Abstract
The convulsions of the EL mouse (EL) were described by Imaizumi et al. in 1954 and were established as epilepsy by Suzuki in 1976. The EL mouse has been kept as an inbred strain and is considered one of the best animal models originated in Japan. The mode of inheritance is autosomal dominant, and environmental risk factors for seizure occurrence are hypothesised to contribute to the polygenic background. Paroxysmal activities in the EL brain arise from the parietal cortex (PCX) and are augmented in the hippocampus, demonstrated by electrophysiology and autoradiography using 2-deoxy glucose when clinical symptoms of seizures appeared. The neurons in the EL PCX, where GABA activity is lower than that of DDY PCX demonstrate increased excitability to proprioceptive sensory input. After repetitive seizure-provoking stimuli, seizures are more easily induced, eventually occurring spontaneously. This phenomenon of “abnormal plasticity” is also observed in the EEG, decreasing GABA activity, expression of the immediately early gene, and various biochemical and molecular processes. This phenomenon is similar to the learning or progressive process of certain neurological diseases.
Keywords: EL mouse, epilepsy, parietal cortex, GABA, neuronal firing, abnormal plasticity
1. Introduction
Epilepsy is a chronic brain disorder caused by various etiologies, that demonstrates paroxysmal recurrent mainly convulsions and/or multiform seizures, accompanied by other neurological symptoms and findings by examinations (EEG). These intractable seizures, known as “morbus sacer,” have made humankind fearful of and cruelly disturbed by the disease since the dawn of history.
Epileptic seizures have two essential physiological mechanisms: abnormality of cellular excitability and network hypersynchronization. Several types of animals with spontaneously occurring seizures have been reported since Studentsov’s report on an audiogenic-seizure mouse in 1924 (cited by Krushinsky et al.1)). The first report of electric discharges during convulsions appeared in the photogenic seizures of a baboon, described in 1972 by Naquet and Meldrum.2) Several reviews on mutant animal models of epilepsy were published by Noebels3) and other authors, including myself.4)
The present paper reviews the work on the EL mouse, particularly on the establishment of an EL* mouse as the gold standard animal model for epilepsy, the basic neuronal mechanism of its seizures and the development of epileptogenesis through abnormal molecular plasticity.
2. Establishment of an EL mouse as an excellent animal model of epilepsy
In 1954, Imaizumi and his colleagues watched the convulsions of several DDY mice that they developed as a model of hydrocephalus type II. These researchers5) reported in 1959 their observation of epileptiform convulsions, named the mice ep and developed them. The mode of inheritance of epilepsy in these mice is autosomal dominant.
A strain of ep mice was provided to us and has been maintained as an inbred strain with the back-crossing procedure onto the parent strain as a genetic animal model of epilepsy in our animal centre. Many investigators (e.g., Kurokawa et al.7)) studied this model from various points of view using, for instance, biochemical and physiological methods, but no significant results were reported because there was no evidence that the animal was epileptic. In 1975, paroxysmal discharges in the animals in the electro-encephalogram (EEG) during seizures and inter-ictal periods were recorded by the author, and the finding was reported in 19768) (Fig. 1). More precise analyses of the EEGs of seizures were reported the following year, demonstrating the EEG was composed of a tiny aura of trembling vibrissae, wild running and tonic-clonic convulsions.9) These findings firmly established the El mouse as an excellent model of epilepsy.
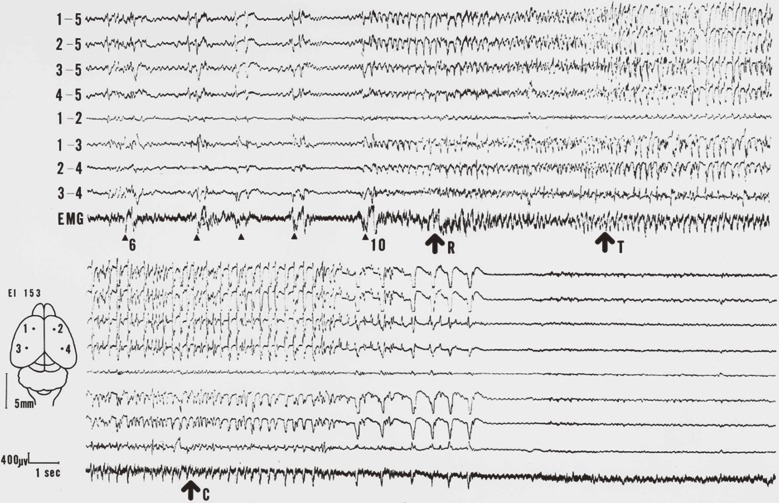
Figure 1.
One of the first records of the seizures of an EL.8) The upper column successively continues to the lower. The upper four traces in each column show monopolar leads (5 means the reference) and the lower, bipolar. Arrowheads represent stimulations. Each arrow represents running (R), tonic convulsion (T) and clonic convulsion (C) respectively.
After the establishment of the EL mouse as an epilepsy model, many domestic and foreign investigators have worked with this animal. Our animal centre provided the animals to many foreign facilities, including the Jackson Laboratory. Significant contributions have been made by Seyfried and his colleagues in Boston. They found eight epilepsy related genes and had worked extensively on this animal using the methods of linkage analysis, micro satellite alley, knocking out, transgenic trials. Finally they proposed that the EL mouse seizure susceptibility was polygenic (Rise et al.10)) and later presented environmental risk factors for seizure occurrence, hypothesising that quantitative trait loci (QTL) were linked to a polygenic background (Todorova et al.11,12)). The details of the genetic aspects of seizure susceptibility still remain unclear.5)
All of the EL/Suz animals used in our experiments were obtained from an inbred strain, now the F128 generation, and the DDY animals were also from an inbred strain, the F62 generation. The mice were carefully reared in our institution and exposed to routine provoking stimulation that consisted of tossing the animal into the air once a week, beginning at 4 to 5 weeks of age. At first, few EL/Suz animals seized. After the inducing stimuli were repeated, all of the EL/Suz easily seized, and eventually, they almost seized spontaneously (Suzuki and Nakamoto13)). The seizure threshold was lowered by repetition of the stimulation, and they were confirmed to be easily prone to seizures. No DDY mice exhibited seizures, even after the provoking stimulation. The seizures of an EL mouse are induced by abrupt accelerating movements, including tossing the animal into the air or performing rotating movements (Fueta et al.14)) beginning at an early stage of development (4 to 5 weeks of age). The effective mode of stimulation is proprioceptive, not vestibular, because these stimuli induce seizures even after unilateral or bilateral destruction of the labyrinths (Suzuki and Nakamoto15,16)).
Some investigators, they say, consider the EL mouse as a model of autism. The author have once analyzed behavior of EL mice and found some abnormal circadian rhythm but not published internationally. However, no symptom which meant autism could not be found.
3. Onset and propagation of paroxysmal discharges in the EL mouse brain
The techniques of recording electrical activities of the brain progressed from the surface to the deep regions of the brain17) (Fig. 2). Following the tossing-up stimuli, the first paroxysmal discharge or the initial spike appeared at the parietal cortex. The paroxysmal activities propagated to the contralateral parietal cortex, frontal cortices, striatum, substantia nigra, thalamus, hippocampus and amygdala17,18) (Fig. 3). The interval time or latency between the first spikes in both parietal cortices was 0.2 msec. The interval time between the temporal cortex and the hippocampus was as long as 4.7 sec. The remarkable behavioural manifestations, including wild running or tonic convulsions, did not occur until autonomous spike activities started in the hippocampus. The parietal cortex and hippocampus are considered to be the focus complex.**
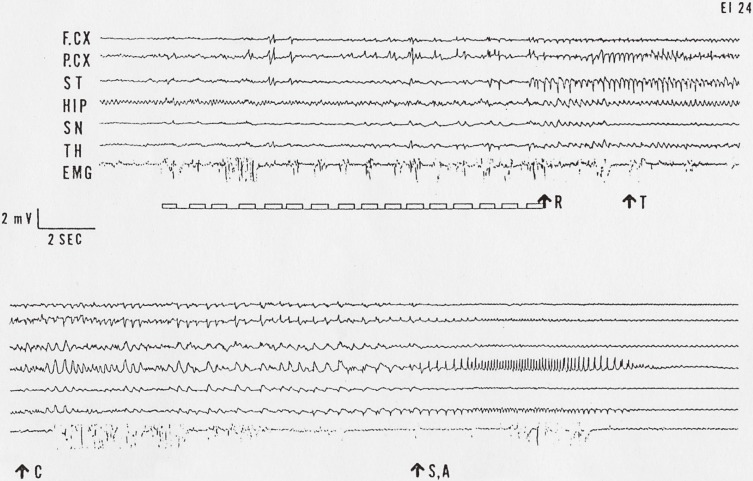
Figure 2.
A typical depth EEG record of full seizure of an EL mouse. The upper 7 traces successively continue to the lower. Small squares under the upper traces represent stimulation procedures. R: wild running, T: tonic convulsions, C: clonic convulsions, S,A: sitting or automatism, F.CX: Frontal cortex, P.CX: Parietal cortex, ST: Striatum, HIP: Hippocampus, SN: Substantia nigra, TH: Thalamus. EMG: electromyogram.
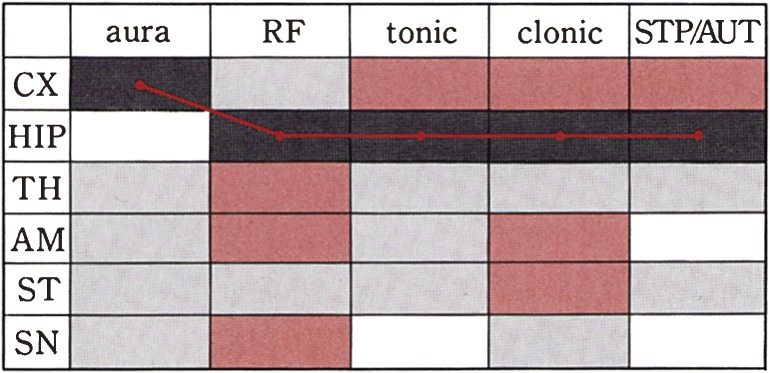
Figure 3.
A schematic representation of sequential propagation of paroxysmal activities to the various regions in the brain. Leading discharges are represented by the red line, starting from CX when the mouse was showing aura, reaching HIP, then running fit, tonic-clonic convulsions and stupor or automatism state. Brown squares represent moderately excitation, grey squares slight excitation.
The [14C] 2-deoxyglucose technique found by Sokoloff et al.19) was applied to elucidate the expected increase in the local metabolic rate through the neural pathway and the epileptic seizure focus of the EL mouse.20,21) The optical density of the autoradiograms was measured with a drum scan densitometer and analyzed with an image-processing system (Fig. 4). After the full seizure and recovery period, the parietal cortex and dorsal hippocampus exhibited a remarkably increased metabolic rate. In contrast, a lower metabolic rate was evident in the thalamic nuclei, central grey, locus coeruleus and several interstitial nuclei.
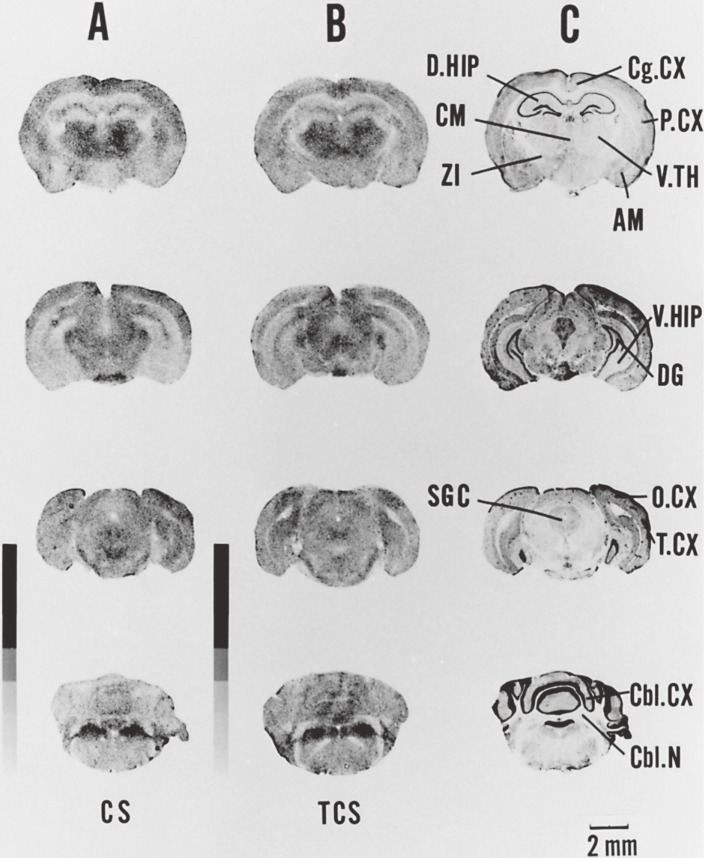
Figure 4.
[14C] Deoxyglucose autoradiographs of coronal sections and cresyl violet-stained sections of EL and DDY mouse brains. The autoradiographs of the [14C] standards are also visualized. A. Sections of DDY mouse after thrown-up stimulation 30 times. B. Sections of EL mouse with full seizure after thrown-up stimulations. C. Nissl sections adjacent to the sections in B. AM: amygdala, Cbl.CX: cerebellar cortex, Cbl.N: cerebellar nuclei, Cg.CX: cingulate cortex, CM: centro-median nucleus, DG: dentate gyrus, D.HIP: dorsal hippocampus, O.CX: occipital cortex, P.CX: parietal cortex, SGC: superior grey cortex, T.CX: temporal cortex, V.HIP: ventral hippocampus, V.TH: ventral thalamus, ZI: zona incerta.
4. Development of epileptogenesis by an abnormal plasticity
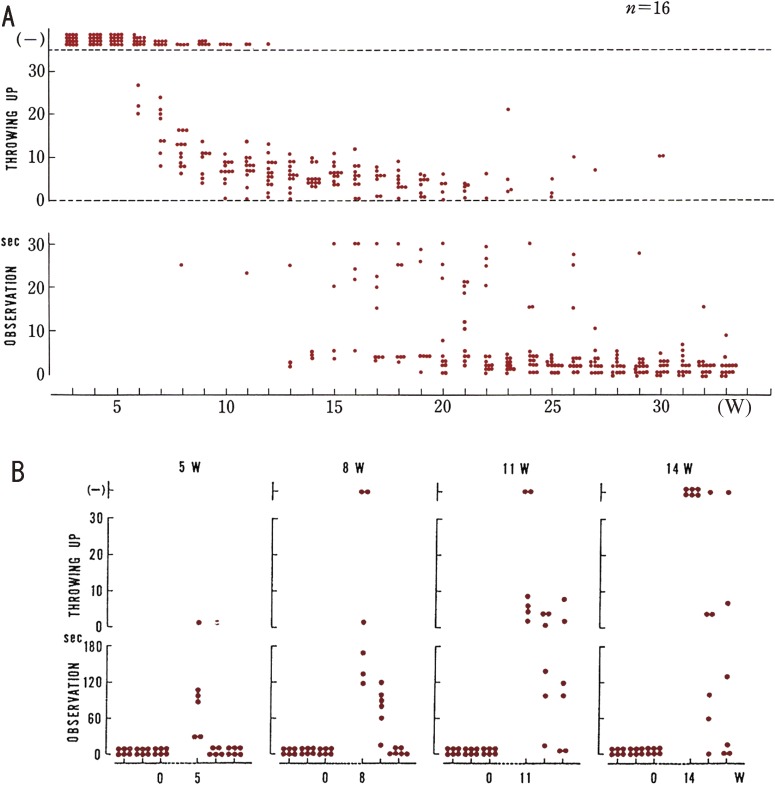
The mice were carefully exposed to routine provoking stimulation once a week, beginning at 4 to 5 weeks of age.13) At first, few EL/Suz animals seized. After the inducing stimuli were repeated, all of the EL/Suz easily seized, and eventually, they seized almost spontaneously (Suzuki and Nakamoto13)). The seizure threshold was lowered by repetition of the stimulation, and the mice were confirmed to be easily prone to seizures (Fig. 5). We named this phenomenon “abnormal plasticity”, which we consider to be an underlying process in the development of seizures in the EL mouse. After a long period without stimulation, the thresholds were raised, or extinction of seizures was observed in mice with fully developed seizures.
Figure 5.
Abnormal plasticity represented by seizure susceptibility or thresholds of seizures. A. Change of seizure susceptibility or thresholds of seizures during development of EL mouse. Each dot denotes one trial of evoking a seizure or one seizure. Upper row (−); no seizure was evoked. Middle row; each dot represents a seizure elicited by tossing-up. Bottom row; each dot represents a seizure occurring during the observation period. The ordinate signify thresholds of seizures, a dot in the lower area indicating a lower threshold. Abscissa, the age of mice in week. B. Effects of cessation and resumption of stimulations. Ordinates and dots are the same as in (A). Numbers at the top of each column indicate length (weeks) of the period of no stimulation. In each experimental group shown in each column, there were six mice with occurring at the lowest threshold.
Reinforcement of stimulation was necessary for the seizures of the EL mice to occur constantly. During recovery of the occurrence of seizures, their incidence increased sooner than in the previous developmental period. This phenomenon appears to resemble the process of learning.
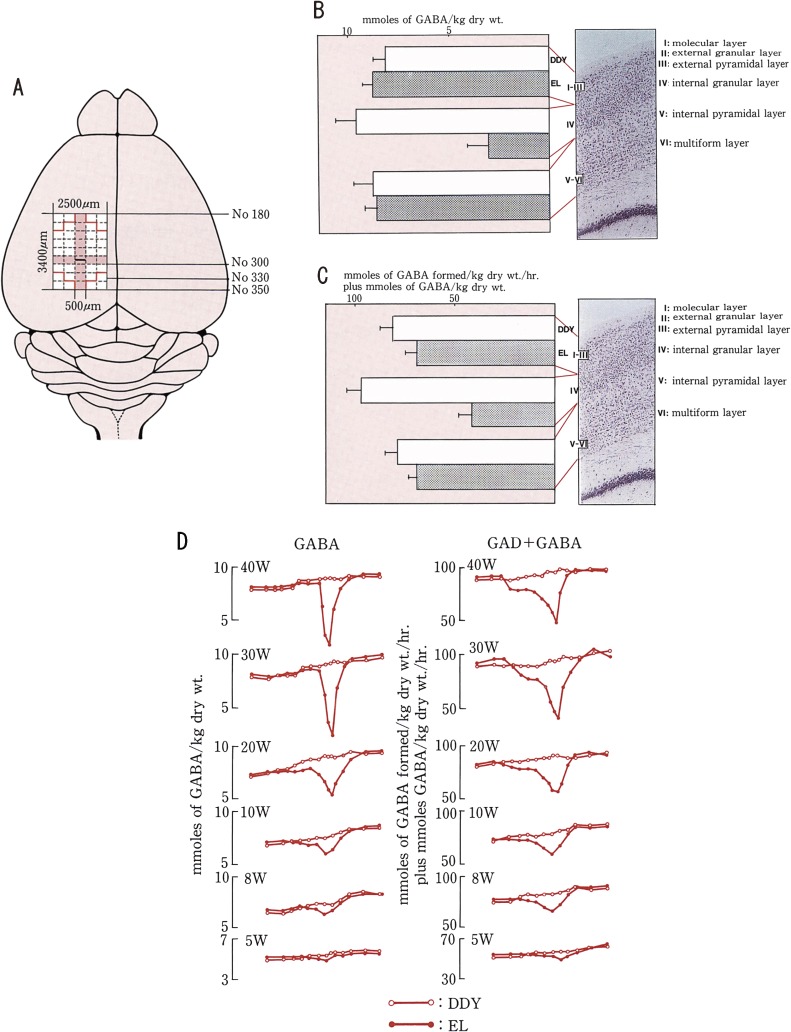
The abnormal plasticity is also represented by growing electroencephalographic discharges (Suzuki and Nakamoto13)) and by decreasing GABA-related inhibitory activity (Murashima et al.22,23)) (Fig. 6). The immediate early gene (IEG) expression is related to the seizure history, seizure threshold and development of EL mouse (Murashima et al.24–26)) (Fig. 7). The EL mouse seizure occurs with development and abnormal plasticity on the genetic bases. The abnormal plasticity is a bridging process from epileptogenesis to ictogenesis. This process may suggest an aggravating process in the development of epilepsy and of certain neuropsychiatric diseases.
Figure 6.
The GABA concentrations and GAD activities in the parietal cortex of EL and DDY. Sample preparation in the parietal cortex. Fifteen micrometer thick serial freeze-dried samples of the brain were obtained according to the number of Sidman atlas. Micro-brain region samples (100–300 ng) were cut from the cortex. Samples of 500 micrometer width were obtained from the rostral to caudal side through 3400 micrometer. The GABA concentrations and GAD activities were quantitatively determined using ultra micro enzymatic chemistry of Lowry. A. Distribution of the GABA concentrations and GAD activities along the rostro-caudal axis of the parietal cortex. B. Distribution of GABA concentrations along the surface to depth axis in the parietal cortex at the age of 30 weeks. The average levels of serial four or eight samples from an animal are represented by open bars (DDY mouse) and solid bars (EL). The average level of GABA concentration in the 4th internal granular layer of EL is the lowest and lower than that of DDY. C. Distribution of GAD activities is similarly shown as GABA concentration in B. The average level of GAD activities in the 4th internal granular layer of EL is the lowest and lower than that of DDY. D. Schematic representation of the distribution of GABA concentrations in the left column and GAD activities in the right column in the parietal cortex of the EL and DDY along the rostro-caudal axis. Open circles connected by solid line show the GABA and GAD plus GABA in the control (DDY). Closed circles connected by solid line show the level of GABA and GAD plus GABA in the EL according to the development at the age of 5, 8, 10, 20, 30 and 40 weeks old. Samples were obtained from the rostral (No. 180 of Sidman Atlas) to the caudal side (No. 350) through 3400 micrometer distance and the width of each samples was 500 micrometer. The data were obtained from the average of serial two, four or eight samples from each animal.
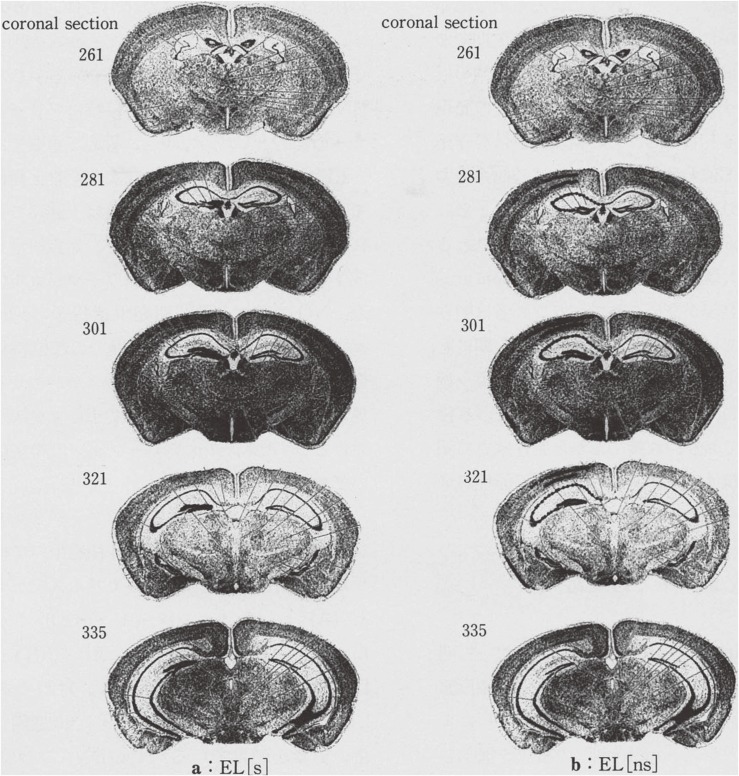
Figure 7.
Response representation of IEG (zif) expressed cell groups by the X-ray emulsion autoradiography which are shown as closed-circle dots on the left side of every serial coronal sections. The right side of every serial coronal sections are Nissle-stained histological samples which are attached as control. a: Samples were obtained from 20 week old EL 30 min after an induced seizure. b: Samples were obtained from 20 week old EL which had few experiences of seizures 30 min after an induced abortive seizure. Numbers of the left side superscript are the numbers of Sidman atlas.
5. Biochemical and molecular aspects in the EL mouse brain
Since the mid-1980s, a number of biochemical works were reported on monoamines in the EL brain. Mori (199827)), Hiramatsu28) and their colleagues worked Glu, 5-hydroxytryptamine and other amines. Mita et al., 199129) measured the developmental changes observed with high values of Gly or Tyr and other substances in many regions of the EL brain. Many other investigations were reported on the EL brain, including slice preparations, but those studies were not in accordance with other published studies because of different developmental conditions or seizure history of the sample animals.
Since the 1980s, Yamagami and his colleagues30,31) have investigated the relationship of nucleic acid, genes and the seizures of the EL mouse, and have obtained many fruitful results.
Using the ultra-micro enzymatic chemistry, Murashima et al.22–24) elucidated the abnormally low distribution of GABA and low GAD (glutamate decarboxylase) activity in the parietal cortex and hippocampus and their developmental changes. These researchers determined precisely the region of low level of GABA metabolism in the EL brain, specifically, 500-micrometer × 400-micrometer area in the parietal cortex and in the 4th layer, the internal granular layer.
Murashima et al.25) reported and summarised their work on the enzymatic and molecular aspects of the hippocampus of EL mouse, including abnormal constitutive neuronal nitric oxide synthetase (n-NOS) and endothelial NOS (e-NOS). These researcher hypothesised the abnormal expression of NOS may play a key role in the pathogenesis of epilepsy.
In spite of various findings of neurophysiological, biochemical and molecular aspects, no definitive morphological or histological abnormal data have been reported.32) More precise or quantitative investigation is required.
6. Seizure mechanism and epileptogenesis of EL mouse
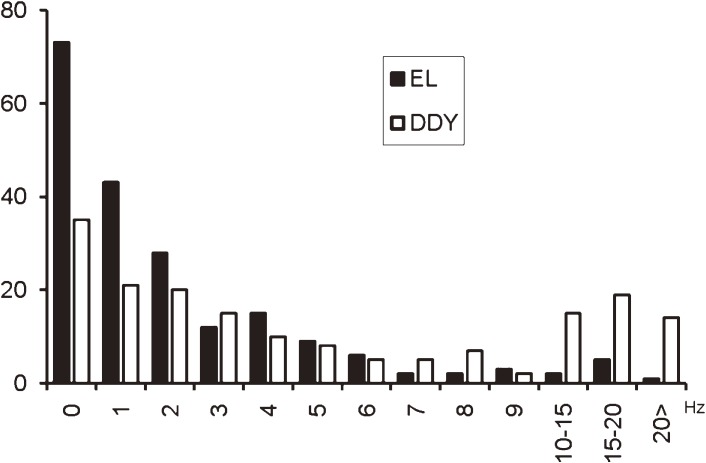
Using an extracellular microelectrode, the units of neuronal activity in the parietal cortex of EL and DDY mice revealed the neurons of EL are less active at rest than those of the DDY mice but the EL neurons responded more actively to proprioceptive afferent input from muscle stimulation than did the DDY neurons (Fig. 8)33).
Figure 8.
The frequencies of the spontaneous firing of the PCX neurons. The filled bars indicate the firing frequency of the EL neurons and the blank bars indicate that of the DDY neurons. The mean frequency, 2.74 (SD 3.45) Hz, of the EL neurons was significantly lower than that of the DDY neurons (7.77 (SD 10.3) Hz, p < 0.001). Ordinates: no. of cells. Abscissa: Hz.
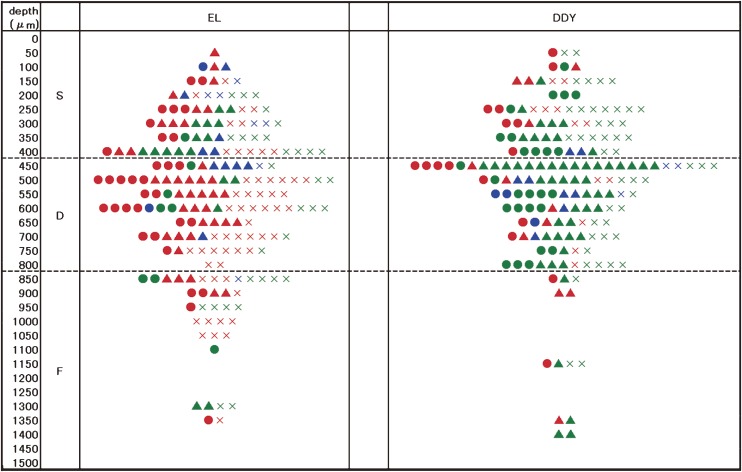
Four patterns of responses to stimulation were observed in the parietal cortex neurons. More excitatory patterns were observed in the EL mice than in the DDY mice. The trans-laminar distribution of cells with different response patterns differed between the EL and DDY mice (Fig. 9). In the EL PCX the facilitated cyclic neurons (red circles in Fig. 9) were found mostly in the D zone, and the facilitated inactive neurons (red crosses in Fig. 9) were located in all the zones (S, D and F). The inhibited cyclic neurons (blue circles in Fig. 9) were rarely found in the upper lamina.33)
Figure 9.
The zonal distribution of the PCX neurons according to each response type. The circle (●) represents the cyclic type (A), the triangle (▲) represents the continuous type (B), and the cross (×) represents the inactive type (C) neurons. Red: facilitative, blue: inhibitory, green: no response.
In the EL PCX, where GABA activity is lower than that of the DDY PCX, the cellular neurophysiological features are consistent with increased neuronal excitability. This activity may provide a basis for the initiation of epileptic discharges in the PCX, i.e., the hyper-synchronised firing of neurons or networks.
7. Novel trial for epilepsy therapy with EL mouse
In the EL mouse brain, positive neurosteroids, including THP (allopregnanolone) that act as allosteric modulators of GABAA receptors and have anticonvulsant properties, were withdrawn before the occurrence of repetitive seizures. This finding may likely indicate a trigger for the ictogenesis and epileptogenesis in the EL mouse.34) Reorganisation of the GABAA receptor subunits may lead to hypersensitivity of the receptor to neurosteroids. GABAA receptor-regulating neurosteroids may be a target for the development of novel antiepileptic agents.
The neural cell adhesion molecule (NCAM) regulates an inwardly rectifying K+ channel. In the EL mouse brain, polysialylated NCAM (PSA-NAM), cadherin, tenascin-R and reelin are upregulated before repetitive seizures, which may trigger the ictogenesis and epileptogenesis by contributing to the abnormal plastic phenomena. NCAM may compensate for the hyper-excitability during development. These functions of THP and NCAM may be useful in developing new approaches to epilepsy treatment.35)
Another project36) is being carried out using transplantation of neural stem cells (NSCs). In EL mice, non-induced NSCs, neuron-induced NSCs (nNSCs), and GABAergic NSCs- (gNSCs), derived from NSCs originating from neural stem spheres were incubated for 24 hrs and were used for the transplantation. These NSCs cells were transplanted to the bilateral dorsal hippocampi. The NSCs transplanted group showed no seizures, while the vehicle control group showed seizures. The GAD-positive transplanted neurons were observed in the NSCs, nNSCs and gNSCs transplanted groups. In vitro, only gNSCs differentiated into GAD positive neurons and showed an elevated GAD gene expression. In the EL mice, the hippocampus and the parietal cortex may exhibit a potency for inducing inhibitory GAD neurons to control seizures.
Recently, Murashima and his colleagues (data are not published because of the application of US patent) have succeeded in controlling the epileptic seizures of EL by transplantation of immature embryonic stem (ES) cells, which were differentiated and formed the inhibitory neuron networks.
8. Conclusions
Since the convulsions of the EL mouse were first observed and established as epilepsy, the inbred strain has been kept, and data were obtained to elucidate the mechanism of epilepsy and abnormal plasticity. These mechanisms can be considered to be a bridging process from epileptogenesis to ictogenesis. In addition to above-mentioned new trials, it is essentially important to elucidate the genomic mechanism of epileptogenesis of an EL mouse. Then one can find a new approaching way to therapy of epilepsy.
Acknowledgment
Many thanks are extended to Dr. Y.L. Murashima, Dr. N. Ozawa, Dr. N. Ishida, Dr. A. Takazawa, Dr. K. Kasamo, Dr. Y. Shinkawa, Dr. T. Shinba, Ms. Y. Nakamoto, Ms. T. Shinozaki, Dr. R. Nonaka, Mr. S. Nakayama, Ms. K. Matuoka, Ms. Y. Niikawa and all staff members at the institute.
Profile
Jiro Suzuki was born in 1936. After he graduated from the Faculty of Medicine at the University of Tokyo in 1961, he started his clinical and research career. He was granted PhD degree for the work “the narcoleptic syndrome and paradoxical sleep”, by which he was awarded the excellent prize of the Japanese Society of Psychiatry and Neurology in 1967. This was one of the pioneering works in sleep research in those days. He worked on the intracellular recording of a cat brainstem in the Brain Institute of the University of Tokyo. He was appointed as an instructor of the Department of Psychiatry. He moved to the Laboratory of Neurophysiology in the Department of Physicians and Surgeons of Columbia University in New York, where he worked on Ca spike in a crayfish giant axon and K channel in an electric plaque of an electric eel. In 1972 he was appointed as a chief staff of the Division of Neurophysiology in Psychiatric Research Institute of Tokyo, where he had started the research on EL mouse until 2012. During these 40 years, he was appointed Professor of Neuropsychiatry in Toho University and later Clinical Professor of Psychiatry in the International University of Health and Welfare. He was elected as the chairman and director of Japanese Society of Psychiatry and Neurology in 1998, the chairman of Japan Epilepsy Society in 2001, and nominated as a council of Japan Medical Association 1997–2000. He convened World Congress of Psychiatry Yokohama 2002 as the executive director. He was nominated as the East Asia representative and honorary staff of World Psychiatric Association from 1999–2004. At present he is the director of Sannou Institute of Psychiatry and Psychology, Japanese Association of Clinical Outpatient Psychiatry and the trustees of several associations.
Footnotes
This mutant mouse strain was first named “ep” by Imaizumi et al.,5) thereafter was called “El” until 1992, when it was renamed “EL” at the International Symposium on Epileptic El Mouse, Neurosciences, Tokyo (President Mori A.). The provider of the animals should identify this strain as such, Suz denotes Suzuki (e.g., Leussis and Heinrichs6)).
Focus means the origin of paroxysmal activities in the epileptic brain.
References
- 1).Krushinsky, L.V., Molodkina, L.N., Fles, D.A., Dobrokhotova, L.P., Steshenko, A.P., Semiokhina, A.F., Zorina, Z.A. and Romanova, L.G. (1970) The Fnctional State of the Brain During Sonic Stimulation. In Physiological Effects of Noise (eds. Welch, B.L. and Welch, A.S.) Plenum Press, New York. [Google Scholar]
- 2).Naquet, R. and Meldrum, B.S. (1972) Photogenic seizures in Baboon. In experimental models of epilepsy (eds. Purpura, D. et al.). Raven Press, New York. [Google Scholar]
- 3).Noebels J.L. (1999) Single-gene models of epilepsy. Adv. Neurol. 79, 227–238 [PubMed] [Google Scholar]
- 4).Suzuki J. (2003) The mutant model for epilepsy — An EL mouse —. J. Jpn. Epil. Soc. 21, 115–145 [Google Scholar]
- 5).Imaizumi K., Ito S., Kutsukake T., Talizawa K., Fujiwara K., Tsuchikawa T. (1959) Epilepsy like anomaly of mice. Exp. Anim. (Jap.) 8, 6–10 [Google Scholar]
- 6).Leussis M.P., Heinrichs S.C. (2007) Temporal ontogeny of circuit activation prior to the onset of seizure susceptibility in EL/Suz mice. Neuroscience 145, 33–41 [DOI] [PubMed] [Google Scholar]
- 7).Kurokawa M., Naruse H., Kato M. (1966) Metabolic studies on ep mouse, a special strain with convulsive predisposition. Prog. Brain Res. 21A, 112–130 [DOI] [PubMed] [Google Scholar]
- 8).Suzuki J. (1976) Paroxysmal discharges in the electroencephalogram of the El mouse. Experientia 32, 336–338 [DOI] [PubMed] [Google Scholar]
- 9).Suzuki J., Nakamoto Y. (1977) Seizure patterns and electroencephalograms of El mouse. Electroencephalogr. Clin. Neurophysiol. 43, 299–311 [DOI] [PubMed] [Google Scholar]
- 10).Rise M.L., Frankel W.N., Coffin J.M., Seyfried T.N. (1991) Genes for epilepsy mapped in the mouse. Science 253, 669–673 [DOI] [PubMed] [Google Scholar]
- 11).Todorova M.T., Burwell T.J., Seyfried T.N. (1999) Environmental risk factors for multifactorial epilepsy in EL mice. Epilepsia 40, 1697–1707 [DOI] [PubMed] [Google Scholar]
- 12).Todorova M.T., Mantis J.G., Le M., Kim C.Y., Seyfried T.N. (2006) Genetic and environmental interactions determine seizure susceptibility in epileptic EL mice. Genes Brain Behav. 5, 518–527 [DOI] [PubMed] [Google Scholar]
- 13).Suzuki J., Nakamoto Y. (1982) Abnormal plastic phenomena of sensory-precipitated epilepsy in the mutant El mouse. Exp. Neurol. 75, 440–452 [DOI] [PubMed] [Google Scholar]
- 14).Fueta Y., Mita T., Matsuoka S. (1983) Experimental animal epilepsy: a new device for the induction of epileptic seizures in the murine, El mouse. J. UOEH 5, 359–364 [DOI] [PubMed] [Google Scholar]
- 15).Suzuki J., Nakamoto Y. (1987) El mouse: A model of sensory-precipitated epilepsy. Excerpta Medica 427, 81–82 [Google Scholar]
- 16).Suzuki, J. (1989) Genetic and physiological mechanisms of epileptic seizures. In Art and Science of Epilepsy (eds. Suzuki, J., Seino, M., Fukuyama, Y. and Komai, S.). Elsevier Science Publishers B.V. (Biomedical Division), New York. [Google Scholar]
- 17).Ishida N., Kasamo K., Nakamoto Y., Suzuki J. (1987) The roles of parietal cortex and hippocampus in the seizure of the El mouse. Jpn. J. Psychiatry Neurol. 41, 498–499 [Google Scholar]
- 18).Suzuki J., Kasamo K., Ishida N., Murashima Y.L., Nakamoto Y., Ozawa N. (1989) The seizure mechanisms of an epileptic mutant animal. Jpn. J. Psychiatry Neurol. 43, 455–457 [DOI] [PubMed] [Google Scholar]
- 19).Sokoloff L., Reivich M., Kennedy C., Des Rosiers M.H., Patlak C.S., Pettigrew K.D., Sakuraba O., Shinohara M. (1977) The 14C deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J. Neurochem. 28, 897–916 [DOI] [PubMed] [Google Scholar]
- 20).Suzuki J., Nakamoto Y., Shinkawa Y. (1983) Local cerebral glucose utilization in epileptic seizures of the mutant El mouse. Brain Res. 266, 359–363 [DOI] [PubMed] [Google Scholar]
- 21).Nakamoto Y., Nakayama K., Suzuki J. (1992) Cerebral uptake of 14C deoxyglucose during the entire seizure and the recovery period in an El mouse. Epilepsy Res. 5, 43–48 [DOI] [PubMed] [Google Scholar]
- 22).Murashima Y.L., Kasamo K., Suzuki J. (1990) Distribution of GABA concentrations and GAD activities in the parietal cortex and the hippocampal CA1 in an EL mouse. Jpn. J. Psychiatry Neurol. 44, 442–444 [PubMed] [Google Scholar]
- 23).Murashima Y.L., Kasamo K., Suzuki J. (1992) Developmental abnormalities of GABAergic system are involved in the formation of epileptogenesis in the El. Neurosciences 18 (Suppl. 2), 63–73 [Google Scholar]
- 24).Murashima Y.L., Kasamo K., Suzuki J. (1996) Developmental and seizure-related regional differences in immediate early gene expression and GABAergic abnormalities in the brain of EL mice. Epilepsy Res. 26, 3–14 [DOI] [PubMed] [Google Scholar]
- 25).Murashima Y.L., Yoshii M., Suzuki J. (2000) Role of nitric oxide in the epileptogenesis of EL mice. Epilepsia 41 (Suppl. 6), S195–S199 [DOI] [PubMed] [Google Scholar]
- 26).Murashima, Y.L., Suzuki, J. and Yoshii, M. (2009) Specific gene expression before and after seizure in epileptic EL mice. In Encyclopedia of Basic Epilepsy Research (ed. Schwartzkroin, P.). Elsevier, New York. [Google Scholar]
- 27).Mori A. (1988) Neurochemical approach to the seizure mechanism. Neurosciences 14, 275–285 [Google Scholar]
- 28).Hiramatsu M., Edamatsu R., Suzuki S., Shimada M., Mori A. (1990) Regional excitatory and inhibitory amino acid levels in epileptic El mouse brain. Neurochem. Res. 15, 821–825 [DOI] [PubMed] [Google Scholar]
- 29).Mita T., Sashihara S., Aramaki I., Fueta Y., Hirano H. (1991) Unusual biochemical development of genetically seizure-susceptible El mice. Brain Res. Dev. Brain Res. 64, 27–35 [DOI] [PubMed] [Google Scholar]
- 30).Yamagami S., Ohno K., Tsuji M., Mori K., Kawakita Y. (1981) Developmental alteration in RNA metabolism of El mouse Brain. Folia Psychiatr. Neurol. Jpn. 35, 253–260 [DOI] [PubMed] [Google Scholar]
- 31).Mui K., Nakanishi A., Kuroda Y., Yokotani N., Kioka T., Onishi H., Yamagami S. (1993) The relationship between translational activity and poly(a)tract of mRNA upon EL mouse brain. Neurosciences 2, 35–42 [Google Scholar]
- 32).Suzuki J., Matsushita M., Nakamoto Y. (1983) Histopathological alterations in the hippocampus of an El mouse. Folia Psychiatr. Neurol. Jpn. 37, 362–363 [Google Scholar]
- 33).Suzuki J., Ozawa N., Murashima Y.L., Shinba T., Yoshii M. (2012) Neuronal activity in the parietal cortex of EL and DDY mice. Brain Res. 1460, 63–72 [DOI] [PubMed] [Google Scholar]
- 34).Murashima, L.Y. and Yoshii, M. (2009) New thrapeutic approaches for epilepsies, focusing on reorganization of the GABAA receptor subunits by neurosteroids. Workshop on neurobiology of epilepsy 2009 (abstract). [DOI] [PubMed] [Google Scholar]
- 35).Murashima, L.Y., Yoshii, M. and Inoue, N. (2011) Cell recognition molecules may trigger the epileptogenesis during development in the hippocampus: Focused on the novel mechanisms for epilepsy therapy. Workshop on neurobiology of epilepsy 2011. Finding novel mechanisms for epilepsy (abstract). [Google Scholar]
- 36).Murashima, Y.L. and Yoshii, M. (2013) Transplantation of embryonic stem cells to dorsal hippocampus of epileptic mutant EL mice to study the epileptic brain. Workshop on neurobiology of epilepsy 2013 (Review). [Google Scholar]