Abstract
The principal objective of this study was to acquire basic data regarding the mycelial growth characteristics for the artificial cultivation of Coprinus comatus. 12 URP primers were employed to evaluate the genetic relationships of C. comatus, and the results were divided into three groups. Among six kinds of mushroom media, MYP medium was selected as the most favorable culture medium for C. comatus. The optimal temperature and pH ranges for the mycelial growth of C. comatus were 23~26℃ and pH 6~8, respectively. The carbon and nitrogen sources for optimal mycelial growth were sucrose and tryptone, respectively.
Keywords: Coprinus comatus, Cultural characteristics, Mycelial growth, RAPD
Coprinus comatus is a member of the Agaricales family. The Agaricales is a common fungus frequently seen on lawns, along gravel roads, and in waste areas all over the world (Park and Lee, 2005). As they age, they deliquesce--i.e. auto-digest themselves--from the bottom of the cap upwards, eventually turning into black ink. Sporophores aid digestion, and have been utilized for the treatment of piles. Its inhibition rates against Sarcoma 180 and Ehrlich carcinoma have been reported as 100% and 90%, respectively (Ying et al., 1987). C. comatus has been identified as containing 'superior'(1→3)-β-glucan contents (Yang et al., 2003), and were determined to harbor ergothioneine, a thiol compound with antioxidant properties (List, 1957). The expected anti-oxidant activity was later confirmed (Badalyan et al., 2003). Additionally, C. comatus has been shown to harbor compounds that kill nematodes (Li and Xiang, 2005), and the mushroom is a delicious and nutritious agaric (Luo et al., 1991) that has been designated as natural, nutritious, and healthy by both the Food and Agriculture Organization and the World Health Organization (Liu and Zhang, 2003). 13 varieties of C. comatus were previously reported in a catalog of books in China, and the optimal growth temperature has been reported as 25℃ (Zhou, 2007). Additionally, many farmers in China cultivate C. comatus, which is also referred to by names such as "shaggy ink cap", "lawyer's wig", and "shaggy mane" (Chen, 2000). Dong et al. (2006) previously reported that the optimal liquid medium composition of C. comatus was (adjusted to pH 8.0): sucrose 3%, corn powder 2%, wheat bran 4%, KH2PO4 0.1% and MgSO4·7H2O 0.05%. Additionally, methods for its cultivation include bag cultures (Luo and Qian, 1999; Zhu, 1998) and bed cultures (Zhu, 1998), and the substrates used for its cultivation include cotton waste, corn cobs, rice straw, urea, ox manure, lime, etc. (Yang and Xue, 2000). Recently, efforts to improve the cultivation techniques for our farm and automated facilities have resulted in an increase in total mushroom production; 55,274 M/T in 1990, and 146,346 M/T in 2007 (Ministry for Food, Agriculture, Forestry and Fisheries in Korea, 2007). However, only about 10 species of mushrooms are currently cultivated on a large scale in Korea. Therefore, we should develop new revenues by assessing the adaptability of mushrooms that are cultivated in China, and use the results as a foundation for the enhancement of international competitiveness in the mushroom industry.
Materials and Methods
Strains and RAPD analysis
We previously collected a total of 7 varieties of C. comatus at the Edible Fungi Institute, Liaoning Academy of Agricultural Sciences, from 1996 to 2006; the mycelia of each isolate were grown on PDA. For RAPD analysis, we performed a static culture of the mycelia into PDB, and then eradicated the media by vacuum culturing the mycelium on Whatman No. 2 filter paper, pouring liquid nitrogen into the sample after lyophilization, and pulverizing it. We then added 1 ml of nucleic lysis buffer and 10 µl of proteinase K (25 mg/ml) into 0.2 g of pulverized. Mycelium, and allowed the reaction to proceed for 1 hour at 60℃ after 2~3 minutes of vortexing, and then divided it into four 1.5 ml microtubes to generate 250 µl supernatants after 10 minutes of centrifugation at 14,000 rpm. We collected the supernatants from the columns after centrifugation at 14,000 rpm on repeat for 4 1-minute cycles in a spin column after mixing 500 µl of DNA binding buffer with the sample. Then, in sequential order, we rinsed the sample with 800 µl of 75% ethanol, applied 1 minute of centrifugation at 14,000 rpm, rinsed again with 300 µl of 75% ethanol, applied 1 additional minute of centrifugation at 14,000 rpm, 2 more minutes at 14,000 rpm after the addition of 100 µl of elution buffer, and finally assessed the DNA concentration via electrophoresis. 12 types of primer were utilized, and PCR was conducted as follows: preheating at 94℃ for 5 minutes, denaturation at 94℃ for 1 minute, annealing at 55℃ for 1 minute, extension at 72℃ for 2 minutes and maintenance at 4℃ after a final extension step at 72℃ for 2 minutes after a total of 35 cycles. The amplified fragments were then electrophoresed at 100 V in 1.8% agarose gel, dyed for 10 minutes with EtBr (ethidium bromide) and investigated for bands using a UV transilluminator lamp. We conducted cluster analysis via UPGMA (unweighted paired group methods with arithmetic average) for RAPD analysis.
Selection of favorable culture media
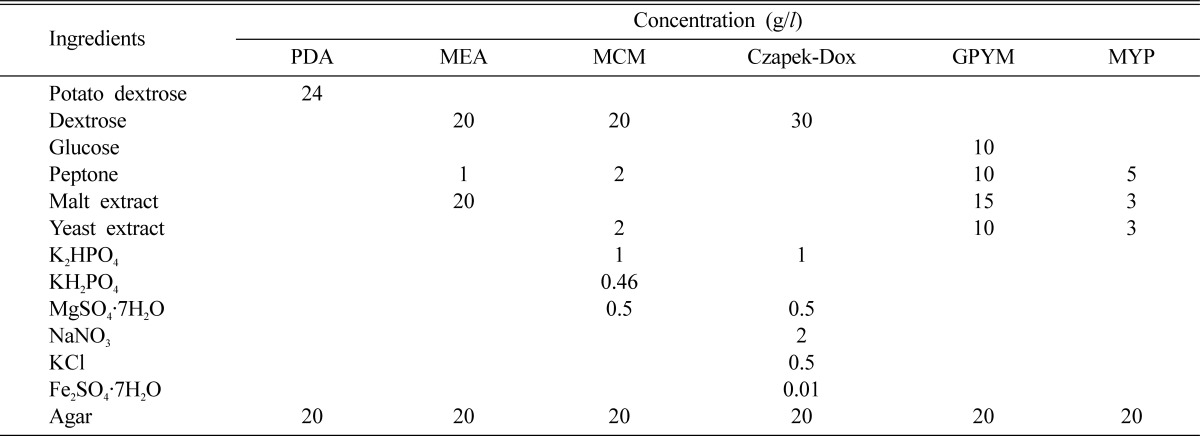
Six different culture media were utilized to assess the mycelial growth of C. comatus (Table 1). The media were adjusted to pH 6 prior to the autoclaving process. After autoclaving (1.5 kg/cm2) for 15 minutes at 121℃, 20 ml of each medium was poured aseptically into a petri dish. A 5 mm cork borer of the inoculum was removed from a 10-day-old culture of C. comatus grown on PDA, and placed in the centre of agar plates, each containing one of six different culture media. After 9 days of incubation at 25 ± 1℃, the mycelial growth of C. comatus was evaluated.
Table 1.
The composition of used media
Effect of temperature for mycelial growth
In order to determine the ideal temperature for the mycelial growth of C. comatus, 6 different temperatures (17, 20, 23, 26, 29 and 32℃) were adopted. A 5mm diameter agar plug was removed from 10-day-old cultures grown on PDA and positioned in the centers of agar plates, each filled with 20 ml of PDA. After 9 days of incubation at 6 different temperatures, the mycelial growth of C. comatus was evaluated.
Effect of pH for mycelial growth
A 5 mm diameter agar plug was removed from 10-day-old cultures grown on PDA, then added to 250 ml Erlenmeyer flasks filled with 100 ml of PDB. The medium was adjusted to pH values of 4, 5, 6, 7 and 8 with the addition of 0.1 N-NaOH or 0.1 N-HCl and incubated for 14 days at 25℃, 125 rpm after inoculation. We filtered the liquid with filter paper (Whatman No.2) and air-dried the samples at 50℃ for 48 hours for dry weight measurements of the mycelial samples after 14 days.
Effect of carbon and nitrogen sources for mycelial growth
The mycelial growth of C. comatus was assessed on each of the basal media supplemented with 10 carbon and eight nitrogen sources, respectively. The basal media for carbon source selection were composed of 11 carbon sources (30 g), tryptone (1 g), MgSO4·7H2O (5.0 g), KCl (0.5 g), FeSO4·7H2O (0.1 g), KH2PO4 (1.0 g), agar (20 g), and distilled water (1000 ml). The basal media for nitrogen source selection were composed of 8 nitrogen sources (30 g), sucrose (30 g), MgSO4·7H2O (5.0 g), KCl (0.5 g), FeSO4·7H2O (0.1 g), KH2PO4 (1.0 g), agar (20 g), and distilled water (1000 ml). In both cases, the basal medium was adjusted to pH 6 and autoclaved for 15 minutes on 121℃ poured plates. The inoculated dishes were replicated four times and incubated for 14 days at 25℃ in darkness. Mycelial growth was measured as previously described.
Results and Discussion
RAPD analysis
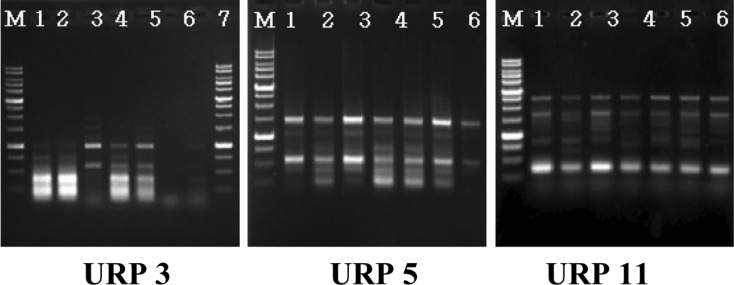
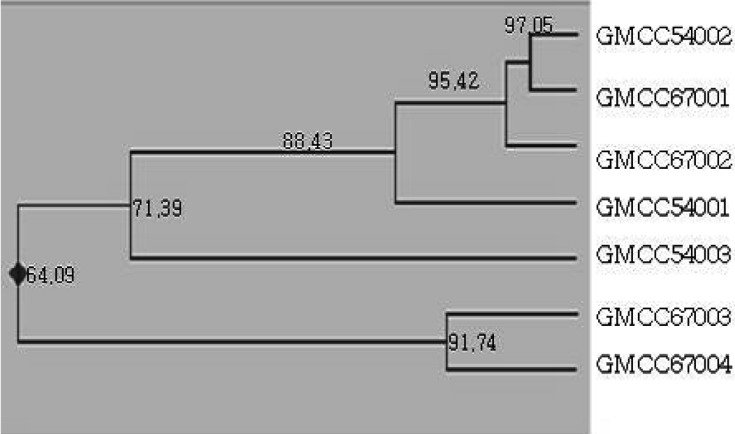
PCR band phase was analyzed with 12 URP primers (Fig. 2), and resulted in seven varieties of different bands at URP 3; in particular, the URP 5 and 11 primers tended to stain similarly, which is characteristic of intraspecificity among equal groups. The RAPD analysis of the collected varieties was divided into 3 groups according to the results of the band phases of URP 3, 5 and 11; however, we noted no morphological differences among strains (Fig. 3).
Fig. 2.
Random amplified polymorphic DNA patterns by primer URP3, URP5 and URP11. M: 1 kb ladder; lane 1, GMCC54002; lane 2, GMCC54003; lane 3, GMCC54004; lane 4, GMCC67001; lane 5, GMCC67002; lane 6, GMCC67003; lane 7, GMCC67004.
Fig. 3.
Grouping of C. comatus strains by URP 3, URP 5 and URP 11 primers. The numbers above each branch indicate the coefficient value. The right number indicates the strain number.
Selection of favorable culture media
Six different culture media were utilized to determine the optimal mycelial growth of seven different strains of C. comatus. Among the 6 culture media utilized, MYP medium was shown to be exceedingly favorable in promoting the mycelial growth of C. comatus, whereas Czapek-Dox medium performed extremely poorly; additionally, among 7 variants of C. comatus, GMCC 54001 evidenced the highest levels of mycelial growth length on MCM medium (Table 2). Chaiyma et al. (2007) previously reported favorable mycelial growth of C. comatus cultivated on Malt extract agar. However, Chaiyma et al. (2007) did not make use of MYP in his experiments. Therefore, we concluded that MYP was the optimal medium for the mycelial growth of C. comatus.
Table 2.
Effect of media on mycelial growth of C. comatus strains
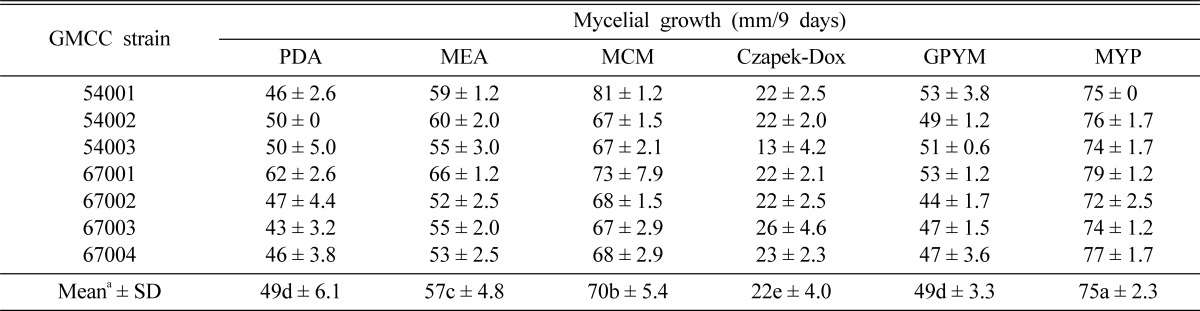
aValues followed by the same letter do not differ significantly at p > 0.05 according to Duncan's multiple range test.
Effect of temperature on mycelial growth
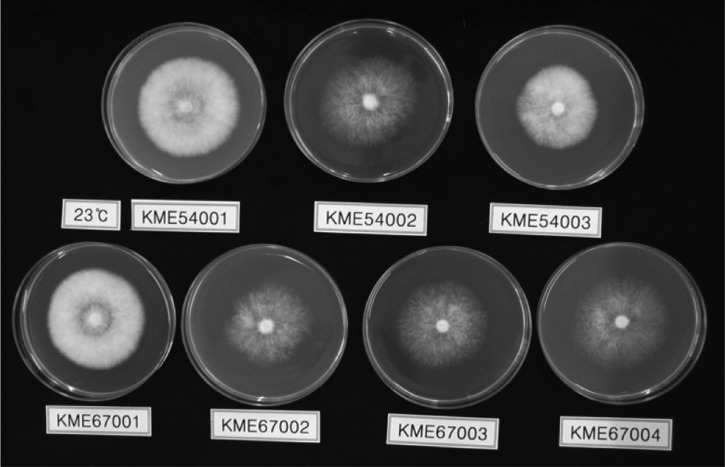
The suitable temperature for the mycelial growth of C. comatus was observed at 23~26℃ and GMCC 67001 evidenced the highest levels of mycelial growth at 63 mm at 26℃ (Table 3, Fig. 4). However, at temperatures above 29℃ or below 20℃, seven strains of C. comatus evidenced poor mycelial growth. With regard to the optimal culture temperature range of C. comatus, Luo and Qian (1999) and Kim (2001) reported it as 25~27℃, Yang and Xue (2000) as 24~28℃ and Shiyong and Zaipei (2007) as 22~28℃. The above results were consistent with the results of this study. Consequently, we selected 26℃ as the optimal mycelial growth temperature of C. comatus.
Table 3.
Effect of temperature on mycelial growth of C. comatus strains
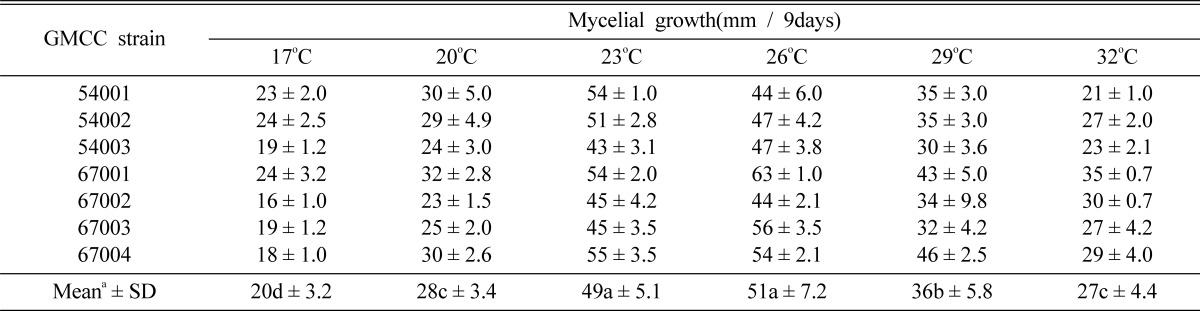
aValues followed by the same letter do not differ significantly at p > 0.05 according to Duncan's multiple range test.
Fig. 4.
Configuration on mycelial growth of Copinus comatus strains incubated at 23℃ for 9 days.
Effect of pH for mycelial growth
Favorable mycelial growth of C. comatus was observed in a pH range of 6~8 (Table 4). Among the seven strains, the optimal mycelial growth was noted at a pH of 7. GMCC 67002 and GMCC 67004 had the highest mycelial dry weight at 75 mm at a pH of 7. Since the mycelial growth of C. comatus was generally favorable in a pH range of 6.5~7.5 (Chen and Yang, 2000; Shiyong and Zaipei, 2007). We observed the highest mycelial dry weight at pH 7. Therefore, it can be seen that the results shown above are similar with the results of the present study.
Table 4.
Effect of pH on mycelial growth of C. comatus strains
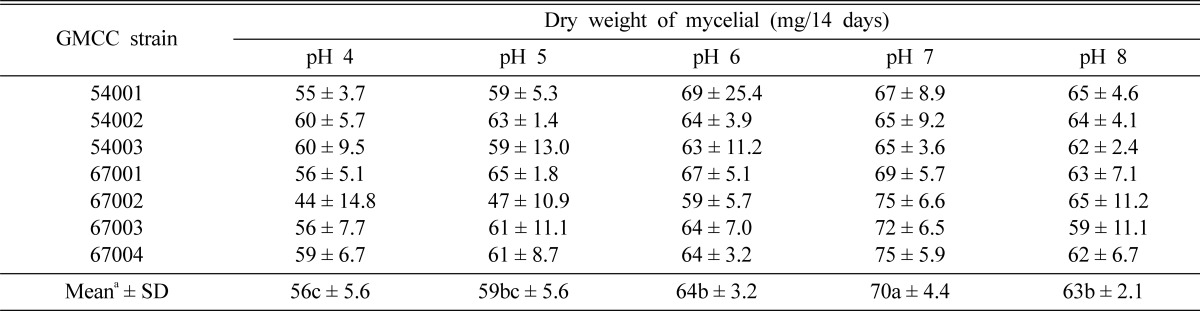
aValues followed by the same letter do not differ signification at p > 0.05 according to Duncan's multiple range test.
Effect of carbon and nitrogen sources for mycelial growth
The carbon sources that most effectively promoted the mycelial growth of C. comatus were maltose and sucrose in disaccharide, and starch in polysaccharide (Table 5). Among the 10 carbon sources utilized herein, GMCC 67001 evidenced the highest mycelial growth (74 mm) in sucrose. Chaiyama et al. (2007) identified the optimal culture carbon sources of C. comatus as mannose and maltose. However, we did not use mannose in this experiment. So, the mycelial growth of C. comatus at amaltose was comparatively good.
Table 5.
Effect of carbon source on mycelial growth of C. comatus strains
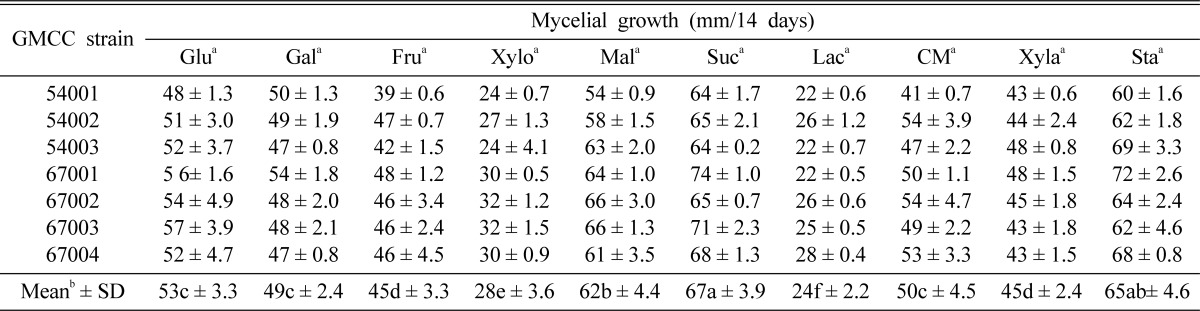
aGlu: glucose, Gal: galactose, Fru: frutose, Xylo: xylose, Mal: maltose, Suc: sucrose, Lac: lactose, CM: CM-cellulose, Xyla: xylan, Sta: starch.
bValues followed by the same letter do not differ significantly at p > 0.05 according to Duncan's multiple range test.
The nitrogen source that most effectively promoted the mycelial growth of C. comatus was tryptone in organic nitrogen (Table 6). Among the eight nitrogen sources utilized herein, GMCC 67002 evidenced optimal mycelial growth (72 mm) in tryptone. However, NaNO2 in mineral nitrogen depressed the mycelial growth of C. comatus, and mycelial growth was weak in NaNO3, NH4NO3, and NH4Cl. Thus, on the basis of our results, we identified tryptone as the optimal nitrogen source for the mycelial growth of C. comatus.
Table 6.
Effect of nitrogen source on mycelial growth of C. comatus strains
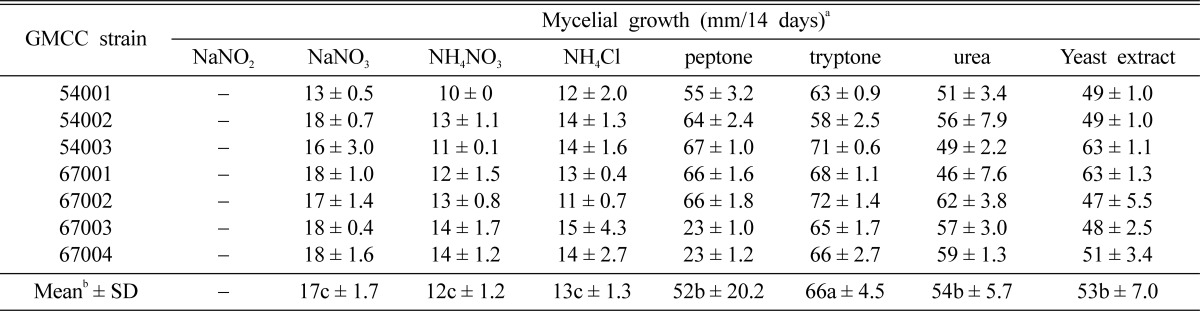
aGrowth rate.
bValues followed by the same letter do not differ significantly at p > 0.05 according to Duncan's multiple range test.
Fig. 1.
Fruit-body of Copinus comatus.
References
- 1.Badalyan CM, Gasparyan AV, Garibyan NG. Investigation of the antioxidant activity of some basidial macromycetes. Mikol Fitopatol. 2003;37:63–68. [Google Scholar]
- 2.Chaiyama V, Petcharat V, Kritsaneepaiboon P. Some morphological and physiological aspects and cultivation of Copinus comatus (O.F.Mull.) Gray. Songklanakarin J Sci Technol. 2007;29:261–274. [Google Scholar]
- 3.Chen MM. Cultivation techniques for Dictyophora, Polyporus umbellata, Copinus comatus. Science and cultivation of edible fungi. Mushroom Science. 2000;2:543–548. [Google Scholar]
- 4.Chen ZZ, Yang J. The new century mushroom cultivation using technology. People's Liberation Army Publishing House; 2000. pp. 414–422. [Google Scholar]
- 5.Dong YH, Wang HQ, Qiu LY. Optimization of a liquid growth medium for Copinus comatus. Acta Edulis Fungi. 2006;13:29–31. [Google Scholar]
- 6.Kim YG. Studies on the morphology characteristics of Coprinus species and artificial cultivation of C. comatus. Korea: Chungnam National University; 2001. Master's degree thesis. [Google Scholar]
- 7.Li Y, Xiang H. Nematicidal activity Coprinus comatus. Acta Phytopathologica Sinica. 2005;35:456–458. [Google Scholar]
- 8.Liu YF, Zhang JS. Recent advances in the study on the medicinal functions of Coprinus comatus. Acta Edible Fungi. 2003;10:60–63. [Google Scholar]
- 9.List PH. Occurrence of ergothioneine in shaggy-mane. Arch Pharm Ber Dtsch Pharm Ges. 1957;62:517–520. doi: 10.1002/ardp.19572901102. [DOI] [PubMed] [Google Scholar]
- 10.Luo T, Qian ZM. The key techniques of the CC100 Copinus comatus. cultivation. Edible Fungi. 1999;4:14–15. [Google Scholar]
- 11.Luo XY, Lü DP, Wang W. Artifical culture of Coprinus comatus Kunyan C-901. Edible Fungi of China. 1991;10:13–15. [Google Scholar]
- 12.Name of writer. Ministry for Food, Agriculture, Forestry and Fisheries in Korea. An actual output of a crop for a special purpose. 2007. pp. 44–81. [Google Scholar]
- 13.Park WH, Lee HD. Wild fungi of Korea. Kyo-Hak Publishing Co., Ltd; 2005. pp. 218–219. [Google Scholar]
- 14.Shiyong J, Zaipei J. Mushroom cultivation techniques. China: Chemical Industry Press; 2007. pp. 118–122. [Google Scholar]
- 15.Yang GL, Xue HB. Specialized cultivation manual about edible and medicinal mushroom. China Agricultural Press; 2000. pp. 361–368. [Google Scholar]
- 16.Yang X, Wan M, Mi K, Feng H, Chan DK, Yang Q. The quantification of (1→3)-β-glucan in edible and medicinal mushroom polysaccarides by using limulus G test. Mycosystema. 2003;22:296–302. [Google Scholar]
- 17.Ying JZ, Mao XL, Ma QM, Zong YC, Wen HA. Icons of medicinal fungi from China. Beijing: Science Press; 1987. p. 313. [Google Scholar]
- 18.Zhou YG. China bacteria directory. Chemical Industry Press; 2007. p. 522. [Google Scholar]
- 19.Zhu JB. The cultivation techniques of Copinus comatus on Shangai Nan-hui County. Edible Fungi. 1998;3:32. [Google Scholar]