Abstract
Purification and characterization of intracellular cellulase produced by A. oryzae ITCC-4857.01 are reported. The enzyme was purified by ion-exchange chromatography using DEAE-cellulose followed by Gel filtration. The purification achieved was 41 fold from the crude extract with yield of 27%. The purified enzyme showed single band on poly acrylamide gel. The molecular weight as determined by SDS-PAGE and gel filtration was 38 KDa and 38.6 KDa respectively and contained only one subunit. The enzyme is glycoprotien as nature and contained 0.67% neutral sugar. The apparent Km value of the enzyme against cellulose was 0.83%. The enzyme showed the highest relative ativities on CMC followed by avicel, salicin and filter paper. The optimum pH of activity was 5.5 and very slight activity was observed at or above pH 7.5 as well as bellow pH 3.5. The optimum tempreture of the activity was 45℃ and the highest activity was exhibited in 35 to 45℃. The enzyme lost their activities almost completely (95~100%) at 80 ℃ or above and as well as bellow 25℃.
Keywords: Aspergillus, DEAE-cellulose chromatography, Gel filtration, Intracellular cellulase, SDS-PAGE
Cellulose is a major polysaccharide constituent of plant cell walls and one of the most abundant organic compounds in the biosphere (Murai et al., 1998; Hong et al., 2001). Cellulose has enormous potential as a renewable source of energy (Coral et al., 2002) and several microorganisms use it as a carbon source. It has also attracted the interest of biotechnologists, who wish to use it as a renewable source of fuels and chemicals (Beguin, 1990). Cellulose is an unbranched glucose polymer composed of glucose units linked by a β-1,4-D-glycosidic bond (Gielkens et al., 1999; Gupta and Gupta, 1979). A number of fungi and bacteria are capable of producing multiple groups of enzymes, which are collectively known as cellulases that act in a synergistic manure to hydrolyze the β-1,4-D-glycosidic bonds within the cellulose molecules (Akiba et al., 1995). Cellulases can be classified into three types: endoglucanases, exoglucanases and β-glucosidases. Endocanases randomly hydrolyze internal β-1,4-glycosidic bonds in cellulose. As a result, the polymer rapidly decreases in length, but the concentration of the reducing sugar increases slowly (Robson and Chamblish, 1989). Exoglucanases hydrolyze cellulose by removing the cellobiose unit from the non reducing end of cellulose; the reducing sugars are rapidly increased, but the polymer length changes little. β-glucosidases cleave cellobiose and oligosaccharides to glucose. Native cellulose is enzymatically hydrolysed by a group of enzymes, endo-β-glucanase, exo-β-glucanase and β-glucosidases, acting synergistically. The cellulolytic enzyme system of distinct microorganisms is often different. Some microorganisms excrete large amounts of cellulolytic enzymes in culture media, whilst others although growing on cellulose, excrete little or no enzymes into the medium. So, it is not clear that the cellulolytic enzymes are truly extracellular or not. In most cases high cellulase activities are found in culture filtrates only in the stationary phase of growth and it can be argued that the enzymes are released by autolysis. Microbial enzymes are classified according to their localization into two groups i.e. cell-bounded (intracellular and surface bound) and extracellular. In the case of enzymes acting on insoluble macromolecules such as cellulose, it is clear that the enzymes must be localized outside the cell but they may still be bound the cell surface. It has indeed been argued that cellulose degradation is most efficient when it takes place through direct contact between microbial cells and the substrate (Hofsten, 1972). By this means a high local enzyme concentration and a favorable spatial arrangement are achieved. Other enzymes such as β-glucosidases which hydrolyse cellobiose and soluble low molecular weight cellodextrins are probably located in the periplasmic region or are truly intracellular.
Fungi are the main cellulase-producing microorganisms, though a few bacteria and actinomycetes have also been recently reported to yield cellulase activity. Microorganisms of the genera Trichoderma and Aspergillus are thought to be major cellulase producers (Peiz et al., 1998), and crude enzymes produced by these microorganisms are commercially available for agricultural use. It was also reported that Trichoderma reesei is capable of increase the production of cellulase from ubstrate and shows both the challenging rheology and a cellulase complex product that is shear-sensitive (Weber and Osborn, 1969). Today these enzymes account for approximately 20% of the world market (Mantyla et al., 1998) mostly from Trichoderma and Aspergillus (Godfrey and West, 1996; Uhlig, 1998).
Although microbes produce both extra and intracellular cellulase but scanty work has been done in later one. So, keeping in mind the above point's intracellular cellulase has been considered for purification and characterization.
Materials and Methods
Organism and culture conditions
Aspergillus oryzae ITCC-4857.01was used in this investigation. The organism was screened from cellulosic waste samples of different places of Rajshahi Metropolitan city, Bangladesh and confirmed by Indian Type Culture Collection (ITCC), Division of Plant pathology, IARI (Begum, 2005). The strain was kept in Plant pathology, Mycology and Microbiology Laboratory, Department of Botany, Rajshahi University and maintained in glycerol at 4℃. Cultivation of the organism was performed in 250-ml Erlenmeyer flasks containing 100 ml of medium. The medium composition (in g l-1) used for growth and enzyme induction was determined previously and it was composed of carboxylmethyl cellulose (Sigma Chemical Co.), 1; sucrose, 30; NaNO3, 2; KH2PO4, 1; MgSO4·7H2O, 0.5; KCl 0.5; FeSO4·7H2O, 0.01. pH 5.6. Inoculums size was 105 spores ml-1. Flasks were shaken on an orbital shaker at 130 rpm for 5 days at 30℃.
Extraction and concentration of intracellular crude enzyme
The culture supernatant and pellet (mycelia mat) were separated by filtration. Supernatant was discarded and 1 g of pellet was suspended in 100 ml of 0.2M sodium acetate buffer, pH 5.2, and homogenized well with hand grinder and kept in an ice bath. The extract was further disrupted by ultrasonification (Ultra-Turax, T-2.5) at 4℃. Disruption process was not continuous, only 5 to 10 seconds at each time to finally 1 minutes. From this solution, cell debris was separated by centrifugation at 10,000 rpm for 10 minutes at 4℃ and filtered. This supernatant is used as crude enzyme solution and concentrated to five-fold by sucrose (with dialysis bag).
Purification of the enzyme DEAE-cellulose chromatography
After dialysis against distilled water and 0.02M aceted buffer, pH 5.2 for 24 hours, 35 ml of the enzyme solution was applied to DEAE-cellulose (Sigma Chemical Co., U.S.A.) column (2.1 × 35 cm) which had been equilibrated with the same buffer at 4℃. The separation of protein from the column was performed by stepwise elution with the buffer containing increasing concentrations of NaCl (0.05M to 1M). About 3.0 ml fraction was collected in different test tubes. The absorbance at 280 nm and enzymatic activity of each fraction was estimated. The fraction (F-1) containing enzyme activity, obtained from DEAE cellulose column was purified further by gel filtration chromatography.
Gel filtration chromatography
The enzyme containing fraction (F-1) was dialyzed against distil water and 0.02 M sodium acetate buffer pH 5.2 for 24 hours at 4℃. After centrifugation, the clear sample was loaded onto the gel bed (Sephadex G-75). After diffusion of the sample, about 1 ml of elute buffer was poured on the top of gel bed and was allowed to diffuse. An additional amount of buffer was then added, so that the space about 3~4 cm above the gel bed was filled with elute. The buffer was allowed to flow continuously through the column and 3 ml fraction of elute was collected by an automatic fraction collector and monitored for enzyme activity as well as for protein concentration at 280 nm.
Determination of protein concentration
Concentration of protein was determined following the method of Lowry et al. (1951) using BSA as standard and the protein in column elute fraction was also monitored by spectrophotometrically at 280 nm.
Assay of cellulase activity
Cellulase activities were assayed by the previous method (Mahadevan and Sridhar, 1982) using cellulose powder (CMC) as substrate (0.05%, w/v) in 0.02M acetate buffer, pH 5.2. The amount of reducing sugar released was determined by dinitrosalicylic acid (DNS) method (Miller, 1959). The enzyme activity was expressed as the amount of reducing sugar released/ml of the sample/unit time.
Polyacrylamide disc gel electrophoresis
Polyacrylamide disc gel electrophoresis was performed in 7.5% polyacrylamide gel at 28℃, pH 8.5 as described by Ornstein (1964).
Molecular weight estimation
Estimation of molecular weight of purified intracellular cellulase was performed by SDS-PAGE according to the method of Weber and Osborn (1969) using 10% gel at 28℃, pH 7.0. Protein was denatured by incubating protein in boiling water for 3 minutes in solution containing 0.15% SDS with and without 0.1% beta-mercaptoethanol and 25% glycerol. The gels were stained with 1% Coomassie Briliant Blue R-250 in 7.5% acetic acid for an hour at room temperature, and destaining was performed by washing the gels in 7% acetic acid (v/v) solution. The standard molecular weight markers (BDH Chem. Ltd. England) used is the following: Lysozyme (14 KDa), Trypsin (20 KDa), Egg albumin (45 KDa), Bovine serum albumin (66 KDa), and b-glactosidase (116 KDa). It was also performed by gel filtration on Sephadex G-150 following the procedure as described by Andrews (1965).
Ultraviolet absorption spectrum of the purified enzyme
Ultraviolet absorption spectrum of enzyme was recorded in aqueous solution with double beam spectrophotometer (Shimadzu Model UV-180) at 28℃.
Analysis of carbohydrate
The total sugar content of purified intracellular cellulase was determined by phenol sulfuric acid method (Dubois et al., 1956) with D-glucose as the standard sugar.
Determination of Km value
Michaelis constant (Km) of purified intracellular cellulase of A. oryzae ITCC-4857.01 was determined by Lineweaver-Burk double reciprocal plot. The initial velocity was equal to the amount of product formed per unit time. The initial velocity (Vi) is determined by quantitavely measuring the amount of the product at various time intervels (Robyt and white, 1990).
Affinity of purified cellulase to different substrates
Percentage of relative activities of purified enzyme was determined by DNS method for detection of affinity of purified cellulase to different substrates viz. carboxyl methyl cellulose (CMC), avicel, salicin and filter paper.
pH effect
The enzyme was incubated for 20 minute at room temperature (28℃) in the following buffers: pH 4.0 to 5.5 sodium acetate; pH 6 to 8 sodium phosphate buffer. The enzyme was then assayed for celllulase activity in the same buffer.
Temperature effect
Enzyme and substrate were equilibrated separately for 5 minute at each test temperatures (10 to 80℃) and then assayed for cellulase activity at the same test temperature.
Results and Discussion
Purification of intracellular cellulase
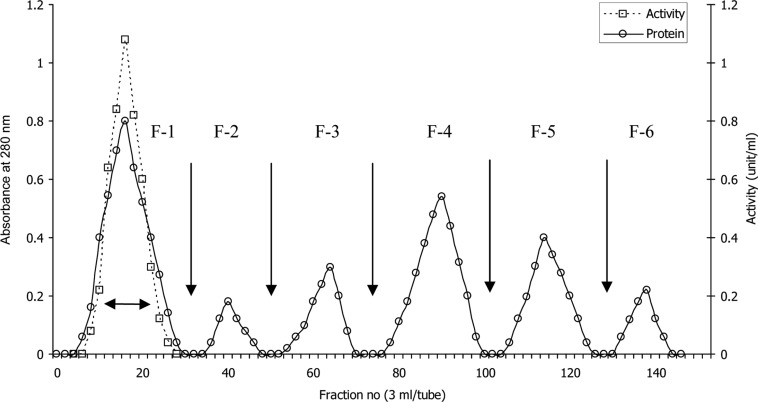
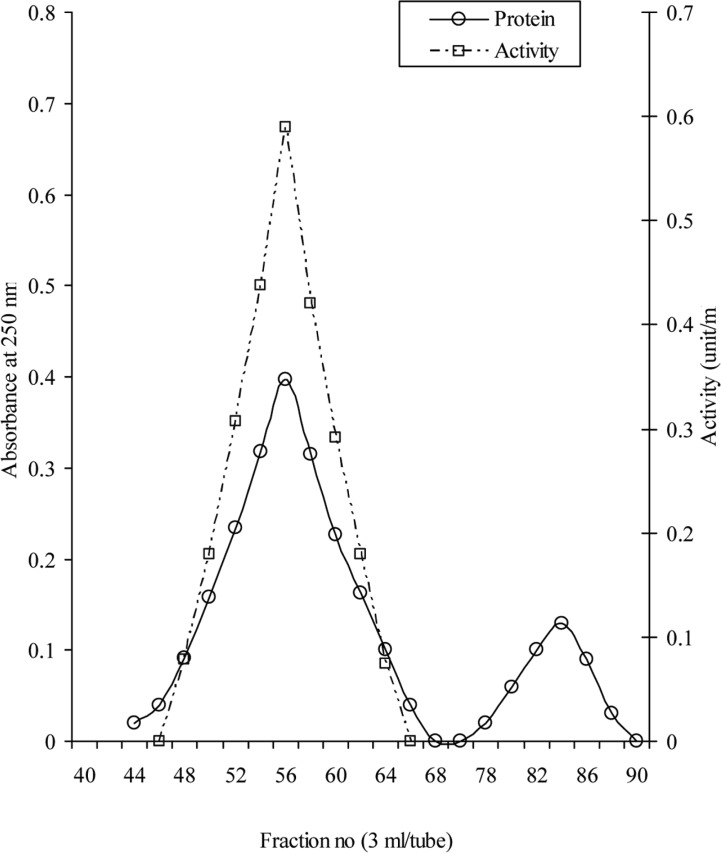
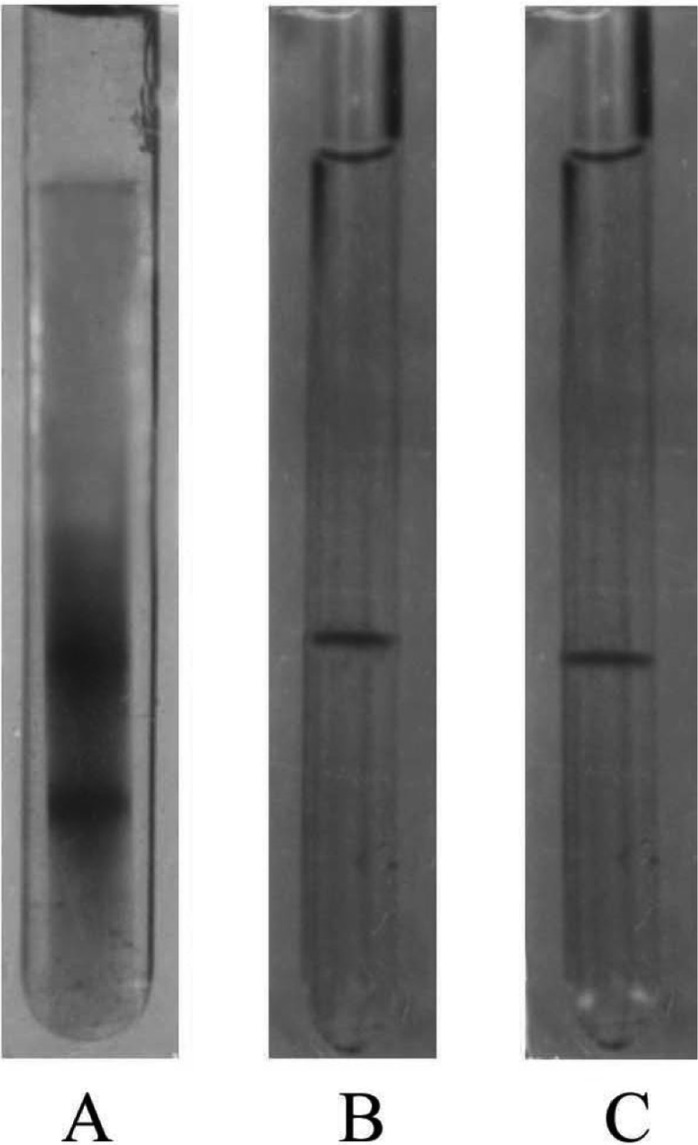
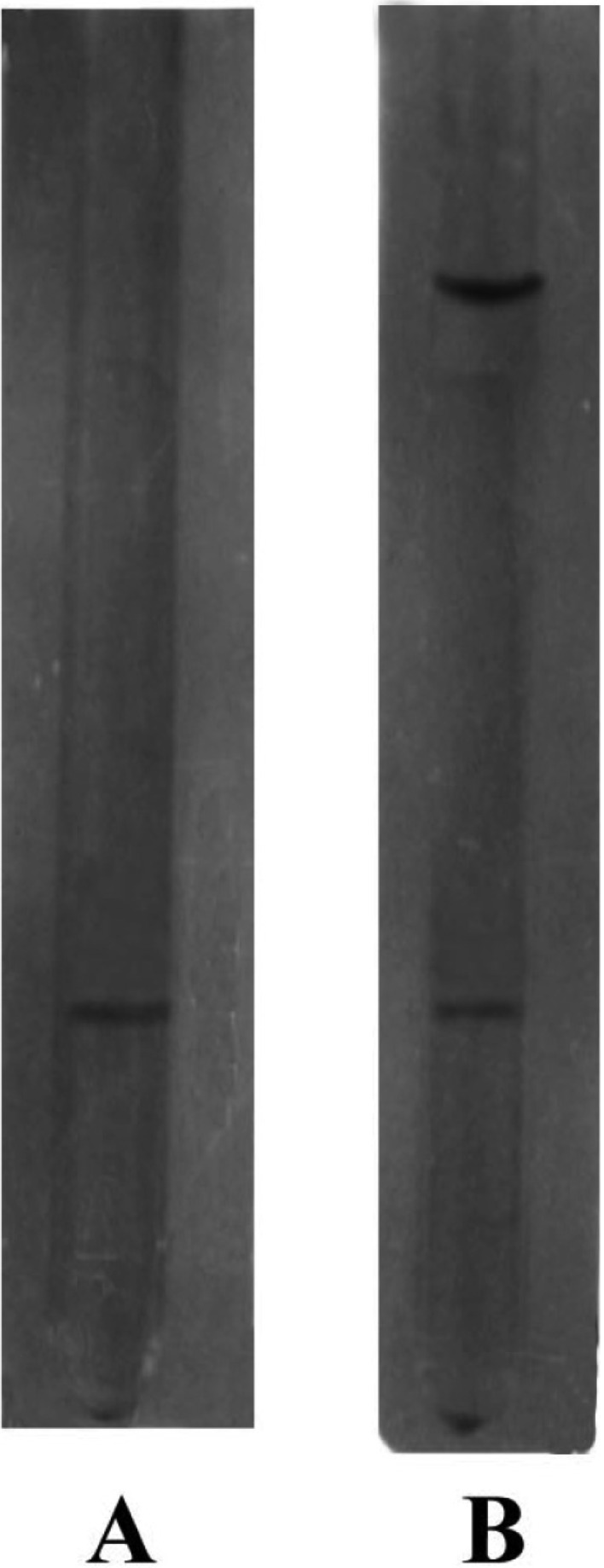
The elution profile of intracellular crude enzyme solution of Aspergillus oryzae ITCC-4857.01 from DEAE-cellulose column is shown in Fig. 1. As shown in Figure, the protein of crude enzyme extract was separated in six fractions as F-1, F-2, F-3, F-4, F-5 and F-6. It was found that only the fraction F-1 contained cellulase activity. This fraction was pooled and subjected to gel filtration for further purification. The fraction (F-1) showed only one peak F-1a (Fig. 2) in gel filtration and contained cellulase activity. This purified enzyme was checked by polyacrylamide disc gel electrophoresis. The enzyme was homogenous and showed single band on polyacrylamide gel (Fig. 3).
Fig. 1.
Ion-exchange chromatography of intracellular crude enzyme solution on DEAE cellulose. The crude enzyme solution (35 mg) was applied to the column (2.1 × 35 cm) preequilibrated with 0.2 M sodium acetate buffer pH 5.2 at 4℃ and eluted by stepwise increases of NaCl concentration in the same buffer. Flow rate 36 ml/hour.
Fig. 2.
Gel filtration of F-1 fraction on Sephadex G-75. Fractions F-1 obtained by DEAE-cellulose chromatography was applied to the column (3 × 120 cm) preequilibrated with 0.2M Sodium acetate buffer, pH 5.2 at 4℃ and developed with the same buffer.
Fig. 3.
Disc electrophoretic pattens of different fractions on 7.5% polyacrylamide gel. A, Crude enzyme; B, F-1 (obtained after DEAE-cellulose); C, F-1a (obtained after gel filtration).
A brief summary of the purification procedure was presented in Table 1. The specific activity of cellulase was 43.9 U/mg, which was increased at each purification step. Although the yield was only about 27% but over 99% of the extracted protein was removed during the purification steps and the enzyme was purified with an increase in purification more than 41 fold. This decrease in yield might be due to denaturation of enzyme during the purification steps or other reasons. Olama et al. (1993) purified cellulase from Trichoderma viride by DEAE-Sephadex A-50 chromatography method followed by CM-Sephadex C-50 and observed 99.8% loss of protein, and the specific activity was increased to about 22.8 fold. Sultana (1997) observed 13.71 U/mg specific activities which were increased to about 32 fold in Aspergillus sp. by DEAE-cellulose chromatography. Po-Jui et al. (2004) reported that the specific activity was 38.22 U/ml increased to about 9.04 fold from Sinorhizobium fredee by DEAESepharose anion-exchange column and followed by Phenyl-Sepharose column. The present results show good similarity with the observation of Olama et al. (1993) and Sultana (1997).
Table 1.
Summary of data on the course of purification of intracellular cellulase from A. oryzae ITCC-4857.01
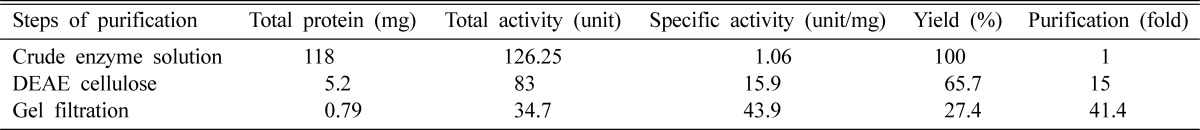
Unit = Release of reducing sugar in mg/min.
Characterization of cellulase molecular weight
The molecular weight of intracellular cellulase was found to be 38 and 38.6 KDa as determined by SDS polyacrylamide gel electrophoresis (Fig. 4) and gel filtration, respectively. This value is very similar to that purified from T viride, M.W. - 42 KDa (Berghem et al., 1975); T. viride, M.W. 38~54 KDa (Ogawa, 1989); T. viride, MW - 58 KDa (Olama et al., 1993); Aspergillus sp. M.W. - 31.2 KDa (Sultana, 1997) and Volvariella volvaceae, M.W. - 42 KDa (Shaojun et al., 2001). Further, the purified intra cellular cellulase might be contained only one subunit as the molecular weight was found to be unchanged both in presence and absence of beta-marcaptoethanol on SDS-polyacrylamide gel electrophoresis. It was also reported that cellulase purified from T. viride (Ogawa, 1989; Olama et al., 1993) and Aspergillus sp. (Sultana, 1997) contained only one subunit.
Fig. 4.
SDS-polyacrylamide gel electrophoretic pattern of F-1a fraction on 7.5% polyacrylamide gel. A, Abscence of beta-mercaptaethanol; B, Presence of beta-mercaptaethanol.
Ultraviolet absorption spectrum of purified intracellular cellulase
Purified intracellular cellulase in aqueous solution gave absorption peak at 270 nm and ranges were 240~270 nm (Fig. 5) that is very similar with the peak (290 nm) and ranges (260~290 nm) of extracellular cellulase (Begum, 2005) that indicates the structural similarity of both enzyme.
Fig. 5.
Ultraviolate absorption spectra of intracellular cellulase.
Analysis of carbohydrate
The purified intracellular cellulase gave slight chocolate color in the presence of phenolsulphuric acid, indicating that the enzyme is glycoprotein in nature and the amount of sugar present was calculated to be 0.67%. Neutral sugar from T. viride was reported to be 6.3~15.1% (Ogawa, 1989) which indicates that the enzymes contained comparatively less amount of neutral sugar.
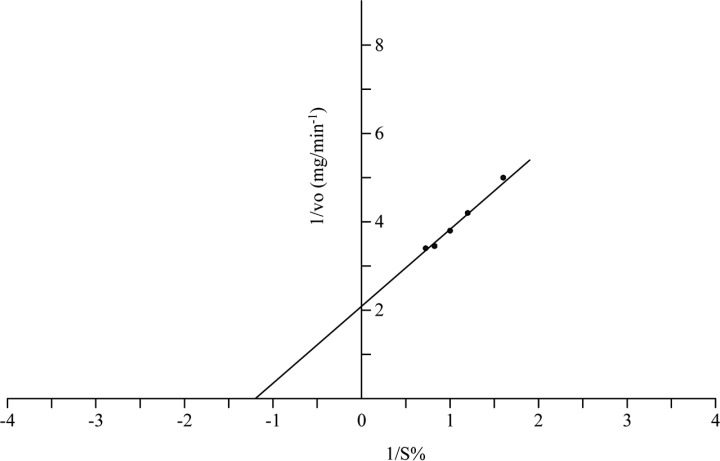
Determination of Km value of intracellular cellulase
Km value of purified intracellular cellulase was determined by Lineweaver-Burk double reciprocal plot (Fig. 6) and was found to be 0.83% using CMC as substrate. The Km value of cellulase from Favouls arcularicas was 0.28% (Enokibara et al., 1991) and 1.32% in T. reesei (Busto et al., 1996). The difference in km value of the presently purified intracellular cellulase may be due to variation of sources from which it was isolated.
Fig. 6.
Lineweaver-Burk double reciprocal plot for the determination of Km value of purified intracellular cellulase form A. oryzae.
Affinity of purified enzyme to different substrates
Activities of the purified enzyme was measured in sodium acetate buffer at pH 5.6 with various substrate at 30℃. The purified intracellular cellulase gave about 100% relative activity in hydrolyzing carboxyl methyl cellulose (CMC) but showed 80 and 60% relative activity when avicel and salicin were used as substrate. Filter paper is accounted as very poor substrate and showed 27% relative activity.
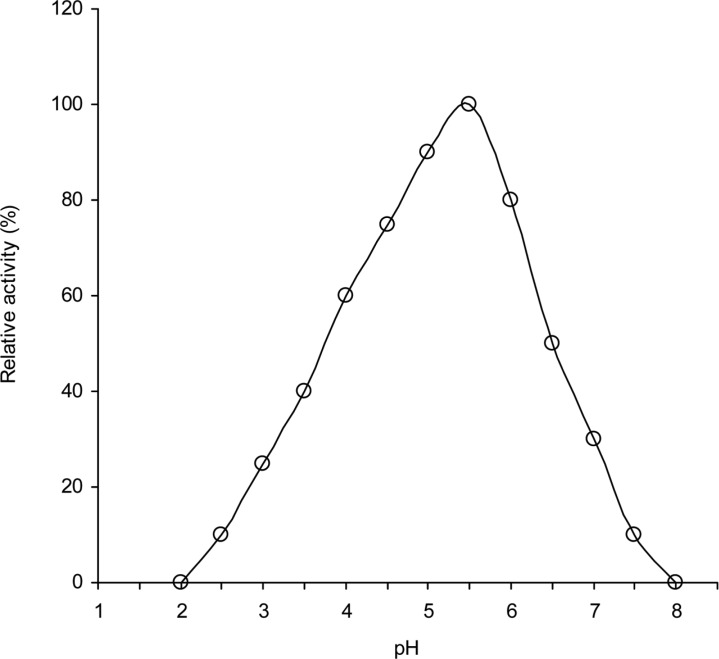
pH effect
The effects of pH on intracellular cellulase activity was determined in different buffers with pH values ranging from 2 to 8 (Fig. 7). Activities of the enzymes were found to be greatly affected by pH changes. The enzyme exhibited high activity in a range spanning 4.0 to 6.0. The optimum enzyme activity was observed at pH 5.5. The activity of the enzyme was found to be decreased more rapidly above or below the optimum pH values and very slight activity was observed at or above, pH 7.5 as well as at or below pH 3.5. Fungal cellulases with pH values of 4.5 to 6.0 are common and have been obtained from Trichodrma viridie (Gupta and Gupta, 1979); Aspergillus niger and A. terreus (Goma et al., 1982); Neurospora crassa (Macris et al., 1987); Aspergillus aureolus and A. clavatus (Mishra, 1988); Rhizopus oryzae (Amadioha, 1993); Volvariella diplasia (Bhadauria et al., 1997); Trichoderma reesei QM 9414 (Wang, 1999). From the results, it is noted that the enzymes are more active in moderate acetic reign than alkaline region. The decrease in activities at the extreme acidic pH or at alkaline pH values might be due to destruction of active site as well as changes in secondary or tertiary structure of cellulase.
Fig. 7.
Effect of pH on the activities of intracellular cellulases of A. oryzae ITCC-4857.01.
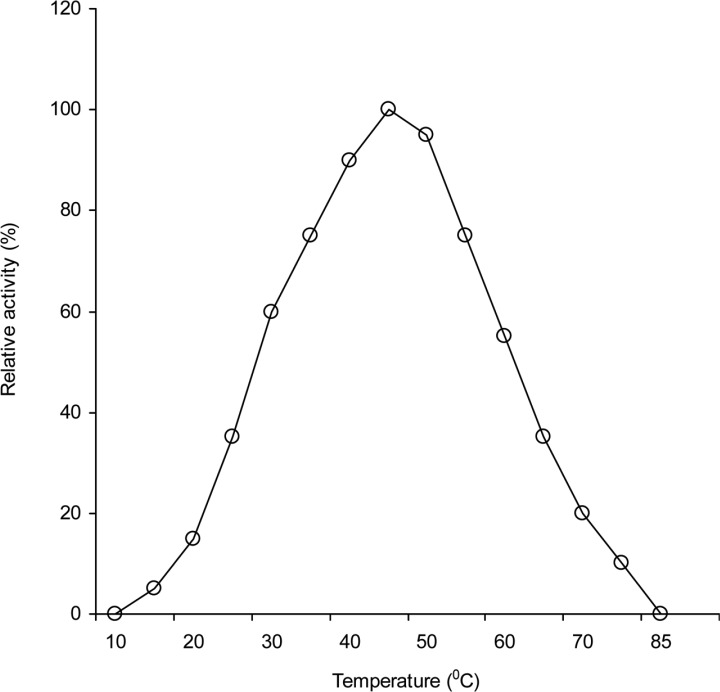
Temperature effect
The activity of the enzyme was determined at various temperatures ranges from 10 to 80℃ and the results are presented in Fig. 8. The activity of the enzyme was increased gradually with the increase or decrease of temperature. The enzyme exhibited high activity at 35 to 55℃ and maximum activity at 45℃. The enzyme lost its activities almost completely (95~100%) at 80℃ or above and as well as at or below 25℃. It is noted that this decrease in activities of enzymes at higher temperature might be due to destruction of secondary or tertiary structure of enzyme. In case of celulase activity the optimum tempreture 40 to 50℃ are obtained from Neurospora crassa (Macris et al., 1987); Aspergillus aureolus and A. clavatus (Mishra, 1988);. Trichodrma viridie (Sandhya, 1992); Morchella conica (Cavazzoni and Manzoni, 1994) and T. reesei QM 9414 (Wang, 1999).
Fig. 8.
Effect of temperature on the activities of intracellular cellulases of A. oryzae ITCC-4857.01.
Acknowledgement
We express thank to Kaji Abdus Salam for his expert assistance with DEAE - cellulose chromatography and gel electrophoresis. We also acknowledge Dr. Ronjit Kumer Saha (Ex-chairmen) and Staff of the Department of Biochemistry and Molecular Biology, Rajshahi university, Bangladesh for their kind support.
References
- 1.Akiba S, Kimura Y, Yamamoto K, Kumagai H. Purification and characterization of a protease-resistant cellulase from Aspergillus niger. J Ferment Bioeng. 1995;79:125–130. [Google Scholar]
- 2.Amadioha AC. Production of cellulolytic enzymes by Rhizopus oryzae in culture and Rhizopus-infected tissues of potato tubers. Mycologia. 1993;88:574–578. [Google Scholar]
- 3.Andrews P. The gel filtration behavior of proteins related to their molecular weights over a wide range. Biochem J. 1965;96:595–605. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beguin P. Molecular biology of cellulose degradation. Annu Rev Microbiol. 1990;44:219–248. doi: 10.1146/annurev.mi.44.100190.001251. [DOI] [PubMed] [Google Scholar]
- 5.Begum MF. Screening of aspergilli from cellulosic waste materials and studies on their cellulolytic properties. Bangladesh: Department of Botany, University of Rajshahi; 2005. Ph.D. thesis. [Google Scholar]
- 6.Berghem L, Pettersson L, Asio-Fredriksson U. The mechanism of enzymatic ecllulose degradation, characterization and enzymatic properties of a β-1-4-glucan cellobiolydrolase from Trichoderma viride. Eur J Biochem. 1975;53:55. [Google Scholar]
- 7.Bhadauria A, Sodhi HS, Kapoor S, Phutela RP. Isolation and characterization of Volvariella diplasia enzyme mutants. Indian J Exp Biol. 1997;35:516–519. [Google Scholar]
- 8.Busto MD, Ortega N, Pereg MM. Location, kinetics. and stability of cellulases induced in Trichoderma rcesei cultures. Biores Tech. 1996;57:187–192. [Google Scholar]
- 9.Cavazzoni V, Manzoni M. Extracellular cellulytic complex from Morchella conica: Production and purification. Lebensmttel-Wissenschaft Technologie. 1994;27:73–77. [Google Scholar]
- 10.Coral G, Arikan B, Unaldi MN, Guvenmes H. Some properties of crude carboxymethyl cellulase of Aspergillus niger Z10 wild-type Strain. Turk J Biol. 2002;26:209–213. [Google Scholar]
- 11.Dubois M, Gilles KA, Hamilton JK, Rebers P, Smith G. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:61–77. [Google Scholar]
- 12.Enokibara S, Mori N, Kitamoto K. Purification and characterization of endoglucanases from Favouls arcularicus. J Fermen Bioeng. 1991;73:230–232. [Google Scholar]
- 13.Gielkens MMC, Dekkers E, Visser J, Graaff LH. Two cellubiohydrolase-encoding genes from Aspergillus niger require D-xylose and the xylanolytic transcriptional activator XlnR for their expression. Appl Environ Microbiol. 1999;65:4340–4345. doi: 10.1128/aem.65.10.4340-4345.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Godfrey T, West S. Industrial Enzymology. 2nd. ed. London: Macmillan Press; 1996. [Google Scholar]
- 15.Goma M, Zein GN, Mahmoud RM, Gibriel A, Abouzied M. Characteristics of cellulolytic enzymes of Aspergillus niger and Aspergillus terreus. Minufiya J Agr Res. 1982;5:299–317. [Google Scholar]
- 16.Gupta JK, Gupta YP. Properties of cellulase from Trichodema viride. Folia Microbiol. 1979;24:269–272. doi: 10.1007/BF02926459. [DOI] [PubMed] [Google Scholar]
- 17.Hofsten BV. Microbial conversion of cellulosic wastes products. Waste recovery by microorganisms. Khalalampur: UNESCO; 1972. May, pp. 1–18. [Google Scholar]
- 18.Hong J, Tamaki H, Akiba S, Yamamoto K, Kumaga H. Cloning of a gene encoding a highly stable endo-β-1, 4-glucanase from Aspergillus niger and its expression in yeast. J Biosci Bioeng. 2001;92:434–441. doi: 10.1263/jbb.92.434. [DOI] [PubMed] [Google Scholar]
- 19.Lowery OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 20.Macris BJ, Ketos D, Evangelidou X, Galiotou-Panayotou M, Rodis P. Solid state fermentation of straw with Neurospora crassa for CMCase and β-glucosidase production. Biotech Lett. 1987;9:661–664. [Google Scholar]
- 21.Mahadevan A, Sridhar R. Methods in physiological plant pathology. Madrus: Sivakami publications; 1982. [Google Scholar]
- 22.Mantyla A, Paloheimo M, Suominen P. Industrial mutants and recombinant strains of Trichoderma reesei. In: Harman GF, Kubicek CP, editors. Trichoderma & Gliocladium-Enzymes, biological control and commercial applications. 2nd ed. London: Taylor & Francis, Ltd; 1998. pp. 291–309. [Google Scholar]
- 23.Miller GL. Use of dinitrosalicylic acid for determination of reducing sugar. Anal Chem. 1959;31:426–428. [Google Scholar]
- 24.Mishra A. Studies an cellulolytic fungi and cellulolytic enzymes with special reference to Aspergilli. India: Department of Botany, Gorakhpur Univ; 1988. Ph.D. Thesis. [Google Scholar]
- 25.Murai T, Ueda M, Kavaguchi T, Arai M, Tanaka M. Assimilation of cellooligosaccharides by a cell surfaceengineered yeast expressing β-glucosidase and carboxymethylcellulase from Aspergillus aculeatus. Appl Environ Microbiol. 1998;64:4857–4861. doi: 10.1128/aem.64.12.4857-4861.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogawa K. Fractionation and purification of cellulases from Trichoderma viride. Bull Fac Agr Myazaki Univ. 1989;36:271–280. [Google Scholar]
- 27.Olama ZA, Hamza MA, El-Sayed MM, Abdel-Fattah M. Purification, properties and factors affecting the activity of Trichoderma viride cellulase. Food Chem. 1993;47:221–226. [Google Scholar]
- 28.Ornstein L. Disc electrophoreses I - Background and theory. Ann NY Acad Sci. 1964;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- 29.Peij N, Gielkens MMC, Veries RP, Visser J, Graaff LH. The transcriptional activator XlnR Regulates both xylanolytic and endoglucanase gene expression in Aspergillus niger. Appl Environ Microbiol. 1998;64:3615–3619. doi: 10.1128/aem.64.10.3615-3619.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Po-Jui C, TaoChun W, YaoTsung C, Liangling L. Purification and characterization of carboxylmethyl cellulase from Sinorhigobium fredii. Bot Bull Acad Sinica. 2004;45:111–118. [Google Scholar]
- 31.Robson LM, Chambliss GH. Cellulases of bacterial origin. Enzyme Microb Technol. 1989;11:626–644. [Google Scholar]
- 32.Robyt JF, White BJ. Biochemical Techniques: Theory and practical. Illinois, U.S.A: Waveland Press. Inc; 1990. [Google Scholar]
- 33.Sandhya S. Effect of temperature on deactivation of cellulase. Asian Environ. 1992;14:78–81. [Google Scholar]
- 34.Shaojun D, Wei G, Busuell JA. Endoglucanase 1 from the edible straw mushroom, Volvariella volvaeeae: Purification, characterization, cloning and expression. Eur J Biochem. 2001;268:5687–5695. doi: 10.1046/j.0014-2956.2001.02503.x. [DOI] [PubMed] [Google Scholar]
- 35.Sultana S. Isolation of cellulolytic microorganism and their activities. Bangladesh: Biochemistry Department, Rajshahi Univ; 1997. M. Phil Thesis. [Google Scholar]
- 36.Uhlig H. Industrial enzymes and their applications. New York: John Wiley & Sons; 1998. p. 435. [Google Scholar]
- 37.Wang JS. Cellulase production by a mutant strain of Trichoderma reesei from bagasse; 23rd ISSCT Congress; New Delhi, India. 1999. pp. 67–76. [Google Scholar]
- 38.Weber K, Osborn M. The reliability of molecular weight determination by SDS-PAGE. J Biol Chem. 1969;244:4406–4412. [PubMed] [Google Scholar]