Abstract
In this study, the antifungal activities of limonene against Trichophyton rubrum were evaluated via broth microdilution and vapor contact assays. In both assays, limonene was shown to exert a potent antifungal effect against T. rubrum. The volatile vapor of limonene at concentrations above 1 µl/800 ml air space strongly inhibited the growth of T. rubrum. The MIC value was 0.5% v/v in the broth microdilution assay. The antifungal activity of limonene against T. rubrum was characterized as a fungicidal effect.
Keywords: Antifungal activities, Limonene, Trichophyton rubrum
Onychomycosis is a common nail infection caused by dermatophytes or by nondermatophytic molds. T. rubrum is known to be the most common causative agent of dermatophytic nail infections in humans (Summerbell et al., 1997). Onychomycosis is difficult to cure due to its high probability of recurrence and prolonged antifungal agent treatment time. As a result of the side effects observed in commercially available antifungal agents, as well as the emergence of drug-resistant isolates, the development of a novel antifungal agent with less profound adverse effects will be necessary for the successful treatment of dermatophytic nail infections.
Essential oils are complex mixtures of naturally occurring compounds-- predominantly monoterpenes and sesquiterpenes-- and are considered alternative natural antimicrobial agents. Essential oils from several plants have been shown to exhibit antifungal properties (Kalemba and Kunicka, 2003). Several studies have demonstrated that the principal components of essential oils, such as eugenol and thymol, exert strong antifungal effects (Silva et al., 2005). Recently, volatile components of essential oils have been reported to exert antifungal effects (Jain and Agrawal, 2002). Limonene is a naturally occurring monoterpene detected in the essential oils of several plants (Pepeljnak et al., 2005; Sonboli et al., 2006). It is a volatile colorless liquid at room temperatures, with an extremely strong smell of oranges. Limonene has been studied and applied as a botanical insecticide (Sfara et al., 2009). To the best of our knowledge, however, only a very few antimicrobial studies of limonene as a single compound in essential oil have been conducted thus far. The principal objective of this study, then, was to evaluate the antifungal activities of limonene against T. rubrum, a major onychomycotic fungus.
Limonene was purchased from the Sigma Co. (USA). T. rubrum (KCTC 6345) was obtained from the Korean Collection of Type Cultures (KCTC). Strains were maintained on the surface of Sabouraud dextrose agar (SDA) at 28℃. In the vapor contact assay, the effects of limonene on the tested fungi were assayed via a modified version of the chamber assay (Jain and Agrawal, 2002). A disposable Phytatray (Sigma, USA) with a sterilized lid was utilized as a chamber containing the limonene and fungus. Limonene at various levels (1, 5, 10, 50, 100, 500 µl) was kept in small vials. SDA plates inoculated with fungal spores, as well as the small limonene vials, were placed in the 800 ml volume Phytatrays. One set of phytatrays, to which no limonene was added, was run as a control. The Phytatrays were incubated for 8 days at 28℃. After incubation, the fungal growth was determined via visual observation. Inhibition was determined by no growth of fungi on the SDA plates after incubation. The minimum inhibitory concentration in vapor contact method was determined as the minimum concentration necessary to inhibit fungal cell growth. In order to determine whether the volatile vapor of limonene evidences fungistatic or fungicidal activity, limonene was removed from the Phytatrays and the plates were grown for an additional 5 days at 28℃. Samples in which the fungi resumed growth were considered examples of fungistatic activity, whereas samples in which the fungi evidenced no additional growth were examples of fungicidal activity. The effects of limonene on the tested fungi in liquid medium were evaluated via broth microdilution assays with some modification. Spore suspensions were prepared as above and adjusted to 1 × 105 ml-1 with sterile distilled water using a Neubauer counting chamber (Haemacytometer). Broth microdilution assays were conducted in 96-well, flat-bottomed microtiter plates. 100 µl of cell suspension was inoculated into 100 µl of RPMI 1640 medium containing various concentrations of limonene (0.5~2% v/v) in 96-well plates. Microtiter plates were incubated for 4 days at 28℃. As a control, an equivalent volume of sterile water, instead of limonene, was added to each well. The minimum inhibitory concentration (MIC) of essential oil was determined by estimating the minimum concentration that inhibited fungal cell growth. This experiment was performed in triplicate. To assess the fungistatic or fungicidal activity, wells evidencing no visible growth were subcultured onto SDA using a 50 µl inoculums and incubated for 5 days at 28℃ to determine whether or not growth resumed. Samples in which no apparent growth was observed were considered examples of fungicidal effects. In an effort to assess the effects of limonene on cell viability, spores and hyphae were stained with a FUN 1 viability kit as well as the Live/Dead FungaLight Yeast viability kit (Molecular Probes, Eugene, OR, USA) and observed under fluorescence microscopy. The spores and hyphae were incubated for 48 h with limonene at MIC and stained at room temperature in darkness, in accordance with the manufacturer's instructions.
Our results demonstrated that the volatile vapor of limonene at concentrations greater than 1 µl/800 ml air space profoundly inhibited the growth of T. rubrum (Fig. 1). After the removal of essential oil from the Phytatrays, no resumption of cell growth was noted after 72 h of incubation, thereby indicating the fungicidal activity of the volatile limonene. Direct application of limonene in the broth microdilution assay also revealed limonene's potent fungicidal effects against T. rubrum. The MIC of limonene against T. rubrum is provided in Table 1. In the broth microdilution assay, spore germination was inhibited by limonene at a concentration of 0.5% v/v. No resumption of growth was observed on the subcultured plates after 4 days of incubation, thus illustrating the profound fungicidal effects of limonene. When the fungal cells were stained using a Live/Dead FungaLight Yeast viability kit, propidium iodide penetrated only the dead cells whose membranes had disintegrated, resulting in an orange-red color.
Fig. 1.

Effect of limonene on the growth of T. rubrum in vapor contact assay. Control Phytatray (left) shows fungal growth. Limonene treated Phytatray (light) shows no fungal growth.
Table 1.
Inhibitory effect of limonene on Trichophyton rubrum
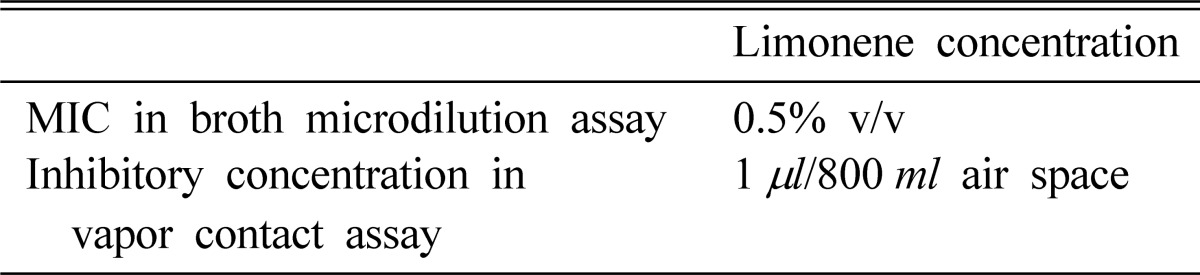
In our results, many spores in the limonene-treated wells evidenced these orange-red spores, which reflected cell death at MIC, whereas the majority of spores in the control wells evidenced green fluorescent cells with intact cell membranes (Fig. 2). Propidium iodide is a fluorescent dye that is used to assess the effects of drugs on cell membrane integrity. Therefore, our results indicated that the treatment of fungal cells with limonene might result in an alteration in the integrity of the cell membrane, which could be visualized by the orange-red staining.
Fig. 2.
Fluorescenct micrographs of spores of T. rubrum. A, Control spores showing green fluorescent, live cells; B, Limonene treated spores at 0.5% v/v showing orange fluorescent, dead cells.
In the FUN 1 viability kit staining, only metabolically active, live cells were marked with orange fluorescent intravacuolar structures, whereas dead cells exhibited a diffuse green-yellow fluorescence. As is shown in Fig. 3, control cells were marked with fluorescent intravacuolar structures, whereas the limonene-treated cells evidenced green-yellow fluorescence without intravacuolar structures, thus identifying them as dead cells with little or no metabolic activity. Complementing the above observations, anomalies in the hyphal structure, such as a reduction in the hyphae diameter and cytoplasmic granulation in the hyphae were observed under light microscopy (Fig. 4). These observations indicated that limonene may affect the hyphal structure. Fries et al. (1973) previously suggested that the volatile compounds of essential oils influence a variety of cell metabolic processes. Although further research will be necessary to determine in detail the mechanisms underlying the antifungal activity of limonene, the results of our studies showed that limonene inhibits the growth of T. rubrum via a fungicidal mode, coupled with induced changes in cell membrane integrity and metabolic activity. Fungicidal agents are frequently preferred over fungistatic agents for the treatment of fungal infections, as the rate of infection recurrence is relatively high when fungistatic agents are utilized. Therefore, the fungicidal properties of limonene may provide knowledge necessary to the development of alternative antifungal agents. Further research will be necessary to evaluate the toxicity and to determine in detail the mechanisms exploited by limonene in its growth inhibitory effects against T. rubrum.
Fig. 3.

Fluorescent micrographs of hyphae of T. rubrum stained with FUN 1 viability kit. A, Limonene treated hyphae showing diffuse green-yellow fluorescent, dead cells without intravacuolar structure; B, Control hyphae showing orange-colored intravacuolar structure (arrows), representing viable cells.
Fig. 4.
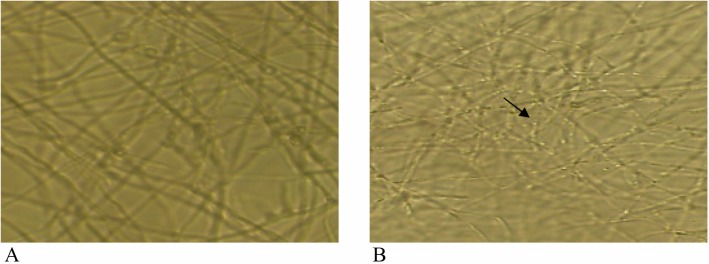
Comparison of hyphae structure of T. rubrum in light microscope (200 ×). A, Hyphae in control; B, Anomaly of hyphae, showing reduction of diameter of hyphae and granulation (arrow) in limonene treatment.
References
- 1.Fries N. Effects of volatile organic compounds on the growth and development of fungi. Trans Br Mycol Soc. 1973;60:1. [Google Scholar]
- 2.Jain SK, Agrawal SC. Fungistatic activity of some perfumes against otomycotic pathogens. Mycoses. 2002;45:88–90. doi: 10.1046/j.1439-0507.2002.00730.x. [DOI] [PubMed] [Google Scholar]
- 3.Kalemba D, Kunicka A. Antibacterial and antifungal properties of essential oils. Curr Med Chem. 2003;10:813–829. doi: 10.2174/0929867033457719. [DOI] [PubMed] [Google Scholar]
- 4.Pepeljnjak S, Kosalec I, Kalodera Z, Blazevic N. Antimicrobial activity of juniper berry essential oil. Acta Pharm. 2005;55:417–422. [PubMed] [Google Scholar]
- 5.Sfara V, Zerba EN, Alzogaray RA. Fumigant insecticidal activity and repellent effect of five essential oils and seven monoterpenes on first-instar nymphs of Rhodnius prolixus. J Med Entomol. 2009;46:511–515. doi: 10.1603/033.046.0315. [DOI] [PubMed] [Google Scholar]
- 6.Silva MRR, Oliveira JG, Femandes OFL, Passos XS, Costa CR, Souza LKH, Lemos JA, Paula JR. Antifungal activity of Ocimum gratissimum towards dermatophytes. Mycoses. 2005;48:172–175. doi: 10.1111/j.1439-0507.2005.01100.x. [DOI] [PubMed] [Google Scholar]
- 7.Sonboli A, Babakhani B, Mehrabian AR. Antimicrobial activity of six constituents of essential oil from Salvia. Z Naturforsch C. 2006;61:160–164. doi: 10.1515/znc-2006-3-401. [DOI] [PubMed] [Google Scholar]
- 8.Summerbell RC. Epidemiology and ecology of onychomycosis. Dermatology. 1997;194:32–36. doi: 10.1159/000246182. [DOI] [PubMed] [Google Scholar]


