Abstract
Noninvasive detection of early β-cell death in type 1 diabetes might identify individuals in whom therapeutic interventions would preserve β-cell mass and prevent hyperglycemia. Recent studies in mice have shown that β-cell death produces a corresponding increase in unmethylated preproinsulin (PPI) DNA in serum. Here, we report the development of a novel assay using dual fluorescent-probe multiplex PCR (TaqMan) to detect differential methylation of circulating PPI DNA. Key assay features include low background signals, linear assay output across a large range of values, and simultaneous detection of methylated and unmethylated PPI DNA in a single reaction. We defined the “unmethylation index” as a summary parameter that reflects the relative amounts of unmethylated vs methylated PPI DNA. To validate this assay's ability to detect β-cell death in vivo, we measured the unmethylation index in the serum of diabetic mouse models, including high- and multiple low-dose streptozotocin-induced diabetes, and the nonobese diabetic mouse model of type 1 diabetes. Our data show a significantly increased unmethylation index concordant with the known timeline of β-cell death that precedes the onset of hyperglycemia. Subsequently, we observed a decrease in the unmethylation index following diabetes development, likely reflecting the absence of further β-cell death in the pancreas. We conclude that simultaneous measurement of methylated and unmethylated PPI DNA using the multiplex PCR method described here is a readily available and sensitive indicator of dying β-cells that may be useful to track diabetes progression and response to therapeutic intervention.
The development of type 1 diabetes is progressive, with loss of pancreatic β-cell function and mass, and development of hyperglycemia over a period that likely spans years (1). Diagnosis, and therefore treatment, occurs only in the final stages of β-cell dysfunction and death, when β-cell mass is nearly completely lost. Earlier identification of β-cell death in the prediabetic state might make β-cell-sparing therapy possible, which ideally would prevent progression to diabetes.
The genes encoding preproinsulin (PPI) in mice and humans undergo differential methylation at specific cytosine bases, which are largely unmethylated in β-cells and methylated in all other cell types (2). Using a SYBR-Green PCR methodology, recent studies correlated the appearance of unmethylated PPI DNA in serum of mice and humans to dying β-cells (3–5). Limitations of the SYBR-Green methodology include the potential for high background signals and the need for several PCRs to assess multiple DNA targets in a single sample. Here, we present a dual fluorescent probe-based multiplex PCR assay (TaqMan) that virtually eliminates background signals, is linear over multiple orders of magnitude, and reduces experimental variability by simultaneously detecting unmethylated and methylated PPI DNA in a single PCR reaction. Our assay allows for a serum-based measurement of β-cell death in multiple diabetic mouse models. This assay therefore represents an important advance in the development of a screening test for β-cell death prior to the development of diabetes.
Materials and Methods
Animals
Male C57Bl/6J (BL6), female nonobese diabetic NOD/ShiLTJ (NOD), and female NOD.CB17-Prkdcscid/J (NOD.SCID) mice were obtained from The Jackson Laboratory (Bar Harbor, Maine). Female CD1 mice were obtained from Charles River Laboratories (Wilmington, Massachusetts). All mice were maintained under protocols approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee. All mice were kept under pathogen-free conditions with a standard light-dark cycle and were fed regular chow diet and water ad libitum. Islets were isolated from pancreas tissue as described elsewhere (6).
Diabetes induction, blood collection, and pancreas staining
BL6 mice (12 wk of age) were given an ip injection of streptozotocin (high-dose STZ; 180 mg/kg) or 5 daily ip injections (multiple low-dose STZ, MLDS; 55 mg/kg). In high-dose STZ treated mice, blood was collected via tail vein on days 1 and 5 after injection and processed to serum through centrifugation. For MLDS, blood was collected repeatedly over time via tail vein. Pancreas tissues were collected at euthanasia, embedded in paraffin, and stained for insulin as described previously (7).
Female NOD mice were obtained at 5 weeks of age. Blood was collected via tail vein and processed to plasma. Blood glucose was measured via tail vein weekly. Mice were considered to have diabetes after 2 consecutive random blood glucose measurements greater than 300 mg/dL. Islet β-cell area was calculated as described previously (8) using 3 sections per pancreas (75 μm apart) from at least 3 mice per group.
DNA extraction and bisulfite treatment
DNA was extracted from MIN6 and NIH3T3 cells or mouse tissues using a genomic DNA extraction kit (Sigma-Aldrich, St. Louis, Missouri). DNA was extracted from 20 μL of serum/plasma samples using the ZR Serum DNA kit (Zymo Research Corp, Orange, California). All DNA samples underwent bisulfite treatment using the EZ DNA Methylation kit (Zymo Research).
Multiplex PCR
The PCR assay was run on bisulfite-treated DNA from each sample in triplicate using a custom designed dual fluorescent probe-based multiplex assay (AHMSN8K; Life Technologies, Gaithersburg, Maryland). For amplification of the mouse 2 PPI promoter, the following primers were used:
5′-AATTGGTTTATTAGGTTATTAGGGTTTTTTGTTAAGATTTTA-3′ (forward); 5′-ACTAAAACTACAATTTCCAAACACTTCCCTAA-3′ (reverse). For detection, the following probes were used: 5′-CTCATTAAACGTCAACACC-3′ (VIC); 5′-CTCATTAAACATCAACACC-3′ (FAM). PCRs were run using TaqMan Universal PCR Master Mix, No AmpErase UNG (Life Technologies) with 60 amplification cycles at 92°C for 15 seconds followed by 60°C for 90 seconds. Water was run as a negative control for all reactions. Amplified products were subcloned into the T/A cloning vector pCR2.1 (Invitrogen, Carlsbad, California), and a minimum of 10 clones were sequenced to confirm the identity of the PCR products.
Statistical analysis
All data are presented as mean ± SEM. A Student's t test was performed for all comparisons involving 2 conditions. For comparisons of more than 2 variables, a one-way ANOVA with a Bonferonni posttest was performed. Prism 5 software (GraphPad Software, San Diego, California) was used for all statistical tests. Statistical significance was assumed at P < .05.
Results
Multiplex PCR detects differentially methylated insulin promoter DNA
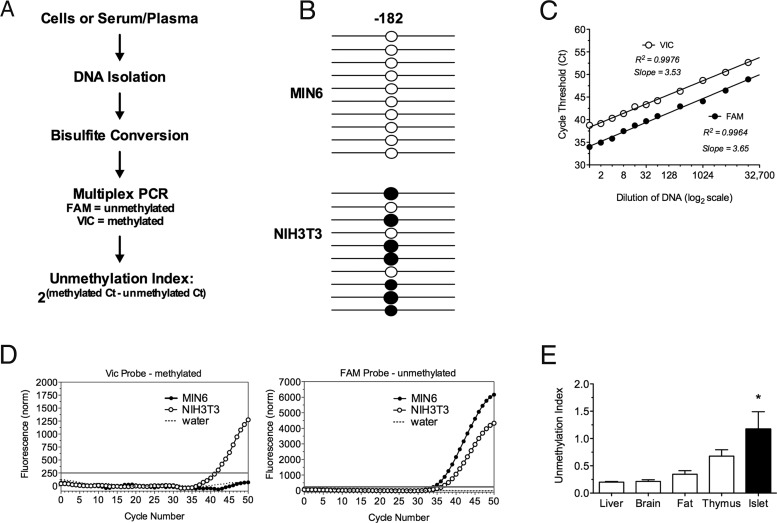
TaqMan-based PCR assays utilize fluorescently labeled probes with a minor groove binder that can be designed to detect single-nucleotide polymorphisms (SNPs) in genes (9, 10). When isolated DNA is treated with bisulfite, unmethylated cytosines are chemically converted to uracils, whereas methylated cytosines are unaffected. Methylated and unmethylated cytosines can be simultaneously detected in the same reaction by TaqMan PCR technology as SNPs. We designed a primer/probe combination to differentiate methylation from unmethylation of the cytosine at −182 bp from the transcriptional start site of the mouse 2 PPI promoter (2). A probe with the VIC fluorophore was designed to detect the methylated base and a probe with the FAM fluorophore was designed to detect the unmethylated base (Figure 1A).
Figure 1.
Unmethylated and Methylated PPI DNA Is Detectable in Mouse Cell Lines and Tissue. A, DNA was extracted from mouse cell lines, tissue, serum or plasma, bisulfite treated, and multiplex PCR was performed. B, Methylation pattern of the −182 CpG site of the PPI promoter with each line representing an individual clone. Open circle, unmethylated; closed circle, methylated. C, Real-time cycle Ct values determining linearity of PCR amplification. D, Quantitative real-time PCR of the methylated product using the VIC-labeled probe and the unmethylated product using the FAM-labeled probe in MIN6, NIH3T3, and water control samples. The gray line indicates the threshold. E, Unmethylation index in mouse liver, brain, fat, thymus, and islet tissues. n = 3–5; *, P < .05.
To validate our multiplex PCR methodology, we assessed the frequency of methylated and unmethylated PPI DNA in the MIN6 mouse β-cell line (which expresses the PPI transcript) and in the NIH-3T3 mouse fibroblast cell line (which does not express the PPI transcript). Bisulfite-treated DNA from both cell lines was subjected to TaqMan PCR amplification, and the resulting products were separated using gel electrophoresis demonstrating a single product; nonbisulfite-treated genomic DNA produced no detectable product (data not shown). Subsequently, we cloned the PCR products and sequenced 10 independent clones from each cell line. In MIN6 cells, 10 of 10 clones showed unmethylated cytosines at position −182, whereas in NIH3T3 cells only 3 of 10 clones showed unmethylated cytosines at this position (Figure 1B). The primer/probe combination was tested for linearity over a range of threshold values observed in mouse serum samples. Both probes exhibited linearity with coefficients of determination (R2) of 0.9976 (VIC) and 0.9964 (FAM) (Figure 1C).
MIN6 and NIH3T3 cell lines were used to optimize the conditions for the multiplex real-time PCR assay (Figure 1D). Bisulfite-treated DNA from NIH3T3 cells exhibited a cycle threshold (Ct) number for the methylated cytosine-specific VIC-labeled probe of 41.61 ± 0.44 cycles, whereas MIN6 cells did not reach detection after 60 amplification cycles. For the unmethylated cytosine-specific FAM-labeled probe, MIN6 DNA reached a detectable Ct (34.01 ± 0.49) as did NIH3T3 DNA (35.99 ± 0.49). These findings affirm the sequencing data.
To determine whether our assay preferentially identifies either necrosis or apoptosis, we treated mouse islets overnight with a mixture of proinflammatory cytokines (IL-1β, TNF-α, interferon-γ) that is thought to cause primarily necrosis (8) or 10 mM thapsigargin, which leads to caspase 3-mediated apoptosis (11). Medium from control, untreated islets exhibited an unmethylated PPI Ct of 46.58 ± 1.9, whereas medium from cytokine-treated islets showed a lower umethylated PPI Ct of 37.6 ± 0.9; similarly, medium from thapsgargin-treated islets showed a lower unmethylated Ct of 41.06 ± 0.8. These data suggest that release of unmethylated PPI DNA occurs under conditions of both necrosis and apotosis.
The unmethylation index can differentiate between insulin-producing and noninsulin-producing tissues
To determine our assay's ability to distinguish insulin-producing from noninsulin-producing tissues, we isolated DNA from different mouse organs. We expressed the assay results as an “unmethylation index,” defined as the ratio of the Ct of the methylated cytosine-specific probe (VIC) to the Ct of the unmethylated cytosine-specific probe (FAM): 2(methylated Ct − unmethylated Ct). As such, a higher unmethylation index reflects a relative abundance of unmethylated PPI DNA. There were no significant differences in the unmethylation indices of liver, brain, fat, or thymus PPI DNA (Figure 1E). However, islets exhibited a significantly higher unmethylation index compared to other tissues, consistent with their containment of β-cells.
Unmethylation indices in the serum increase prior to hyperglycemia in mouse models of diabetes
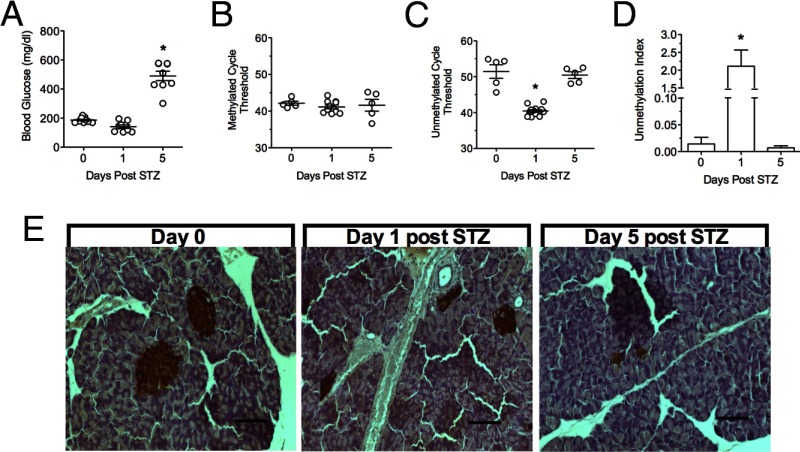
To measure differentially methylated PPI DNA in vivo, we examined 3 mouse models of diabetes mellitus. First, we evaluated the rapid onset of β-cell death and diabetes in the high-dose STZ model (12). Random blood glucose values were slightly below normal 1 day after injection, consistent with release of insulin from dying β-cells, and became significantly elevated after 5 days (Figure 2A). DNA was extracted from serum samples taken at 1 and 5 days post-STZ and analyzed using our multiplex assay. Whereas the methylated PPI DNA Ct values remained unchanged, the unmethylated PPI DNA Ct values decreased significantly 1 day post-STZ and returned to baseline levels 5 days post-STZ (Figure 2, B and C). The unmmethylated PPI DNA Ct decrease at day 1 represents a 100-fold increase in the unmethylation index (from ∼0.02 to 2.0) (Figure 2D), suggesting an acute release of unmethylated PPI DNA from dying β-cells. Immunohistochemical staining of pancreata at day 1 showed distinctly reduced insulin staining, consistent with a reduction in β-cell mass (Figure 2E). By day 5, insulin staining in the pancreas was rare.
Figure 2.
Blood Glucose and Unmethylation Index Values in Mice Treated with High-Dose STZ. A, Random longitudinal blood glucose values from high-dose STZ-treated BL6 mice. B, Ct values of methylated PPI DNA. C, Ct values of unmethylated PPI DNA. D, Unmethylation index at 0, 1, and 5 days post-STZ injection. E, Insulin (brown) and hematoxylin (blue) staining of pancreas tissue at 0, 1, and 5 days post-STZ injection (scale bar, 100 μm). n = 5, *, P < .05 compared with time 0.
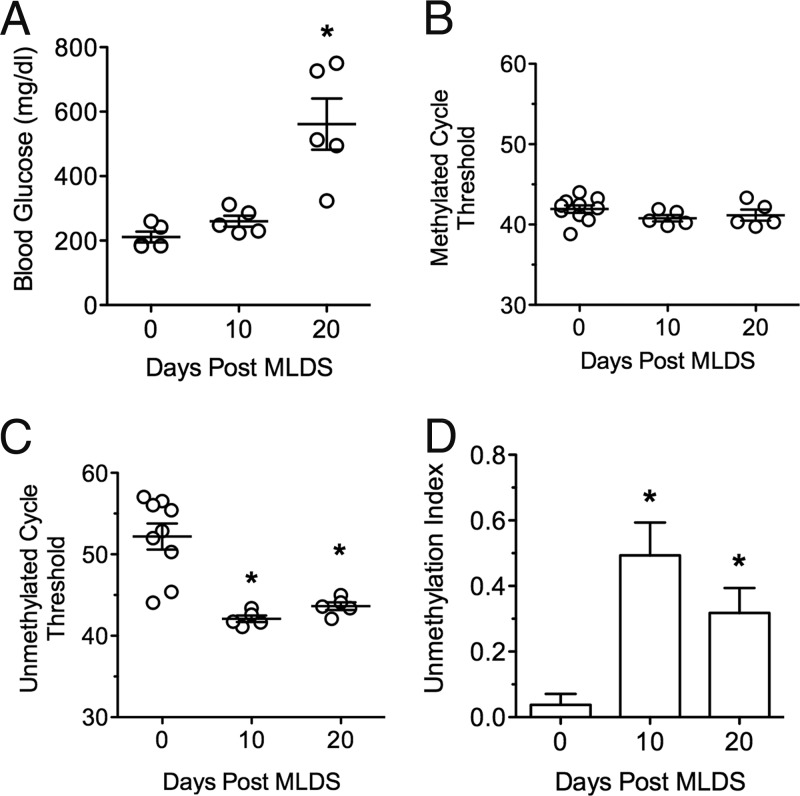
Multiple low-dose STZ (MLDS) administration leads to a gradual loss of β-cells with hyperglycemia development over weeks (13). In the MLDS mice, random blood glucose values were statistically unchanged 10 days after the initial injection, but significantly increased by 20 days (Figure 3A). The Ct values for methylated PPI DNA did not change, but the Ct values for unmethylated PPI DNA showed a significant decrease by 10 days, leading to an approximately 10-fold increase in the unmethylation index (Figure 3, B–D). By 20 days, when blood glucose values were diagnostic of diabetes, the unmethylation index decreased, but not to baseline values.
Figure 3.
Blood Glucose and Unmethylation Index Values in Mice Treated with MLDS. A, Random longitudinal blood glucose values from MLDS-treated BL6 mice at 0, 10, and 20 days after first injection. B, Ct values of methylated PPI DNA. C, Ct values of unmethylated PPI DNA. D, Unmethylation index at 0, 10, and 20 days post-MLDS treatment. n = 5; *, P < .05 compared with time 0.
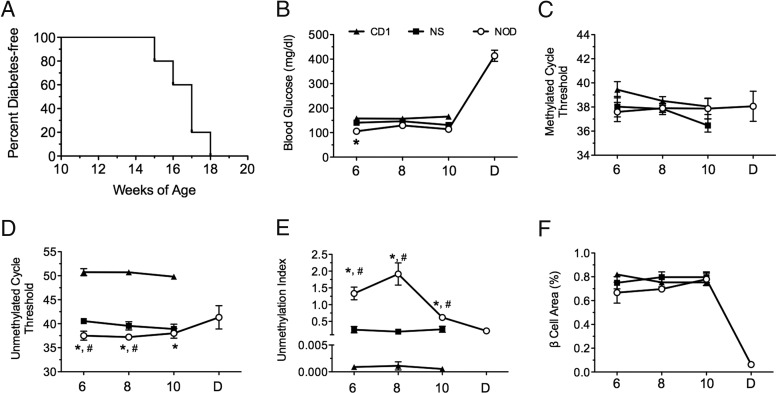
Next, we studied the NOD mouse model of autoimmune diabetes. Female NOD mice develop spontaneous islet inflammation at approximately 4 weeks of age, and frank diabetes between 12 and 20 weeks of age (14) (see Figure 4A). We compared female NOD mice with female CD1 and NOD.SCID mice, neither of which develop islet inflammation or diabetes. Figure 4B shows random blood glucose values at 6, 8, and 10 weeks. The Ct values for methylated PPI DNA did not differ between any of the mouse groups studied (Figure 4C), but the unmethylated PPI DNA Ct values were significantly different between strains (Figure 4D). NOD mice exhibited an unmethylation index of just greater than 1.0 at 6 and 8 weeks of age and just less than 1.0 at 10 weeks, whereas NOD.SCID mice and CD1 mice were significantly lower (<0.4 and < 0.005, respectively, Figure 4E). Notably, relative β-cell area in the prediabetic NOD mice was similar to NOD.SCID and CD1 (Figure 4F), suggesting that β-cell death in NOD mice during this period was likely balanced by an increased proliferative response (15).
Figure 4.
Diabetes Incidence, Blood Glucose, and Methylation Index Values in NOD, NOD.SCID, and CD1 Mice. A, Diabetes incidence curve and (B) random longitudinal blood glucose values. C, Ct values of methylated PPI DNA. D, Ct values of unmethylated PPI DNA. E, Unmethylation index at 6, 8, and 10 weeks of age and after diabetes diagnosis in NOD mice, NOD.SCID, and CD1 mice. F, Percent β-cell area. n = 5; *, P < .05 compared with CD1 mice; #, P < .05 compared with NOD.SCID mice by one-way ANOVA.
Discussion
Type 1 diabetes is characterized by early islet inflammation followed by frank β-cell death. During the early phases of disease in mice and humans, there is a subclinical period in which β-cell death occurs prior to hyperglycemia (14, 16, 17). Identification of β-cell death in high-risk individuals in this early phase might allow for the initiation of therapies that preserve β-cell mass, and thereby delay or prevent diabetes onset. Recent studies reported that appearance of increased unmethylated PPI DNA in the serum might reflect the onset of dying β-cells (3, 5). In this study, we report a new methodology based on this hypothesis, with some advantages over prior methods.
Methylated cytosines at CpG sites correlate with gene silencing (18, 19). Accordingly, studies have examined the distribution of methylated and unmethylated cytosines in the insulin gene as a mechanism for silencing non-β or activating β-cells (2). We surmised that the discrete unmethylated cytosines in the PPI promoter in β-cells could be detected using the TaqMan SNP genotyping assay methodology (10). Here, we showed the ability to simultaneously detect methylated and unmethylated cytosines at position −182 bp in the mouse 2 PPI promoter in cell lines and primary mouse tissues. Compared with the methylation-specific PCR using SYBR-Green introduced previously (3–5), positive attributes of this assay include simultaneous detection of both methylated and unmethylated cytosines in a single reaction without use of a nested PCR, low background levels, and linearity over a range of Ct values observed in serum.
Use of the unmethylation index allows for minimization of interassay variability by simultaneous normalization to the methylated PPI DNA. In the mouse models of diabetes studied here, the methylated PPI DNA Ct did not appear to change. This finding is not surprising, given that most PPI DNA in the serum derives from non-β-cells, which are mostly methylated at the −182 bp cytosine (2). A notable finding in our study is that the unmethylation index rises prior to the development of hyperglycemia in all 3 animal models. The subsequent fall in the unmethylation index upon diabetes development likely reflects the minimal β-cells remaining (no further release of new unmethylated DNA). What is unknown is the clearance rate of PPI DNA from the serum. This information will be crucial in determining the “window” of time in which the assay is likely to detect underlying β-cell death. Nevertheless, our findings show that we can detect the appearance of unmethylated DNA in the serum prior to the onset of frank diabetes, suggesting that subclinical β-cell death can be detected with minimally invasive techniques. An additional observation in our study is that NOD.SCID mice, which never spontaneously develop diabetes, exhibit an unmethylation index intermediate between CD1 and NOD mice. We believe this observation may reflect the inherent stress that exists in β-cells from the NOD background, as described in our earlier report (14). Although we have not studied it here, our method may also be useful in detecting β-cell-cell death in the progression from prediabetes to overt type 2 diabetes.
Important assumptions and caveats to our assay should be noted. Our assay assumes that the bisulfite reaction is similarly efficient from sample to sample, that the reaction is uniform across the entire stretch of a DNA strand, and that the reaction is independent of the frequency of methylated cytosine bases in a given strand. These are reasonable assumptions, because the reaction is chemical in nature and is conducted on all samples simultaneously. Most importantly, our assay likely does not detect true abundances of methylated vs unmethylated PPI DNA, because the fluorescence intensity of the probes (VIC vs FAM) is substantially different (Figure 1D), and the threshold fluorescence that is assigned is identical for both probes in a single reaction. As such, the unmethylation index is not necessarily a ratio of abundance of the 2 species. Nevertheless, the linearity of the Ct values suggests that the index reflects actual changes in the relative abundance of each species. Also, we do not know whether our assay identifies necrotic death differently than apoptotic death, although both types of mechanisms may be operating in type 1 diabetes however our data suggest that both can be measured. Future studies will focus on use of this assay in human samples to detect early β-cell death in diabetes.
Acknowledgments
We thank the Indiana University Diabetes Research Center Islet Core for assistance with obtaining islets. We thank Mr Andrew Templin (Indiana University) and Ms Aarthi Maganti (Indiana University) for their assistance with cell culture.
This work was supported by a Clinical and Translational Sciences Award KL2 grant (KL2 TR000163 to S.A.T.) and grant R01 DK083583 (to R.G.M.) from the National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Ct
- cycle threshold
- MLDS
- multiple low-dose STZ
- PPI
- preproinsulin
- SNP
- single-nucleotide polymorphism
- STZ
- streptozotocin.
References
- 1. Harjutsalo V, Sjöberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet. 2008;371:1777–1782 [DOI] [PubMed] [Google Scholar]
- 2. Kuroda A, Rauch TA, Todorov I, et al. Insulin gene expression is regulated by DNA methylation. PloS one. 2009;4:e6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akirav EM, Lebastchi J, Galvan EM, et al. Detection of β cell death in diabetes using differentially methylated circulating DNA. Proc Natl Acad Sci USA. 2011;108:19018–19023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lebastchi J, Deng S, Lebastchi AH, et al. Immune therapy and β-cell death in type 1 diabetes. Diabetes. 2013;62:1676–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Husseiny MI, Kuroda A, Kaye AN, Nair I, Kandeel F, Ferreri K. Development of a quantitative methylation-specific polymerase chain reaction method for monitoring β cell death in type 1 diabetes. PloS one. 2012;7:e47942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stull ND, Breite A, McCarthy R, Tersey SA, Mirmira RG. Mouse islet of Langerhans isolation using a combination of purified collagenase and neutral protease. J Vis Exp. 2012;67:e4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tersey SA, Carter JD, Rosenberg L, Taylor-Fishwick DA, Mirmira RG, Nadler JL. Amelioration of type 1 diabetes following treatment of non-obese diabetic mice with INGAP and lisofylline. J Diabetes. 2012;2:251–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maier B, Ogihara T, Trace AP, et al. The unique hypusine modification of eIF5A promotes islet β cell inflammation and dysfunction in mice. J Clin Invest. 2010;120:2156–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Livak KJ. Allelic discrimination using fluorogenic probes and the 5′ nuclease assay. Genet Anal. 1999;14:143–149 [DOI] [PubMed] [Google Scholar]
- 10. De la Vega FM, Lazaruk KD, Rhodes MD, Wenz MH. Assessment of two flexible and compatible SNP genotyping platforms: TaqMan SNP genotyping assays and the SNPlex genotyping system. Mutat Res. 2005;573:111–135 [DOI] [PubMed] [Google Scholar]
- 11. Robbins RD, Tersey SA, Ogihara T, et al. Inhibition of deoxyhypusine synthase enhances islet β cell function and survival in the setting of endoplasmic reticulum stress and type 2 diabetes. J Biol Chem. 2010;285:39943–39952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Antkowiak PF, Tersey SA, Carter JD, Vandsburger MH, Nadler JL, Epstein FH, Mirmira RG. Noninvasive assessment of pancreatic β-cell function in vivo with manganese-enhanced magnetic resonance imaging. Am J Physiol Endocrinol Metab. 2009;296:E573–E578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lukić ML, Stosić-Grujicić S, Shahin A. Effector mechanisms in low-dose streptozotocin-induced diabetes. Dev Immunol. 1998;6:119–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tersey SA, Nishiki Y, Templin AT, et al. Islet β-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model. Diabetes. 2012;61:818–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sherry NA, Kushner JA, Glandt M, Kitamura T, Brillantes AM, Herold KC. Effects of autoimmunity and immune therapy on β-cell turnover in type 1 diabetes. Diabetes. 2006;55:3238–3245 [DOI] [PubMed] [Google Scholar]
- 16. Ize-Ludlow D, Lightfoot YL, Parker M, et al. Progressive erosion of β-cell function precedes the onset of hyperglycemia in the NOD mouse model of type 1 diabetes. Diabetes. 2011;60:2086–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ferrannini E, Mari A, Nofrate V, Sosenko JM, Skyler JS, DPT-1 Study Group Progression to diabetes in relatives of type 1 diabetic patients: mechanisms and mode of onset. Diabetes. 2010;59:679–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baylin SB, Ohm JE. Epigenetic gene silencing in cancer—a mechanism for early oncogenic pathway addiction? Nat Rev Cancer. 2006;6:107–116 [DOI] [PubMed] [Google Scholar]
- 19. Robertson KD. DNA methylation and human disease. Nat Rev Genet. 2005;6:597–610 [DOI] [PubMed] [Google Scholar]