S, Watson D, Rueda L, Engber B, et al. Host-feeding habits of Culex and other mosquitoes (Diptera: Culicidae) in the borough of Queens in New York City, with characters and techniques for identification of Culex mosquitoes. J. Med. Entomol. 2002;39:777–785. doi: 10.1603/0022-2585-39.5.777. [DOI] [PubMed] [Google Scholar]
- Arlettaz R. Feeding behaviour and foraging strategy of free-living mouse-eared bats, Myotis myotis and Myotis blythii . Anim. Behav. 1996;51:1–11. [Google Scholar]
- Brack V, Whitaker JO. Foods of the northern myotis, Myotis septentrionalis, from Missouri and Indiana, with notes on foraging. Acta Chiropterol. 2001;3:203–210. [Google Scholar]
- Broders HG, Findlay CS, Zheng L. Effects of clutter on echolocation call structure of Myotis septentrionalis and M. lucifugus . J. Mammal. 2004;85:273–281. [Google Scholar]
- Buchler ER. Prey selection by Myotis lucifugus (Chiroptera: Vespertilionidae) Am. Nat. 1976;110:619–628. [Google Scholar]
- Caceres M, Barclay R. Myotis septentrionalis. Mammal. Species. 2000;634:1–4. [Google Scholar]
- Candy S. The application of generalized linear mixed models to multi-level sampling for insect population monitoring. Environ. Ecol. Stat. 2000;7:217–238. [Google Scholar]
- Canyon D, Hii J. The gecko: an environmentally friendly biological agent for mosquito control. Med. Vet. Entomol. 1997;11:319–323. doi: 10.1111/j.1365-2915.1997.tb00416.x. [DOI] [PubMed] [Google Scholar]
- Carter TC, Menzel MA, Owen SF, Edwards JW, Menzel JM, Ford WM. Food habits of seven species of bats in the Allegheny Plateau and Ridge and Valley of West Virginia. Northeast. Nat. 2003;10:83–88. [Google Scholar]
- Clements A. The biology of mosquitoes. London, United Kingdom: CABI; 1999. [Google Scholar]
- Dabrowska-Prot E, Luczak J, Tarwid K. The predation of spiders on forest mosquitoes in field experiments. J. Med. Entomol. 1968;5:252–256. doi: 10.1093/jmedent/5.2.252. [DOI] [PubMed] [Google Scholar]
- Darsie R, Ward R. Identification and geographical distribution of the mosquitoes of North America, north of Mexico. Gainesville, FL: The University of Florida Press; 1981. [Google Scholar]
- Diggle P, Heagerty P, Liang K, Zeger S. Analysis of longitudinal data. 2nd ed. Oxford, United Kingdom: Oxford University Press; 2002. [Google Scholar]
- Fenton MB, Fullard JH. The inβuence of moth hearing on bat echolocation strategies. J. Comp. Physiol. A. 1979;132:77–86. [Google Scholar]
- Fox I. Predation on Aedes aegypti (Diptera: Culicidae) by Theridion rufipes (Araneae: Theridiidae) in Puerto Rico. J. Med. Entomol. 1998;35:611–613. doi: 10.1093/jmedent/35.4.611. [DOI] [PubMed] [Google Scholar]
- Fukui D, Masashi M, Nakano S, Aoi T. Effect of emergent aquatic insects on bat foraging in a riparian forest. J. Anim. Ecol. 2006;75:1252–1258. doi: 10.1111/j.1365-2656.2006.01146.x. [DOI] [PubMed] [Google Scholar]
- Fullard JH. Auditory sensitivity of Hawaiian moths (Lepidoptera: Noctuidae) and selective predation by the Hawaiian hoary bat (Chiroptera: Lasiurus cinereus semotus) Proc. R. Soc. Lond. B. 2001;268:1375–1380. doi: 10.1098/rspb.2001.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullard JH, Napoleone N. Diel flight periodicity and the evolution of auditory defences in the Macrolepidoptera. Anim. Behav. 2001;62:349–368. [Google Scholar]
- Griffin DR, Webster FA, Michael CR. The echolocation of flying insects by bats. Anim. Behav. 1960;8:141–154. [Google Scholar]
- Humphrey SR, Richter AR, Cope JB. Summer habitat and ecology of the endangered Indiana bat, Myotis sodalis . J. Mammal. 1977;58:334–346. [Google Scholar]
- Jackson RR, Nelson XJ, Sune GO. A spider that feeds indirectly on vertebrate blood by choosing female mosquitoes as prey. Proc. Natl. Acad. Sci. U.S.A. 2005;102:15155–15160. doi: 10.1073/pnas.0507398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurta A. Mammals of the Great Lakes Region. Ann Arbor, MI: University of Michigan Press; 1995. revised edition. [Google Scholar]
- Lamborn R. Dragonflies vs. mosquitoes: can the mosquito pest be mitigated? New York City, NY: D. Appleton and Company; 1890. [Google Scholar]
- Lee YF, McCracken GF. Flight activity and food habits of three species of Myotis bats (Chiroptera: Vespertilionidae) in sympatry. Zool. Stud. 2004;43:589–597. [Google Scholar]
- Lollar A, French BS. Captive care and medical reference for the rehabilitation of insectivorous bats. Mineral Wells, TX: Bat World Publication; 1998. [Google Scholar]
- MacDonald R, Madder D, Surgeoner G. Diel periodicity of oviposition by Culex pipiens and Culex restuans in southern Ontario. Proc. Entomol. Soc. Ont. 1981;112:39–40. [Google Scholar]
- Merritt R, Cummins K. An introduction to the aquatic insects of North America. Dubuque, IA: Kendall/Hunt Publishing Company; 1996. [Google Scholar]
- Ratcliffe J, Fullard J. The adaptive function of tiger moth clicks against echolocating bats: an experimental and synthetic approach. J. Exp. Biol. 2005;208:4689–4698. doi: 10.1242/jeb.01927. [DOI] [PubMed] [Google Scholar]
- Ratcliffe JM, Dawson JW. Behavioral flexibility: the little brown bat, Myotis lucifugus, and the northern long-eared bat, M. septentrionalis, both glean and hawk prey. Anim. Behav. 2003;66:847–856. [Google Scholar]
- Reddy MR, Lepore TJ, Pollack RJ, Kiszewski AE, Spielman A, Reiter P. Early evening questing and oviposition activity by the Culex (Diptera: Culicidae) vectors of West Nile virus in northeastern North America. J. Med. Entomol. 2007;44:211–214. doi: 10.1603/0022-2585(2007)44[211:eeqaoa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Reiskind MH, Wilson ML. Culex restuans (Diptera: Culicidae) oviposition behavior determined by larval habitat quality and quantity in southeastern Michigan. J. Med. Entomol. 2004;41:179–186. doi: 10.1603/0022-2585-41.2.179. [DOI] [PubMed] [Google Scholar]
- Roitberg B, Mondor E, Tyerman J. Pouncing spider, flying mosquito: blood acquisition increases predation risk in mosquitoes. Behav. Ecol. 2003;14:736–740. [Google Scholar]
- Rydell J, MCNeill DP, Eklof J. Capture success of little brown bats (Myotis lucifugus) feeding on mosquitoes. J. Zool. 2002;256:379–381. [Google Scholar]
- Schoeman MC, Jacobs DS. Support for the allotonic frequency hypothesis in an insectivorous bat community. Oecologia (Berl.) 2003;134:154–162. doi: 10.1007/s00442-002-1107-1. [DOI] [PubMed] [Google Scholar]
- Siemers BM. Finding prey by associative learning in gleaning bats: experiments with a Natterers bat Myotis nattereri . Acta Chiropterol. 2001;3:211–215. [Google Scholar]
- Siemers BM, Ivanova T. Ground gleaning in horseshoe bats: comparative evidence from Rhinolophus blasii. In: euryale R, editor. R. mehelyi. Behav. Ecol. Sociobiol. Vol. 56. 2004. pp. 464–471. [Google Scholar]
- Sih A, Crowley P, McPeek M, Petranka J, Strohm-eier K. Predation, competition, and prey communities: a review of field experiments. Annu. Rev. Ecol. Syst. 1985;16:269–311. [Google Scholar]
- Strickman D, Sithiprasasna R, Southard D. Bionomics of the spider, Crossopriza lyoni (Araneae, Pholcidae), a predator of dengue vectors in Thailand. J. Arachnol. 1997;25:194–201. [Google Scholar]
- Svensson AM, Danielsson I, Rydell J. Avoidance of bats by water striders (Aquarius najas, Hemiptera) Hydrobiologia. 2002;489:83–90. [Google Scholar]
- Takagi M, Sugiyama A, Maruyama K. Survival of newly emerged Culex tritaeniorhynchus (Diptera: Culicidae) adults in field cages with or without predators. J. Med. Entomol. 1996;33:699–701. doi: 10.1093/jmedent/33.4.698. [DOI] [PubMed] [Google Scholar]
- Whitaker JO, Lawhead B. Foods of Myotis lucifugus in a maternity colony in central Alaska. J. Mammal. 1992;73:646–648. [Google Scholar]
- Whitaker JOJ. Prey selection in a temperate zone insectivorous bat community. J. Mammal. 2004;85:460–469. [Google Scholar]
- Wund MA. Learning and the development of habitat-specific bat echolocation. Anim. Behav. 2005;70:441–450. [Google Scholar]
- Yuval B, Bouskila A. Temporal dynamics of mating and predation in mosquito swarms. Oecologia (Berl.) 1993;95:65–69. doi: 10.1007/BF00649508. [DOI] [PubMed] [Google Scholar]