Abstract
Elevated levels of circulating proinflammatory cytokines are associated with obesity and increased risk of type 2 diabetes, but the mechanism is unknown. We tested whether proinflammatory cytokines IL-1B+IL-6 at low picogram per milliliter concentrations (consistent with serum levels) could directly trigger pancreatic islet dysfunction. Overnight exposure to IL-1B+IL-6 in islets isolated from normal mice and humans disrupted glucose-stimulated intracellular calcium responses; cytokine-induced effects were more severe among islets from prediabetic db/db mice that otherwise showed no signs of dysfunction. IL-1B+IL-6 exposure reduced endoplasmic reticulum (ER) calcium storage, activated ER stress responses (Nos2, Bip, Atf4, and Ddit3 [CHOP]), impaired glucose-stimulated insulin secretion, and increased cell death only in islets from prediabetic db/db mice. Furthermore, we found increased serum levels of IL-1B and IL-6 in diabetes-prone mice at an age before hyperglycemia was exhibited, suggesting that low-grade systemic inflammation develops early in the disease process. In addition, we implanted normal outbred and inbred mice with subcutaneous osmotic mini-pumps containing IL-1B+IL-6 to mimic the serum increases found in prediabetic db/db mice. Both IL-1B and IL-6 were elevated in serum from cytokine-pump mice, but glucose tolerance and blood glucose levels did not differ from controls. However, when compared with controls, isolated islets from cytokine-pump mice showed deficiencies in calcium handling and insulin secretion that were similar to observations with islets exposed to cytokines in vitro. These findings provide proof of principle that low-grade systemic inflammation is present early in the development of type 2 diabetes and can trigger ER stress-mediated islet dysfunction that can lead to islet failure.
Obesity is a key environmental risk factor for developing type 2 diabetes (T2D), yet most obese individuals do not develop T2D. Genome-wide association studies (1) suggest that the failure of β-cells to adequately compensate for increased insulin resistance plays a key role in the progression of T2D (2, 3). A number of obesity-related factors, including glucotoxicity and lipotoxicity (4, 5), amyloid deposition (6), and inflammation (7, 8), can trigger oxidative stress and/or endoplasmic reticulum (ER) stress that can lead to β-cell dysfunction and death (5, 9).
Among contributing factors, low-grade systemic inflammation precedes the development of hyperglycemia and is perhaps the earliest potential trigger of islet decline. The added metabolic stress of obesity, particularly on adipose tissue, is associated with secretion of cytokines such as IL-6, IL-8, monocyte chemoattractant protein-1 and TNF-α (10). Monocyte recruitment and differentiation by monocyte chemoattractant protein-1 results in macrophage accumulation in adipose tissue of obese individuals, further contributing to cytokine production (10). Recent work has connected excess nutrient intake with inflammasome activation mediated by innate immune cell sensors in adipose tissue (11). Inflammasome activation leads to IL-1B secretion, which can be prevented by reducing caloric intake (11). These processes collectively create a state of chronic, low-grade systemic inflammation (12). By contrast, the islet cytokine levels associated with the inflammatory processes in T1D are thought to be much higher due to infiltrating immune cells that directly secrete cytokines near the β-cell (13, 14). Increases in islet-associated macrophage infiltration and high levels of local, islet-derived cytokine production have been reported in GK rats, db/db mice, high-fat–fed mice, and human T2D patients (15). However, this form of localized pancreatic inflammation differs from the insulitis observed in T1D and appears to follow the onset of hyperglycemia.
Individuals with elevated serum levels of certain proinflammatory cytokines show increased risk for developing T2D (16–19). Of particular interest, increased serum levels of IL-6 in combination with detectable levels of IL-1B result in a significantly elevated risk for developing T2D that is not observed with either cytokine alone (18). In normal healthy adults, the serum IL-1B and IL-6 concentrations range from 0.5 to 12 and 0.5 to 5 pg/mL, respectively (16–20). Although obesity tends to increase circulating levels of cytokines by ∼50% to 100% (16–19), serum concentrations remain within the low picograms per milliliter range. Considerably higher concentrations of ∼50 to 150 pg/mL of IL-1B combined with nanograms per milliliter concentrations of IFN-γ and TNF-α are necessary to reproduce the inhibition of insulin secretion and β-cell apoptosis that are key features of T1D in the islet (21–23). The actual cytokine concentrations that islets are exposed to during insulitis in T1D have never been directly measured. For our purposes, we employ cytokines that are 1) at concentrations in the general range of circulating levels in mice and 2) linked with T2D development in humans (18).
ER stress may provide the link between inflammation and the development of β-cell dysfunction in T2D. Proinflammatory cytokines, specifically IL-1B, IFN-γ, and TNF-α in nanograms per milliliter concentrations, downregulate expression of sarco(endo)plasmic calcium ATPase 2b (SERCA2b), reduce ER calcium storage, and induce ER stress, suggesting a possible mechanism of islet dysfunction and apoptosis (24). Calcium-imaging studies of the leptin-receptor–deficient db/db mouse, a model of T2D, demonstrated that dysfunctional insulin secretion and intracellular calcium responses to glucose stimulation were closely associated with deficiencies in ER calcium sequestration in islets (25). Additional work has shown that blocking SERCA pumps depletes calcium from the ER, which results in ER stress and apoptosis in normal β-cells (26, 27). Furthermore, islets from db/db mice and human donors have reduced SERCA2b expression. Depleted ER calcium also triggers the unfolded protein response (UPR), which upregulates ER chaperones to attenuate synthesis of new proteins, correct inappropriate protein folding, and enhance protein-folding capacity (28). Chronic ER stress can hinder overall insulin production and trigger apoptosis (9, 24).
We hypothesize that obesity-induced increases in circulating cytokines are sufficient to disrupt normal β-cell function early in the disease process. In the absence of a susceptible genetic background, these disruptions are minor and may not have long-term consequence for the β-cell, but for those with a genetic risk for developing T2D, these mild disruptions could instead induce pronounced β-cell dysfunction. In this study, we examined islets from db/db mice at an age in which islet function and glycemia were normal, yet adiposity and serum levels of IL-1B and IL-6 were elevated compared with heterozygous controls. The monogenic leptin receptor defect in db/db mice produces deficiencies in islet function that coincide with the early stages of β-cell decline in human T2D (2, 3). We exposed islets from normal mice and prediabetic mice to IL-6+IL-1B in vitro to mimic serum concentrations found in obesity and then examined changes in islet function. Overnight exposure to IL-6+IL-1B was sufficient to trigger ER stress, impair ER calcium handling, impair glucose-stimulated insulin release, and increase cell death in islets from db/db mice with limited effects on islets from heterozygous controls.
Materials and Methods
Mice
All mice used in these studies were housed in a pathogen-free facility at the University of Virginia (UVA), and all protocols were approved by the Institutional Animal Care and Use Committee. Male 4- to 5-week-old BKS.Cg-Dock7m+/+ Leprdb/J (db/db) and age-matched heterozygous control mice (The Jackson Laboratory, Bar Harbor, Maine) were used for all studies unless otherwise stated. Blood glucose was taken with a One-Touch Ultra2 glucose monitor (Lifescan Inc, Milpitas, California) upon arrival as well as before euthanasia for islet isolation. Although most 4- to 5-week-old mice had normal blood glucose, hyperglycemia was observed occasionally. Mice with nonfasting blood glucose levels >300 mg/dL were excluded from this study to maintain focus on the prediabetic state. Male CD1 and C57BL6/J mice were also used for some studies as described in detail below.
Serum cytokine measurements
Blood was collected by heart puncture immediately after euthanasia from db/db and heterozygous mice, and serum samples were frozen at −20°C until assay. Serum IL-6, TNF-α, and IL-1B were measured by ELISA following the manufacturer's instructions using standard diluent buffers designed for use with mouse serum or plasma (Life Technologies, Carlsbad, California). Each cytokine was measured from different populations because each mouse typically had enough serum to measure only 1 cytokine. All samples were measured on a single 96-well plate for each cytokine, so no interassay variability is reported for this study. The mean intra-assay variability was <10% for each cytokine ELISA (IL-1B = 9.5%, TNF-α = 9.8%, and IL-6 = 8.8%). Assay sensitivity was defined as the lowest standard curve concentration with an intra-assay coefficient <20%. Based on that criterion, all cytokine values for each mouse serum sample examined were above the limit of detection and within the reportable range of each assay.
Islet isolation
Pancreatic islets were isolated and cultured as previously described (29).
Cytokine and drug treatments
Mouse cytokines (R&D Systems, Inc, Minneapolis, Minnesota) were prepared in PBS (Life Technologies) with 0.1% BSA (Roche Diagnostics, Indianapolis, Indiana) at stock concentrations of 5 μg/mL for IL-1B and 10 μg/mL for IL-6. Unless otherwise stated, all other drugs were purchased from Sigma-Aldrich (St Louis, Missouri), prepared in dimethyl sulfoxide, and kept at −80°C.
Intracellular calcium
Intracellular calcium [Ca2+]i was measured using the ratiometric [Ca2+]i indicator fura-2 AM as previously described (30). To convert fura-2 ratios to nanomolar calcium concentrations, we calibrated our calcium measurements using the method of Grynkiewicz et al (31).
Glucose-stimulated insulin secretion
Sets of 20 islets per replicate were preincubated at 37°C and 5% CO2 for 1 hour in a standard Krebs-Ringer bicarbonate solution and then tested for insulin release as previously described (32). Inter- and intra-assay variability was <10%.
Cell death measurements
We incubated islets for 15 minutes with 2 μg/mL propidium iodide (PI) (Sigma-Aldrich) plus 50 μl/mL annexin V fluorescein conjugate (Life Technologies). Islets were imaged once under bright-field illumination to determine the islet borders, then reimaged using 480-nm excitation and 535-nm emission to measure annexin V fluorescence, followed by 535-nm excitation and 610-nm emission to measure PI fluorescence. Fluorescent signals were averaged over the surface area of each islet, thus accounting for differences in islet size or cell number that could impact the results. The Cell Death Detection ELISA Plus kit (Roche Diagnostics) was also used following the manufacturer's instructions to assess cytoplasmic histone-associated DNA fragments after induction of apoptosis.
RT-PCR procedure
Total RNA from islets was isolated with RNeasy mini kit (QIAGEN, Valencia, California) and reverse-transcribed using Quantitect reverse transcription Kit (QIAGEN) according to the manufacturer's protocol. The resulting cDNA was subjected to RT-PCR using SYBR Green-based technology and gene-specific primers (for primer sequences, see Supplement Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). For the thermal cycle reaction Stratagene Mx3000P Real-Time PCR System (Agilent Technologies, Santa Clara, California) and Sensimix SYBR Low ROX master mix (Bioline, Taunton, Massachusetts) were used. Homogeneity of products from each reaction was confirmed by melting curve analysis and the threshold cycle (CT) methodology was used to calculate relative quantities of mRNA products from each sample. Note that xbp1 and xbp1s were tested as published previously (33). All samples were corrected for total RNA input by normalizing CT values to the CT value of Actb message. All data represent the average of triplicate samples, each consisting of pooled islets from 3 mice.
Osmotic mini-pump experiments
ALZET (Cupertino, California) osmotic mini-pumps (model 1007d, rate 0.5 μl/h for 7 days) were used for sc delivery of proinflammatory cytokines in vivo to 5-week-old male CD1 mice for one trial and 11-week-old C57BL6/J mice for an additional trial. Carrier-free murine IL-1B and IL-6 (R&D Systems) were loaded into mini-pumps according to the manufacturer's instructions at concentrations of 32 μg/mL for IL-1B and 4 μg/mL for IL-6 and placed in sterile saline at 37°C overnight (18–22 hours) to prime the pump. Mice were anesthetized using isoflurane, and an incision was made at the nape of the neck to insert a mini-pump containing either IL-1B+IL-6 or saline into the sc space. Sham surgery was used as a secondary control. Wound clips were then used to close the incision. Mice were given pain medication, allowed to recover, and checked for health twice per day for the first 3 days. Glucose tolerance tests were performed on day 4 after surgery by the UVA Animal Characterization Core as published previously (34), and mice were euthanized for islet isolation on day 6 after surgery.
Data analysis
A 2-tailed Student's t test was used for all comparisons related to studies shown in Figures 1, 6, and 8. A Wilcoxon signed rank test was used to compare the effects of cytokine exposure on insulin secretion in Figure 2 against each strain's respective untreated control. We used a Wilcoxon test for this comparison because a normality assumption of error distribution was not satisfied for the 3mM glucose condition and because we are primarily interested in insulin release differences between untreated and cytokine-exposed groups in each strain. A 1-way ANOVA with Tukey's posttest was used for all calcium studies shown in Figures 3 to 5 and cell death studies in Figure 7 to examine statistical differences across multiple conditions. P values <.05 indicate statistical significance.
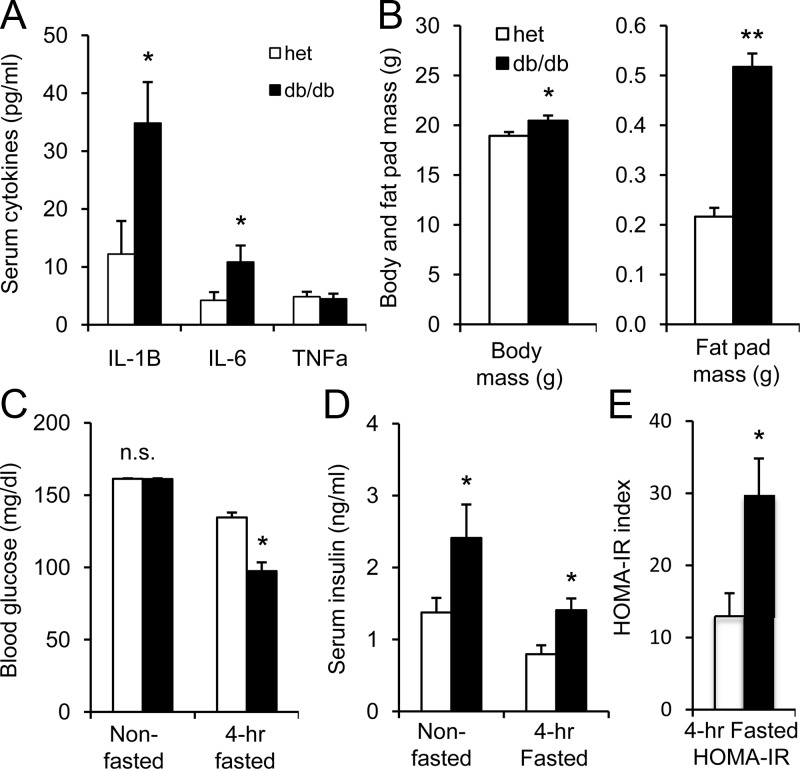
Figure 1.
Comparison of db/db vs heterozygous mice at 4 to 5 weeks of age. A, Serum levels of IL-1B, IL-6, and TNF-α in heterozygous (het; white bars) mice vs db/db mice (black bars); n = 9–12 mice per strain for each cytokine measured. B, Body mass and fat pad mass from heterozygous control mice (n = 67; white) and db/db mice (n = 53; black). C, Nonfasted (heterozygous, n = 67; db/db, n = 53) and fasted (heterozygous, n = 31; db/db, n = 27) blood glucose levels (n.s., not significant). D, Nonfasted (heterozygous, n = 8; db/db, n = 9) and fasted (heterozygous, n = 4; db/db, n = 3) serum insulin levels. *, P < .05; **, P < .01.
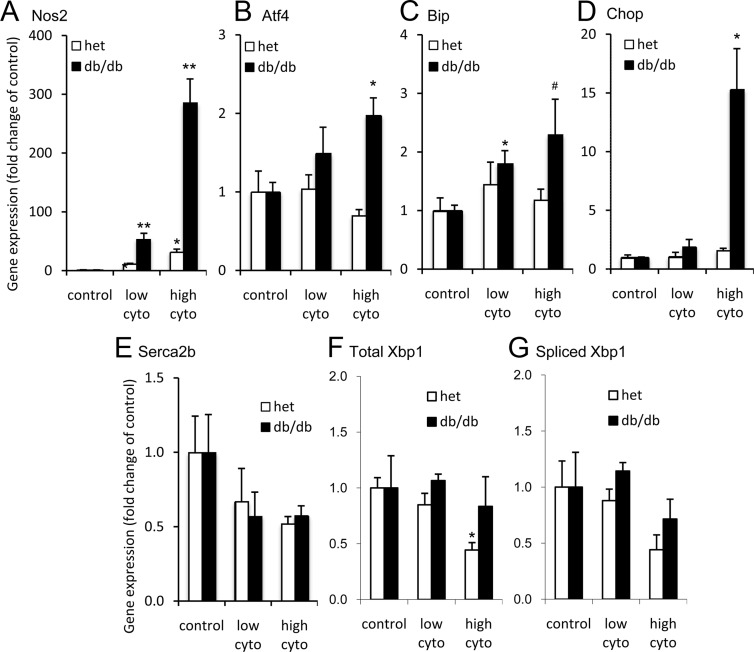
Figure 6.
ER stress genes activated in db/db islets. A–E, Islet mRNA expression in untreated (control [con]), low-cytokine (cyto) (5 pg/mL IL-1B plus 10 pg/mL IL-6) and high-cytokine (100 pg/mL IL-1B plus 200 pg/mL IL-6) conditions for Nos2 (A), Atf4 (B), Bip (C), Chop (D), Serca2b (E), total xbp1 (F), and spliced xbp1 (G). Three sets of islets per condition were pooled from 2 to 4 heterozygous mice (het; white bars) or 2 to 4 db/db mice (black bars) for each set. Asterisks indicate cytokine effects determined by Student's t test against untreated control islets from each respective strain. *, P < .05; **, P < .01; #, P = .10.
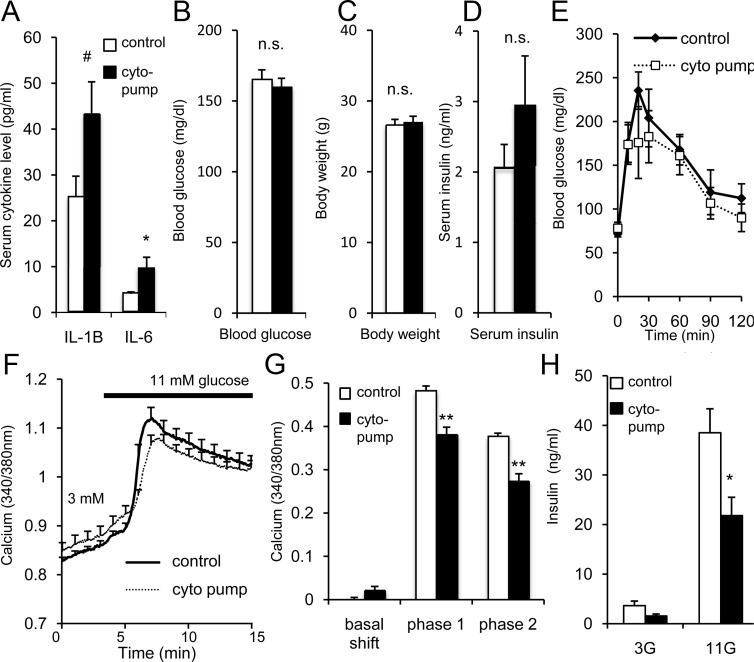
Figure 8.
In vivo cytokine exposure mimics low-grade inflammation. A, Mean serum levels of IL-1B and IL-6 for mice implanted with cytokine-loaded osmotic mini-pumps (cyto pump, n = 7) or controls (saline pump, n = 4; and sham surgery, n = 9; control groups did not differ and were combined for analysis). B–D, Corresponding mean blood glucose (B), body weight (C), and serum insulin levels (D). For A–D, white bars indicate control and black bars indicate cytokine pump. E, Glucose tolerance test comparing cyto-pump (solid line, n = 4) mice and sham-surgery control mice (dashed line, n = 5). F, GSCa traces from 1 representative trial conducted on islets isolated from cyto pumps vs control. G, Mean GSCa results for basal calcium (displayed relative to control values), phase 1, and phase 2 among 4 trials using >5 islets for each condition for each trial. H, GSIS for islets from cyto-pump mice (n = 4) vs sham-surgery control mice (n = 4). *, P < .05; **, P < .01; #, P < .10; n.s., not significant. 3G, 3 mM glucose; 11G, 11 mM glucose.
Figure 2.
Overnight exposure to IL-1B+IL-6 reduces GSIS in islets from db/db mice compared with heterozygous (het) controls. A and B, Mean insulin release in 1 hour from islets in modified Krebs-Ringer bicarbonate solutions containing 3mM glucose (A) or 11mM glucose (B). Data represent 4 trials of 2 to 3 replicates for a total of 13 replicates per condition. Twenty islets were used for each replicate of each condition. Controls (white bars) were untreated; cytokine treatment (cyto; black bars) was 5 pg/mL IL-1B plus 10 pg/mL IL-6. *, P < .05.
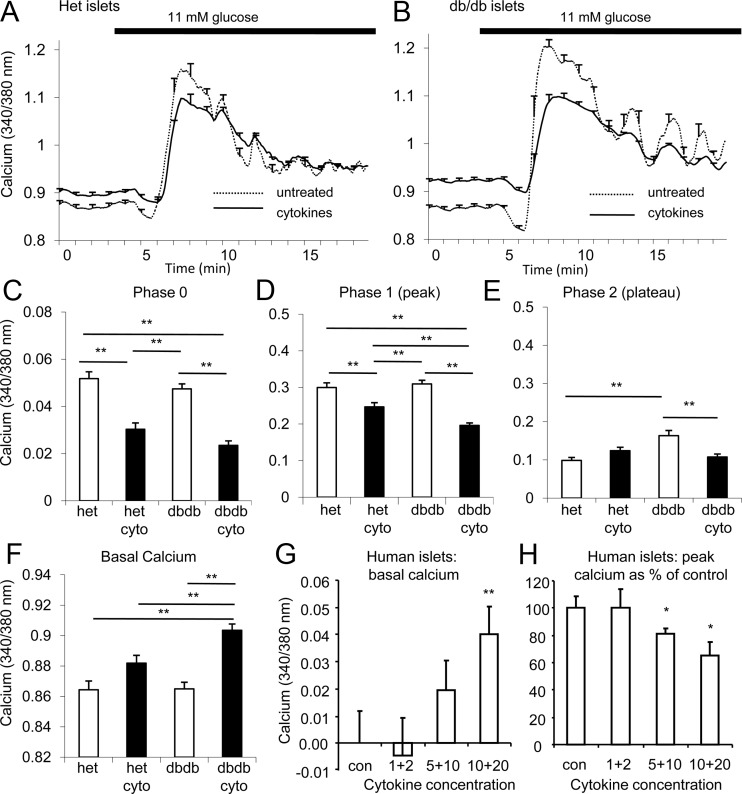
Figure 3.
Overnight exposure to IL-1B+IL-6 magnifies effects on islets from prediabetic mice compared with nondiabetes-prone controls. A and B, GSCa traces among islets isolated from heterozygous (het; A) and db/db (B) mice at 4 to 5 weeks of age that were either untreated (dashed line) or exposed to 5 pg/mL IL-1B plus 10 pg/mL IL-6 (solid line, cytokines). C–F, Mean values for phase 0 (C), phase 1 (D), phase 2 (E), and basal calcium (F) after overnight cytokine (cyto) exposure. G and H, Cytokine effects on basal calcium relative to untreated controls (G) and phase 1 (H) in human islets. *, P < .05; **, P < .01. Bars with asterisks indicate what groups were statistically significant in C–F. For G and H, asterisks indicate differences compared with untreated control islets. At least 3 trials were performed using >5 islets for each condition for each trial.
Figure 5.
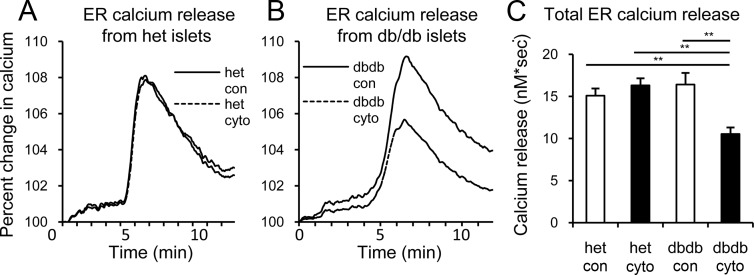
Cytokine-induced changes in ER calcium. A and B, Representative traces of islet calcium release from the ER in response to acute SERCA blockade with CPA for islets from heterozygous (het; A) or db/db (B) mice. C, Mean values for total CPA-induced calcium release determined by area under the curve. **, P < .01. Two trials were performed using >5 islets for each condition for each trial. Abbreviations: cyto, cytokine; con, control.
Figure 7.
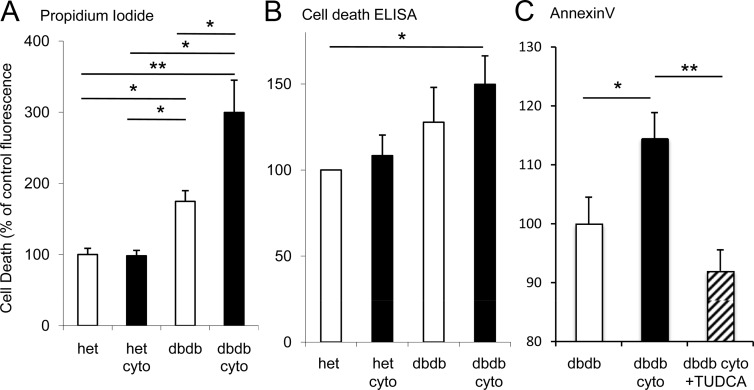
Increased cell death rates among cytokine-exposed db/db mouse islets. A and B, Cell death is reported as percentage of values for untreated islets from heterozygous mice (het) as measured by PI fluorescence (A), Cell Death Detection ELISA (B), or annexin V fluorescence (C). Cyto indicates islets exposed overnight to 5 pg/mL IL1-B plus 10 pg/mL IL-6. The number of islets assessed for each condition ranged from 80 to 105 for PI and 39 to 46 for annexin V and was 50 per condition for each of 4 trials for ELISA. *, P < .05; **, P < .01.
Results
Prediabetic db/db mice have increased circulating cytokine levels
Increased levels of the proinflammatory cytokines IL-1B and IL-6 together are associated with increased risk of T2D in humans (18), so we examined whether these cytokines were also elevated in db/db mice. As shown in Figure 1A, increased serum levels of IL-1B and IL-6, but not TNF-α (a cytokine commonly associated with obesity), were observed in 4- to 5-week-old db/db mice. Increased serum cytokine levels were associated with small body weight differences (<10%) in a large sample size of db/db mice (n = 53) and heterozygous control mice (n = 67); gonadal fat tissue weights used as an estimate of body fat, in contrast, were markedly greater among db/db mice (Figure 1B). Nonfasted blood glucose values did not differ between strains, but 4-hour fasted blood glucose levels were significantly lower (Figure 1C), and serum insulin levels were elevated among db/db mice (Figure 1D).
Although reduced fasting blood glucose might suggest the db/db mice are more insulin-sensitive, homeostasis assessment model of insulin resistance (HOMA-IR) calculations (HOMA-IR = [blood glucose × insulin (ng/mL) × 26 μU/mL]/405) indicated that insulin resistance for db/db mice was more than double that of heterozygous mice (Figure 1E), suggesting increases in both insulin release and insulin resistance in the early stages of T2D (2, 3). Because of the short fast, the HOMA-IR values for both heterozygous and db/db mice are much higher than normally observed with an overnight fast, but the point is that db/db mice have significantly higher levels of insulin resistance. An alternative possibility is that elevated serum IL-1B and IL-6 in db/db mice might directly stimulate increased insulin release as suggested by some in vitro studies (35, 36); however, this does not agree with our observed cytokine effects on insulin secretion as shown below.
Cytokines reduce glucose-stimulated insulin secretion in islets from prediabetic mice
We first examined the effects of low-dose IL-1B+IL-6 on glucose-stimulated insulin secretion (GSIS) because these cytokines are associated with human T2D development (18) and elevated in db/db mice. A unique effect of 18 to 22 hours of IL-1B+IL-6 exposure on calcium handling at basal glucose was observed (see Supplemental Figure 1), which was not observed with either cytokine alone, whereas a synergistic effect of the 2 cytokines was observed at 11mM glucose. As shown in Figure 2A, IL-1B+IL-6 had no effect on insulin release in low (3mM) glucose conditions, as previously reported for other low-dose cytokines (32). Islets from untreated db/db mice appeared to release more insulin in low glucose compared with islets from untreated heterozygous mice (not significant), consistent with left-shifted glucose sensitivity, which is a hallmark of β-cell compensation in early T2D (2, 3). In contrast, cytokines significantly inhibited insulin secretion in 11mM glucose as determined by Wilcoxon signed rank test (Figure 2B) only in islets from db/db mice, with no significant effect in the control heterozygous strain. These cytokine effects were similar for 4 separate trials; trial-to-trial differences in insulin release rates contributed to the observed variance. Additionally, key genes involved with GSIS and other aspects of islet function, such as glucokinase (Gck), glucose transporter 2 (Slc2a2), pancreatic and duodenal homeobox 1 (Pdx1), or Wolfram syndrome-1 (Wfs1), were unchanged among both mice strains, as shown in Supplemental Figure 2.
Cytokines impair calcium handling in murine and human islets
We next examined the glucose-stimulated calcium (GSCa) response at 5-second intervals using the fluorescent probe fura-2 AM. This technique provides enhanced sensitivity (30) and additional insight into the kinetics of glucose stimulation that static insulin secretion measurements cannot. A GSCa response consists of 3 phases (26, 30): phase 0, a brief dip in [Ca2+]i caused primarily by increased calcium uptake by the ER; phase 1, a sharp increase in calcium influx due to the closure of KATP channels and subsequent opening of voltage-gated calcium channels that triggers release of docked insulin granules; and phase 2, a sustained elevation in [Ca2+]i that corresponds with ongoing insulin secretion.
As shown in Figure 3, A and B, untreated islets isolated from 4- to 5-week-old db/db mice showed similar GSCa responses to heterozygous controls, indicating that islets from db/db mice at this age are capable of normal or even enhanced responses to glucose stimulation. However, overnight exposure with 5 pg/mL IL-1B plus 10 pg/mL IL-6 disrupted islet calcium handling in islets from db/db mice more substantially than in islets from heterozygous controls. Cytokine exposure reduced phase 0 (Figure 3C, measured from baseline to minimum) and phase 1 (Figure 3D, measured from baseline to peak) in islets from both strains; phase 1 was even more inhibited in cytokine-exposed islets from db/db mice compared with cytokine-exposed heterozygous controls. The phase 2 [Ca2+]i response (Figure 3E, measured from baseline to mean plateau value) was significantly larger for untreated islets from db/db mice compared with heterozygous controls, which is consistent with the elevated insulin levels typically observed at this early stage of the disease. After overnight cytokine exposure, islets from db/db mice displayed a significant decrease, and islets from heterozygous mice showed no significant effect (if anything, a mild increase). These opposing cytokine effects on phase 2 are similar to what we observed for static insulin release.
Cytokine effects on basal [Ca2+]i were perhaps the most striking. As shown in Figure 3F, basal calcium in cytokine-exposed islets from db/db mice was substantially elevated against all other treatment groups with no other significant differences observed among the other groups (P < .01, ANOVA). Because [Ca2+]i is so tightly regulated within cells, this seemingly small change represents an increase in basal [Ca2+]i of ∼30% globally and could indicate much larger shifts within calcium microdomains (37). These data suggest deficiencies in calcium buffering within islets from db/db mice. The effects of IL-1B+IL-6, especially on basal [Ca2+]i, were more pronounced in islets from a small subset of 4- to 5-week-old db/db mice that were already showing evidence of hyperglycemia (Supplemental Figure 3). In addition, we found a significant elevation in basal [Ca2+]i among untreated islets from the hyperglycemic group as compared with untreated islets from normoglycemic db/db and heterozygous mice, thus associating basal [Ca2+]i disruptions with hyperglycemia (Supplemental Figure 3).
To demonstrate that these cytokine effects are β-cell–specific, we developed a technique to identify individual dispersed islet cells that respond to glucose stimulation like β-cells. As with islets, overnight exposure to IL-1B+IL-6 resulted in increased basal calcium levels and reduced peak calcium response to glucose stimulation in these dispersed islet cells (see Supplemental Figure 4).
Recombinant human IL-1B+IL-6 had similar effects on basal and stimulated calcium in human islets from 3 donors, as shown in Figure 3, G and H, using concentrations in the range of published serum values (20). Note that for the studies with human islets, stimulation with 28mM glucose plus 20mM KCl was used to maximize the calcium response. This tends to obscure the phase 0 and phase 2 responses, so only basal and peak calcium are reported.
ER stress and the effects of cytokine exposure on intracellular calcium handling
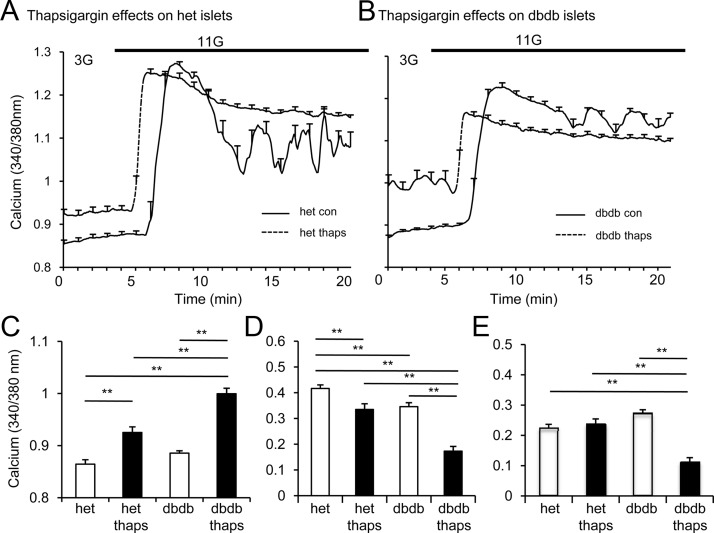
Several studies implicate the ER as a mediator of cytokine action (24, 38), and our previous work indicates that elevated basal calcium is associated with reduced calcium buffering within the ER (32, 39). We thus examined whether disrupting ER calcium handling could reproduce cytokine effects. In Figure 4, A and B, overnight treatment with the SERCA pump Ca2+-ATPase inhibitor thapsigargin raised the basal [Ca2+]i and inhibited the peak calcium response to glucose stimulation, as reported previously (25, 32). We found thapsigargin-induced effects were more severe in db/db mouse islets in terms of basal calcium (Figure 4C), phase 1 (Figure 4D), and phase 2 (Figure 4E), similar to effects observed under low-dose cytokine exposure (compare with Figure 3). Thapsigargin entirely eliminates phase 0, so values were not reported. Note that thapsigargin effects on islet function were generally greater than low-dose cytokine effects (compare Figures 3 and 4), suggesting that thapsigargin treatment may not be an appropriate model for ER stress caused by low-grade inflammation. Nevertheless, the key point is that islets from db/db mice are more susceptible to ER dysfunction, whether from exposure to thapsigargin or low-dose cytokines.
Figure 4.
SERCA blockade mimics cytokine effects. A and B, GSCa traces from 1 representative trial of islets from heterozygous (het; A) and db/db (B) mice after overnight treatment with 1μM thapsigargin (thaps; solid line) or untreated (control [con]; dashed line). C–E, Mean values among islets from heterozygous or db/db mice for basal calcium (C) and phase 1 (D) and phase 2 (E) calcium responses to glucose stimulation after treatment with 1μM thapsigargin (thaps) or untreated. *, P < .05; **, P < .01. At least 3 trials were performed using >5 islets for each condition for each trial.
Cytokines disrupt ER calcium buffering capacity
Cytokine impact on ER calcium storage was assessed in islets from db/db and control mice using cyclopiazonic acid (CPA) after overnight exposure to IL-1B+IL-6. CPA is a reversible SERCA inhibitor that prevents calcium uptake into the ER, thus permitting ER calcium release into the cytosol. As shown in Figure 5, A and B, cytokines had negligible effects on CPA-induced release of ER calcium in islets from heterozygous mice but substantially reduced ER calcium in db/db islets. Note that all data are normalized to baseline calcium for comparisons of CPA-induced calcium release. CPA-induced calcium release in Figure 5C showed an ∼40% drop in ER calcium exclusively in the cytokine-exposed db/db islets, as measured by area under the curve.
Cytokines activate ER stress genes in prediabetic murine islets
We further investigated the differences between islets from db/db and control mice by examining the expression of genes involved in the UPR to ER stress. In Figure 6A, both low-dose and high-dose cytokine exposure triggered greater increases in the expression of inducible nitric oxide synthase (Nos2, a key mediator of cytokine signaling) in db/db islets than in control islets. Only islets from db/db mice displayed dose-dependent increases in activating transcription factor-4 (Atf4, involved in restoring normal ER function, Figure 6B) and binding Ig protein (Bip, a chemical chaperone, Figure 6C), 2 protective components of the UPR. Importantly, high-dose cytokines caused a 15-fold increase in DNA-damage inducible transcript 3 (Ddit3), also known as CAAT/enhancer binding protein homologous protein (CHOP, an execution signal triggered by prolonged ER stress) in db/db mouse islets, with no response from control islets (Figure 6D). No significant changes were observed in Serca2b (Figure 6E). Cytokine exposure did not increase expression of total or spliced Xbp1 in islets from either heterozygous or db/db islets (Figure 6, F and G). In fact, exposure to high-dose cytokines reduced total Xbp1 for the heterozygous strain. No other significant differences were observed. These findings suggest that this combination of cytokines preferentially activates the Atf4/Chop arm of the ER stress pathway, but only in islets from the diabetes-prone db/db mice.
Cytokines increase cell death in prediabetic murine islets but not in control islets
Elevated CHOP expression levels suggest increased susceptibility to cell death. Islet cell death was measured using several approaches after overnight exposure to a low dose of IL-1B+IL-6. As shown in Figure 7A, untreated islets from db/db mice showed a modest increase in PI, whereas cytokine-exposed islets from db/db mice nearly tripled PI fluorescence. These findings were supported by Cell Death Detection ELISA data (Figure 7B), which showed significant increases in DNA fragmentation indicative of apoptosis only among cytokine-exposed islets from db/db mice. Similar observations were made using annexin V (Figure 7C), which measures early stages of apoptosis by phosphatidylserine translocation. Cytokine-induced cell death was rescued by relieving ER stress using chaperone molecule tauroursodeoxycholic acid (TUDCA, 50μM), as shown in Figure 7C. Similar results were observed for PI staining after TUDCA treatment (data not shown). Because cytokine exposure did not increase cell death significantly in islets from heterozygous mice, there were no notable effects upon TUDCA treatment (data not shown). Of interest, the TUDCA rescue of cell death appears to be specific to reducing ER stress rather than a general effect in reducing nitric oxide synthase. As shown in Supplemental Figure 5, TUDCA actually increased Nos2 expression in islets from outbred CD1 mice instead of decreasing it. However, TUDCA diminished cytokine-induced increases in Bip expression, suggesting alleviation of ER stress. These data suggest a role for cytokine-induced ER dysfunction as a trigger for islet cell death in a diabetes-prone background.
In vivo low-grade systemic inflammation mimics in vitro low-dose cytokine effects on islet function
To demonstrate the role of low-grade inflammation on islet function in vivo, mice were implanted with sc osmotic mini-pumps containing either saline (control) or IL-1B+IL-6. Two trials were conducted using different strains: trial 1 consisted of 11-week-old C57BL6/J mice implanted with saline pumps (n = 3), sham surgery (n = 4) or cytokine pumps (n = 3); trial 2 consisted of 8-week-old CD1 mice given saline pump (n = 1), sham surgery (n = 5), or cytokine pumps (n = 4). No differences were observed between sham and saline-pump controls in body weight, blood glucose, or serum levels of IL-1B and IL-6. Sham-surgery and saline-pump implanted mice were combined as a control group for comparison with cytokine-pump implanted mice.
Mice implanted with cytokine pumps in Figure 8A had approximately 2 to 3 times the serum levels of IL-1B and IL-6 compared with controls; serum cytokine levels were comparable to levels found in db/db and heterozygous mice. Similar findings were observed in both outbred CD1 mice and inbred C57BL6/J mice; Figure 8 represents combined data from both trials unless otherwise noted. No differences were observed in nonfasted blood glucose (Figure 8B), body weight (Figure 8C), serum insulin (Figure 8D; data from CD1 mice only), or glucose tolerance (Figure 8E; data from CD1 mice only) between cytokine-pump–implanted mice and controls after surgery. The data show that chronic low levels of inflammation are sufficient to affect β-cell function even in normal inbred (C57BL/6J) and outbred (CD1) mice. The db/db mice were not used in these studies because IL-1B and IL-6 are inherently elevated in circulation. Fasted blood glucose levels obtained at the start of the glucose tolerance test did not differ (time 0 in Figure 8E; blood glucose = 71.8 ± 3.6 mg/dL for controls and 76.3 ± 5.2 mg/dL for the cytokine pump). Islets isolated from cytokine-pump mice, however, showed mild elevations in basal calcium and reduced phase 1 and phase 2 calcium response (Figure 8, F and G) as well as reduced GSIS (Figure 8H, data from CD1 mice only) in comparison with islets from control mice. As seen in our various model systems, low-grade inflammation is capable of impacting islet function in normal healthy mice without detectable changes in overall physiological function of the animal.
Discussion
In this study, we provide evidence that cytokines associated with low-grade systemic inflammation 1) are elevated in the serum of diabetes-prone mice before developing hyperglycemia, 2) are sufficient to trigger changes in calcium homeostasis in normal murine and human islets, and 3) induce ER stress, reduce insulin secretion, and increase cell death in islets from diabetes-prone mice. We simulated the increased levels of IL-1B and IL-6 observed in prediabetic db/db mice in vivo by implanting healthy mice with sc osmotic mini-pumps containing cytokines. Islets from these cytokine-exposed mice had similar perturbations in function as islets exposed to cytokines in vitro. Together, these data suggest that low-grade inflammation could trigger β-cell decline early in the development of T2D.
IL-1B and IL-6 are sufficient, but other factors may contribute
Our rationale for examining IL-1B+IL-6 as a model of chronic low-grade systemic inflammation is based primarily on a report linking increased risk of developing T2D with the combination of increased IL-6 and increased/detectable levels of IL-1B in the bloodstream in humans (18). IL-1B is well established as a proinflammatory cytokine involved with the demise of the β-cell (40, 41). IL-6 serves as both an immunoregulator and mediator of glucose homeostasis, transmitting endocrine and/or paracrine signals, leading to protective or pathogenic activity (10, 42). Effects of IL-6 on β-cells are complex and not fully understood (10, 42). In our hands, in vitro exposure to a combination of IL-1B and IL-6 produced significant disruptions in basal calcium levels that were not observed in islets exposed to either cytokine alone (see Supplemental Figure 1). In addition, we observed increased serum levels of both IL-1B and IL-6, but not TNF-α, in db/db mice before developing hyperglycemia, indicating that these cytokine differences are present early in the disease state.
IL-1B+IL-6 thus provides a minimal model for low-grade inflammation in T2D. A number of other cytokines, chemokines, and circulating factors that are associated with obesity and T2D could also play contributing roles in β-cell decline. For example, leptin, adiponectin, resistin, and visfatin are associated with β-cell failure in T2D (43). Some cytokines act in concert to amplify effects such as the combination of TNF-α, IFN-γ, and IL-1B in models of T1D (21, 44). The primary role of IL-6 in this capacity may be to amplify the effects of IL-1B because IL-6 does not appear to affect islet function independently at low doses. In addition, glucose is known to impact cytokine effects on islets (36, 45), so the compounding factor of glucotoxicity could amplify the damaging effects of low-grade inflammation in islets as the disease progresses. Glucose and other factors can also activate inflammasomes to stimulate IL-1B production within the islet (46, 47). Note that we did not examine possible contributions of glucose in the present study because we were focusing on prediabetic db/db mice for which hyperglycemia would not yet be a factor. In summary, the full range of circulating factors that may trigger β-cell decline in T2D have yet to be determined.
Calcium as a marker of cytokine effects
Not only is calcium central to regulating ER function, insulin secretion, and other cellular processes in islets (48–50), but also in pathophysiological conditions, calcium is a key second messenger involved in cytokine signaling that can lead to β-cell dysfunction and death (24, 51–53). In this study, we used the GSCa response as a marker of cytokine effects on glucose stimulation in islets. In normal healthy islets, we found that IL-1B+IL-6 caused a mild elevation of basal [Ca2+]i, a reduction in the phase 0 dip in [Ca2+]i, and a reduction in the phase 1 (peak) response to glucose stimulation, as we have described for other cytokine combinations (32). Cytokines at circulating levels thus have physiological effects on islets.
Cytokine effects were mild enough as to not significantly disrupt insulin secretion in islets from normal healthy mice; they did, however, inhibit insulin secretion in islets from diabetes-prone mice. Static GSIS measurements largely represent insulin release associated with ongoing phase 2 in calcium signaling. Thus, the phase 2 component of the GSCa may elucidate this discrepancy because it was significantly reduced in islets from db/db mice and mildly increased (although not significantly) in control islets. Phase 2 was the only phase of the GSCa response that displayed seemingly opposite responses between strains to cytokine exposure and to thapsigargin-induced ER stress. This suggests ER involvement in the observed differences in insulin release.
ER stress and other intrinsic defects in β-cells
The ER is a major intracellular source and buffer for calcium and a key component of calcium homeostasis. We showed that cytokine exposure reduces ER calcium storage capacity in islets, induces the ER stress response and UPR genes, and has GSCa dynamics similar to those seen under thapsigargin exposure. These findings are consistent with the well-established effects of cytokine action on the ER (24, 54). Additionally, we demonstrated that circulating levels of cytokines present during prediabetes are 1) sufficient to alter ER calcium handling and 2) express activation of ER stress genes only in islets from diabetes-prone mice. Cytokine-induced cell death was found uniquely in islets from db/db mice and could be reversed by pretreatment with the chemical chaperone TUDCA, further implicating the ER in these processes.
Although ER stress appears to play a role, oxidative stress, mitochondrial dysfunction, and ion-channel defects among other factors could contribute to cytokine-induced dysfunction. For example, disruptions in mitochondrial membrane potential precede cytokine-induced β-cell death (55–57) There is also evidence that cytokines require calcium influx across the plasma membrane to induce cell death (44, 58, 59). However, these other studies used much higher (nanograms per milliliter) cytokine concentrations as compared with the concentrations that we used. Our initial characterization of cytokine exposure on GSCa responses suggested potential issues with ER calcium handling at circulating levels. We previously showed that reduced insulin release correlates with reduced ER calcium buffering, but not mitochondrial activity or plasma membrane ion channel activity (39). Furthermore, ER stress is closely associated with cytokine action in β-cells (24, 54, 57). However, we cannot disregard the possible contribution of these factors in β-cell decline in T2D.
Chronic low-grade inflammation and T2D
Whether chronic low-grade inflammation contributes to T2D development in humans remains an open question. Several studies have provided associations between serum cytokine levels and T2D (16–19), but these studies do not speak to causality. Perhaps more instructive are studies examining anti-inflammatory approaches to treating T2D. For example, antagonizing IL-1 receptor-mediated activity reduces hyperglycemia and tissue inflammation in diabetic mice (60) and humans (61) and favors the possibility of β-cell regeneration in addition to normalizing insulin secretion (41). Salsalate, a precursor of the anti-inflammatory aspirin, also improved hemoglobin A1c in patients with T2D (62, 63). Although these drug treatments suggest that anti-inflammatory approaches may be beneficial, to our knowledge, our study is the first to show that chronic low-grade inflammation may play a causal role in T2D by triggering β-cell dysfunction.
To what extent our studies reproduce the chronic state of low-grade inflammation that occurs in obesity and T2D over a period of months to years is also an open question. The effects of cytokine exposure are dose-dependent and time-dependent and are reversible up to a point (64, 65). Although our overnight studies of cytokine exposure do not nearly approach the duration of chronic inflammation in the gradual progression of T2D, we found similar effects of 7-day cytokine exposure in vivo as we did with overnight cytokine exposure in vitro. This suggests that the effects of cytokine exposure on islet function likely remain as long as chronic low-grade inflammation remains. Importantly, this supports a case for adaptation in normal islets vs maladaptation in islets from diabetes-prone individuals. Whereas islets from heterozygous mice appear to maintain viability and function, the short-term maladaptation in islets from db/db mice after overnight exposure could lead to a continuing decline in function under chronic low-grade inflammatory conditions, which would be accompanied by increased workload and additional environmental stressors as T2D progresses, leading eventually to islet failure.
Conclusions
Our observations thus support low-grade inflammation as one of the earliest factors to impact the islet's environment and function before developing T2D. Future work will focus on identifying genes that are differentially regulated between db/db and heterozygous mice in response to low-grade inflammation as well as identifying additional circulating cytokines and chemokines that may directly impact the endocrine pancreas in the early stages of T2D. Although beyond the scope of this study, the mechanism of low-grade systemic inflammation could apply to other disorders in a similar manner. For example, obesity can impact the severity of rheumatoid arthritis (66) and may contribute to specific types of asthma (67) and cancer (68). Thus, low-grade systemic inflammation caused by obesity may contribute to numerous disease states, including, but not limited to, T2D.
Acknowledgments
We thank the UVA Human Islet Isolation Laboratory for access to human islets, and we also thank Linda Langman and Kenneth Brayman for their personal support of these studies. Thanks to Lauren Wilson and Meredith Orseth for their technical assistance with data collection.
This work was supported by National Institutes of Health Grants K01 DK081621 and R01 DK089182 to C.S.N. Mouse islets were acquired through the UVA Cell and Islet Isolation Core facility. Glucose tolerance tests were performed with the help of the UVA Animal Characterization Core, Susanna R. Keller, and Stefan Hargett.
C.M.O., C.L., K.L.C., P.R.S., S.B.D., J.D.C., and J.W.R., implemented the research, interpreted results, and contributed conceptually to the manuscript. W.X. and J.K.L. interpreted results and contributed conceptually. C.M.O. and C.S.N. designed experiments, analyzed and interpreted the results, and wrote the manuscript. All authors approved the final manuscript.
Disclosure Summary: The authors declare that there is no duality of interest associated with this manuscript.
Footnotes
- [Ca2+]i
- intracellular calcium
- CPA
- cyclopiazonic acid
- CT
- threshold cycle
- ER
- endoplasmic reticulum
- GSCa
- glucose-stimulated calcium
- GSIS
- glucose-stimulated insulin secretion
- HOMA-IR
- homeostasis assessment model of insulin resistance
- PI
- propidium iodide
- SERCA2b
- sarco(endo)plasmic calcium ATPase 2b
- T2D
- type 2 diabetes
- TUDCA
- tauroursodeoxycholic acid
- UPR
- unfolded protein response.
References
- 1. Diabetes Genetics Initiative of Broad Institute of Harvard and MIT; Lund University; Novartis Institutes of BioMedical Research; Saxena R, Voight BF, Lyssenko V, et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336 [DOI] [PubMed] [Google Scholar]
- 2. Weir GC, Bonner-Weir S. Five stages of evolving β-cell dysfunction during progression to diabetes. Diabetes. 2004;53(Suppl 3):S16–S21 [DOI] [PubMed] [Google Scholar]
- 3. Leahy JL, Hirsch IB, Peterson KA, Schneider D. Targeting β-cell function early in the course of therapy for type 2 diabetes mellitus. J Clin Endocrinol Metab. 2010;95:4206–4216 [DOI] [PubMed] [Google Scholar]
- 4. Roduit R, Morin J, Massé F, et al. Glucose down-regulates the expression of the peroxisome proliferator-activated receptor-α gene in the pancreatic β -cell. J Biol Chem. 2000;275:35799–35806 [DOI] [PubMed] [Google Scholar]
- 5. Robertson RP, Harmon J, Tran PO, Poitout V. β-Cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes. 2004;53(Suppl 1):S119–S124 [DOI] [PubMed] [Google Scholar]
- 6. Haataja L, Gurlo T, Huang CJ, Butler PC. Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocr Rev. 2008;29:303–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vozarova B, Weyer C, Lindsay RS, Pratley RE, Bogardus C, Tataranni PA. High white blood cell count is associated with a worsening of insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51:455–461 [DOI] [PubMed] [Google Scholar]
- 8. Donath MY, Schumann DM, Faulenbach M, Ellingsgaard H, Perren A, Ehses JA. Islet inflammation in type 2 diabetes: from metabolic stress to therapy. Diabetes Care. 2008;31(Suppl 2):S161–S164 [DOI] [PubMed] [Google Scholar]
- 9. Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29:42–61 [DOI] [PubMed] [Google Scholar]
- 10. Gustafson B. Adipose tissue, inflammation and atherosclerosis. J Atheroscler Thromb. 2010;17:332–341 [DOI] [PubMed] [Google Scholar]
- 11. Vandanmagsar B, Youm YH, Ravussin A, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kolb H, Mandrup-Poulsen T. The global diabetes epidemic as a consequence of lifestyle-induced low-grade inflammation. Diabetologia. 2010;53:10–20 [DOI] [PubMed] [Google Scholar]
- 13. Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic β-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54(Suppl 2):S97–S107 [DOI] [PubMed] [Google Scholar]
- 14. Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and β-cell loss in type 1 diabetes. Nat Rev Endocrinol. 2009;5:219–226 [DOI] [PubMed] [Google Scholar]
- 15. Ehses JA, Perren A, Eppler E, et al. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes. 2007;56:2356–2370 [DOI] [PubMed] [Google Scholar]
- 16. Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334 [DOI] [PubMed] [Google Scholar]
- 17. Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53:693–700 [DOI] [PubMed] [Google Scholar]
- 18. Spranger J, Kroke A, Möhlig M, et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–817 [DOI] [PubMed] [Google Scholar]
- 19. Alexandraki KI, Piperi C, Ziakas PD, et al. Cytokine secretion in long-standing diabetes mellitus type 1 and 2: associations with low-grade systemic inflammation. J Clin Immunol. 2008;28:314–321 [DOI] [PubMed] [Google Scholar]
- 20. O'Gorman MR, Donnenberg AD. Handbook of Human Immunology. Boca Raton, FL: CRC Press, Taylor & Francis Group; 2008 [Google Scholar]
- 21. Eizirik DL, Sandler S, Welsh N, et al. Cytokines suppress human islet function irrespective of their effects on nitric oxide generation. J Clin Invest. 1994;93:1968–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Corbett JA, Sweetland MA, Wang JL, Lancaster JR, Jr, McDaniel ML. Nitric oxide mediates cytokine-induced inhibition of insulin secretion by human islets of Langerhans. Proc Natl Acad Sci U S A. 1993;90:1731–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Karlsen AE, Rønn SG, Lindberg K, et al. Suppressor of cytokine signaling 3 (SOCS-3) protects β -cells against interleukin-1β- and interferon-γ-mediated toxicity. Proc Natl Acad Sci U S A. 2001;98:12191–12196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cardozo AK, Ortis F, Storling J, et al. Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic β-cells. Diabetes. 2005;54:452–461 [DOI] [PubMed] [Google Scholar]
- 25. Roe MW, Philipson LH, Frangakis CJ, et al. Defective glucose-dependent endoplasmic reticulum Ca2+ sequestration in diabetic mouse islets of Langerhans. J Biol Chem. 1994;269:18279–18282 [PubMed] [Google Scholar]
- 26. Roe MW, Mertz RJ, Lancaster ME, Worley JF, 3rd, Dukes ID. Thapsigargin inhibits the glucose-induced decrease of intracellular Ca2+ in mouse islets of Langerhans. Am J Physiol. 1994;266:E852–E862 [DOI] [PubMed] [Google Scholar]
- 27. Zhou YP, Teng D, Dralyuk F, et al. Apoptosis in insulin-secreting cells. Evidence for the role of intracellular Ca2+ stores and arachidonic acid metabolism. J Clin Invest. 1998;101:1623–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shameli A, Yamanouchi J, Thiessen S, Santamaria P. Endoplasmic reticulum stress caused by overexpression of islet-specific glucose-6-phosphatase catalytic subunit-related protein in pancreatic Beta-cells. Rev Diabet Stud. 2007;4:25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carter JD, Dula SB, Corbin KL, Wu R, Nunemaker CS. A practical guide to rodent islet isolation and assessment. Biol Proced Online. 2009;11:3–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Corbin KL, Hall TE, Haile R, Nunemaker CS. A novel fluorescence imaging approach for comparative measurements of pancreatic islet function in vitro. Islets. 2011;3:14–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450 [PubMed] [Google Scholar]
- 32. Dula SB, Jecmenica M, Wu R, et al. Evidence that low-grade systemic inflammation can induce islet dysfunction as measured by impaired calcium handling. Cell Calcium. 2010;48:133–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lipson KL, Fonseca SG, Ishigaki S, et al. Regulation of insulin biosynthesis in pancreatic β-cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell Metab. 2006;4:245–254 [DOI] [PubMed] [Google Scholar]
- 34. Cole BK, Keller SR, Wu R, Carter JD, Nadler JL, Nunemaker CS. Valsartan protects pancreatic islets and adipose tissue from the inflammatory and metabolic consequences of a high-fat diet in mice. Hypertension. 2010;55:715–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Spinas GA, Mandrup-Poulsen T, Molvig J, et al. Low concentrations of interleukin-1 stimulate and high concentrations inhibit insulin release from isolated rat islets of Langerhans. Acta Endocrinol (Copenh). 1986;113:551–558 [DOI] [PubMed] [Google Scholar]
- 36. Palmer JP, Helqvist S, Spinas GA, et al. Interaction of β-cell activity and IL-1 concentration and exposure time in isolated rat islets of Langerhans. Diabetes. 1989;38:1211–1216 [DOI] [PubMed] [Google Scholar]
- 37. Rutter GA, Tsuboi T, Ravier MA. Ca2+ microdomains and the control of insulin secretion. Cell Calcium. 2006;40:539–551 [DOI] [PubMed] [Google Scholar]
- 38. Oyadomari S, Takeda K, Takiguchi M, et al. Nitric oxide-induced apoptosis in pancreatic β-cells is mediated by the endoplasmic reticulum stress pathway. Proc Natl Acad Sci U S A. 2001;98:10845–10850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jahanshahi P, Wu R, Carter JD, Nunemaker CS. Evidence of diminished glucose stimulation and endoplasmic reticulum function in nonoscillatory pancreatic islets. Endocrinology. 2009;150:607–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maedler K, Dharmadhikari G, Schumann DM, Størling J. Interleukin-1β targeted therapy for type 2 diabetes. Expert Opin Biol Ther. 2009;9:1177–1188 [DOI] [PubMed] [Google Scholar]
- 41. Dinarello CA, Donath MY, Mandrup-Poulsen T. Role of IL-1β in type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17:314–321 [DOI] [PubMed] [Google Scholar]
- 42. Kristiansen OP, Mandrup-Poulsen T. Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes. 2005;54(Suppl 2):S114–S124 [DOI] [PubMed] [Google Scholar]
- 43. Dunmore SJ, Brown JE. The role of adipokines in β-cell failure of type 2 diabetes. J Endocrinol. 2013;216:T37–T45 [DOI] [PubMed] [Google Scholar]
- 44. Chang I, Cho N, Kim S, et al. Role of calcium in pancreatic islet cell death by IFN-γ/TNF-α. J Immunol. 2004;172:7008–7014 [DOI] [PubMed] [Google Scholar]
- 45. Zawalich WS, Zawalich KC, Rasmussen H. Interleukin-1α exerts glucose-dependent stimulatory and inhibitory effects on islet cell phosphoinositide hydrolysis and insulin secretion. Endocrinology. 1989;124:2350–2357 [DOI] [PubMed] [Google Scholar]
- 46. Maedler K, Sergeev P, Ris F, et al. Glucose-induced β cell production of IL-1β contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002;110:851–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Masters SL, Dunne A, Subramanian SL, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol. 2010;11:897–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Satin LS. Localized calcium influx in pancreatic β-cells: its significance for Ca2+-dependent insulin secretion from the islets of Langerhans. Endocrine. 2000;13:251–262 [DOI] [PubMed] [Google Scholar]
- 49. Mears D. Regulation of insulin secretion in islets of Langerhans by Ca2+ channels. J Membr Biol. 2004;200:57–66 [DOI] [PubMed] [Google Scholar]
- 50. Wiederkehr A, Wollheim CB. Impact of mitochondrial calcium on the coupling of metabolism to insulin secretion in the pancreatic β-cell. Cell Calcium. 2008;44:64–76 [DOI] [PubMed] [Google Scholar]
- 51. Levy J. Abnormal cell calcium homeostasis in type 2 diabetes mellitus: a new look on old disease. Endocrine. 1999;10:1–6 [DOI] [PubMed] [Google Scholar]
- 52. Chandra J, Zhivotovsky B, Zaitsev S, Juntti-Berggren L, Berggren PO, Orrenius S. Role of apoptosis in pancreatic β-cell death in diabetes. Diabetes. 2001;50(Suppl 1):S44–S47 [DOI] [PubMed] [Google Scholar]
- 53. Ramadan JW, Steiner SR, O'Neill CM, Nunemaker CS. The central role of calcium in the effects of cytokines on β-cell function: implications for type 1 and type 2 diabetes. Cell Calcium. 2011;50:481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Allagnat F, Fukaya M, Nogueira TC, et al. C/EBP homologous protein contributes to cytokine-induced pro-inflammatory responses and apoptosis in β-cells. Cell Death Differ. 2012;19:1836–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Grunnet LG, Aikin R, Tonnesen MF, et al. Proinflammatory cytokines activate the intrinsic apoptotic pathway in β-cells. Diabetes. 2009;58:1807–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Barbu A, Welsh N, Saldeen J. Cytokine-induced apoptosis and necrosis are preceded by disruption of the mitochondrial membrane potential (Δψm) in pancreatic RINm5F cells: prevention by Bcl-2. Mol Cell Endocrinol. 2002;190:75–82 [DOI] [PubMed] [Google Scholar]
- 57. Luciani DS, Gwiazda KS, Yang TL, et al. Roles of IP3R and RyR Ca2+ channels in endoplasmic reticulum stress and β-cell death. Diabetes. 2009;58:422–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Maedler K, Størling J, Sturis J, et al. Glucose- and interleukin-1β-induced β-cell apoptosis requires Ca2+ influx and extracellular signal-regulated kinase (ERK) 1/2 activation and is prevented by a sulfonylurea receptor 1/inwardly rectifying K+ channel 6.2 (SUR/Kir6.2) selective potassium channel opener in human islets. Diabetes. 2004;53:1706–1713 [DOI] [PubMed] [Google Scholar]
- 59. Fei H, Zhao B, Zhao S, Wang Q. Requirements of calcium fluxes and ERK kinase activation for glucose- and interleukin-1β-induced β-cell apoptosis. Mol Cell Biochem. 2008;315:75–84 [DOI] [PubMed] [Google Scholar]
- 60. Sauter NS, Schulthess FT, Galasso R, Castellani LW, Maedler K. The antiinflammatory cytokine interleukin-1 receptor antagonist protects from high-fat diet-induced hyperglycemia. Endocrinology. 2008;149:2208–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Larsen CM, Faulenbach M, Vaag A, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526 [DOI] [PubMed] [Google Scholar]
- 62. Yuan M, Konstantopoulos N, Lee J, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkβ. Science. 2001;293:1673–1677 [DOI] [PubMed] [Google Scholar]
- 63. Goldfine AB, Silver R, Aldhahi W, et al. Use of salsalate to target inflammation in the treatment of insulin resistance and type 2 diabetes. Clin Transl Sci. 2008;1:36–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Eizirik DL, Bendtzen K, Sandler S. Short exposure of rat pancreatic islets to interleukin-1β induces a sustained but reversible impairment in β-cell function: influence of protease activation, gene transcription, and protein synthesis. Endocrinology. 1991;128:1611–1616 [DOI] [PubMed] [Google Scholar]
- 65. Hughes KJ, Chambers KT, Meares GP, Corbett JA. Nitric oxides mediates a shift from early necrosis to late apoptosis in cytokine-treated β-cells that is associated with irreversible DNA damage. Am J Physiol Endocrinol Metab. 2009;297:E1187–E1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Derdemezis CS, Voulgari PV, Drosos AA, Kiortsis DN. Obesity, adipose tissue and rheumatoid arthritis: coincidence or more complex relationship? Clin Exp Rheumatol. 2011;29:712–727 [PubMed] [Google Scholar]
- 67. Farah CS, Salome CM. Asthma and obesity: a known association but unknown mechanism. Respirology. 2012;17:412–421 [DOI] [PubMed] [Google Scholar]
- 68. Sethi G, Shanmugam MK, Ramachandran L, Kumar AP, Tergaonkar V. Multifaceted link between cancer and inflammation. Biosci Rep. 2012;32:1–15 [DOI] [PubMed] [Google Scholar]