Abstract
Xylaria, belonging to the Ascomycotina, is known to produce diverse classes of bioactive substances. In an effort to identify the chemical constituents of the fruiting bodies of Xylaria polymorpha, linoleic acid (1), linoleic acid methyl ester (2), ergosterol (3), 4-acetyl-3,4-dihydro-6,8-dihydroxy-3-methoxy-5-methyl-1H-2-benzopyran-1-one (4), and 4-hydroxyscytalone (5) were isolated from its methanolic extract. Their structures were assigned on the basis of various spectroscopic studies.
Keywords: Ergosterol, 4-Hydroxyscytalone, Linoleic acid, Mushroom, Xylaria polymorpha
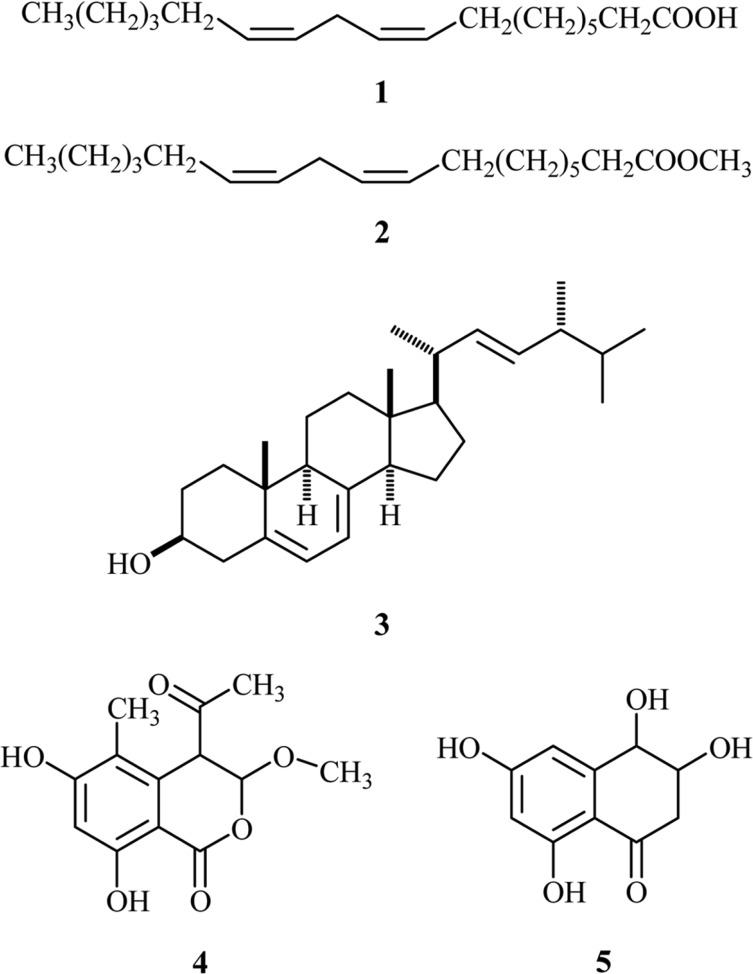
Mushrooms produce a large variety of secondary metabolites with unique chemical structures and interesting biological activities. Xylaria, belonging to Ascomycotina, is known to produce diverse classes of bioactive compounds including cytochalasin analogs (Jayasuriya et al., 2004), antifungal metabolites multiplolides A and B (Boonphong et al., 2001), NPY Y5 receptor antagonists xyarenals A and B (Smith et al., 2002), acetylcholine esterase inhibitors xyloketals A-E (Lin et al., 2001), xylariamide A (Davis, 2005), and xanthones (Healy et al., 2004). In a previous study for antimicrobial agents from Korean native mushroom extracts, we found that the fruiting body extracts of X. polymorpha exhibited potent antifungal activity against plant pathogenic fungi. By using antifungal activity-guided fractionation, we isolated and reported two new antifungal polypropionates, xylarinic acids A and B (Jang et al., 2007), and two new 2-benzoxepin derivatives, xylarinols A and B (Lee et al., 2009). Further investigation on the chemical constituents of the fruiting body of X. polymorpha led to the isolation of five compounds, linoleic acid (1), linoleic acid methyl ester (2), ergosterol (3), 4-acetyl-3,4-dihydro-6,8-dihydroxy-3-methoxy-5-methyl-1H-2-benzopyran-1-one (4), and 4-hydroxyscytalone (5) (Fig. 1). In this study, we describe the isolation and structure determination of these compounds.
Fig. 1.
Structures of compounds 1~5.
Materials and Methods
General methods
ESI-mass was taken on a Navigator mass spectrometer in positive and negative modes. NMR spectra were obtained on a Varian UNITY Inova NMR spectrometer with 1H NMR at 400MHz and 13C NMR at 100MHz in CDCl3 or CD3OD. Chemical shifts were given in ppm (δ) using tetramethylsilane (TMS) as internal standard.
Fungal materials
The dried mushroom Xylaria polymorpha (285 g) was collected in the Gwangneung forest in Gyeonggi province, Korea, in October 2006, and identified by the staff at the Korea Research Institute of Bioscience and Biotechnology (KRIBB), according to the taxonomic key of Imazeki and Hongo (Imazeki et al., 1988).
Extraction and isolation
Dried fruiting bodies of X. polymorpha (285 g) were extracted twice with methanol at room temperature. The solvent partitioning was carried out by using hexane, chloroform, and ethyl acetate. Silica gel column chromatographies were performed by a stepwise elution with increasing amount (2, 10 and 33%, stepwise) of ethyl acetate in hexane and then by an increasing (2%→90%) gradient of methanol in chloroform. Sephadex LH-20 column chromatography was done with an elution solvent of chloroform:methanol (1 : 1, v/v). Preparative reversed-phase HPLC was carried out using an ODS column (20 × 150 mm), eluting with 40% aqueous MeOH at a flow rate of 6 ml/min, monitoring at UV 250 nm. C18 Sep-pak cartridge was done by washing with 30% aqueous methanol and eluting with 40% aqueous methanol.
Results and Discussion
Purification of compounds 1~5
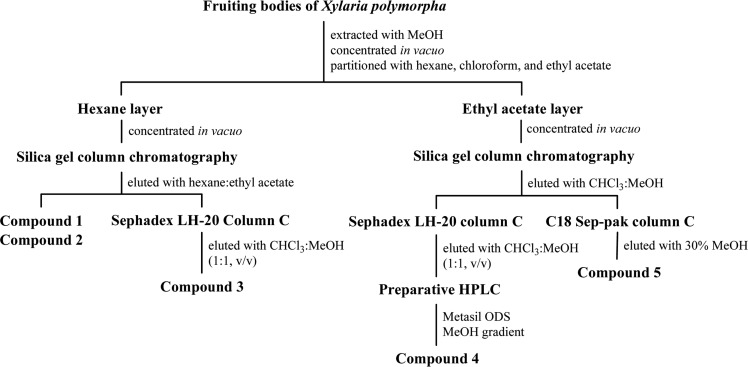
Compounds 1~5 were isolated as shown in Fig. 2. The dried fruiting bodies of X. polymorpha were extracted twice with methanol at room temperature. After the removal of the methanol under reduced pressure, the resulting solution was partitioned consecutively with hexane, chloroform, and ethyl acetate. The hexane-soluble fraction was chromatographed on a column of silica gel eluted with increasing amount (2, 10 and 33%, stepwise) of ethyl acetate in hexane. Compounds 1 and 2 were obtained from initial fractions. Fraction 4 was further purified by Sephadex LH-20 column chromatography with chloroform:methanol (1 : 1, v/v) to provide compound 3. Compounds 4 and 5 were purified from the ethyl acetate-soluble portion. The ethyl acetate-soluble fraction was chromatographed on a column of silica gel eluted by a gradient with increasing amount (2%→90%) of methanol in chloroform. A initial fraction was subjected to a column of Sephadex LH-20 eluted with chloroform:methanol (1 : 1, v/v), followed by preparative HPLC to afford compound 4. Compound 5 was purified from middle fractions by using C18 Sep-pak cartridge that was washed by 30% aqueous methanol and eluted with 40% aqueous methanol.
Fig. 2.
Isolation procedures of compounds 1~5.
Determining the structures of compounds 1~5
The fruiting bodies of the fungus X. polymorpha were extracted with methanol. The methanolic extract was partitioned by using organic solvents. Repeated chromatographic separations of the hexane- and ethyl acetatesoluble fractions led to the purification of compounds 1~5.
The 1H NMR spectrum of compound 1 in CDCl3 exhibited signals due to olefinic protons at δ 5.3~5.4, triplet methylene protons at δ 2.8, 11 methylene protons at δ 2.3, 2.0, 1.6 and 1.3, and methyl protons at δ 0.8. These signals were well matched to corresponding signals of linoleic acid, suggesting that this compound is linoleic acid or an unsaturated fatty acid. In the ESI-mass measurement, its molecular weight was determined to be 280 by a quasi-molecular ion peak at m/z 279 [M-H]- in the negative mode. This molecular weight was consistent with linoleic acid. Therefore, compound 1 was identified as linoleic acid.
The 1H NMR spectrum of compound 2 in CDCl3 was very similar to that of compound 1, except for an additional methyl peak at δ 3.7 in compound 2. The 1H NMR spectrum implied that compound 2 was linoleic acid methyl ester. Comparison of 1H NMR spectrum of 2 with linoleic acid methyl ester revealed that compound 2 was identical to linoleic acid methyl ester.
The 1H NMR spectrum of compound 3 in CDCl3 revealed that this compound was a member of triterpenoids. The 1H NMR spectrum showed four olefinic protons at δ 5.56, 5.37, 5.25, and 5.18, an oxygenated methine at δ 3.6, many methines and methylene protons between δ 1.0 and 2.7 and six methyl protons. On the basis of this spectral data, a database survey in the triterpene pool suggested that compound 3 was very similar to ergosterol. Direct comparison of the 1H NMR spectrum of compound 3 with ergosterol revealed that compound 3 was identical to ergosterol.
The molecular weight of compound 4 was established by ESI-mass measurements, which provided quasi-molecular ion peaks at m/z 267 [M+H]+ and 289 [M+Na]+ in positive mode and at m/z 265 [M-H]- in negative mode, suggesting the molecular weight to be 266. The 1H NMR spectrum measured in CD3OD exhibited an aromatic methine singlet at δ 6.32, two methine singlets at δ 5.71 and 4.31, a methoxyl methyl at δ 3.52, and two methyl singlets at δ 2.17 and 2.03. In the 13C NMR spectrum, 13 carbon peaks were evident. Each carbon peaks was assigned as a ketone carbonyl carbon at δ 203.3, an ester carbonyl carbon at δ 168.1, two oxygenated sp2 carbons at δ 163.9 and 162.5, one aromatic methine carbon at δ 101.1, three sp2 quaternary carbons at δ 134.9, 116.1, and 99.8, an acetal carbon at δ 102.0, a methine carbon at δ 53.6, a methoxyl methyl carbon at δ 55.9, and two methyl carbons at δ 27.9 and 9.9.
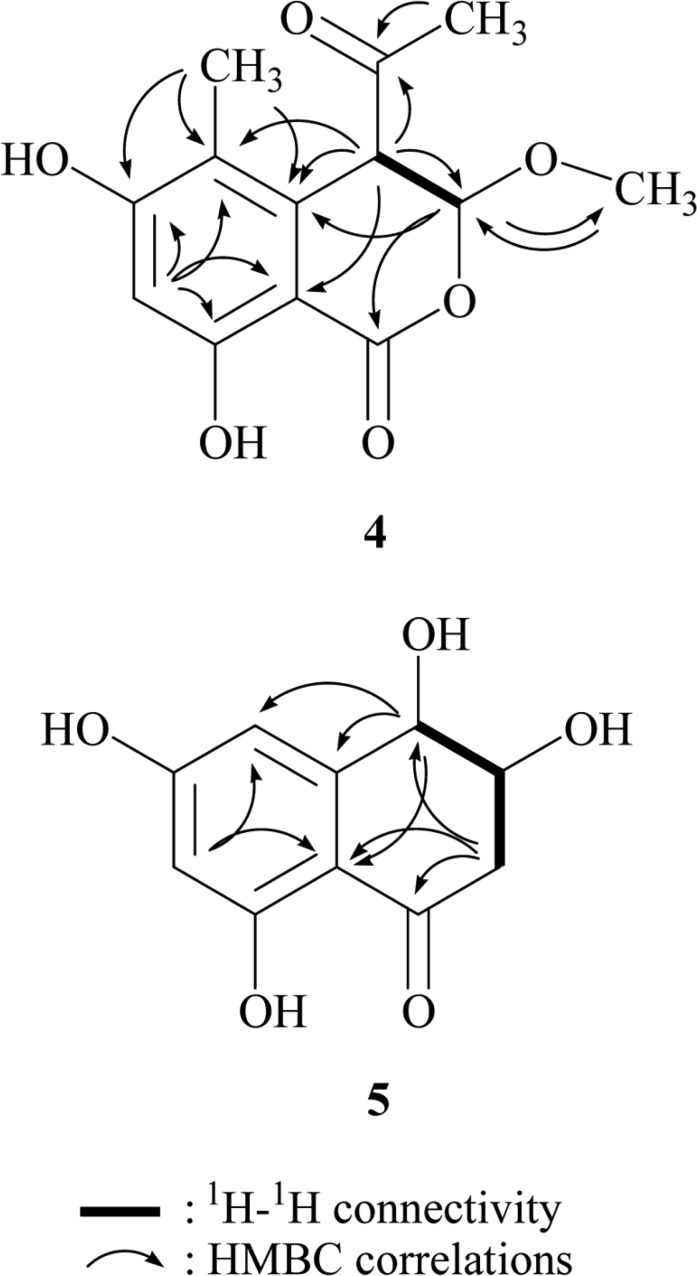
The structure of 4 was determined on the basis of the HMBC experiment, summarized in Fig. 3. HMBC showed correlations from a methine proton at δ 6.32 to carbons at δ 163.9, 162.5, 116.1, and 99.8, from aromatic methyl protons at δ 2.03 to carbons at δ 163.9, 134.9, and 116.1, from an acetal proton at δ 5.71 to carbons at δ 168.1 and 134.9, and 55.9, from a methine proton at δ 4.31 to carbons at δ 203.3, 134.9, 116.1, 102.0, and 99.8, from methyl protons at δ 3.52 to an acetal carbon at δ 102.0, and from methyl protons at δ 2.17 to carbonyl carbon at δ 203.3. Based on these HMBC correlations, the structure of 4 was determined as 4-acetyl-3,4-dihydro-6,8-dihydroxy-3-methoxy-5-methyl-1H-2-benzopyran-1-one (Krohn et al., 2001, 2004).
Fig. 3.
HMBC correlations of compounds 4 and 5.
The structure of compound 5 was determined by 1H and 13C NMR, 1H-1H COSY, HMQC, and HMBC experiments. 1H NMR spectrum measured in CD3OD showed signals due to two aromatic methines at δ 6.17 and 6.60, two oxygenated methines at δ 4.48 and 3.96, and one methylene at δ 2.94 and 2.62. In the 13C NMR spectrum, ten carbons were observed. The 1H-1H COSY spectrum established a partial structure, -CH(-OH)-CH(-OH)-CH2-. All proton-bearing carbons were assigned by the HMQC spectrum. The structure of compound 5 were determined by the HMBC experiment, which showed the long-range correlations from the methine proton at δ 4.48 to carbons at δ 148.4, 110.0, and 108.5, from the methylene protons at δ 2.94 and 2.62 to carbons at δ 201.3, 110.0, and 73.7, and from an aromatic proton at δ 6.17 to carbons at δ 110.0 and 108.5 as shown in Fig. 3. These HMBC cross-peaks assigned the structure of compound 5 as 4-hydroxyscytalone, which was previously reported from tricyclazole-treated cultures of Leptosphaeria maculans (Dahiya and Rimmer, 1988).
Although Xylaria has been known to produce diverse bioactive substances, this is first report on the chemical constituents of the fruiting bodies of X. polymorpha. In this study, we found that X. polymorpha produced linoleic acid, linoleic acid methyl ester, ergosterol, 4-acetyl-3,4-dihydro-6,8-dihydroxy-3-methoxy-5-methyl-1H-2-benzopyran-1-one, and 4-hydroxyscytalone. Of the compounds isolated, linoleic acid methyl ester, and ergosterol were ubiquitous in the higher fungi. However, 4-acetyl-3,4-dihydro-6,8-dihydroxy-3-methoxy-5-methyl-1H-2-benzopyran-1-one and 4-hydroxyscytalone were rare in fungi and isolated from the fruiting bodies of X. polymorpha for the first time.
References
- 1.Boonphong S, Kittakoop P, Isaka M, Pittayakhajonwut D, Tanticharoen M, Thebtaranonth Y. Multiplolides A and B, new antifungal 10-membered lactones from Xylaria multiplex. J Nat Prod. 2001;64:965–967. doi: 10.1021/np000291p. [DOI] [PubMed] [Google Scholar]
- 2.Dahiya JS, Rimmer SR. Accumulation of flaviolin, 4-hydroxyscytalone and 2-hydroxyjuglone in tricyclazoletreated cultures of Leptosphaeria maculans. Phytochemistry. 1988;27:3481–3482. [Google Scholar]
- 3.Davis RA. Isolation and structure elucidation of the new fungal metabolite (-)-xylariamide A. J Nat Prod. 2005;68:769–772. doi: 10.1021/np050025h. [DOI] [PubMed] [Google Scholar]
- 4.Healy PC, Hocking A, Tran-Dinh N, Pitt JI, Shivas RG, Mitchell JK, Kotiw M, Davis RA. Xanthones from a microfungus of the genus Xylaria. Phytochemistry. 2004;65:2373–2378. doi: 10.1016/j.phytochem.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 5.Imazeki R, Otani Y, Hongo T. Fungi of Japan. Tokyo: Yama-kei publishers; 1988. [Google Scholar]
- 6.Jang YW, Lee IK, Kim YS, Lee S, Lee HJ, Yu SH, Yun BS. Xylarinic acids A and B, new antifungal polypropionates from the fruiting body of Xylaria polymorpha. J Antibiot (Tokyo) 2007;60:696–699. doi: 10.1038/ja.2007.89. [DOI] [PubMed] [Google Scholar]
- 7.Jayasuriya H, Herath KB, Ondeyka JG, Polishook JD, Bills GF, Dombrowsky AW, Springer MS, Siciliano S, Malkowitz L, Sanchez M, Guan Z, Tiwari S, Stevenson DW, Borris RP, Singh S. Isolation and structure of antagonists of chemokine receptor (CCR5) J Nat Prod. 2004;67:1036–1038. doi: 10.1021/np049974l. [DOI] [PubMed] [Google Scholar]
- 8.Krohn K, Florke U, Rao MS, Steingrover K, Aust HJ, Draeger S, Schulz B. Metabolites from fungi 15. new isocoumarins from an endophytic fungus isolated from the Canadian thistle Cirsium arvense. Nat Prod Lett. 2001;15:353–361. doi: 10.1080/10575630108041303. [DOI] [PubMed] [Google Scholar]
- 9.Krohn K, Sohrab MH, Aust HJ, Draeger S, Schulz B. Biologically active metabolites from fungi, 19: new isocoumarins and highly substituted benzoic acids from the endophytic fungus, Scytalidium sp. Nat Prod Res. 2004;18:277–285. doi: 10.1080/14786410310001620637. [DOI] [PubMed] [Google Scholar]
- 10.Lee IK, Jang YW, Kim YS, Yu SH, Lee KJ, Park SM, Oh BT, Chae JC, Yun BS. Xylarinols A and B, two new 2-benzoxepin derivatives from the fruiting bodies of Xylaria polymorpha. J Antibiot (Tokyo) 2009;62:163–165. doi: 10.1038/ja.2008.20. [DOI] [PubMed] [Google Scholar]
- 11.Lin Y, Wu X, Feng S, Jiang G, Luo J, Zhou S, Vrijmoed LL, Jones EB, Krohn K, Steingrover K, Zsila F. Five unique compounds: xyloketals from mangrove fungus Xylaria sp. from the South China Sea coast. J Org Chem. 2001;66:6252–6256. doi: 10.1021/jo015522r. [DOI] [PubMed] [Google Scholar]
- 12.Smith CJ, Morin NR, Bills GF, Dombrowski AW, Salituro GM, Smith SK, Zhao A, MacNeil DJ. Novel sesquiterpenoids from the fermentation of Xylaria persicaria are selective ligands for the NPY Y5 receptor. J Org Chem. 2002;67:5001–5004. doi: 10.1021/jo011054+. [DOI] [PubMed] [Google Scholar]