Abstract
Comparative effects of oyster mushrooms on plasma and fecal lipid profiles and on liver and kidney function were evaluated in hyper and normocholesterolemic rats. Feeding of hypercholesterolemic rats a 5% powder of oyster mushrooms (Pleurotus ostreatus, P. sajor-caju and P. florida) reduced the plasma total cholesterol level by 37%, 21% and 16%, respectively and reduced the triglyceride level by 45%, 24% and 14%, respectively. LDL/HDL ratio decreased by 64%, 45% and 41% for P. sajor-caju, P. ostreatus and P. florida fed rats, respectively. Mushroom feeding also reduced body weight in hypercholesterolemic rats. However, it had no adverse effect on plasma bilirubin, creatinin and urea nitrogen level. Mushroom feeding also increased the total lipid and cholesterol excretion in the feces. The present study reveals that feeding of 5% oyster mushroom powder does not have detrimental effects on the liver and kidneys rather may provide health benefits for the cardiovascular-related complication by decreasing the atherogenic lipid profiles.
Keywords: Hypercholesterolemic rats, Lipid profile, Liver and kidney function, Oyster mushrooms
Mushrooms are increasingly being recognized as an important food for their significant role in human health, nutrition and disease. Mushrooms provide a wide variety of physiologically active components: Pleurotus sajorcaju inhibits hypertensive effects through its active ingredients, which affect the renin-angiotensin system (Chang, 1996), Tricholoma magnivelare produces vasorelaxation because of its lectin content (Wang et al., 1996), P. ostreatus possesses antitumor activity (Yoshioka et al., 1985) and hypoglycaemic effects on experimentally induced diabetic rat (Chorvathova et al., 1993), Lentinus edodes and Grifola frondosa have antihypertensive effects in spontaneously hypertensive rats (Kabir et al., 1987) and Agaricus bisporus decreases low-density lipoprotein-cholesterol (LDL-C) in serum by increasing the expression of LDL receptor at mRNA level and LDL receptor activity (Fukushima et al., 2000).
Experimental evidence suggests that one of the most important food components that help to reduce serum cholesterol is its polyunsaturated fatty acid (PUFA) content (Hashimoto et al., 1999, 2001; Gamoh et al., 1999, 2001; Hossain et al., 1999). Arachidonic acid exacerbates platelet functions (Hossain et al., 1999a), whereas linolenic acid (LNA) acts as a precursor of the physiologically important PUFA, such as eicosapentaenoic acid (EPA; C20:5, ω-3) and docosahexaenoic acid (DHA; C22:6, ω3) (Schmidt et al., 2001). There is considerable data supporting the hypothesis that the health benefit obtained through the lowering of blood cholesterol may be derived from the effects of EPA and DHA (Hashimoto et al., 1998). In addition to their roles in the development and function of the central nervous system, these two fatty acids play an important role in the physiological functions of the cardiovascular system (Hashimoto et. al., 1999a). Thus, one of the objectives of the present study was to generate awareness of the beneficial effects of edible mushrooms, particularly of oyster mushrooms, on hypercholesterolaemia, which poses serious health problems in both developed and developing countries.
Materials and Methods
Animals
Forty young Long Evans rats (Rattus rattus) of 114 ± 12 g (mean ± SD) were used in the present study. Rats were housed in an animal room at 23 ± 2℃, under 12 h dark-light cycles and then divided randomly into five groups. Rats were fed a basal diet supplemented with, no cholesterol or mushroom (normocholesterolemic control rats; NC), 1% cholesterol (hypercholesterolemic rats; HC), 1% cholesterol and 5% powder of Pleurotus ostreatus (HC + PO group rats), 1% cholesterol and 5% powder of Pleurotus sajor-caju (HC + PS group rats), 1% cholesterol and 5% powder of Pleurotus florida (HC + PF group rats).
Diet composition and feeding
The composition of the basal diet was as follows (g/100 g). Wheat flower 50, rice powder 11, wheat bran 19, casein (non fat) 8, egg white 10, soybean oil 1, table salt 0.5, vitamin mixture 0.25 and mineral mixture 0.25. The composition of the vitamin mixture in the diet was as follows (gram/100g vitamin mixture): retinyl acetate 9.5 × 10-4, cholecalciferol 1.2 × 10-3, α-tochoferol acetate 0.05, thiamin hydrochloride 2.4, nicotinic acid 12, riboflavin 2.4, D-calcium pantothenate 9.6, pyridoxine hydrochloride 1.2, folic acid 9.5 × 10-2, vitamin K 0.25, cyanocobalamine 9.5 × 10-3, inositol 47.95 and ascorbic acid 24.0. The composition of the mineral mixture added to diet was as follows (g/100 g of mineral): calcium gluconate 28.5, K2HPO4 17.3, CaCO3 26, MgSO4 12.6, KCl 12.6, CuSO4 0.06, FeSO4 0.3, MnSO4 0.55, NaF 2.5 × 10-4, KI 9 × 10-4, sodium molybdate 3 × 10-4, SeO2 3 × 10-4 and CrSO2 1.5 × 10-3. Rats were feed for 40 days.
Collection of oyster mushrooms
Mature fruiting bodies of Pleurotus ostreatus, P. sajor-caju, and P. florida were collected from the National Mushroom Development and Extension Centre, Savar, Dhaka, Bangladesh. The fruiting bodies were dried in sunlight and crushed into powder. The powder was mixed with the basal diet.
Plasma TG estimation
Plasma triglyceride (TG) was measured enzymatically using the glycerophosphate oxidase assay (Burtis and Ashwood, 2006). In this method, lipase catalyzed the hydrolysis of triglycerides to yield glycerol and free fatty acids. Glycerol concentration was then determined with the Trinder reaction using glycerol kinase, glycerol-3 phosphate oxidase and peroxidase. The end product was the quinoneimine dye (red). Its absorbance was measured spectrophotometrically at 546 nm which was directly proportional to triglyceride concentration.
Plasma TC estimation
Plasma total cholesterol (TC) was measured enzymatically using the cholesterol oxidase assay (Burtis and Ashwood, 2006). In this method, cholesterol esterase (ChE) first catalyzed the hydrolysis of cholesterol which was oxidized by cholesterol oxidase (ChO) to yield hydrogen peroxide. In a coupled reaction catalyzed by peroxidase (POD), quinoneimine dye (red) was formed from hydrogen peroxide, 4-aminoantipyrine and phenol. The absorbance of the dye was measured spectrophotometrically at 546 nm which was directly proportional to cholesterol concentration.
Estimation of plasma lipoproteins
High-density lipoprotein cholesterol (HDL-C) was measured by the same procedure of cholesterol estimation after precipiting low-density lipoprotein cholesterol (LDL-C) and very low density lipoprotein cholesterol (VLDL-C) using magnesium sulfate and phosphotungstic acid.
Low-density lipoprotein cholesterol was calculated as follows (Burtis and Ashwood, 2006):
LDL-C = [TC - (HDL-C + TG/5)]
And very low density lipoprotein cholesterol was calculated as:
VLDL-C = [TC - (HDL-C + LDL-C)]
Estimation of plasma total bilirubin
Plasma total bilirubin was measured with a colourimetric test- Jendrassic- Grof method. Bilirubin first reacted with diazotized sulphuric acid (DSA) and in the presence of an accelerator (caffeine) forms a red azo dye. The absorbance was measured spectrophotometrically at 546 nm which was directly proportional to bilirubin concentration.
Estimation of plasma creatinine
Plasma creatinine was measured using the Jaffe reaction (Burtis and Ashwood, 2006). In the Jaffe reaction, creatinine reacts with alkaline picrate to produce a reddish-orange colored complex. The absorbance was measured spectrophotometrically at 520 nm which was directly proportional to the creatinine concentration.
Estimation of plasma urea
Plasma urea was measured using the Barthelot method (Burtis and Ashwood, 2006). In this method, after urea was hydrolyzed with urease, the ammonium ion formed was reacted with phenol and hypochlorite in alkaline medium to form indophenol. Nitropruside was used to catalyze the reaction. The absorbance of the dissociated indophenol, a blue chromogen, was measured spectrophotometrically at 560 nm which was directly proportional to the concentration of ammonia formed from urea.
Estimation of plasma uric acid
Plasma uric acid was measured using uricase method (Burtis and Ashwood, 2006). Uricase catalyzed the oxidation of uric acid to allantoin and H2O2. In the presence of peroxidase, H2O2 reacted with 4-aminoantipyrine and 3,5-dichloro-2-hydroxybenzenesulphonate to form a quinoneimine dye. The absorbance was measured spectrophotometrically at 520 nm, which was directly proportional to the uric acid concentration.
Analysis of plasma enzyme profile
The activity of the plasma transaminases, glutamate pyruvate transaminase (GPT) and glutamate oxaloacetate transaminase (GOT) were determined using the kinetic method (Burtis and Ashwood, 2006). The oxoacids formed in the transaminase reaction were measured indirectly by enzymatic reduction to the corresponding hydroxyacids. The accompanying change in NADH concentration was monitored spectrophotometrically at 340 nm. Plasma alkaline phosphatase (ALP) activity was determined using 4-nitrophenyl phosphate. ALP catalyzed the hydrolysis of 4-nitrophenyl phosphate, forming phosphate and free 4-nitrophenol, which in dilute acid solutions was colorless but under alkaline condition 4-nitrophenol was converted to the 4-nitrophenoxide ion, which is an intense yellow color. The absorbance of this color compound was measured spectrophotometrically at 420 nm for the determination of plasma alkaline phosphatase activity.
Analysis of fecal cholesterol and total lipid
Feces of rats were collected every day and dried. Total lipid was extracted by using chloroform: methanol (Folch et al., 1956). One gram of dried (powdered) feces was mixed with 10 ml of chloroform: methanol (2 : 1) solution and allowed to stand for 3 days with regular mixing. Then, the solution was filtered and the methanol was aspirated and the chloroform evaporated. The extracted lipid was then weighed. 2 ml of H2O was then added and made suspension by using bath sonicator. This suspension was then used to estimate cholesterol content in feces. Cholesterol was estimated by enzymatic method using the cholesterol oxidase assay.
Statistical analysis
Results were expressed as mean ± SEM. All parameters for inter group differences were analyzed by one-way ANOVA followed by post hoc operations. The statistical program used was SPSS 11.5. (P < 0.05 was considered statistically significant).
Results and Discussion
Mushroom feeding reduced body weight in hypercholesterolemic rats. Feeding of P. sajor-caju, P. ostreatus and P. florida reduced body weight significantly in hypercholesterolemic rats by 17.36%, 23.37% and 24.13%, respectively (Table 1). This finding is of special significance because obesity is associated with numerous diseases including diabetes, atherosclerosis, coronary heart disease and others (Simopoulus and Pavlou, 1997).
Table 1.
Effects of P. sajor-caju, P. ostreatus and P. florida feeding on the body weight of hypercholesterolemic rats
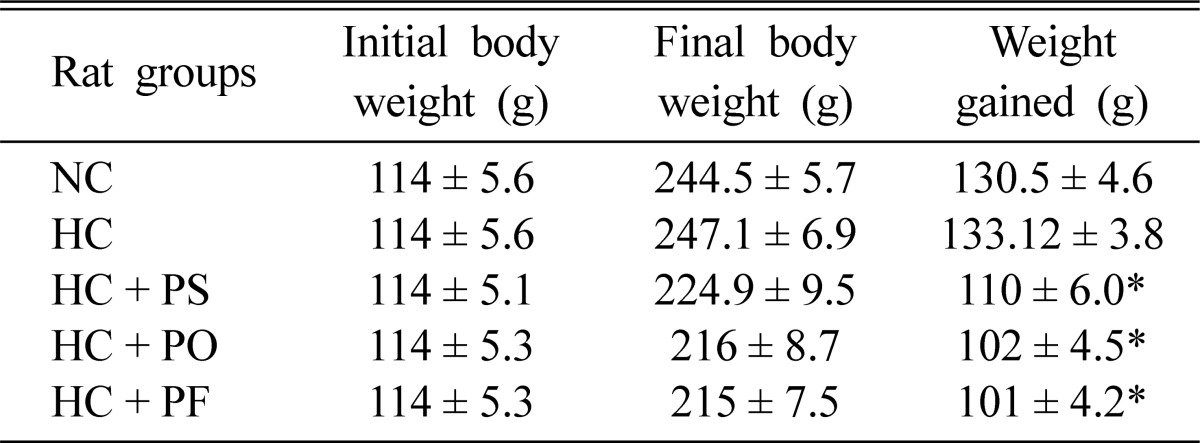
The results are the mean ± SEM. Data was analyzed by one way ANOVA and then post hoc LSD test. *Indicates significant difference at P0.05 level. NC, normo-cholesterolemic rats; HC, hypercholesterolemic rats; HC + PS, P. sajor-caju fed hypercholesterolemic rats; HC + PO, P. ostreatus fed hypercholesterolemic rats; HC + PF, P. florida fed hypercholesterolemic rats.
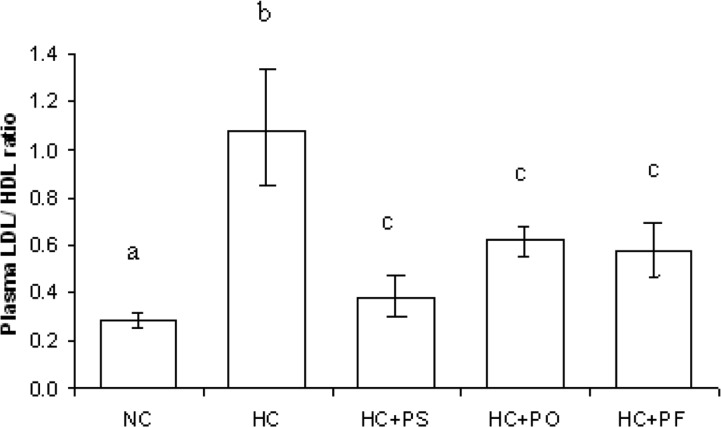
Plasma TC, TG, HDL-C, LDL-C, VLDL-C in NC, HC, HC + PS, HC + PO, HC + PF rats after mushroom feeding for 40 days are presented in Table 2 and bilirubin, creatinin and blood urea nitrogen (BUN) levels are presented in Table 3. Table 4 shows the plasma enzyme profiles. In HC rats, plasma TC increased by 21% compared to levels in NC rats. Plasma TC concentrations decreased by 21% in HC + PS rats, by 37% in HC + PO rats and by 16% in HC + PF rats compared to HC rats. In HC rats, plasma TG increased by 55% compared to levels in NC rats. Plasma TG concentrations decreased by 24% in HC + PS rats, by 45% in HC + PO and by 14% in HC+PF rats compared to HC rats. In HC rats, plasma HDL-C levels decreased by 35% and plasma LDL-C level increased by 144% compared to levels in NC rats. Plasma HDL-C level increased slightly, but not significantly in HC + PS and HC + PF rats compared to HC rats. But HC + PS, HC + PO and HC + PF rats showed significant decreases in plasma LDL-C levels by 59%, 47% and 41%, respectively compared to HC rats. The ratio of plasma LDL-C to HDL-C is shown in Fig. 1. In HC rats, this ratio increased by 266%, compared to NC rats. But mushroom feeding reduced the ratio significantly in HC + PS, HC + PO and HC + PF rats by 64%, 45% and 41% respectively compared to HC rats. There was no significant difference in plasma bilirubin, creatinin and BUN levels in the hypercholesterolemic and mushroom-fed hypercholesterolemic rats. Also, the enzyme profiles of different rat groups are not significantly different, although all three types of mushroom feeding have slightly reduced the GOT, GPT and ALP activity in plasma.
Table 2.
Effects of P. sajor-caju, P. ostreatus and P. florida mushrooms on plasma lipid profiles of hypercholesterolemic rats
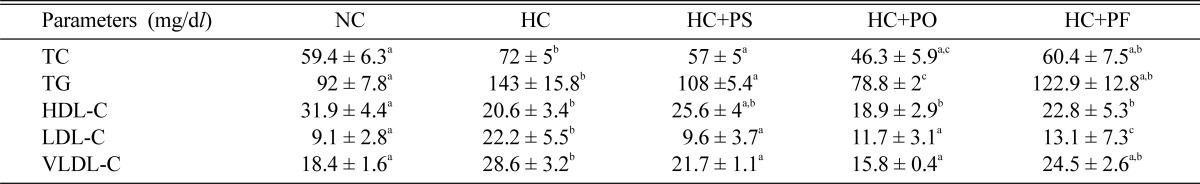
The results are the mean±SEM. Values in the same row that do not share a common superscript are significantly different at P < 0.05 (one way ANOVA then LSD post hoc comparison). TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; VLDL-C, very low-density lipoprotein cholesterol.
Table 3.
Effects of P. sajor-caju, P. ostreatus and P. florida mushrooms on liver and kidney function parameters of hypercholesterolemic rats

The results are the mean ± SEM. Values in the same row that do not share a common superscript are significantly different at P < 0.05 (one way ANOVA then LSD post hoc comparison); BUN, blood urea nitrogen.
Table 4.
Effects of P. sajor-caju, P. ostreatus and P. florida mushrooms on plasma enzyme profile related to liver and kidney function of hypercholesterolemic rats

The results are the mean ± SEM. Values in the same row that do not share a common superscript are significantly different at P < 0.05 (one way ANOVA then LSD post hoc comparison). GOT, glutamate oxaloacetate transaminase; GPT, glutamate pyruvate transaminase; ALP, alkaline phosphatase.
Fig. 1.
Effects of P. sajor-caju, P. ostreatus and P. florida on plasma LDL-C/HDL-C ratio of hypercholesterolemic rats. Results are mean ± SEM. Bars with different symbol (a, b and c) indicate significant differences at P < 0.05.
The present study provides evidence that feeding 5% oyster mushrooms to rats significantly ameliorates the plasma atherogenic lipid profiles in experimentally induced hypercholesterolemic rats. Rats are particularly resistant to the development of hypercholesterolaemia and atherosclerosis (Wissier et al., 1954; Fillias et al., 1956) and have a strong capability to maintain their plasma cholesterol (Fujioka et al., 1995; Spady and Cuthbert, 1992; Roach et al., 1993). Therefore, in order to induce hypercholesterolaemia or atherosclerosis in rats, cholesterol feeding is associated with other additives, including bile acids and propylthiouracil (an anti-thyroid drug), which increase the intestinal absorption of cholesterol (Dolphin and Forsyth, 1983; Pathe and Chevallier, 1976). However, in the present study, the addition of 1% cholesterol to the basal diet without bile acids and/or anti-thyroid drugs produced hypercholesterolaemia in the rats, because cholesterol feeding itself increases bile acid secretion by approximately three to four folds in rats (Uchida et al., 1996). The 21% increase in plasma cholesterol in the hypercholesterolemic rats in the present study was comparable to that reported by Bobek et al. (1995), who fed rats cholesterol (0.3%) diet with added bile acids (0.5%) and showed a 1.7 fold higher cholesterolaemia in their cholesterol fed rats compared with normal rats.
In this experiment, 5% mushroom feeding on rats significantly repressed the increment of plasma cholesterol. The mechanism by which mushrooms reduce plasma TC levels in hypercholesterolemic rats is not clearly understood. Mushrooms contain the hypocholesterolaemic agent mevnolin (monacolin K, lovastatin) (Gunde-Cimermann et al., 1993), which may be involved in decreasing the activity of the 3-hydroxy-3-methylglutaryl coenzyme A (HMGCoA) reductase enzyme (Bobek et al., 1995), the rate-limiting enzyme for cholesterol biosynthesis. Thus, mushroom feeding may involve the suppression of endogenous cholesterol biosynthesis by inhibiting the activity of HMG-CoA reductase activity. In addition, mushroom contains water soluble gel forming substances including β-1, 3-D-glucan and pectin, which bind to bile acids, thereby inhibiting cholesterol-bile micelle formation and cholesterol resorption
The low level of LDL-C in control rats suggests that the principal cholesterol carrying lipoprotein in normocholesterolemic rodents, including rats are not the LDL particles; rather they are HDL and VLDL. However, when rats become hypercholesterolemic, the LDL-C level increased 2~3 fold over normocholesterolemic rats, again demonstrating that the principal cholesterol carrying lipoprotein in hypercholesterolemic rats was LDL and the carriers were probably HDL and VLDL. Our results of reduced LDL-C after mushroom feeding are consistent with a similar report suggesting decrease of LDL from rat blood (Chorvathova et al., 1993). Usually, a high level of LDL-C and a low level of HDL-C indicate an imbalance between cholesterol transport from the liver to extrahepatic tissues and back to the liver. Mushroom feeding significantly decreased LDL/HDL ratio in hypercholesterolemic rats. Thus, mushrooms may provide an important health benefit by increasing plasma HDL-C and decreasing plasma LDL-C. The process of excreting cholesterol from the body begins with the hydrolysis of LDL-C and HDL-C ester into free cholesterol in the liver. The free cholesterol is then either, secreted immediately or converted into bile acids in the bile ducts and then secreted. The fruiting bodies of mushrooms increase fecal cholesterol (Table 5). Thus, the decreased plasma cholesterol may also be attributed to such a mechanism. The higher level of plasma HDL-C indicates that more cholesterol from peripheral tissues was returning to the liver for catabolism and subsequent excretion. Plasma VLDL-C and TG content in mushroom fed hypercholesterolemic rats were lower compared to the hypercholesterolemic control rats. VLDL-C is the major transport vehicle for the TG from the liver to extrahepatic tissues, whereas LDL-C is not secreted as such the liver; rather, it seems to be formed from VLDL-C after partial removal of TG by lipoprotein lipase (Mayes, 1997). After feeding cholesterol to rats, LDL-C became the prime carrier for cholesterol, then consequently leading to a decreased cholesterol content of VLDL-C and HDL-C in mushroom fed hypercholesterolemic rats.
Table 5.
Effects of P. sajor-caju, P. ostreatus and P. florida on fecal total lipid and cholesterol

The results are the mean ± SEM. Values in the same row that do not share a common superscript are significantly different at P < 0.05 (one way ANOVA then LSD post hoc comparison).
The present results suggest that oyster mushroom ingestion has significant health benefits through the modulation of physiological functions that include various atherogenic lipid profiles in hypercholesterolaemia. Therefore, oyster mushroom may be a good source of nutrition that may also act as a prophylactic against hypercholesterolaemia, hyperlipidaemia and related complications, which are the risk factors of atherosclerosis.
References
- 1.Bobek P, Hromadova M, Ozdin L. Oyster mushroom (Pleurotus ostreatus) reduces the activity of 3-hydroxy-3-methyl-glutaryl coA reductase in rat liver microsomes. Experientia. 1995;51:589–591. doi: 10.1007/BF02128749. [DOI] [PubMed] [Google Scholar]
- 2.Burtis CA, Ashwood ER. Teitz Fundamentals of Clinical Chemistry. New Delhi, India: Reed Elsevier India Private Limited; 2006. pp. 348–488. [Google Scholar]
- 3.Chang R. Functional properties of edible mushroom. Nutr Rev. 1996;54:91–93. doi: 10.1111/j.1753-4887.1996.tb03825.x. [DOI] [PubMed] [Google Scholar]
- 4.Chorvathoba V, Bobek P, Ginter E, Klvanova J. Effect of the oyster fungus on glycemia and cholesterolemia in rats with insulin depended diabetes. Physol Res. 1993;42:175–179. [PubMed] [Google Scholar]
- 5.Dolphin PJ, Forsyth SJ. Nascent hepatic lipoproteins in hypothyroid rats. J Lipid Res. 1983;24:541–551. [PubMed] [Google Scholar]
- 6.Fillias LC, Andrus SB, Mann GV, Stare FJ. Experimental production of gross atherosclerosis in the rat. J Exp Med. 1956;104:539–554. doi: 10.1084/jem.104.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Folch J, Lees M, Sloane-Stanely GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1956;226:497–509. [PubMed] [Google Scholar]
- 8.Fujioka T, Nara F, Tsujita Y, Fukushige J, Fukami M, Kuroda M. The mechanism of lack of hypocholesterolemic effects of pravastatin sodium, a 3-hydroxy-3-methyl reductase inhibitor in rats. Biochim Biophys Acta. 1995;1254:7–12. doi: 10.1016/0005-2760(94)00154-q. [DOI] [PubMed] [Google Scholar]
- 9.Fukushima M, Nakano Y, Morii Y, Ohashi T, Fujiwara Y, Sonoyama K. Hepatic receptor mRNA in rats is increased by dietary mushroom (Agaricus bisporus) fiber and sugar beet fiber. J Nutr. 2000;130:2151–2156. doi: 10.1093/jn/130.9.2151. [DOI] [PubMed] [Google Scholar]
- 10.Gamoh S, Hashimoto M, Sugioka K. Chronic administration of docosahexaenoic acid improves reference memory-related ability in young rats. Neuroscience. 1999;129:70–79. doi: 10.1016/s0306-4522(99)00107-4. [DOI] [PubMed] [Google Scholar]
- 11.Gamoh S, Hashimoto M, Hossian MS, Masumura S. Chronic administration of docosahxaenoic acid improves the performance of radial arm maze task in aged rats. Clin Exp Pharmacol Physiol. 2001;28:266–270. doi: 10.1046/j.1440-1681.2001.03437.x. [DOI] [PubMed] [Google Scholar]
- 12.Gunde-Cimerman N, Plemanitas A, Cimerman A. Pleurotus fungi produce mevinolin and inhibitor of HMG CoA reductase. FEMS Microbiol Lett. 1993;111:333–337. doi: 10.1111/j.1574-6968.1993.tb06536.x. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto M, Shinozuka K, Shahdat HM. Antihypertensive effect of all-cis-5, 8, 11, 14, 17-icosapentaenoate of aged rats is associated with an increase in the release of ATP from caudal artery. J Vasc Res. 1998;35:55–62. doi: 10.1159/000025565. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto M, Shinozuka K, Tanabe Y. Hypotension induced by exercise is associated with enhanced release of adenyl purines from aged rat artery. Am J Physiol. 1999;276:970–975. doi: 10.1152/ajpheart.1999.276.3.H970. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto M, Shinozuka K, Gamoh S. The hypotensive effect of docosahexaenoic acid is associated with the enhanced released of ATP from the caudal artery of aged rats. J Nutr. 1999a;129:70–76. doi: 10.1093/jn/129.1.70. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto M, Hossain MS, Shimada T, Yamasaki H, Fujii Y, Shido O. Effects of docosahexacnoic acid on annular lipid fluidity of the rat bile canalicular plasma membrane. J Lipid Res. 2001;42:1160–1168. [PubMed] [Google Scholar]
- 17.Hossian MS, Hashimoto M, Gamoh S, Masumura S. Antioxidative effects of docosahexaenoic acid in the cerebrum versus cerebellum and brain stem of aged hypercholesterolemic rats. J Neurochem. 1999;72:1133–1138. doi: 10.1046/j.1471-4159.1999.0721133.x. [DOI] [PubMed] [Google Scholar]
- 18.Hossain MS, Hashimoto M, Gamoh S, Masumura S. Association of age-related decrease in platelet membrane fluidity with platelet lipid peroxide. Life Sci. 1999a;64:135–143. doi: 10.1016/s0024-3205(98)00543-8. [DOI] [PubMed] [Google Scholar]
- 19.Kabir Y, Yamaguchi M, Kimura S. Effect of shiitake (Lentinus edodes) and maitake (Grifola frondosa) mushrooms on blood pressure and plasma lipids of spontaneously hypertensive rats. J Nutr Sci Vitaminol. 1987;33:341–346. doi: 10.3177/jnsv.33.341. [DOI] [PubMed] [Google Scholar]
- 20.Mayes PA. Metabolism of Lipids. In: Harper HA, Rodwell VW, Mayes PA, editors. Reviews of physiological chemistry. Los Altos: Lange publications; 1997. pp. 280–321. [Google Scholar]
- 21.Pathe D, Chevallier F. Effects of thyroid state on cholesterol metabolism in the rat. Biochim Biophys Acta. 1976;441:155–164. doi: 10.1016/0005-2760(76)90290-3. [DOI] [PubMed] [Google Scholar]
- 22.Roach PD, Balasubramaniam S, Hirata F. The low density lipoprotein receptor and cholesterol synthesis are affected differently by dietary cholesterol in the rat. Biochim Biophys Acta. 1993;1170:165–172. doi: 10.1016/0005-2760(93)90067-j. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt EB, Christensen JH, Ardestrup L. Marine ω-3 fatty acid. Basic features and background. Lipids. 2001;36:65–68. doi: 10.1007/s11745-001-0684-x. [DOI] [PubMed] [Google Scholar]
- 24.Simopoulus AP, Pavlou KN. Nutrition and Fitness, Metaboilic and Behavioral Aspects in Health and Disease. Basel: World Review of Nutrition and Dietetics. Karger; 1997. pp. 1–266. [PubMed] [Google Scholar]
- 25.Spady DK, Cuthbert JA. Regulation of hepatic sterol metabolism in the rat: parallel regulation of activity andmRNA for 7α-hydroxylase but not 3-hydroxy-3-methyl-glutaryl-coenzymeA reductase or low density lipoprotein receptor. J Biol Chem. 1992;267:5584–5591. [PubMed] [Google Scholar]
- 26.Uchida K, Satoh T, Chikai T. Influence of cholesterol feeding on the bile acid metabolism in young and aged germ free rats. Jpn J Pharmacol. 1996;71:113–118. doi: 10.1254/jjp.71.113. [DOI] [PubMed] [Google Scholar]
- 27.Wang HX, Ooi VE, Ng TB, Chiu KW, Cang ST. Hypotensive and vasorelaxing activities of a lectin from the edible mushroom Tricholoma magnivelare. Pharmacol Toxicol. 1996;79:318–323. doi: 10.1111/j.1600-0773.1996.tb00016.x. [DOI] [PubMed] [Google Scholar]
- 28.Wissier RW, Eilert ML, Schroeder MA, Cohen L. Production of lipomatous and atheromatous arterial lesions in the albino rat. Am Med Assoc Arch Pathol. 1954;57:333–351. [PubMed] [Google Scholar]
- 29.Yoshioka Y, Tabeta R, Saito H, Uehara N, Fukuoka F. Antitumor polysaccharides from P. ostreatus (Fr.) Quel. isolation and structure of a â-glucan. Carbohydrate Res. 1985;140:93–100. doi: 10.1016/0008-6215(85)85052-7. [DOI] [PubMed] [Google Scholar]