Abstract
In this study, dual culture, poison agar, and direct methods were used to assess the ability of Trichoderma virens IMI-392430, T. pseudokoningii IMI-392431, T. harzianum IMI-392432, T. harzianum IMI-392433, and T. harzianum IMI-392434 to control Ceratocystis paradoxa, which causes the pineapple disease of sugarcane. The highest percentage inhibition of radial growth (PIRG) values were observed with T. harzianum IMI-392432 using two dual culture methods, 63.80% in Method I and 80.82% in Method II. The minimum colony overgrowth time was observed with T. harzianum IMI-392432 and the maximum was observed with T. pseudokoningii IMI-392431. Different concentrations of different day-old metabolites of Trichoderma isolates were tested against mycelial growth of C. paradoxa. The highest PIRG (84.685%) exhibited at 80% concentration of 30-day-old metabolites of T. harzianum IMI-392432 using the modified bilayer poison agar method. In the direct assay method the maximum mycelial growth weight (PIGW) was observed at the same concentration and the same day-old metabolites of T. harzianum IMI-392432. This study showed that Trichoderma isolates have a good antagonistic effect on C. paradoxa mycelial growth and T. harzianum IMI-392432 has the most potential to control the pineapple disease pathogen.
Keywords: Ceratocystis paradoxa, PIGW, PIRG, Secondary metabolites, Trichoderma
Pineapple disease caused by Ceratocystis paradoxa results in a considerable loss in sett germination and can reduce cane yield by 31~35% (Anonymous, 2000). The disease is severe in heavy textured soils and poorly drained fields, and it can reduce germination by up to 47% (Anonymous, 1999). The affected setts emit a smell resembling that of the mature pineapple fruit (Went, 1896), which is due to ethyl acetate formed by metabolic activity of the pathogen. The ethyl acetate content in the infected tissue may rise to 1%, which is sufficient to inhibit germination of buds (Kuo et al., 1969). The fungus is essentially soilborne and is transmitted to cane setts via two types of spores: thin-walled cylindrical conidia (6~24 µm × 2~5.5 µm) and thick-walled oval chlamydospores (10~25 µm × 7.5~20 µm). The latter ensures the long-term survival of the pathogen in the soil. Infection occurs mainly through cut ends but also through wounds caused by insects and through cracks in cuttings. The fungus spreads rapidly through the parenchyma, which becomes red and breaks down leading to a hollow and blackened interior. The economic importance of the disease is also significant.
When shoot population is reduced, yield is directly reduced. Furthermore, grapy stands necessitate costly replanting or recruiting, and weed problems may also result if leaf canopy development is retarded. Fungicide is most commonly used as a treatment to manage, but there is a need for non-chemical methods of control to reduce the adverse effects of toxic chemicals on the environment, particularly the sugarcane ecosystem. Biological control of plant pathogens by microorganisms has been considered a more natural and environmentally acceptable alternative to the existing chemical treatment methods (Baker and Paulitz, 1996). The antagonistic activity of Trichoderma species against plant pathogens has been studied extensively (Burgess and Hepworth, 1996; Burns and Benson, 2000; Etabarian, 2006; Hjeljord et al., 2001). Knowledge about the behavior of these fungi as antagonists is essential for their effective use because they can act against pathogens in several ways like produce lytic enzymes, antibiotics etc (KüçüK and Kivanç, 2003). A number of commercial formulations, based on T. harzianum and T. virens, are available for the control of soil borne and foliar disease in a range of horticulture crops (Etabarian, 2006; Samuels, 1996). T. harzianum isolate, T39, is the active ingredient of Trichodex, which is reported to control botrytis grey mold on a range of crops (Elad, 1994). T. harzianum has been evaluated for the control of black seed rot disease of oil palm sprouted seeds in Nigeria (Eziashi et al., 2007), but there is little information on the efficacy of T. virens and other isolates of T. harzianum against Ceratosystis paradoxa, the pathogen that causes pineapple disease in sugarcane. Therefore, the focus of this investigation was to evaluate the potential of select Trichoderma isolates to biologically control C. paradoxa. The physical mode of antagonism and the effect of secondary metabolites produced by selective Trichoderma strains on the growth and development of C. paradoxa were also investigated.
Materials and Methods
Source of Trichoderma isolates
Five Trichoderma isolates, T. virens (Miller) IMI-392430, T. pseudokoningii IMI-392431, T. harzianum (Rifai) IMI-392432, T. harzianum (Rifai) IMI-392433, and T. harzianum (Rifai) IMI-392434, were collected from the Biotechnology and Microbiology Laboratory, Department of Botany, Rajshahi University, Bangladesh. These isolate were previously verified by CABI Bioscience, Surrey, U.K. (Rahman, 2009).
Isolation and identification of C. paradoxa
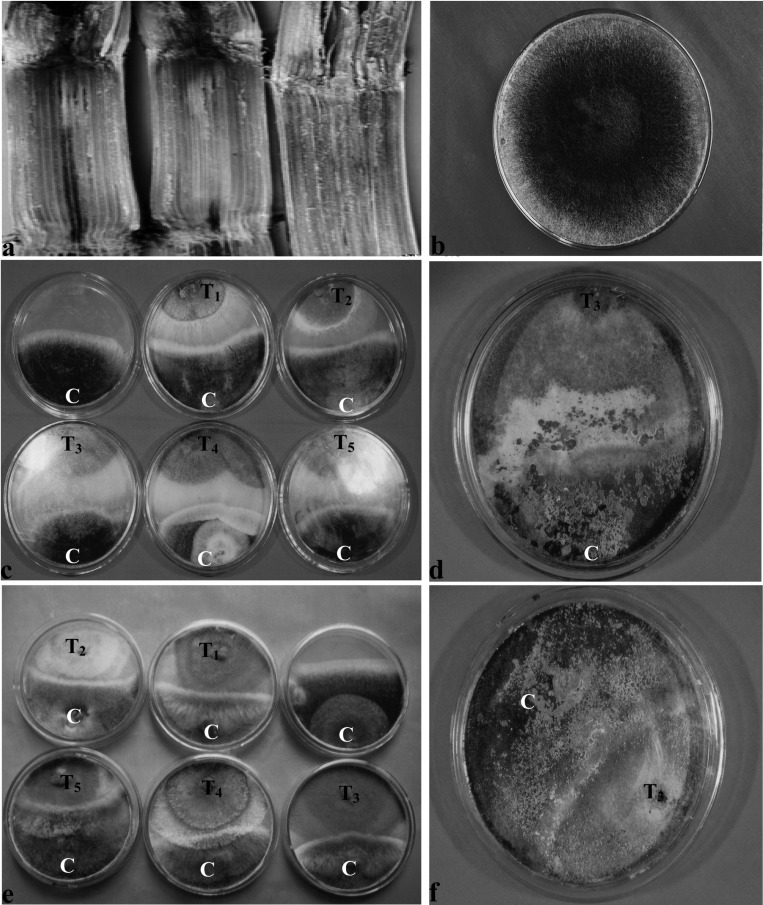
Pineapple disease infected setts with typical symptoms were collected (Fig. 1a) from experimental fields of the Bangladesh Sugarcane Research Institute, Ishurdi, Pabna, Bangladesh. The samples were washed with tap water to remove sand and soil particles, split open longitudinally with a sharp knife, and surface sterilized with a 1:1000 mercuric chloride solution for 1 minute. The specimens were then washed three times with sterile distilled water. The blackish spores of C. paradoxa were collected with a sterile needle, transferred to Potato Dextrose Agar (PDA) medium, incubated at room temperature (28 ± 1℃), and observed regularly to monitor fungi growth. The fungi that grew on PDA (Fig. 1) were isolated and purified by the hyphal tip culture method. The fungi were identified following the key outline by Edgerton (1959). Pure culture of C. paradoxa was preserved on a PDA slant at 4℃. The pathogenicity of the C. paradoxa isolate was confirmed on a local sugarcane cultivar. All the cultures were stored at 4℃ until further study.
Fig. 1.
a & b. Symptoms of pineapple disease of sugarcane and C. paradoxa colonies on PDA. c & e, Antagonistic effects of Trichoderma isolates against C. paradoxa in dual culture Method I and Method II, respectively; d & f, Shows overgrowth of Trichoderma covering the C. paradoxa colony after 7 and 6 days of inoculation in dual culture (Method I and Method II). C, T1, T2, T3, T4, and T5 indicate C. paradoxa, T. virens IMI-392430, T. pseudokoningii IMI-392431, T. harzianum, IMI-392432, T. harzianum, IMI-392433, and T. harzianum IMI-392434, respectively.
Screening by dual culture method
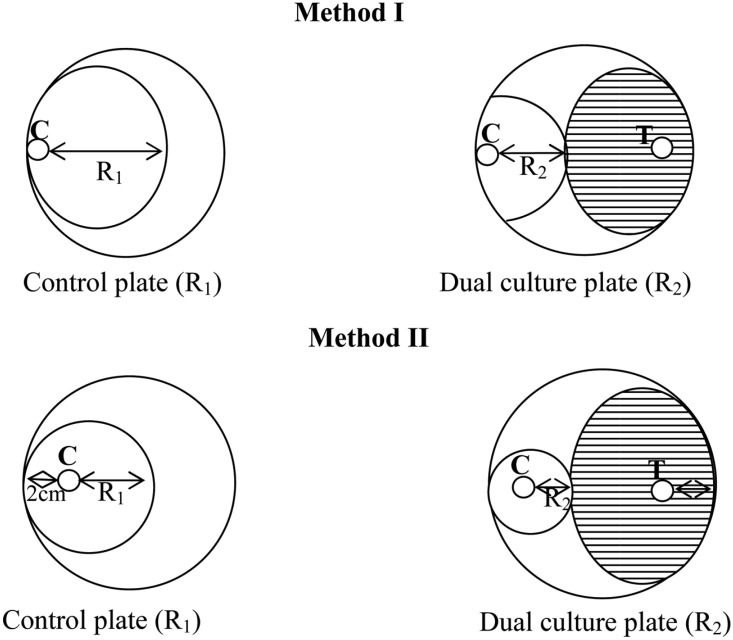
Two methods were followed for dual culture technique. In the first method (Method-I), an agar disc (6 mm) was taken from 4-day-old PDA culture plates of each Trichoderma isolate and placed at the periphery of the PDA plates (9 mm). Another agar disc of the same size of C. paradoxa was also placed at the periphery but on the opposing end of the same Petri dish (Fig. 2). In the second method (Method-II), an agar disc (6-mm) of the antagonist, Trichoderma (T), was placed 2 cm away from the periphery of the Petri dish, and a same sized agar disc of the test fungus, C. paradoxa (C), was similarly placed 2 cm away from the edge of the Petri plate but on the end opposite of Trichoderma sample. As a control, C. paradoxa was placed in a similar manner on a fresh PDA plate (Fig. 2). All pairings were carried out in quadruplicate and incubated at 28℃. Antagonistic activity was tested 4 days after incubation by measuring the radius of the C. paradoxa colony in the direction of the antagonist colony (R2) and the radius of the C. paradoxa colony in the control plate (R1). The two readings were transformed into percentage inhibition of radial growth (PIRG) using the formula developed by Skidmore and Dickinson (1976),
Fig. 2.
Measurement of radial growth of Ceratocystis paradoxa mycelia by Method I where culture plug placement was at the margin and Method II where placement was 2 cm away from the margin. Note: R1, Radius of C. paradoxa colony in control plate; R2, Radius of C. paradoxa colony in dual culture plate; C, Ceratocystis paradoxa isolate; T, Trichoderma isolate.
Observations were continued on the dual culture plates after 4 days of incubation and PIRG was calculated. The number of days required for the antagonist to overgrow the whole colony of C. paradoxa was recorded.
Screening by Poison Agar Technique Using Crude Metabolites
Preparation of culture filtrates of Trichoderma
Two hundred milliliters of Richard's solution (KNO3: 1.0 g, KH2PO4: 0.5 g, MgSO4·7H2O: 0.25 g, glucose: 34 g, and trace amounts of FeCl3 in 1 l distilled water, pH 6.5) was prepared and poured into 500 ml conical flasks and autoclaved for 15 minute at 121℃/1.05 kg/cm2 pressure. Six pieces of agar discs (6 mm) were kept in a flask (with media) for each strain of Trichoderma with four replicates. The flasks were incubated on a Gallenkamp orbital incubator at 100 rpm at 28℃ (Dennis and Webster, 1971). The culture filtrates were collected after 10, 20, and 30 days of incubation. These were then concentrated to about 50% using a vacuum evaporator at 38~40℃ and filtered by sterilized membrane filter.
Preparation of poison agar plate
Initially, 20, 40, 60, and 80% of PDA medium was prepared and kept in 250 ml conical flask for each medium and autoclaved for 15 minutes. The sterilized metabolites were then added to this PDA medium respectively. The molten PDA, with different concentrations of metabolites, was poured into Petri plates and allowed to solidify. As a control, Richard's solution was mixed with PDA in the same concentrations as used for Trichoderma metabolites.
Screening technique
For normal poison agar method, seven-day-old culture discs (6 mm) of C. paradoxa were inoculated at the centre of previously prepared poison agar plates and incubated at room temperature (28 ± 2℃) for 10 days. For the modified bilayer poison agar method, an agar disc (6 mm) of C. paradoxa was inoculated on the center of a normal PDA plate for 4 days. Afterwards a second layer of molten PDA, incorporated with ascending concentrations of sterilized metabolites of Trichoderma, was poured over the C. paradoxa colony. As a control, a second layer of molten PDA, incorporated with only sterilized Richard's solution instead of Trichoderma metabolites, was poured over the C. paradoxa colony. Observation was made on radial extension of the mycelia on culture plates for both the experimental treatment and control. Data were recorded on the mycelial extension of colony diameter after 4 to 10 days of inoculation. The readings were calculated for the percentage inhibition of radial growth (PIRG) based on the formula by Skidmore and Dickinson (1976), the same as for the dual culture experiment.
Direct Assay of Trichoderma Metabolites
Two methods were carried out to assess the inhibition of mycelial growth of C. paradoxa. In the first method, the PDB was prepared in quadruplicate at concentrations of 20, 40, 60, and 80%. Previously prepared sterilized Trichoderma metabolites were added proportionally into each conical flask. Then C. paradoxa mycelial discs were placed in each flask and incubated at room temperature (28℃) for 7 days. As a control, Richard's solution without the Trichoderma culture filtrates was added to the same concentrations of PDB media. In the second method, C. paradoxa mycelial discs were cultured in PDB as described in first method. On the 7th day of culture, Trichoderma filtrates at 20, 40, 60, and 80% were incorporated respectively into particular C. paradoxa cultures and incubated for another 7 days. As a control, Richard's solution without Trichoderma metabolites was added as earlier described. On the 7th day of mycelial growth, C. paradoxa mycelia was harvested from the flask, gently washed with distilled water, and oven dried at 60℃. The mean mycelial weight of the treatments was compared to the dry mean weight of C. paradoxa mycelia from the control flask. Data on mycelial weight for treatment flask at different concentrations and control flasks were recorded. The differences between the two readings multiplied by 100 were taken as the percentages of inhibition of mycelial growth weight (PIWG) following the modified method of Skidmore and Dickinson (1976).
PIWG = (A1-A2)/A1 × 100, where A1 = mycelial weight of C. paradoxa in control flasks and A2 = mycelial weight of C. paradoxa mycelia in treatment flasks.
Results
Screening by dual culture technique
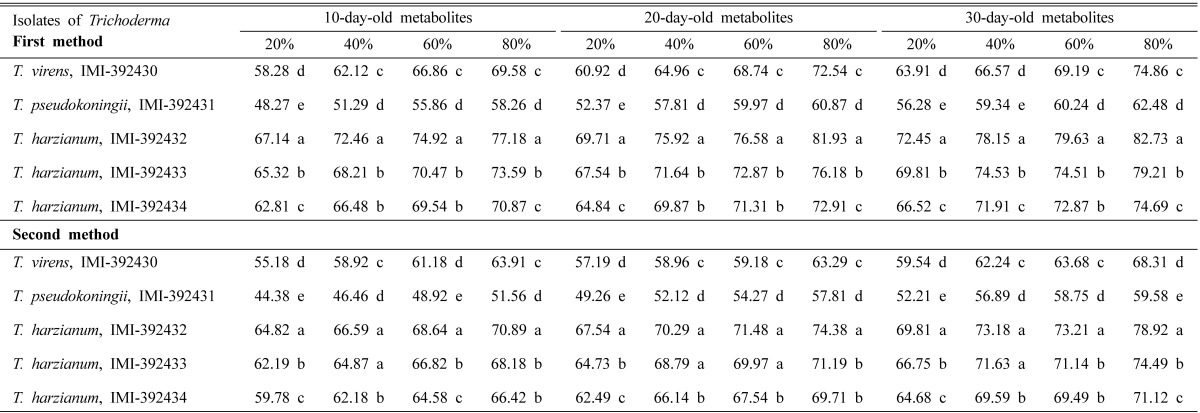
Each Trichoderma isolate inhibited the radial mycelial growth of C. paradoxa. The percentage inhibition of radial growth (PIRG) values ranged from 40.47 to 63.80% for first method and 58.12 to 80.82% for second method (Table 1 and Fig. 1c & e). The highest PIRG values (63.80% and 80.82%) were observed with T. harzianum IMI-392432 and the lowest recorded (40.47% and 58.12%) were observed with T. pseudokoningii IMI-392431 for first and second methods, respectively. With both methods, the highest PIRG values recorded were for T. harzianum IMI-392432, which was significantly different (P ≤ 0.05) from the others. Colony overgrowth times varied from 7 to 12 days for the first method and 6 to 10 days for the second method (Table 1 and Fig. 1d & f). For both methods, the minimum colony overgrowth time recorded was for T. harzianum IMI-392432 and the maximum colony overgrowth time recorded was for T. pseudokoningii IMI-392431 for both methods.
Table 1.
Mean PIRG values and colony overgrowth time of Trichoderma isolates against C. paradoxa by dual culture method
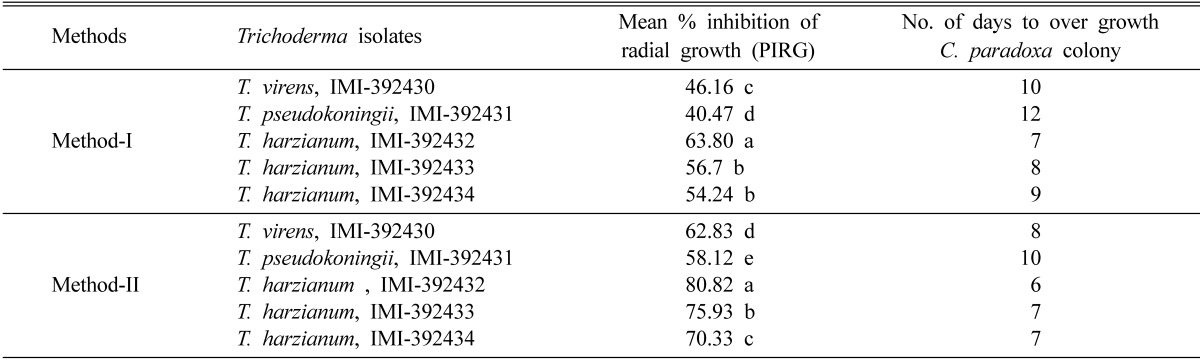
In a column the same letters are not significantly different by DMRT at 5% level.
Screening by poison agar technique
The PIRG values by metabolites of Trichoderma strains varied significantly (p ≤ 0.05) at different concentrations and different days. With the normal poison agar method, the highest PIRG values (82.63%) were achieved at 80% concentration on the 4th day by 30-day-old metabolites of T. harzianum IMI-392432 using the normal poison agar method (Table 2). However, with the modified bilayer poison agar method, the highest PIRG values (84.68%) were achieved at 80% concentration of 30-day-old metabolites of T. harzianum IMI-392432 (Table 3). The lowest PIRG values, 4.02 and 7.81%, were recored at 20% concentration on the 10th day of 10-day-old metabolites of T. pseudokoningii IMI-392431 using the normal poison agar and modified bilayer poison agar methods, respectively. The observed PIRG values of each strain were significant (P ≤ 0.05) among different concentrations and different days of metabolites in both methods.
Table 2.
Mean PIRG values by normal poison agar method using Trichoderma metabolites
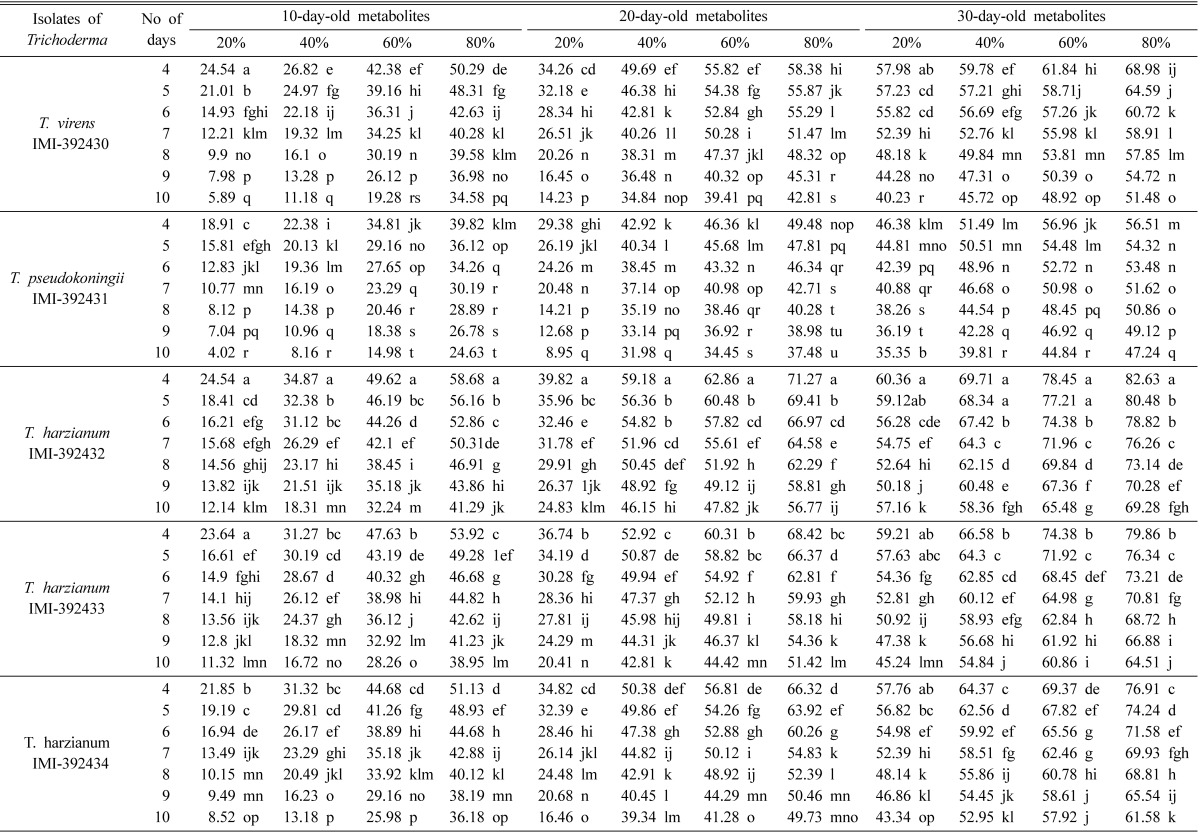
In a column the same letters are not significantly different by DMRT at 5% level.
Table 3.
Mean PIRG values by modified bilayer poison agar method using Trichoderma metabolites
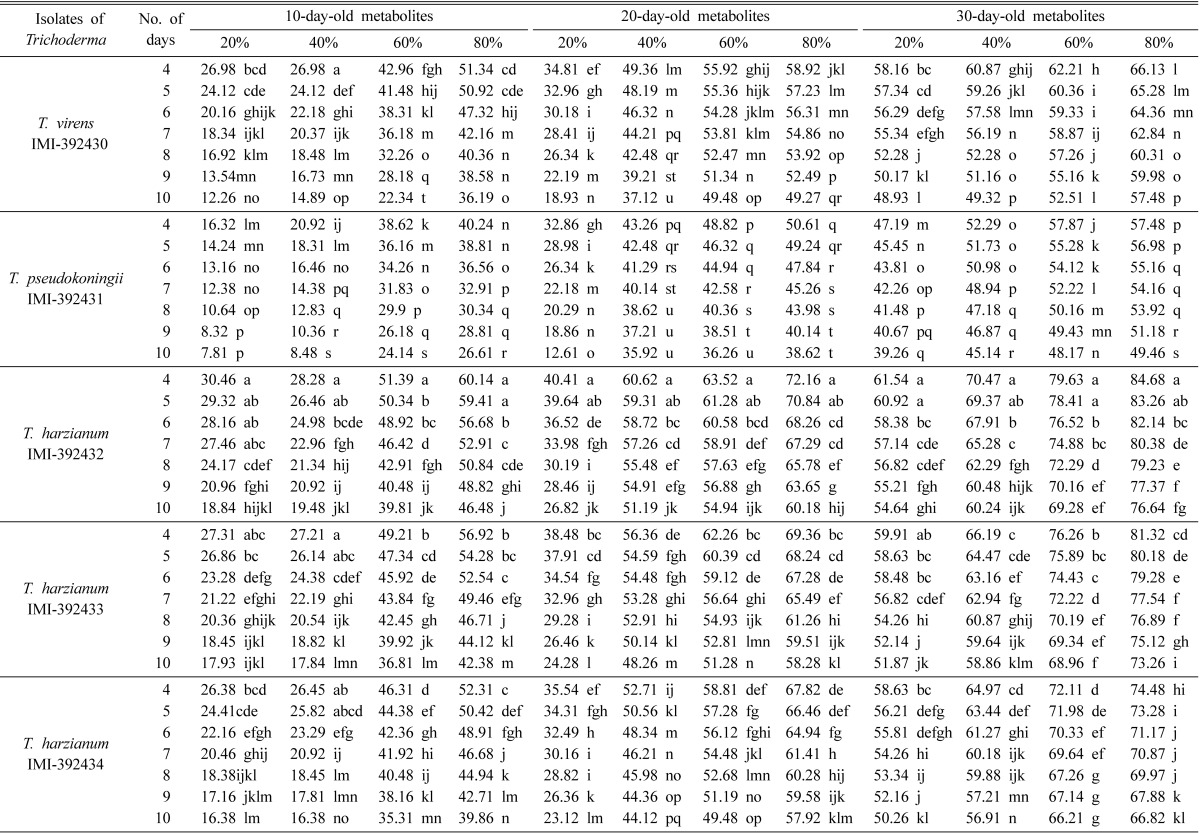
In a column the same letters are not significantly different by DMRT at 5% level.
Direct assay of Trichoderma metabolites on growth of C. paradoxa
Trichoderma metabolites significantly (P ≤ 0.05) inhibited mycelial growth of C. paradoxa at different concentrations and on different days. The highest percentage of inhibition of mycelial growth weight (PIGW) recorded were 82.73 and 78.92% with T. harzianum IMI-392432 at 80% metabolite concentration for the first and second method, respectively. The lowest PIGW values, 48.27 and 44.38%, were recorded at 20% metabolites on 10-day-old metabolites of T. pseudokoningii IMI-392431 by the first and second method, respectively (Table 4). It was also observed that PIGW values were statistically significant (P ≤ 0.05) for each isolate of Trichoderma at different concentrations for both methods.
Table 4.
Mean PIGW values in direct assay method using Trichoderma metabolites
In a column the same letters are not significantly different by DMRT at 5% level.
Discussion
Mycelial interaction is a basic method to assess antagonistic properties of microorganisms. These results revealed that all strains of Trichoderma antagonized C. paradoxa growth to various degrees and that different isolates within the same species also showed different degrees of inhibition. Jinantara (1995) reported that all nine isolates of T. harzianum possessed different abilities to attack Sclerotium rofsii, which was also in agreement with Henis et al. (1983) who found that different isolates of T. harzianum parasitized sclerotia of S. rolfsii with varying percentages of inhibition. Two comparative methods were used to test for variation in screening results in the placement of fungal inocula on discs. Results showed that although the percentage of inhibition values varied but the ranking of antagonisticity remained in the same order.
However, the first method is recommended for an accurate radius measurement of the test fungi within the dual culture plate because when test fungi are placed on the margin of plate it is easy to take measurements from margin towards the centre. Based on two criteria, the highest percentage inhibition of radial growth (PIRG values) and minimum colony overgrowth time, the T. harzianum IMI-392432 isolate was the best antagonist. Dharmaputra et al. (1994) tested two isolates of T. harzianum and one isolate of T. viride against Ganoderma and found that all isolates inhibited the mycelial growth of the pathogen, but T. harzianum (isolate B10-1) showed the best performance. Etabarian (2006) reported that T. viridie (MO) reduced the colony area of Macrophomina phaseoli by 19.2 and 34.9% using the dual culture and cellophane methods, respectively. Other than mycelial interaction and hyperparasitism by the Trichoderma species, scientists have also considered the action use of antibiotic metabolites as a contributing mechanism in the biocontrol of plant pathogens (Ghisalberti and Rowland, 1993). This study showed that secondary metabolites produced by Trichoderma strains were effective inhibitors of growth of C. paradoxa. The ability of Trichoderma species to produce inhibitory substances against microorganisms has been described by Dennis and Webster (1971) and Jinantara (1995). In this study, T. harzianum IMI-392432 displayed the best performance using the poison agar method at different concentrations of metabolites and on different days. To know whether the antibiotic action of secondary metabolites of Trichoderma were diffusible as well as antifungal, the modified bilayer agar experiment was carried out. The inhibition of radial growth of C. paradoxa was very pronounced compared to the growth of the uninoculated control bilayers. It is clear that the presence or absence of Trichoderma metabolites can have a significant role on the outcome of C. paradoxa mycelia. This experiment confirmed that the metabolites produced by T. harzianum are diffusible and can prevent, inhibit, or suppress the growth of C. paradoxa in culture. Therefore, Trichoderma has a large potential as a biocontrol agent against C. paradoxa. In previous studies, Schoeman et al. (1996) reported that metabolites of T. harzianum could influence the outcome of the decay caused by Basidiomycetes in freshly-felled pine. Eziashi et al. (2007) reported T. polysporum significantly reduced the growth of C. paradoxa followed by T. viridie, T. hamatum and T. aureoviride. The actual effect and mechanism involved is not known, but Trichoderma spp. are known to produce a range of metabolites that may affect the growth of microorganisms and plants (Ghisalberti and Rowland, 1993).
The antifungal properties of Trichoderma strains against C. paradoxa were confirmed where cultures filtrates of Trichoderma were used to control C. paradoxa in both experiments.The high PIGW value recorded at 80% metabolite concentration indicates that a high percentage of culture filtrate makes inhibition more effective. Eziashi et al. (2007) also reported that C. paradoxa was inhibited at high concentrations of 100% and 70% of metabolites by T. polysporum and T. viride, respectively. Based on the PIGW values, T. harzianum IMI-392432 showed the best inhibitory effect on C. paradoxa growth. Filtrates from Trichoderma species have been reported to exhibit antifungal activities (Calistru et al., 1997). Doi and Mori (1994) successfully found antifungal potential of culture filtrates of two Trichoderma species on wood decay fungi. Papavizas (1982) demonstrated that the culture filtrates of various T. harzianum strains suppressed growth of the white rot pathogen, Sclerotium cepivorum. Our results that culture filtrates of Trichoderma inhibited mycelial growth of C. paradoxa were very similar to above findings. This result also suggests that, secondary metabolites of T. harzianum IMI-392432 produced antifungal compounds and such compounds may play an active role in the inhibitory effects on C. paradoxa colony growth.
Due to variable antagonistic potential of individual Trichoderma isolates, it is important that they be screened first to select for the most active antagonist against a particular pathogen thus a particular Trichoderma species can be considered as a biocontrol agent.
In vitro results obtained using different techniques suggest that T. harzianum IMI-392432 was the best at inhibiting the mycelial growth of C. paradoxa. So, it might be use as a potential biocontrol agent in future.
Acknowledgements
The author would like to thank Dr. Ibrahim Talukder, Principal Scientific Officer, Bangladesh Sugarcane Research Institute, Ishurdi, Pabna, Bangladesh for providing the facilities to isolate and identify C. paradoxa.
References
- 1.Anonymous . BSRI Annual Report (1997) Pabna: Bangladesh Sugarcane Research Institute; 1999. p. 89. [Google Scholar]
- 2.Anonymous . Investigation of varietal resistance/susceptibility against pineapple disease. BSRI Annual Report (1998-99) Pabna: Bangladesh Sugarcane Research Institute; 2000. p. 86. [Google Scholar]
- 3.Baker R, Paulitz TC. Theoretical basis for microbial interactions leading to biological control of soilborne plant pathogens. In: Hall R, editor. Principles and Practice of Managing Soilborne Plant Pathogens. St. Paul: The American Phytopathological Society; 1996. pp. 50–79. [Google Scholar]
- 4.Burgess DR, Hepworth G. Biocontrol of sclerotinia stem rot (Sclerotinia minor) in sunflower by seed treatment with Gliocladium virens. Plant Pathol. 1996;45:583–592. [Google Scholar]
- 5.Burns JR, Benson DM. Biocontrol of damping-off of Catharanthus roseus caused by Pythium ultimum with Trichoderma virens and binucleate Rhizoctonia fungi. Plant Dis. 2000;84:644–648. doi: 10.1094/PDIS.2000.84.6.644. [DOI] [PubMed] [Google Scholar]
- 6.Calistru C, McLean M, Berjak P. In vitro studies on the potential for biological control of Aspergillus flavus and Fusarium moniliforme by Trichoderma species: a study of the production of extracellular metabolites by Trichoderma species. Mycopathologia. 1997;137:115–124. doi: 10.1023/A:1006802423729. [DOI] [PubMed] [Google Scholar]
- 7.Dennis C, Webster J. Antagonistic properties of species-groups of Trichoderma. I. Production of non-volatile antibiotics. Trans Brit Mycol Soc. 1971;57:25–39. [Google Scholar]
- 8.Dharmaputra OS, Purba RY, Sipayung A. In: Holderness M, editor. Research activities on the biology and control of Ganoderma at SEAMEO BIOTROP and IOPRI Marihat; Proceedings of First International Workshop on Perennial Crop Diseases Caused by Ganoderma; Selangor: Universiti Pertanian Malaysia; 1994. [Google Scholar]
- 9.Doi S, Mori M. Antifungal properties of metabolites produced by Trichoderma isolates from sawdust media of edible fungi against wood decay fungi. 1994;28:143–151. [Google Scholar]
- 10.Edgerton CW. Sugarcane and Its Diseases. Baton Rouge: Louisiana State University Press; 1959. p. 290. [Google Scholar]
- 11.Elad Y. Biological control of grape grey mold by Trichoderma harzianum. Crop Prot. 1994;13:35–38. [Google Scholar]
- 12.Etabarian HR. Evaluation of Trichoderma isolates for biological control of charcoal stem rot in melon caused by Macrophomina phaseolina. J Agric Sci Technol. 2006;8:243–250. [Google Scholar]
- 13.Eziashi EI, Omamor IB, Odigie EE. Antagonism of Trichoderma viride and effects of extracted water soluble compounds from Trichoderma species and benlate solution on Ceratocystis paradoxa. Afr J Biotechnol. 2007;6:388–392. [Google Scholar]
- 14.Ghisalberti EL, Rowland CY. Antifungal metabolites from Trichoderma harzianum. J Nat Prod. 1993;56:1799–1804. doi: 10.1021/np50100a020. [DOI] [PubMed] [Google Scholar]
- 15.Henis Y, Adams PB, Lewis JA, Papavizas GC. Penetration of sclerotia of Sclerotium rolfsii by Trichoderma spp. Phytopathology. 1983;73:1043–1046. [Google Scholar]
- 16.Hjeljord LG, Stensvand A, Tronsmo A. Antagonism of nutrient-activated conidia of Trichoderma harzianum (atroviridae) P1 against Botrytis cinerea. Phytopathology. 2001;91:1172–1180. doi: 10.1094/PHYTO.2001.91.12.1172. [DOI] [PubMed] [Google Scholar]
- 17.Jinantara J. Evaluation of Malaysian isolates of Trichoderma harzianum Rifai and Glicocladium virens Miller, Giddens and Foster for the biological control of Sclerotium foot rot of chilli. Selangor, Malaysia: Universiti Putra Malaysia; 1995. Ph.D. Thesis. [Google Scholar]
- 18.KüçüK C, Kivanç M. Isolation of Trichoderma spp. and determination of their antifungal, biochemical and physiological features. Turk J Biol. 2003;27:247–253. [Google Scholar]
- 19.Kuo TT, Chien MM, Li HW. Ethyl acetate produced by Ceratocystis paradoxa and C. adiposum and its role in inhibition of germination of sugarcane buds. Can J Bot. 1969;47:1459–1463. [Google Scholar]
- 20.Papavizas GC. Survival of Trichoderma harzianum in soil and in pea and bean Rhizospheres. Phytopathology. 1982;72:121–125. [Google Scholar]
- 21.Rahman MA. Screening of Trichoderma spp. and their efficacy as a bio conversion agent of municipal solid waste through appropriate technique of solid state fermentation. Rajshahi, Bangladesh: University of Rajshahi; 2009. Ph.D. Thesis. [Google Scholar]
- 22.Samuels GJ. Tricoderma: a review of biology and systematics of the genus. Mycol Res. 1996;100:923–935. [Google Scholar]
- 23.Schoeman MW, Webber JF, Dickinson DJ. The effect of diffusible metabolites of Trichoderma harzianum on in vitro interactions between basidiomycete isolates at two different temperature regimes. Mycol Res. 1996;100:1454–1458. [Google Scholar]
- 24.Skidmore AM, Dickinson CH. Colony interactions and hyphal interference between Septoria Nodorum and phylloplane fungi. Trans Brit Mycol Soc. 1976;66:57–64. [Google Scholar]
- 25.Went FAFC. Notes on sugarcane diseases. Ann Bot. 1896;10:583–600. [Google Scholar]