Abstract
The effect of fermentation parameters and medium composition on the simultaneous mycelial growth and exo-polymer production from submerged cultures of Ganoderma applanatum was investigated in shake-flask cultures. The optimum initial pH for mycelial growth and exo-polymer production was 5.0 and 6.0, respectively. The optimum temperature was 25℃ and the optimum inoculum content was 3.0% (v/v). The optimal carbon and nitrogen sources were glucose and corn steep powder, respectively. After 12 days fermentation under these conditions, the highest mycelial growth was 18.0 g/l and the highest exo-polymer production was 3.9 g/l.
Keywords: Exo-polymer, Ganoderma applanatum, Mycelia, Submerged culture
Mushrooms are highly nutritious and may exhibit medicinal properties. In recent years, mushrooms have emerged as an important source of bioactive chemicals that have properties such as antitumor (Suzuki et al., 1989), immunological (Luettig et al., 1989), anti-complementary (Zhao et al., 1993), anti-inflammatory (Chihara et al., 1992), anticoagulant (Nishino et al., 1991), hypoglycemic (Yamada et al., 1989), hypolipidemic activity (Yang et al., 2000), mitogenic activity on lymphocyte (Jeon et al., 1998) and anti-viral (Rym et al., 1999).
Ikekawa et al. (1969) published one of the first scientific reports on the antitumor activities of Polyporacease mushrooms. The three kinds of antitumor agent from mushroom polymers were developed: Krestin from cultured mycelia of Trametes versicolor (Ohno et al., 1976), Lentinan from fruiting bodies of Lentinus edodes (Chihara, 1992; Maeda and Chihara, 1971), and Schizophyllan from the liquid cultured broth product of Schizophyllum commune (Komatsu et al., 1969),. Mushroom polymers are mostly glucans with different types of glycosidic linkages, such as (1→3), (1→6)-β-glucans and (1→3)-α-glucans, but some are heteroglycans.
Elfvingia applanata is a species of Basidiomycete, also called "Ganoderma applanata", which has been used in folk medicine for treating various ailments, including cancer (Makino, 1989). Recently, components of the modulate humoral immune response were detected in a purified fraction obtained from G. applanatum (Kim et al., 1994c). Later, anti-bacterial and antiviral activities of the aqueous extract of G. applanatum was reported (Kim et al., 1994b; Rym et al., 1999) without any toxicity (Kim et al., 1994a).
Biologically active polymers can be obtained not only in fruiting bodies of mushrooms but also in submerged mycelial fermentations. Recently, the production of polymers from submerged mycelial cultures have been extensively exploited because they require fewer steps and because the purification process is simpler (Bae et al., 2000; Cavazzoni and Adami, 1992; Jong and Birmingham, 1992; Tseng, 1984).
In the present investigation, therefore, the optimum culture conditions of Ganoderma applanatum for the mycelial growth and exo-polymer production were studied.
Materials and Methods
Strain and basal medium
The G. applanatum (KACC 50174) was obtained from the Korean Agricultural Culture Collection (KACC). It was maintained on potato dextrose agar (PDA, Difco) slants at 4℃ and subcultured every 3 months. The mushroom complete medium (MCM) was used to perform submerged mycelial culture for mycelial growth and exo-polymer production. The composition of MCM (g/l) is as follows: glucose (20), MgSO4·7H2O (0.5), KH2PO4 (0.46), K2HPO4 (1.0), yeast extract (2) and peptone (2). The pH was adjusted to 4.0 (with HCl) before sterilization.
Preparation of inoculum
The inoculum was prepared using the method described by Song and Cho (1987). The G. applanatum was initially grown on potato dextrose agar medium in a petri-dish. The seed culture of G. applanatum was grown in 250-ml flasks containing 100 ml of potato dextrose broth at 25℃ on rotary shaker at 150 rpm. The pH was adjusted to 4.0 before sterilization. After an incubation period of 10 days, 100 ml of the culture broth with mycelial pellets were homogenized aseptically in a Sorvall omni-mixer for 3 min in an ice bath. A 2% (v/v) mycelial suspension was used as inoculum for the subsequent experiments.
Submerged culture for mycelial growth and exo-polymer production
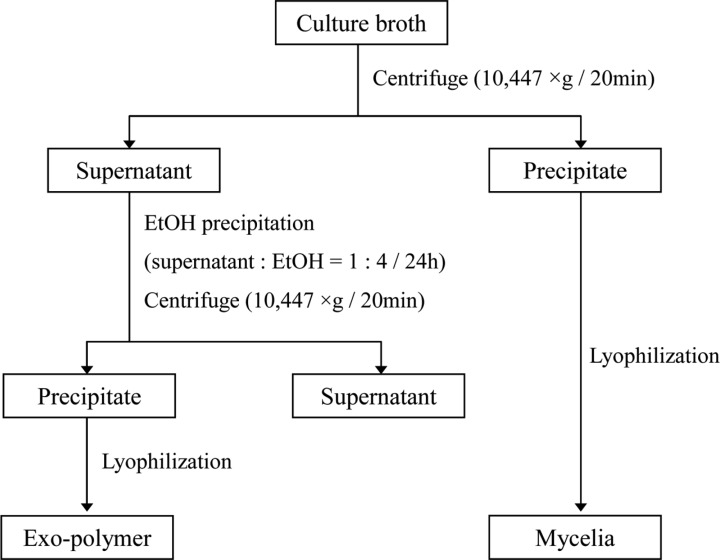
The submerged mycelial cultures were carried out in 250-ml flasks containing 50 ml of the media (MCM) on a rotary shaker (150 rpm). After harvest, the cultured mycelia were collected by centrifugation (10,447 ×g, 20 min). The cell free exo-polymer was precipitated by adding 4 volumes of ethanol to the supernatent. Mycelia and exo-polymer were lyophilized. This recovery process is shown in Fig. 1.
Fig. 1.
Schematic diagram of mycelial growth and exo-polymer 4. The maximum yield of production in submerged mycelial cultures of G. applanatum.
Optimization of culture condition
The submerged mycelial cultures were grown in 250-ml culture flasks containing 50 ml of MCM medium on a rotary shaker (150 rpm). To determine the optimal medium pH, we grew cultures at 3, 4, 5, 6, 7, and 8 by adding 1 N HCl or NaOH before sterilization. To determine optimal temperature, we grew cultures at 20, 25 and 30℃ for 7 days. To determine the optimum concentration of inoculum, we grew cultures from seed amounts of 1, 2, 3, 4, and 5% (v/v).
Carbon and nitrogen source
The optimum carbon source for the mycelial growth and exo-polymer production were determined using either 1, 2, 4 ,6, or 8% (w/v) individually glucose, maltose, arabinose, mannose and molasess. The optimum nitrogen source was determined using medium containing either 0.2, 0.4, 0.6, 0.8, or 1.0% (w/v) individually yeast extract, peptone, malt extract, meat extract, corn steep liquor and corn steep powder.
Results and Discussion
Effect of pH, temperature and inoculum content
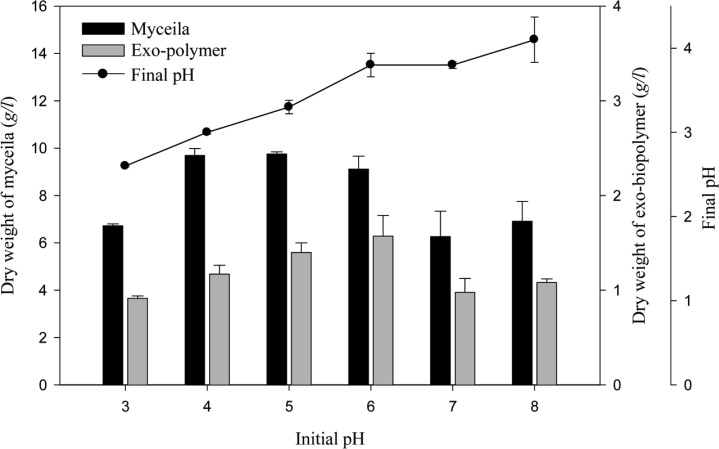
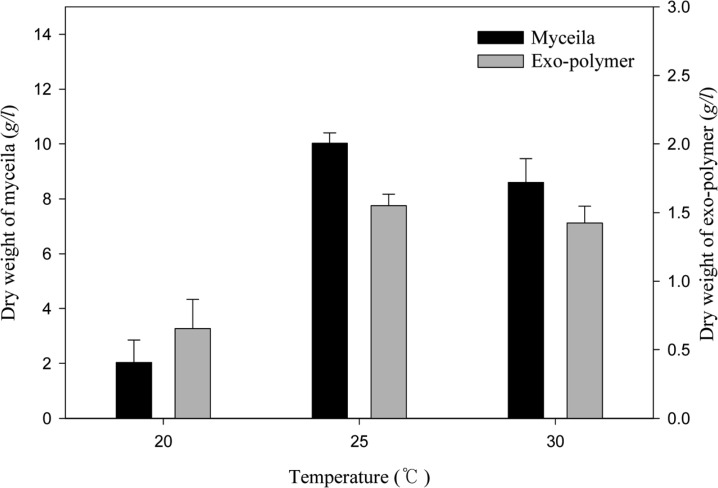
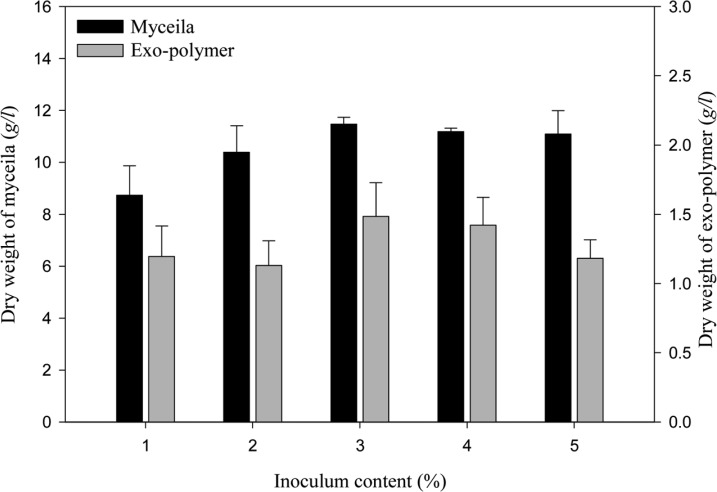
The effect of pH on mycelial growth and exo-polymer production is presented in Fig. 2. The growth of G. applanatum was highest in a pH of 4 to 6. Maximum mycelia yield (9.76 g/l) was achieved at pH 5 and maximum exo-polymer production (1.57 g/l) was obtained at pH 6. The effect of temperature on mycelial growth and exo-polymer production is presented in Fig. 3. Maximum yield of mycelia (10.03 g/l) and exo-polymer (1.55 g/l) were achieved at 25℃. Optimal pH and temperature for the mycelial growth of Ganoderma sp. is approximately 4 and 25 to 30℃, respectively (Yang and Liau, 1998). The effect of inoculum content on mycelial growth and exo-polymer production is presented in Fig. 4. The maximum yield of mycelia (11.47 g/l) and maximum exo-polymer production (1.48 g/l) were achieved using a seed content of 3% (v/v).
Fig. 2.
Effect of pH on mycelial growth and exo-polymer production in submerged mycelial cultures of G. applanatum in shake flasks. All experimental data are mean ± SD of three replicates. Culture conditions: 150 rpm, 25℃, 7 days and 2% inoculum.
Fig. 3.
Effect of temperature on mycelial growth and exo-polymer production in submerged mycelial cultures of G. applanatum in shake flasks. All experimental data are mean ± SD of three replicates. Culture conditions: 150 rpm, pH 5.0, 7 days and 2% inoculum.
Fig. 4.
Effect of inoculum content on mycelial growth and exo-polymer production in submerged mycelial cultures of G. applanatum in shake flasks. All experimental data are mean ± SD of three replicates. Culture conditions: 150 rpm, 25℃, pH 5.0, 7 days.
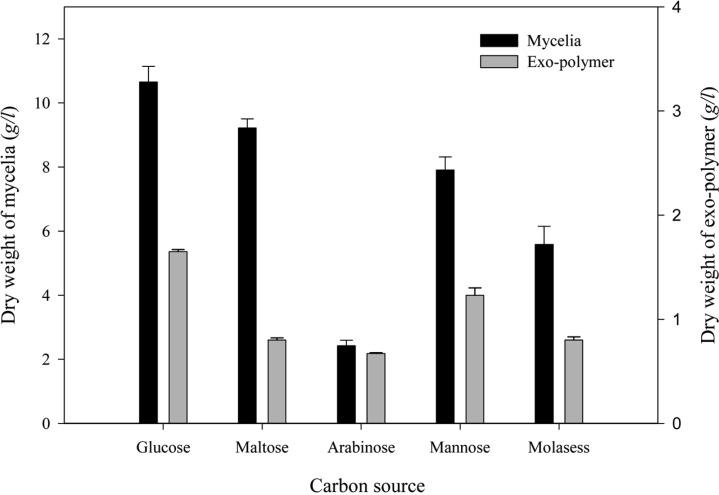
Effect of carbon source
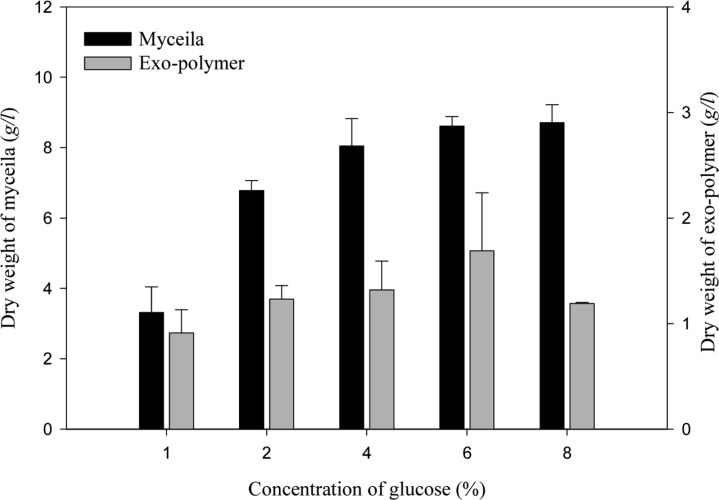
The effect of carbon source on mycelial growth and exo-polymer production were determined using medium containing various carbon sources. When five different carbon sources (glucose, maltose, arabinose, mannose and molasess) were examined (Fig. 5), the highest mycelial growth (10.65 g/l) and exo-polymer production (1.65 g/l) were obtained using glucose as a carbon source. The optimum concentrations of glucose are shown in Fig. 6. The highest mycelial growth (8.71 g/l) was obtained using 8% (v/v) glucose and the maximum exo-polymer production (1.69 g/l) was obtained using 6% (v/v) glucose. When glucose concentration exceeded 8% (v/v), exo-polymer production decreased. Lee et al. (2007) also reported that exopolysaccharide production in G. applanatum was highest in cultures with glucose by airlift bioreactor, and exopolysaccharide production was enhanced by an increase in glucose concentration.
Fig. 5.
Effect of various carbon sources on mycelial growth and exo-polymer production in submerged mycelial cultures of G. applanatum in shake flasks. All experimental data are mean ± SD of three replicates. Culture conditions: 150 rpm, 25℃, pH 5.0, 7 days and 3% inoculum. Each carbon source was supplemented to 2% in basal medium.
Fig. 6.
Effect of various concentrations of glucose on mycelial growth and exo-polymer production in submerged mycelial cultures of G. applanatum in shake flasks. All experimental data are mean ± SD of three replicates. Culture conditions: 150 rpm, 25℃, pH 5.0, 7 days and 3% inoculum.
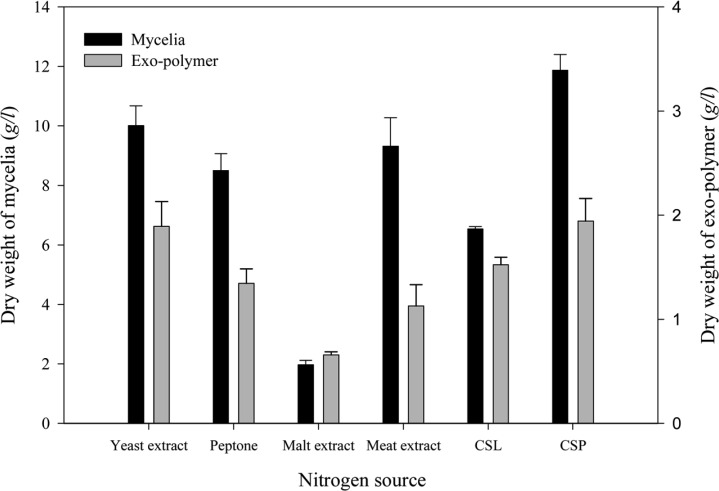
Effect of nitrogen source
The effect of nitrogen source on mycelial growth and exo-polymer production were determined using medium containing various nitrogen sources (yeast extract, peptone, meat extract, malt extract, corn steep liquor and corn steep powder; Fig. 7). The highest mycelial growth (11.87 g/l) and exo-polymer production (1.94 g/l) were obtained using corn steep powder (CSP) as a nitrogen source. Kim et al. (1997) reported a higher production of mycelia and exo-polysaccharides using organic nitrogen than inorganic nitrogen.
Fig. 7.
Effect of various nitrogen sources on mycelial growth and exo-polymer production in submerged mycelial cultures of G. applanatum in shake flasks. All experimental data are mean ± SD of three replicates. Culture conditions: 150 rpm, 25℃, pH 5.0, 7 days and 3% inoculum. Each nitrogen source was supplemented at a rate of 0.4% in basal medium (CSL: Corn steep liquor, CSP: Corn steep powder).
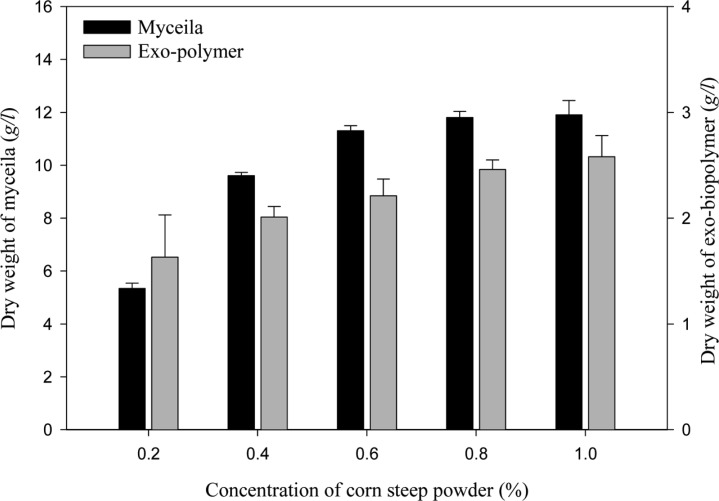
The optimum concentration of CSP for mycelial growth and exo-polymer production is shown in Fig. 8. The highest mycelial growth (11.87 g/l) and exo-polymer production (2.58 g/l) were obtained using 1.0% (v/v) CSP.
Fig. 8.
Effect of various concentrations of corn steep powder on mycelial growth and exo-polymer production in submerged mycelial cultures of G. applanatum in shake flasks. All experimental data are mean ± SD of three replicates. Culture conditions: 150 rpm, 25℃, pH 5.0, 7 days and 3% inoculum.
Effect of culture period for exo-polymer production and mycelial growth
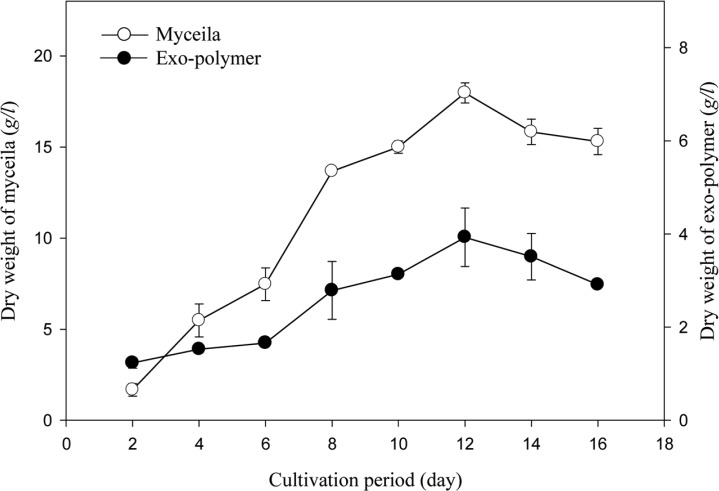
The time courses for the mycelial growth and the exo-polymer production in shake flasks are shown in Fig. 9. The maximum yield of mycelia (18.0 g/l) and exo-polymers (3.9 g/l) was achieved at the end of log phase (12 days), with the specific growth rate (µ) of 0.01 h-1. The pH value decrease to pH 3.7 (data not shown). This decrease in pH seems to be due to the production of organic acid as metabolites.
Fig. 9.
Time profiles of mycelial growth and the exo-polymer production in submerged mycelial cultures of G. applanatum in shake flasks. All experimental data are mean ± SD of three replicates. Culture conditions: 150 rpm, 25℃, pH 5.0, 7 days and 3% inoculum. Medium composition (g/l): glucose (60), MgSO4 (0.5), KH2PO4 (0.46), K2HPO4 (1.0), corn steep powder (10).
Acknowledgment
This work was supported by the Daegu University Research Grant (sabbatical research for 6 month), 2008.
References
- 1.Bae JT, Sinha J, Park JP, Song CH, Yun JW. Optimization of submerged culture conditions for exo-biopolymer production by Pacilomyces japonica. J Microbiol Biotechnol. 2000;10:482–487. [Google Scholar]
- 2.Cavazzoni V, Adami A. Exopolysaccharides produced by mycelial edible mushroom. Ital J Food Sci. 1992;1:9–15. [Google Scholar]
- 3.Chihara G. Immunopharmacology of lentinan, a polysaccharide isolated from Lentinus edodes: its application as a host defense potentiator. Int J Orient Med. 1992;17:57–77. [Google Scholar]
- 4.Hara C, Kiho T, Tanaka Y, Ukai S. Anti-inflammatory activity and conformational behavior of a branched (1→3)-α-D-glucan from an alkaline extract of Dictyophora indusiata Fish. Carbohydr Res. 1982;110:77–82. doi: 10.1016/0008-6215(82)85027-1. [DOI] [PubMed] [Google Scholar]
- 5.Ikekawa T, Uehara N, Maeda Y, Nakanishi M, Fukuoka F. Antitumor activity of aqueous extracts of edible mushrooms. Cancer Res. 1969;29:734–735. [PubMed] [Google Scholar]
- 6.Jeon KJ, Suh JS, Oh CH, Yum JY, Eun JS. Effect of ethyl alcohol fraction of Cervus nippon on mouse T-lymphocyte. Korean J Pharmacogn. 1998;29:312–317. [Google Scholar]
- 7.Jong SC, Birmingham JM. Medicinal benefits of the mushroom Ganoderma. Adv Appl Microbiol. 1992;37:101–134. doi: 10.1016/s0065-2164(08)70253-3. [DOI] [PubMed] [Google Scholar]
- 8.Kim HK, Cheong JC, Chang HH, Kim GP, Cha DY, Moon BJ. The artificial cultivation of Pleurotus eryngii (I) - Investigation of mycelial growth conditions. Korean J Mycol. 1997;25:305–310. [Google Scholar]
- 9.Kim YS, Kang JK, Lee CK, Han SS. Effect of Elfvingia applanata extract on the acute toxicity in mice. Yakhak Hoeji. 1994a;38:756–762. [Google Scholar]
- 10.Kim YS, Ryu KH, Lee CK, Han SS. Antimicrobial activity of Elfvingia applanata extract alone and in combination with some antibiotics. Yakhak Hoeji. 1994b;38:742–748. [Google Scholar]
- 11.Kim YS, Ryu KH, Mo YK, Lee CK, Han SS. Effects of the antitumor component, F-D-P, isolated from Elfvingia applanata on the immune response. Korean J Pharmacogn. 1994c;25:348–355. [Google Scholar]
- 12.Komatsu N, Okubo S, Kikumoto S, Kimura K, Saito G, Sakai S. Host mediated antitumor action of shizophyllan, a glucan produced by Schizophyllum commune. Gann. 1969;60:137–144. [PubMed] [Google Scholar]
- 13.Lee WY, Park Y, Ahn JK, Ka KH, Park SY. Factors influencing the production of endopolysaccharide and exopolysaccharide from Ganoderma applanatum. Enzyme Mirob Technol. 2007;40:249–254. [Google Scholar]
- 14.Luettig B, Steinmuller C, Gifford GE, Wagner H, Lohmann-Matthes ML. Macrophage activation by the polysaccharide arabinogalactan isolated from plant cell cultures of Echinacea purpurea. J Natl Cancer Inst. 1989;81:669–675. doi: 10.1093/jnci/81.9.669. [DOI] [PubMed] [Google Scholar]
- 15.Maeda YY, Chihara G. Lentinan, a new immunoaccelerator of cell mediated response. Nature. 1971;229:634. doi: 10.1038/229634a0. [DOI] [PubMed] [Google Scholar]
- 16.Makino T. Makino's new illustrated flora of Japan. Tokyo: The Hokuryukan Co. LTD; 1989. [Google Scholar]
- 17.Nishino T, Kiyohara H, Yamada H, Nagumo T. An anticoagulant fucoidan from brown seaweed Ecklonia kurome. Phytochemistry. 1991;30:535–540. doi: 10.1016/0031-9422(91)83722-w. [DOI] [PubMed] [Google Scholar]
- 18.Ohno R, Yokomatsu S, Wakayama K, Sugiura S, Imaik K. Effect of protein-polysaccharide preparations, PS-K, on the immune response of mice to sheep red blood cells. Gann. 1976;67:97–99. [PubMed] [Google Scholar]
- 19.Rym KH, Eo SK, Kim YS, Lee CK, Han SS. Antiviral activity of water soluble substance from Elfvingia applanata. Korean J Pharmacogn. 1999;30:25–33. [Google Scholar]
- 20.Song CH, Cho KY. A synthetic medium for the production of submerged cultures of Lentinus edodes. Mycologia. 1987;76:866–876. [Google Scholar]
- 21.Suzuki I, Hashimoto K, Oikawa S, Sato K, Osawa M, Yadomaf T. Antitumor and immunomodulating activities of a β-glucan obtained from liquid cultured Grifola frondosa. Chem Pharm Bull. 1989;37:410–413. doi: 10.1248/cpb.37.410. [DOI] [PubMed] [Google Scholar]
- 22.Tseng TC. Studies on Ganoderma lucidum. Liquid culture and chemical composition of mycelium. Bot Bull Acad Sinica. 1984;23:149–154. [Google Scholar]
- 23.Yamada H, Kiyohara H. Bioactive polysaccharides from Chinese herbal medicines. Abs Chinese Med. 1989;3:104–124. [Google Scholar]
- 24.Yang BK, Ha JY, Jeong SC, Das S, Yun JW, Lee YS, Choi JW, Song CH. Production of Exopolymers by submerged mycelial culture of Cordyceps militaris and its hypolipidemic effect. J Microbiol Biotechnol. 2000;10:784–788. [Google Scholar]
- 25.Yang FC, Liau CB. The influence of environmental contitions on polysaccharide formation by Ganoderma lucidum in submerged cultures. Process Biochem. 1998;33:547–553. [Google Scholar]
- 26.Zhao IF, Kiyohar H, Matsumoto T, Yamada H. Anti-complementary acidic polysaccharides from roots of Lithosperum euchromum. Phytochemistry. 1993;34:719–723. doi: 10.1016/0031-9422(93)85346-s. [DOI] [PubMed] [Google Scholar]