Abstract
Antifungal activity of celery essential oil against Malassezia furfur was investigated using broth microdilution and vapor contact methods. Potent antifungal activity was evident using both methods. Fungicidal activity was revealed in the vapor contact method.
Keywords: Antifungal activities, Celery essential oils, In vitro, Malassezia furfur
Malassezia species are yeasts that comprise part of the normal microflora of human skin. Especially, they are abundant in regions supplied with sebaceous glands because of their lipid requirement for growth. Several skin problems including pityriasis versicolor, dandruff, folliculitis, and atopic dermatitis can be caused by Malassezia species under suitable environmental conditions. Taxonomic revision has divided the genus Malassezia into seven different species (Guillot and Gueho, 1995). Among these, Malassezia furfur is one of the main causative agents of pityriasis versicolor and dandruff. Colonies of M. furfur on Sabouraud Dextrose Agar (SDA) are creamy-yellow in color and oval in shape. The increasing incidence of fungal infections and antifungal resistance of fungal pathogens has prompted the search for novel and effective antifungal agents. Essential oils are mixtures of volatile secondary metabolites of plant. They are isolated from different parts of aromatic plants. The potential of essential oils as antimicrobial agents has been reported (Barrtta et al., 1998). However, relatively little is known of the antifungal activity of essential oils against Malassezia. Nenoff et al. (1996) demonstrated that the essential oil of the tea tree inhibits the growth of M. furfur. The aim of this study is to investigate the essential oil of celery and its volatile vapor as an antifungal agent against M. furfur.
Celery essential oil was purchased from a cosmetic company in Korea. M. furfur Korean Collection for Type Culture (KCTC) 7545 was maintained on SDA covered with corn oil at 35℃. The antifungal effects of the oil's volatile vapor on M. furfur growth were determined by the modification of a chamber assay (Jain and Agrawal, 2002). Disposable Phytatrays (Sigma-Aldrich, St. Louis, MO, USA) with sterilized lids was used as the chamber for the essential oil and M. furfur. Essential oil (0.5, 1, 1.5 ml) contained in a small vial and SDA inoculated with M. furfur were placed in the 800ߢ volume of a Phytatray. A set of trays lacking essential oil was run as a control. Each Phytatray was incubated at 35℃ for 5 days. After incubation, growth of M. furfur was determined by microscopic observation. Absence of cells on SDA was evidence of growth inhibition. To demonstrate the sporostatic or sporocidal activity of the volatile vapor, essential oil was removed and the inoculated SDA was incubated for 5 days at 35℃. After incubation, yeast growth was investigated as described above. Resumption of growth during the 5-day incubation was evidence of fungistatic activity, while the absence of growth was indicative of fungicidal activity. To estimate the effect of direct exposure of essential oil on M. furfur, a broth microdilution assay was performed. Briefly, cell suspensions prepared as described were adjusted with modified Leeming & Notman broth medium (MLNB) to an optical density of 0.35 at 620 nm. Cell suspension of M. furfur was diluted in modified MLNB. A 100 µl aliquot of the cell suspension was inoculated into 100 µl of fresh MLNB containing a mixture of essential oil (0.5~2% v/v) and 0.05% Tween-40 in wells of a 96-well dish (Falcon, Lincoln Park, NJ, USA). Cell growth was investigated by measuring absorbance at 620 nm after 96 h incubation at 35℃. In controls, sterile water and an equivalent concentration of Tween 40 were added to each well instead of essential oil. Minimum inhibitory concentration (MIC) of essential oil was determined by estimating the minimum concentration that inhibited the growth of M. furfur.
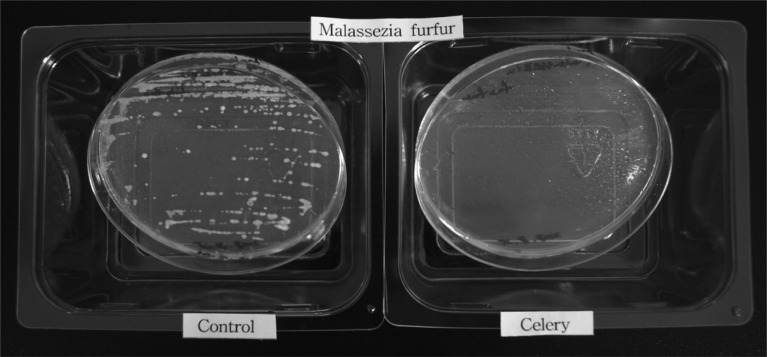
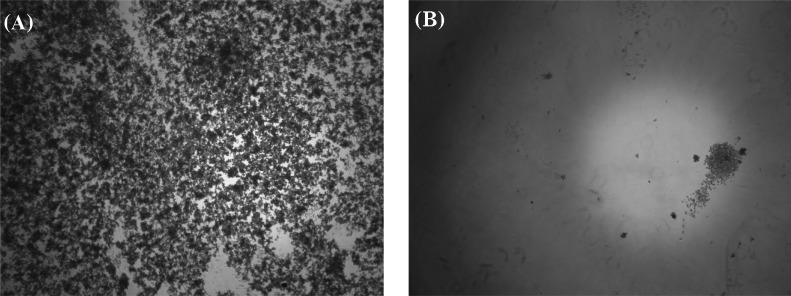
The volatile vapor of celery essential oil at a level exceeding 1 ml/800 ml air space strongly inhibited M. furfur growth (Fig. 1). After the removal of essential oil, cell growth was not evident after 72 h incubation indicating the fungicidal activity of the volatile vapor of the celery essential oil. Direct application of celery essential oil in the broth micodilution assay revealed the potent antifungal activity against M. furfur (Fig. 2). Average absorbance levels of control wells at 0 h and 96 h were 0.168 and 0.670, respectively. In contrast, the absorbance in the presence of 1% celery essential oil after 96 h incubation was 0.170. Therefore, celery essential oil at 1% and its volatile vapor at 1 ml/800 ml air space strongly inhibited growth of M. furfur (Table 1).
Fig. 1.
Phytatray chamber assay of effect of celery essential oil on growth of Malassezia furfur. M. furfur grown in the absence and presence of celery oil are displayed in the left and right trays, respectively.
Fig. 2.
Inhibition effect of celery essential oil on growth of Malassezia furfur assessed by broth microdilution after 72 h incubation. A, control culture not exposed to essential oil; B, culture treated with 1% (v/v) essential oil.
Table 1.
Inhibitory effect of celery essential oil against Malassezia furfur

Jain and Agrawal (2002) demonstrated the fungistatic activity of the volatile vapor of several essential oils against fungi. To our knowledge, no other report had described antifungal activity of volatile vapour of celery essential oil against M. furfur. Although the exact nature of the volatile vapor-mediated growth inhibition has not been described, volatile compounds of essential oils may influence a variety of cell metabolic events (Fries et al., 1973). Essential oils are widely used in folk medicine and cosmetic industry, but only in recent years they have been recognized as a potential antimicrobial agent. Further research will be needed to investigate the mechanism of antifungal activity of celery essential oil and its volatile vapor. Such studies may lead to the application of the antifungal agents in aromatherapy and cosmetic formulations.
References
- 1.Barrtta MT, Dorman HJD, Deams SG, Figueiredo C, Barroso JG, Ruberto G. Antimicrobial and antioxidant properties of some commercial essential oils. Flav Fragr J. 1998;13:235–244. [Google Scholar]
- 2.Fries N. Effects of volatile organic compounds on the growth and development of fungi. Trans Br Mycol Soc. 1973;60:1. [Google Scholar]
- 3.Guillot J, Gueho E. The diversity of Malassezia yeast confirmed by rRNA sequence and nuclear DNA comparison. Antonie Van Leeuwenhoek. 1995;67:297–314. doi: 10.1007/BF00873693. [DOI] [PubMed] [Google Scholar]
- 4.Jain SK, Agrawal SC. Fungistatic activity of some perfumes against otomycotic pathogens. Mycoses. 2002;45:88–90. doi: 10.1046/j.1439-0507.2002.00730.x. [DOI] [PubMed] [Google Scholar]
- 5.Nenoff P, Haustein UF, Brandt W. Antifungal activity of the essential oil of tea tree against pathogenic fungi in vitro. Skin-Pharmacol. 1996;9:388–394. doi: 10.1159/000211450. [DOI] [PubMed] [Google Scholar]