Abstract
A phytochemical study on the flowe r of Calotropis gigantea (Linn.) using silica gel column chromatography and preparative thin layer chromatography, led to the first time isolation of Di-(2-ethylhexyl) phthalate (compound 1) and anhydrosophoradiol-3-acetate (compound 2). The structures of these compounds were confirmed by spectroscopic analyses (IR, HRTOFMS and NMR). The antibacterial and antifungal activities of ethyl acetate extract, compound 1 and compound 2 were measured using the disc diffusion method. Ethyl acetate extract and compound 1 presented better results than compound 2. The minimum inhibitory concentrations (MICs) of the extract and compounds were found to be in the range of 16~128 µg/ml. The cytotoxicity (LC50) against brine shrimp nauplii (Artemia salina) were also evaluated and found to be 14.61 µg/ml for ethyl acetate, 9.19 µg/ml for compound 1 and 15.55 µg/ml for compound 2.
Keywords: Anhydrosophoradiol-3-acetate, Antimicrobial, Calotropis gigantea and Di-(2-ethylhexyl) phthalate
The emergence of human pathogenic microorganisms that are resistant to major classes of antibiotics has been increased in recent years, due to the indiscriminate use of antimicrobial drugs (Karaman et al., 2003). But this has caused many clinical problems in the treatment of infectious diseases and the antibiotics commonly used are sometimes associated with adverse effects on the host, which include hypersensitivity, allergic reaction and immunosuppression (Mukherjee et al., 2002). Plants are known to produce some chemicals, which are naturally toxic to bacteria and fungi (Basile et al., 1999). Therefore, research for development of new antimicrobial agents from plants is an urgent need.
Calotropis gigantea L. (Asclepiadaceae) is a laticiferous shrub widely distributed in Bangladesh, India, Burma, Pakistan and sub Himalayan tract (Kartikar and Basu, 1994). The roots and leaves of Calotropis gigantea are used traditionally for treatment of abdominal tumors, boils, syphilis, leprosy, skin diseases, piles, wounds, rheumatism, insect-bites, ulceration and elephantiasis (Ghani, 2003). Various parts of this plant have been reported to possess multiple therapeutic properties like anti-inflammatory, analgesic, anticonvulsant, anxiolytic, sedative, antidiarrhoeal and antipyretic (Adak and Gupta, 2006; Argal and Pathak, 2006; Chitme et al., 2004; Chitme et al., 2005). A literature review showed that Calotropis gigantea contained cardenolide glycosides (Mueen et al., 2005; Lhinhatrakool and Sutthivaiyakit, 2006; Kiuchi et al., 1998), pregnanes (Kitagawa et al., 1992; Shibuya et al., 1992), a nonprotein amino acid (Pari et al., 1998), terpenes (Gupta and Ali, 2000; Thakur et al., 1984; Anjaneyulu and Row, 1968), Flavonoids (Sen et al., 1992) and steroids (Habib et al., 2007; Basu and Nath, 1934). Powdered flowers of Calotropis gigantea, in small doses, are also useful in the treatment of colds, cough, asthma, catarrh, indigestion, inflammatory diseases and loss of appetite (Ghani, 2003). Stomachic, digestive and analgesic properties of Calotropis gigantea flowers have been reported in literature (Kartikar and Basu, 1994; Pathak and Argal, 2007). People in Indian-subcontinent including Bangladesh used Calotropis gigantea flowers as a traditional flock medicine in small pox, muscular pain, convulsions, scabies, and a number of ailments (Mueen et al., 2005; Ghani, 2003). The present study was undertaken to investigate the in vitro antimicrobial activity of isolated compounds from Calotropis gigantea flower against some pathogenic bacteria and fungi as well as cytotoxic activity against brine shrimp nauplii.
Materials and Methods
General methods
IR-spectra were taken on FTIR-8900 spectrophotometer (Shimadzu Kyoto, Japan) and High Resolution TOF Mass Spectra were obtained using a Waters LCT Premier mass spectrometer (UK) coupled with a Waters AQUITY HPLC system, with data acquisition achieved using MassLynx software, version 4.0. GCMS-QP2010S (Shimadzu Kyoto, Japan) spectrometer was used for taking GC-MS. NMR spectra were recorded on Bruker 400MHz FT spectrometer (DPX-400, Switzarland). All the spectra were taken in Analytical Research Division, Bangladesh Council of Scientific and Industrial Research (BCSIR) Laboratories, Dhaka-1205, Bangladesh.
Plant material
The flowers (flower's petal) of Calotropis gigantea were collected in April, 2008 from the relevant area (Meherchandi) of Rajshahi University campus and authenticated by Professor A. T. M. Naderuzzaman, Department of Botany, University of Rajshahi. A voucher specimen (No. 1A. Alam, Collection date 15.08.2004) was preserved in the Department of Botany, University of Rajshahi, Bangladesh.
Extraction and isolation
The shed-dried powdered flower (1.0 kg) of Calotropis gigantea was extracted with ethyl acetate (1.5 l) at room temperature. The solvent was completely removed by rotary vacuum evaporator from the crude extract to yield a residue of 38 g. Then crude ethyl acetate extract (10 g) was applied on silica gel (60~120 mesh) chromatography using n-hexane with a gradient of ethyl acetate up to 100% and followed by chloroform. Sixty four (64) fractions were collected. Among these fractions, fraction 21~30 afforded compound 2 as white crystals (75 mg). Fractions 40~48 were subjected to preparative TLC (n-hexane-methanol: 20 : 0.1) to afford the pure compound 1 (98 mg) as colorless oily liquid. The purity of the isolated compounds was checked on TLC plates.
Microorganisms
Four Gram positive (Staphylococcus aureus ATCC25923, Bacillus subtilis QL40, Bacillus megaterium QL38 and Sarcina lutea QL166), four Gram negative (Escherichia coli ATCC27853, Shigella sonnei C182, Shigella shiga C180 and Shigella dysenteriae ATCC26131) pathogenic bacterial strains and four fungal strains (Aspergillus niger ATCC235561, Aspergillus flavus ATCC10558, Aspergillus fumigatus ATCC10231, and Fusarium sp ATCC56390) were collected from the Institute of Biological Science (IBS), University of Rajshahi, Bangladesh.
Antibacterial and antifungal study
The ethyl acetate extract, compound 1 and compound 2 were tested separately for antibacterial and antifungal activity by disc diffusion assay method (Rois et al., 1988). Kanamycin disc (30 µg/disc) and Nystatin disc (100 µg/disc) were used as positive antibacterial and antifungal control, respectively. Blank disc impregnated with the respective solvent was used as negative control. The antibacterial activity of each sample was tested against each bacterium at concentrations of 30 µg/disc, 60 µg/disc and 90 µg/disc. For antifungal screening, each sample was tested at concentrations of 100 µg/disc, 200 µg/disc and 400 µg/disc. Antibacterial assay plates were incubated at 37 ± 1℃ for 24 h and antifungal assay plates were incubated at 37 ± 1℃ for 48 h. Each experiment was carried out in triplicates, and diameter of the zone of inhibition surrounding each disc was recorded. The minimum inhibitory concentration (MIC) for the samples having antimicrobial activity, were also determined by serial dilution technique (Reiner, 1982).
Brine shrimp lethality bioassay
The experiment was carried out using the method described by Meyer (Meyer et al., 1982). In brief, Artemia salina Leach (brine shrimp eggs) was allowed to hatch and mature as nauplii (Larvae) in seawater for 48 h at 25℃. Serially diluted test solutions (80 µl in DMSO from a stock solution of 5 mg/ml DMSO) were added to the seawater (5 ml), containing 10 nauplii. After incubation for 24 h at 25℃, the number of survivors was counted. The LC50 (50% lethal concentration, µg/ml) was determined from triplicate experiments. Ampicillin trihydrate was used as positive control.
Results and Discussion
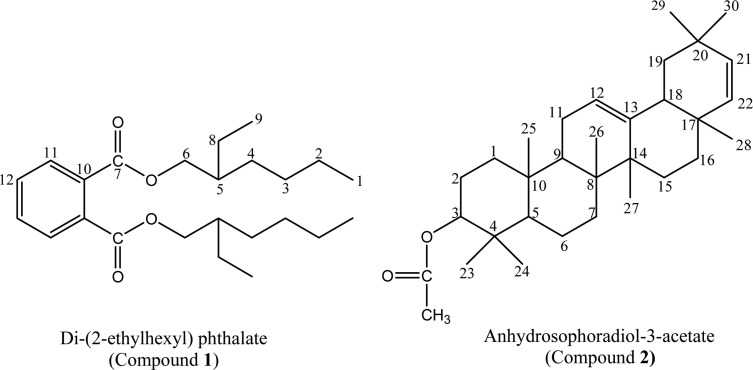
Isolated and purified compounds 1 and 2 were characterized by IR, Mass and NMR spectral data. Molecular formula for compound 1 was deduced as C24H38O4 through EI-MS which showed the molecular ion (M+) peak at m/z 390.3617 (calcd for C24H38O4). The presence of a phthalate was inferred from the EI-MS peaks at m/z 167 (48) and m/z 149 (100). The IR spectrum revealed a carbonyl band observed at 1739 cm-1 and strong C-O bands in the range 1047~1250 cm-1. The aromatic signals between 6.96 (dd) and 7.11 (dd) ppm on the 1H-NMR spectrum of compound 1 have reasonable coupling constants for protons at the ortho-substituted ring. Signal at 4.15 (m) ppm is assigned to a methylene group geminal to the ester alcohol group. The 13C-NMR spectrum of compound 1, confirming the symmetry of the molecule, exhibited the expected 12 carbon resonances (Table 1) assigned by DEPT experiment to two quaternary, three methine and five methylene carbons with two methyl groups. By comparison of 1H and 13C-NMR data to those published in literature (Rao et al., 2000; Amade et al., 1994), compound 1 was identified as Di-(2-ethylhexyl) phthalate (DEHP). DEHP (compound 1) is a well known synthetic plasticizer, already reported to be present in Alchornea cordifolia (Mavar-Manga et al., 2008), Aloe vera (Lee et al., 2000), Euphorbia cyparissias and Euphorbia seguieriana (Toth-Soma et al., 1993) and may have a taxonomic significance. The effective presence of compound 1 in flowers, not as a contaminant from solvents, was further confirmed by GC-MS analysis. The plant flowers were not conserved in plastic bags, so these could be discounted as a source of DEHP. The present study could not determine if DEHP is synthesized by the plant, absorbed by the roots or adsorbed from external atmosphere, but this compound, whatever its origin, appears likely to be present in preparations of Calotropis gigantea flower.
Table 1.
1H- and 13C-NMR spectral data of Compound 1 and 2
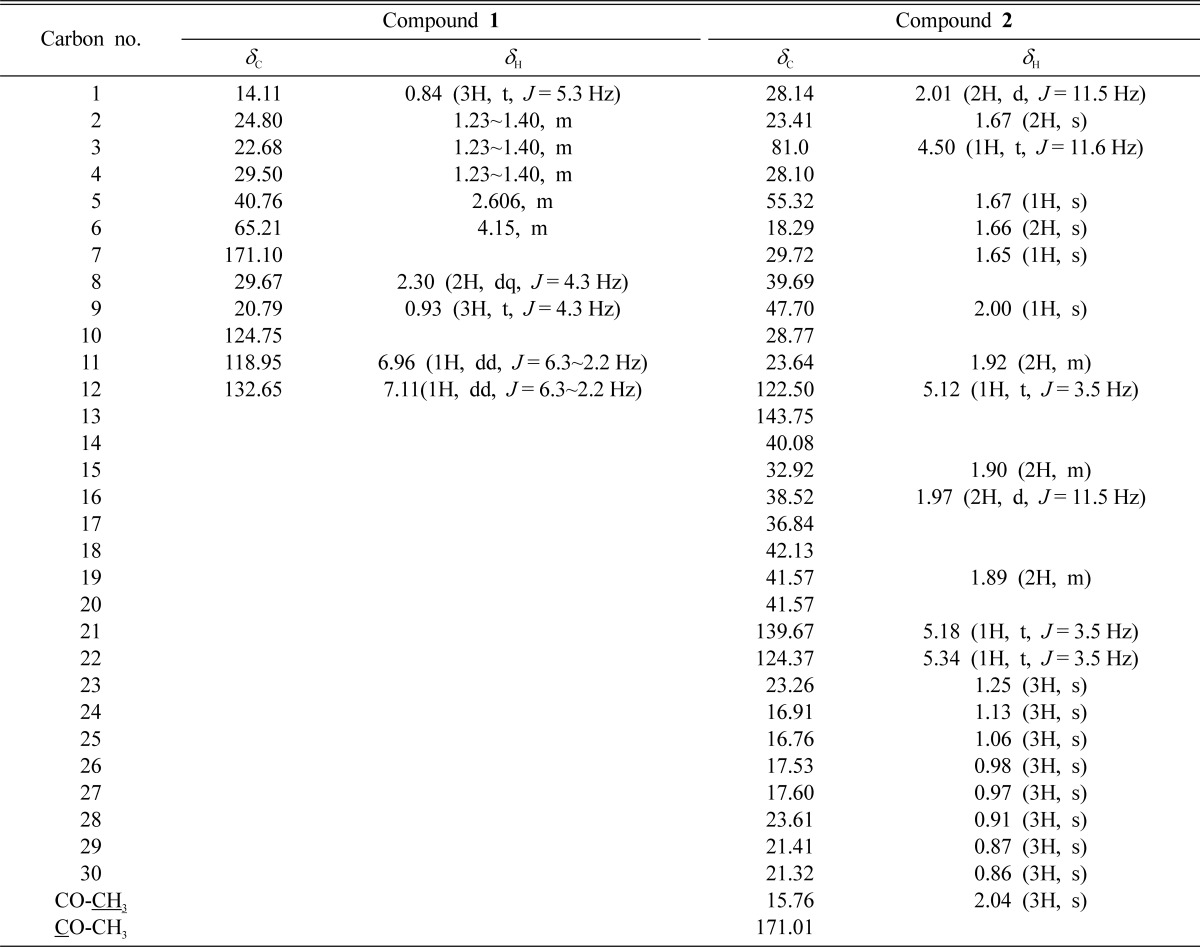
Proton resonance integral, multiplicity, and coupling constant (J = Hz) are in parentheses.
Similarly, compound 2 was obtained as white crystal. Its EI-MS showed a molecular ion (M+) peak at m/z 466.4023. Its 1H-NMR spectrum exhibited three olefinic protons at δ 5.34, 5.14 and 5.12 and an acetyl methyl protons at δ 2.04. The 13C-NMR showed an acetyl carbonyl carbon at δ 171.01, four olefinic carbons at δ 122.5 (C-12), 143.75 (C-13), 139.67 (C-21) and 124.37 (C-22) and acetyl methyl carbon at 15.76 (Table 1). Based on the foregoing observations and a comparison of the data with the literature (Kim et al., 2004), compound 2 was determined to be anhydrosophoradiol-3-acetate. Isolation of both DEHP and anhydrosophoradiol-3-acetate is reported for the first time from this plant.
In vitro antibacterial activity study, both ethyl acetate extract and compound 1 showed a better broad spectrum of antibacterial activity against both Gram positive (Staphylococcus aureus, Bacillus subtilis, and Sarcina lutea) and Gram negative (Escherchia coli, Shigella sonnei, Shigella shiga and Shigella dysenteriae) bacteria, with inhibition zones in the range of 07~20 mm (Table 2). Although ethyl acetate extract showed activity, compound 1 was inactive against Bacillus megaterium. Compound 2 showed moderate activity against Staphylococcus aureus, Sarcina lutea and Escherchia coli. It produced inhibition zone ranging from 08 to 15 mm (Table 2). Minimum inhibitory concentration (MIC) values were also evaluated against four Gram positive and four Gram negative bacteria. The lowest MIC values were observed for ethyl acetate extract (16 µg/ml) and compound 1 (32 µg/ml) against Bacillus subtilis and Sarcina lutea (Table 3). Compound 2 showed lowest MIC value (64 µg/ml) against Staphylococcus aureus (Table 3). In antifungal activity test, ethyl acetate extract produced zone of inhibition between 07 to 15 mm against Aspergillus flavus and Aspergillus fumigatus whereas compound 1 exhibited activity against Aspergillus flavus (Table 4). Compound 2 had no antifungal activity.
Table 2.
In vitro antibacterial activity of the extract and isolated compounds
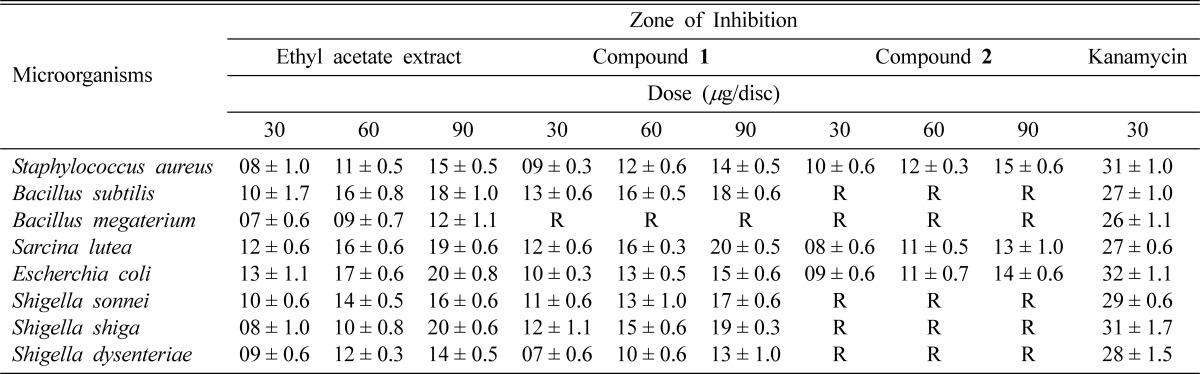
Data are expressed as mean ± S.E (Standard error); R = Resistance.
Table 3.
Minimum inhibitory concentrations (MICs) of the extract and isolated compounds
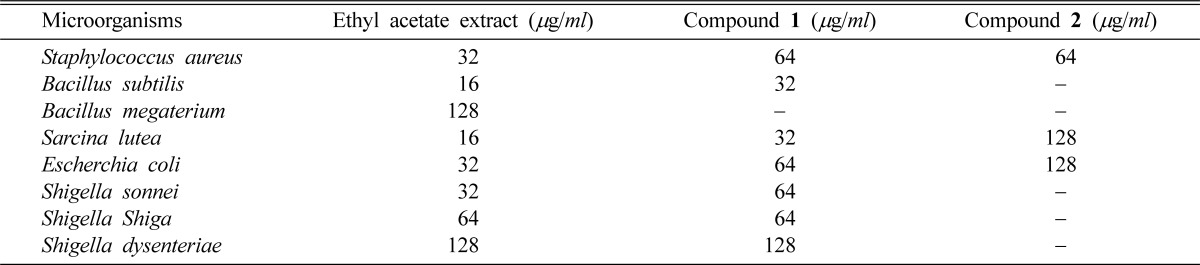
Table 4.
In vitro antifungal activity of the extract and isolated compounds
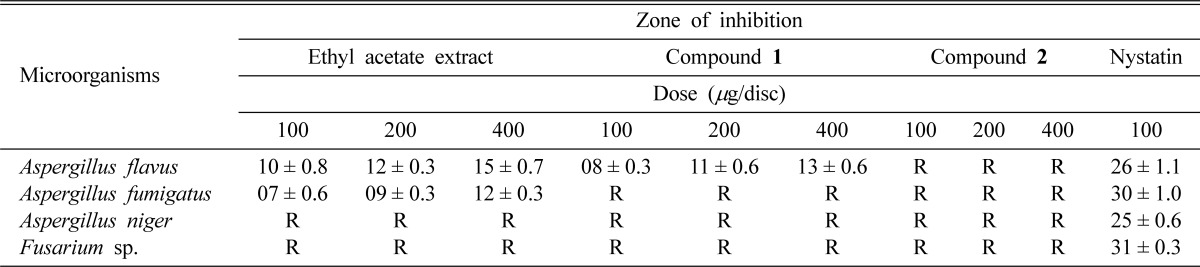
Data are expressed as mean ± S.E (Standard error); R = Resistance.
Ethyl acetate extract, compound 1 and compound 2 showed toxicity against brine shrimp nauplii (Artemia salina). Among the samples, compound 1 showed the highest toxicity and LC50 value was 9.19 (µg/ml). Ethyl acetate extract and compound 2 exhibited moderate activity in comparison with ampicillin trihydrate (Table 5).
Table 5.
Cytotoxicity of the extract and isolated compounds against brine shrimp nauplii
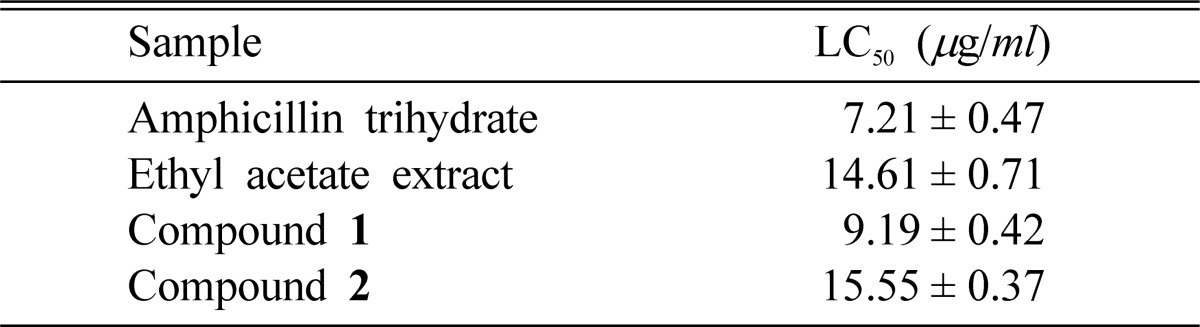
Data are expressed as mean ± S.E (Standard error)
In previous study, Sastry and Rao (Sastry and Rao, 1995) showed the activity of DEHP (compound 1) against Staphylococcus aureus, Proteus vulgaris, Salmonella typhi, Salmonella paratyphi, Salmonella typhimurium and Pseudomonas aurioginosa. The present study revealed the antishigellosis activity of DEHP because it had better activity against Shigella shiga, Shigella sonnei and Shigella dysenteriae. The DEHP is considered as pro inflammatory agent in other studies (Gourlay et al., 2003; Oie et al., 1997). Researchers also showed that anhydrosophoradiol-3-acetate (compound 2) exhibited potent cytotoxicity against A549, SK-OV-3, SK-MEL-2, MES-SA and HCT-15 tumour cell lines (Kim et al., 2004). So the overall findings of this study make important contribution in proper use of Calotropis gigantea flower for better health care system of common people in Bangladesh.
Fig. 1.
Structures of compounds 1 and 2.
References
- 1.Adak M, Gupta JK. Evaluation of anti-inflammatory activity of Calotropis gigantea (AKANDA) in various biological systems. Nepal Med Coll J. 2006;8:156–161. [PubMed] [Google Scholar]
- 2.Anjaneyulu V, Row LR. The triterpenes of Calotropis gigantea Linn. Curr Sci. 1968;6:156–157. [Google Scholar]
- 3.Amade P, Mallea M, Bouaicha N. Isolation, structure identification and biological activity of two metabolites produced by Penicillum olsonii Bainier and Sartory. J Antibiot. 1994;47:201–207. doi: 10.7164/antibiotics.47.201. [DOI] [PubMed] [Google Scholar]
- 4.Argal A, Pathak AK. CNS activity of Calotropis gigantea roots. J Ethnopharmacol. 2006;106:142–145. doi: 10.1016/j.jep.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 5.Basile A, Giordano S, Lopez-Saez JA, Cobianchi RC. Antibacterial activity of pure flavonoids isolated from mosses. Phytochemistry. 1999;52:1479–1482. doi: 10.1016/s0031-9422(99)00286-1. [DOI] [PubMed] [Google Scholar]
- 6.Basu KP, Nath MC. Calosterol, a sterol present in the milky juice of Calotropis gigantea. Biochem J. 1934;28:1561–1564. doi: 10.1042/bj0281561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chitme HR, Chandra R, Kaushik S. Evaluation of antipyretic activity of Calotropis gigantea (Asclepiadaceae) in experimental animals. Phytother Res. 2005;19:454–456. doi: 10.1002/ptr.1672. [DOI] [PubMed] [Google Scholar]
- 8.Chitme HR, Chandra R, Kaushik S. Studies on anti-diarrhoeal activity of Calotropis gigantea R.Br. in experimental animals. J Pharm Pharm Sci. 2004;7:70–75. [PubMed] [Google Scholar]
- 9.Ghani A. Medicinal Plants of Bangladesh. Dhaka: Asiatic Society of Bangladesh; 2003. p. 141. [Google Scholar]
- 10.Gourlay T, Samartzis I, Stefanou D, Taylor K. Inflammatory response of rat and human neutrophils exposed to di-(2-ethyl-hexyl)-phthalate-plasticized polyvinyl chloride. Artificial Organs. 2003;27:256–260. doi: 10.1046/j.1525-1594.2003.07107.x. [DOI] [PubMed] [Google Scholar]
- 11.Gupta J, Ali M. Rare chemical constituents from Calotropis gigantea roots. Indian J Pharm Sci. 2000;62:29–32. [Google Scholar]
- 12.Habib MR, Nikkon F, Rahman M, Haque ME, Karim MR. Isolation of stigmasterol and β-sitosterol from methanolic extract of root bark of Calotropis gigantea (linn) Pakistan J Biol Sci. 2007;10:4174–4176. doi: 10.3923/pjbs.2007.4174.4176. [DOI] [PubMed] [Google Scholar]
- 13.Karaman I, Sahin F, Gulluce M, Ogutcu H, Sngul M, Adiguzel A. Antimicrobial activity of aqueous and methanol extracts of Juniperus oxycedrus L. J Ethnopharmacol. 2003;85:231–235. doi: 10.1016/s0378-8741(03)00006-0. [DOI] [PubMed] [Google Scholar]
- 14.Kartikar KR, Basu N. Indian Medicinal Plants. Allahabad, India: Lolit Mohan Basu; 1994. p. 1607. [Google Scholar]
- 15.Kim M, Lee H, Hahm K, Moon Y, Woo E. Pentacyclic triterpenoids and their cytotoxicity from the stem bark of Styrax japonicas. Arch Pharm Res. 2004;27:283–286. doi: 10.1007/BF02980060. [DOI] [PubMed] [Google Scholar]
- 16.Kitagawa I, Zhang RS, Park JD, Baek NI, Takeda Y, Yoshikawa M, Shibuya H. Indonesian medicinal plants. I. Chemical structures of calotroposides A and B, two new oxypregnane-oligoglycosides A and B, from the root of Calotropis gigantea (Asclepiadaceae) Chem Pharm Bull (Tokyo) 1992;40:2007–2013. doi: 10.1248/cpb.40.2007. [DOI] [PubMed] [Google Scholar]
- 17.Kiuchi F, Fukao Y, Maruyama T, Obata T, Tanaka M, Sasaki T, Mikage M, Haque ME, Tsuda Y. Cytotoxic principles of a Bangladeshi crude drug, akond mul (roots of Calotropis gigantea L.) Chem Pharm Bull (Tokyo) 1998;46:528–530. doi: 10.1248/cpb.46.528. [DOI] [PubMed] [Google Scholar]
- 18.Lee KH, Kim JH, Lim DS, Kim CH. Anti-leukaemic and anti-mutagenic effects of Di-(2-ethylhexyl) phthalate isolated from Aloe vera Linn. J Pharm Pharmacol. 2000;52:593–598. doi: 10.1211/0022357001774246. [DOI] [PubMed] [Google Scholar]
- 19.Lhinhatrakool T, Sutthivaiyakit S. 19-Nor- and 18, 20-epoxy-cardenolides from the leaves of Calotropis gigantea. J Nat Prod. 2006;69:1249–1251. doi: 10.1021/np060249f. [DOI] [PubMed] [Google Scholar]
- 20.Mavar-Manga H, Haddad M, Pieters L, Baccelli C, Penge A, Quetin-Lectercq J. Anti-inflammatory compounds from leaves and root of Alchornea cordifolia (Schumach. & Thonn.) Müll. Arg J Ethnopharmacol. 2008;115:25–29. doi: 10.1016/j.jep.2007.08.043. [DOI] [PubMed] [Google Scholar]
- 21.Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, Mclaughlin JL. Brine shrimp: a convenient general bioassay for active plant constituents. Planta Med. 1982;45:31–33. [PubMed] [Google Scholar]
- 22.Mueen AKK, Rana AC, Kixit VK. Calotropis species (Asclepiadaceae)-A comprehensive review. Pharmacognosy Magazine. 2005;1:48–52. [Google Scholar]
- 23.Mukherjee PK, Saritha GS, Suresh B. Antimicrobial potential of two different Hypericum species available in India. Phytother Res. 2002;16:692–695. doi: 10.1002/ptr.1016. [DOI] [PubMed] [Google Scholar]
- 24.Oie L, Hersoug LG, Madsen JO. Residential exposure to plasticizers and its possible role in the pathogenesis of asthma. Environ Health Perspect. 1997;105:972–978. doi: 10.1289/ehp.97105972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pari K, Rao PJ, Devakumar C, Rastogi JN. A novel insect antifeedant nonprotein amino acid from Calotropis gigantea. J Nat Prod. 1998;61:102–104. doi: 10.1021/np970255z. [DOI] [PubMed] [Google Scholar]
- 26.Pathak AK, Argal A. Analgesic activity of Calotropis gigantea flower. Fitoterapia. 2007;78:40–42. doi: 10.1016/j.fitote.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 27.Rao GN, Kumar PM, Dhandapani VS, Krishna TR, Hayashi T. Constituents of Cassia auriculata. Fitoterapia. 2000;71:82–83. doi: 10.1016/s0367-326x(99)00108-2. [DOI] [PubMed] [Google Scholar]
- 28.Reiner R. Antibiotic - An Introduction. Switzerland: Roche Scientific Services; 1982. Detection of antibiotic activity; pp. 21–27. [Google Scholar]
- 29.Rois JJ, Reico MC, Villar A. Antimicrobial Screening of natural products. J Enthopharmocol. 1988;23:127–149. doi: 10.1016/0378-8741(88)90001-3. [DOI] [PubMed] [Google Scholar]
- 30.Sastry VMVS, Rao GRK. Dioctyl phthalate and antibacterial compound from the marine brown alga-Sargassum wightii. J Appl Phycol. 1995;7:185–186. [Google Scholar]
- 31.Sen S, Sahu NP, Mahato SB. Flavonol glycosides from Calotropis gigantea. Phytochemistry. 1992;31:2919–2921. doi: 10.1016/0031-9422(92)83668-o. [DOI] [PubMed] [Google Scholar]
- 32.Shibuya H, Zhang R, Park JD, Back NI, Takeda Y, Yoshikawa M, Kitagawa I. Chemical structures ofCalotroposides C, D, E, F and G, five additional new oxypregnane-oligoglycosides from the root of Calotropis gigantea (Asclepiadaceae) Chem Pharm Bull. 1992;40:2647–2653. doi: 10.1248/cpb.40.2007. [DOI] [PubMed] [Google Scholar]
- 33.Thakur S, Das P, Itoh T, Imai K, Matsumoto T. Latex extractables of Calotropis gigantea. Phytochemistry. 1984;23:2085–2087. [Google Scholar]
- 34.Toth-Soma LT, Gulyas S, Szegletes Z. Functional connection between and extracellular secretion in species of Euphorbia genus. Acta Biol Hung. 1993;44:433–443. [PubMed] [Google Scholar]