Abstract
Fibroblast growth factor 21 (FGF21) is a potent regulator of glucose and lipid metabolism and is currently being pursued as a therapeutic agent for insulin resistance and type 2 diabetes. However, the cellular mechanisms by which FGF21 modifies insulin action in vivo are unclear. To address this question, we assessed insulin action in regular chow– and high-fat diet (HFD)–fed wild-type mice chronically infused with FGF21 or vehicle. Here, we show that FGF21 administration results in improvements in both hepatic and peripheral insulin sensitivity in both regular chow– and HFD-fed mice. This improvement in insulin responsiveness in FGF21-treated HFD-fed mice was associated with decreased hepatocellular and myocellular diacylglycerol content and reduced protein kinase Cϵ activation in liver and protein kinase Cθ in skeletal muscle. In contrast, there were no effects of FGF21 on liver or muscle ceramide content. These effects may be attributed, in part, to increased energy expenditure in the liver and white adipose tissue. Taken together, these data provide a mechanism by which FGF21 protects mice from lipid-induced liver and muscle insulin resistance and support its development as a novel therapy for the treatment of nonalcoholic fatty liver disease, insulin resistance, and type 2 diabetes.
Nonalcoholic fatty liver disease (NAFLD) is now the most prevalent chronic liver disease in the United States and affects approximately 1 of 3 individuals (1). NAFLD is also a major risk factor for the development of hepatic insulin resistance and type 2 diabetes (2, 3). However, there are currently no known therapies for NAFLD. Previous studies have demonstrated that chronic administration of fibroblast growth factor 21 (FGF21) reverses hepatic steatosis and improves insulin sensitivity in obese mice, but the cellular mechanisms by which FGF21 reverses insulin resistance remain unknown (4).
To understand the physiological mechanisms by which FGF21 modulates insulin resistance, we used miniosmotic pumps to chronically infuse FGF21 in age- and weight-matched wild-type (WT) mice fed a regular chow (RC) or high-fat diet (HFD) and assessed whole-body glucose turnover by hyperinsulinemic-euglycemic clamp. We combined this technique with measurements of radiolabeled glucose uptake to assess insulin action in liver, muscle, and adipose tissue and assessed whole-body energy expenditure by indirect calorimetry. To investigate the cellular mechanisms by which FGF21 reverses insulin resistance, we also assessed liver lipid intermediates (diacylglycerols [DAGs] and ceramides) and downstream signaling pathways that have been implicated in causing insulin resistance in liver and skeletal muscle (5–7).
Materials and Methods
Animals
Male WT C57BL/6J mice were individually housed under controlled temperature (23°C) and lighting (12-h light/dark cycle, lights on at 7:00 am) with free access to water and food. To study the effects of FGF21 treatment, miniosmotic pumps (Alzet, Cupertino, California) containing human recombinant FGF21 (Novo Nordisk, Måløv, Denmark) or vehicle (saline) were implanted subcutaneously. FGF21 was released at a concentration of 1 mg/(kg body weight-d) for 7 days, treatment that efficiently increased plasma FGF21 by ∼4 times compared with the vehicle group (Table 1). Mice were fed with RC or a HFD (55% calories from fat, TD93075; Harlan Laboratories, Madison, Wisconsin) for 7 days. To address the question of whether brown adipose tissue (BAT) is necessary for FGF21-mediated effects on whole-body metabolism, mice were subjected to BAT lipectomy or sham operated 4 days before pump implantation and institution of HFD feeding. BAT lipectomy was performed in mice at ∼10 weeks of age under isoflurane anesthesia. The back of the neck was sterilized with alcohol, and then a dorsal midline incision was made between the shoulder blades (∼1 cm). The Sulzer vein was tied and cut. Then, the interscapular BAT was carefully removed from the mice. After 4 days of recovery, the HFD feeding was started, and pump implants were performed. Body composition was assessed by 1H magnetic resonance spectroscopy using a Bruker Minispec analyzer (Bruker BioSpin, Billerica, Massachusetts). Energy expenditure, respiratory quotient (RQ), oxygen consumption (Vo2), carbon dioxide production (Vco2), locomotor activity, and food intake were measured using a comprehensive laboratory animal metabolic system (CLAMS; Columbus Instruments, Columbus, Ohio). Drinking was measured as described previously (8). Mice were ∼3 months old during the experiments. All procedures were approved by the Yale University Institutional Animal Care and Use Committee.
Table 1.
Physiological Parameters and Plasma Analysis
| Vehicle (RC) | FGF21 (RC) | Vehicle (HFD) | FGF21 (HFD) | |
|---|---|---|---|---|
| Initial body weight, g | 25.5 ± 0.7 | 25.4 ± 0.5 | 26.0 ± 0.5 | 25.4 ± 0.4 |
| Body weight, g | 27.3 ± 0.8 | 24.5 ± 0.7a | 30.1 ± 0.6 | 25.9 ± 0.9a |
| Body fat, % | 6.8 ± 0.7 | 4.3 ± 0.6a | 9.7 ± 0.7 | 4.8 ± 0.3a |
| Caloric intake, kcal/(kg-h) | 21.2 ± 2.0 | 19.9 ± 1.6 | 20.1 ± 0.5 | 20.6 ± 1.0 |
| RQ | 0.92 ± 0.1 | 0.91 ± 0.1 | 0.81 ± 0.01 | 0.80 ± 0.01 |
| Drinking, mL/(kg-h) | 1.49 ± 0.1 | 2.47 ± 0.2a | 0.9 ± 0.07 | 2.9 ± 0.35a |
| Activity, counts/h | 145 ± 9.5 | 169 ± 26 | 183 ± 10 | 153 ± 14 |
| Fasting glucose, mg/dL | 109 ± 2.6 | 101 ± 3.5 | 114.0 ± 6.4 | 100.5 ± 3.4 |
| Fasting insulin, μU/mL | 8.2 ± 0.6 | 7.3 ± 1.0 | 15.3 ± 1.4 | 8.4 ± 1.2a |
| HOMA-IR | 2.2 ± 0.1 | 1.9 ± 0.1 | 4.3 ± 0.5 | 2.0 ± 0.2b |
| Clamp insulin, μU/mL | 45.3 ± 4.9 | 44.4 ± 2.4 | 60.9 ± 3.6 | 53.2 ± 5.6 |
| Plasma FGF21, ng/mL | 0.83 ± 0.1 | 3.55 ± 0.2b | 0.87 ± 0.1 | 3.67 ± 0.3b |
| Fasting glucagon, pg/mL | 31.5 ± 4.6 | 28.6 ± 3.5 | 27.8 ± 2.1 | 26.4 ± 4.2 |
| Fasting corticosterone, ng/mL | 105 ± 19 | 173 ± 32 | ||
| Fasting growth hormone, ng/mL | 1.62 ± 0.8 | 13.7 ± 2.9b | ||
| Plasma adiponectin, μg/mL | 36.5 ± 4.3 | 101 ± 11b | 38.1 ± 4.8 | 100 ± 9.8b |
Data are presented as means ± SEM; n = 8 mice/group.
P < .05 vs vehicle.
P < .01 vs vehicle.
In addition, a homeostasis model assessment of insulin resistance (HOMA-IR) was used to evaluate insulin resistance (Tables 1 and 2) and was calculated with the following formula: fasting serum insulin (microunits/milliliter) × fasting plasma glucose (millimoles per liter)/22.5 (9).
Table 2.
Physiological Parameters and Plasma Analysis from BAT Lipectomized Mice
| Vehicle (HFD) | FGF21 (HFD) | |
|---|---|---|
| Body weight, g | 29.0 ± 0.5 | 25.5 ± 0.4a |
| Body fat, % | 8.0 ± 1.4 | 5.0 ± 0.4b |
| Caloric intake, kcal/(kg-h) | 19.7 ± 1.5 | 21.1 ± 1.0 |
| RQ | 0.80 ± 0.01 | 0.76 ± 0.01a |
| Drinking, mL/(kg-h) | 2.9 ± 0.5 | 4.1 ± 0.8 |
| Activity, counts/h | 143.9 ± 14.7 | 124.9 ± 9.2 |
| Fasting glucose, mg/dL | 109.0 ± 5.7 | 99.5 ± 3.4 |
| Fasting insulin, μU/mL | 15.4 ± 1.4 | 6.6 ± 0.4a |
| HOMA-IR | 4.2 ± 0.4 | 1.7 ± 0.1a |
Data are presented as means ± SEM; n = 8 mice/group.
P < .01 vs vehicle.
P < .05 vs vehicle.
Plasma assays
Blood samples were collected by cardiac puncture in heparinized syringes and centrifuged at 12 000 rpm for 2 minutes. Plasma was then either immediately used or frozen at −20°C for further analyses. Plasma glucose (10 μL/sample) was measured using a YSI 2700D glucose analyzer (YSI Inc., Yellow Springs, Ohio). Plasma fatty acids were determined with a NEFA C kit (Wako Pure Chemical Industries, Osaka, Japan). Plasma insulin, corticosterone, GH, adiponectin, and glucagon concentrations were measured by ELISA kits (Millipore, Billerica, Massachusetts). Plasma FGF21 was measured by ELISA kit (Quantikine ELISA; R&D Systems, Minneapolis, Minnesota). For plasma glucagon measurements, aprotinin was added to the blood in precooled tubes during collection to prevent glucagon degradation. Mice were fasted for 6 hours before blood was collected for plasma adiponectin and plasma FGF21 measurements or overnight fasted for insulin, glucagon, and GH.
Liver and musle lipid intermediates measurements
Mice were fasted for 6 hours before tissue harvesting for lipid extraction. Tissue triglycerides were extracted using the method of Bligh and Dyer (10) and measured using a commercial triglyceride reagent (Diagnostic Chemicals Limited, Oxford, Connecticut). Cytosolic and membrane DAG were measured as described previously (11, 12). Total DAG content is expressed as the sum of individual species. Ceramide content was measured as described previously (12).
Hyperinsulinemic-euglycemic clamp studies and intraperitoneal glucose tolerance tests
Jugular venous catheters were implanted 6–7 days before the hyperinsulinemic-euglycemic clamp experiments. Miniosmotic pumps for these mice were implanted during the catheter implantation surgery. Hyperinsulinemic-euglycemic clamps were performed as described previously (11). After overnight fasting, to measure basal glucose turnover, restrained mice were infused with [3-3H]glucose at a rate of 0.05 μCi/min for 120 minutes. After the basal period, a hyperinsulinemic-euglycemic clamp was performed in those mice for 120 minutes with a 4-minute primed insulin (20 mU/kg) followed by a continuous infusion (2 mU/kg/min). [3-3H]Glucose was infused at a rate of 0.1 μCi/min, and 20% dextrose was infused at variable rates to maintain euglycemia (∼120 mg/dL). A 10-μCi bolus of 2-deoxy-d-[1-14C]glucose (PerkinElmer Life and Analytical Sciences, Waltham, Massachusetts) was injected after 85 minutes to estimate the insulin-stimulated tissue glucose uptake. Blood samples were collected by tail bleeding (at 0, 25, 50, 65, 80, 90, 100, 110, and 120 min). These clamps were performed according to standard operating procedures adopted by the Mouse Metabolic Phenotyping Center consortium (13). At the end of the clamp, mice were anesthetized with pentobarbital sodium injection (150 mg/kg), and all tissues were taken within 4 minutes, snap-frozen in liquid nitrogen using aluminum tongs, and stored at −80°C for subsequent analysis. Biochemical analysis and calculations for the hyperinsulinemic-euglycemic clamps were performed as described previously (11, 14). Intraperitoneal glucose tolerance tests were conducted as described previously (15). In brief, after overnight fasting, mice were injected intraperitoneally with glucose (1 mg/kg body weight–10% dextrose). Blood samples were taken by tail bleeding at 0, 15, 30, 45, 60, 90, and 120 minutes after the injection.
Liver and muscle novel PKC activation
Protein kinase C (PKC) ϵ and PKCθ activation was assessed in liver and skeletal muscle protein extracts, respectively, as described previously (16).
Immunoblot analysis
Immunoblots were performed as described previously (17). Membranes were incubated overnight with primary antibodies for phospho-Akt2 (Ser474) (Cell Signaling Technology, Danvers, Massachusetts). After further washings, membranes were incubated with horseradish peroxidase–conjugated secondary antibody (Bio-Rad, Hercules, California) and visualized by enhanced chemiluminescent substrate (Pierce, Rockford, Illinois). Membranes were stripped and reblotted with anti–total Akt2 antibody (Cell Signaling Technology) or glyceraldehyde-3-phosphate dehydrogenase (Santa Cruz Technology, Santa Cruz, California). Bands were then quantified using ImageJ (National Institutes of Health, Bethesda, Maryland).
Oxygen consumption measurement in primary hepatocytes and in adipose tissue
Primary hepatocytes from vehicle- or FGF21-treated mice were isolated at the Yale Liver Center. Cells were washed 3 times with recovery media (DMEM with high glucose plus 10% fetal bovine serum), and equal amounts of cells (12 000) were seeded in each well of a Seahorse XF24 cell culture plate (Seahorse Bioscience, North Billerica, Massachusetts). These experiments were repeated 6 times under the same conditions as described previously (18). In brief, cells were kept in recovery media for 4–6 hours and then were washed with DMEM media (low glucose plus 10% fetal bovine serum) and incubated overnight. The following morning, cells were washed with prewarmed (∼37°C) XF24 Assay medium. XF24 Assay medium (600 μL) was then added to each well. Immediately before measurements, cells with Assay media were placed in an unbuffered, humidified incubator at 37°C for 1 hour to allow temperature and pH equilibration. Three measurements of basal oxygen consumption rates (picomoles per minutes) were recorded using an instrument protocol of 3-minute mix, 2-minute wait, and 3-minute measure. Next, palmitate was injected at a final concentration of 200 μM, and oxygen consumption was recorded using the same instrument protocol. Three measurements of oxygen consumption rate were taken, and the average of 3 measurements was used for analysis.
In addition, freshly isolated mouse gonadal white adipose tissue (WAT) or BAT was rinsed with XF-DMEM (containing 25 mM HEPES), and cut into pieces (∼10 mg). After extensive washing, 1 piece of tissue was placed in each well of a XF24 Islet Capture Microplate (Seahorse Bioscience) and covered with the islet capture screen that allows free perfusion while minimizing tissue movement. XF Assay medium (500 μL) was added, and samples were analyzed in the XF24 analyzer.
Oxygen consumption measurements in skeletal muscle cells
Muscle fibers were prepared according to a protocol as described earlier (19). In brief, a subsample of the dissected soleus muscle of 5 mg wet weight was immediately transferred onto a small Petri dish on ice containing 2 mL of ice-cold organ preservation solution (BIOPS) comprising 2.77 mM CaK2 EGTA buffer, 7.23 mM K2 EGTA buffer, 0.1 μM free calcium, 20 mM imidazole, 20 mM taurine, 50 mM 2-(N-morpholino)ethanesulfonic acid hydrate (MES), 0.5 mM dithiothreitol, 6.56 mM MgCl2 · 6H2O, 5.77 mM ATP, and 15 mM phosphocreatine (pH 7.1). The muscle sample was then gently dissected using forceps with sharp tips. To ensure complete permeabilization, the fibers were incubated on a shaker at 4°C in BIOPS solution containing 50 μg/mL saponin for 30 minutes. Fibers were washed for 10 minutes at 4°C in ice-cold mitochondrial respiration medium 5 (MiR05: 0.5 mM EGTA, 3 mM MgCl2, 60 mM potassium lactobionate, 20 mM taurine, 10 mM KH2PO4, 20 mM HEPES, 110 mM sucrose, and 1 g/L BSA essentially fatty acid free, adjusted to pH 7.1), and the wet weight of the fibers was measured on a microbalance (Mettler Toledo, Greifensee, Switzerland). Subsequent respiration measurements were performed in MiR05 at 37°C using the high-resolution respirometer Oxygraph-2k (Oroboros, Innsbruck, Austria). All respirometric experiments were performed in a hyperoxygenated oxygraph chamber (250–400 μM) to prevent any potential oxygen diffusion limitation to the muscle fiber bundle. A substrate-uncoupler-inhibitor titration protocol was used to assess leak respiration (state 2 respiration) after addition of pyruvate (5 mM, all concentrations are final), malate (2 mM), and glutamate (10 mM). Subsequent addition of saturating ADP concentrations (3 mM) yielded maximal oxidative phosphorylation capacity (state 3 respiration at saturating [ADP]). State 4 respiration was induced by addition of oligomycin (2 μg/mL).
Citrate synthase (CS) activity was assessed in homogenates of the muscle samples. On average, 8 mg wet weight of muscle sample was weighed out and 20 times the volume of cell lysis reagent was added (CelLytic; Sigma-Aldrich, St Louis, Missouri). The sample was homogenized for 2 minutes at 30 Hz in a tissue lyser (TissueLyser II; Qiagen, Valencia, California) and centrifuged for 10 minutes at 12 000 rpm (4°C). The supernatant was removed, and CS activity was measured fluorometrically at 412 nm and 25°C (Citrate Synthase Assay Kit; Sigma-Aldrich), according to the guidelines of the manufacturer. Respiration per CS activity normalized for protein content was calculated by dividing mass-specific respiration [picomoles/(milligrams wet tissue · minute)] by the corresponding CS activity [micromoles/(minute − milligrams protein)].
Statistical analysis
All data are expressed as means ± SEM. Results were assessed using a two-tailed unpaired Student t test or 2-way ANOVA (Prism 5; GraphPad Software, Inc, La Jolla, California). A P value <.05 was considered significant.
Results
FGF21 regulates energy expenditure
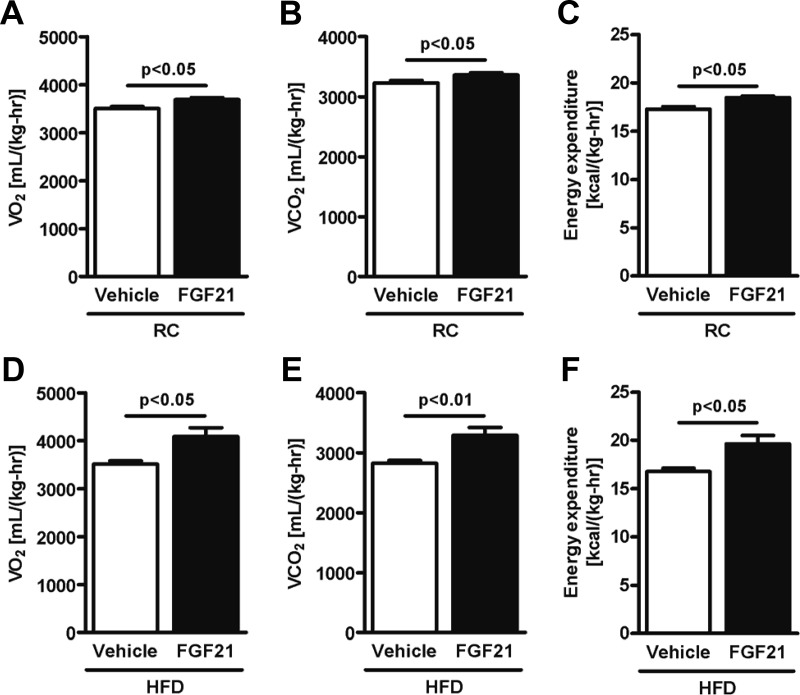
Chronic FGF21 administration led to ∼11% and ∼14% reductions in body weight and ∼35% and ∼50% reductions in fat mass in mice fed RC and HFD, respectively (Table 1). In RC-fed mice, Vo2 (Figure 1A), Vco2 (Figure 1B), and energy expenditure (Figure 1C) were ∼5% higher in FGF21-treated mice, without any difference in locomotor activity and caloric intake (Table 1). In HFD-fed mice, an increase by ∼15% in Vo2 (Figure 1D), Vco2 (Figure 1E), and energy expenditure (Figure 1F) was also observed in FGF21-treated mice, without any change in locomotor activity and caloric intake (Table 1). Because body weight differed between vehicle-treated mice and FGF21-treated mice, analysis of covariance was performed to determine whether the difference observed in energy expenditure was independent of body weight as suggested by Tschop et al (20). Analysis of covariance revealed that FGF21 increased energy expenditure independent of body weight changes (Supplemental Table 1 published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). We therefore assessed oxygen consumption in isolated hepatocytes and found an ∼20% increase in basal respiratory rate in hepatocytes isolated from FGF21-treated mice (Figure 2A). Moreover, palmitate oxidation was higher in hepatocytes from FGF21-treated mice (Figure 2A). Consistent with these results, we found that peroxisome proliferator–activated receptor-γ coactivator 1α (PGC-1α), carnitine palmitoyltransferase 1 (CPT-1α), and uncoupling protein (UCP)-2 mRNA expression were increased in liver from FGF21-treated mice (Figure 2B). Adipose tissue oxygen consumption was also measured. We observed an ∼30% increase in BAT O2 consumption and an ∼80% increase in WAT O2 consumption in FGF21-treated mice (Figure 2C). Consistent with the increase in oxygen consumption in WAT, we observed increased mRNA expression of UCP-1, PGC-1α, Cidea, and PRDM16 in WAT (Figure 2D) from FGF21-treated mice, suggesting that FGF21 promoted the “browning” of WAT. However, oxygen consumption measurements in skeletal muscle adjusted for CS activity normalized for protein content, a biomarker of mitochondrial content and total cristae area, revealed that leak respiration as well as oxidative phosphorylation capacity was not different between the FGF21-treated group and the control group (Figure 2E). Consistent with this, mRNA expression of PGC-1α, UCP-3, and CPT-1α in muscle was not different between groups (Figure 2F).
Figure 1.
FGF21 regulates energy expenditure. Vo2 (A), Vco2 (B), and energy expenditure per body weight (C) were significantly higher in FGF21-treated mice (n = 8/group) fed RC. Vo2 (D), Vco2 (E), and energy expenditure per body weight (F) were significantly higher in FGF21-treated mice (n = 8/group) fed a HFD. Data are presented as means ± SEM.
Figure 2.
FGF21 increases oxygen consumption in hepatocytes and adipose tissue. A, Oxygen consumption rate (OCR) in isolated hepatocytes. B, PGC-1α, CPT-1α, and UCP-2 mRNA expression in liver. C, BAT and WAT oxygen consumption rate. D, mRNA expression of UCP-1, PGC-1α, Cidea, and PRDM16 in WAT. E, Oxygen consumption rate in muscle. F, PGC-1α, UCP-3, and CPT-1α mRNA expression in muscle. Data are presented as means ± SEM (n = 6–8/group). These experiments were performed in RC-fed mice.
FGF21 regulates whole-body insulin responsiveness
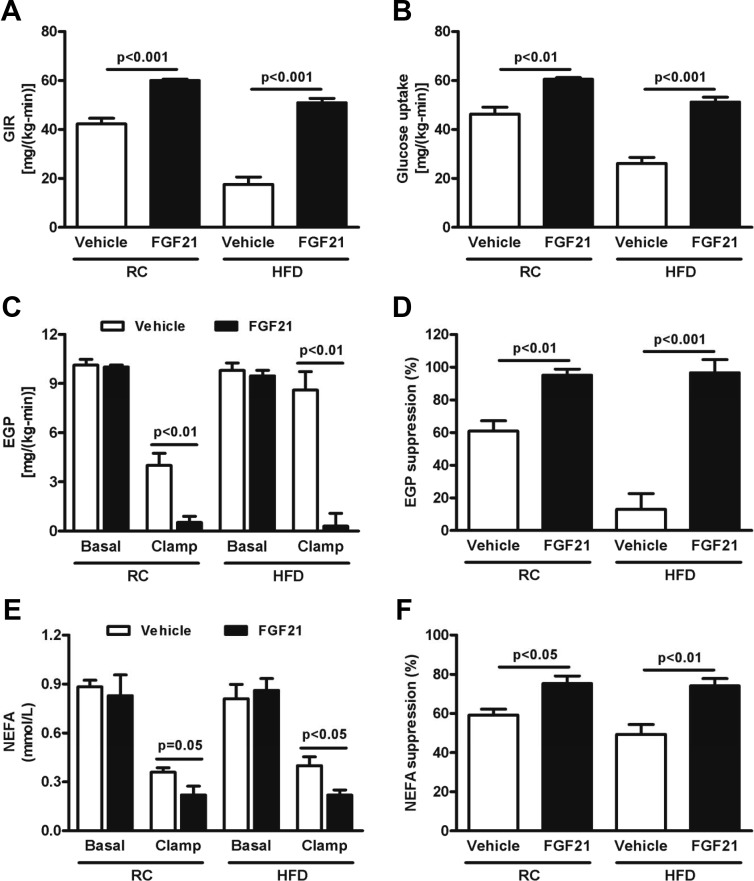
Although FGF21 is known to improve insulin sensitivity when injected in HFD-fed mice, the cellular mechanisms responsible for this are unknown (4, 21). To examine the physiological mechanisms by which FGF21 enhances insulin sensitivity in vivo, we performed hyperinsulinemic-euglycemic clamps to assess insulin action in liver, muscle, and adipose tissue in FGF21-treated mice. Exogenous administration of FGF21 resulted in a marked improvement in whole-body insulin sensitivity as reflected by ∼40% and ∼200% increases in the glucose infusion rate in RC- and HFD-fed mice, respectively (Figure 3A and Supplemental Figure 1). This improvement in whole-body insulin sensitivity was accounted for partially by an increase in whole-body glucose uptake during the hyperinsulinemic-euglycemic clamp in FGF21-treated mice (Figure 3B). Specifically, increases in skeletal muscle (Supplemental Figure 2A), WAT (Supplemental Figure 2B), and BAT (Supplemental Figure 2C) glucose uptake were observed. Although basal endogenous glucose production (EGP) was similar between RC and HFD groups treated with FGF21 or not (Figure 3C), measurement of EGP in hyperinsulinemic conditions revealed marked suppression of EGP in FGF21-treated mice compared with controls, indicating enhanced hepatic insulin sensitivity and protection from HFD-induced hepatic insulin resistance (Figure 3, C and D). Basal plasma fatty acid concentrations were similar between groups, but the ability of insulin to suppress plasma fatty acid concentrations during the hyperinsulinemic-euglycemic clamp was significantly increased in the FGF21-treated mice (Figure 3, E and F), suggesting that insulin suppression of WAT lipolysis was also improved in these mice. Interestingly, FGF21 also increased mRNA expression of GLUT-1 in WAT (Supplemental Figure 3A), with no change in GLUT-4 mRNA expression.
Figure 3.
FGF21 regulates insulin sensitivity. Glucose infusion rate (GIR) during the last 40 minutes of the hyperinsulinemic-euglycemic clamp (A) and whole-body glucose uptake during the hyperinsulinemic-euglycemic clamp (B) were significantly higher in FGF21-treated mice, with both RC and the HFD. Basal EGP (C) was similar between groups, whereas suppression of EGP during the hyperinsulinemic-euglycemic clamp (D) was higher in FGF21-treated mice, with both diets. Basal nonesterified fatty acids (NEFAs) (E) were similar between groups, whereas insulin-stimulated NEFA suppression during the hyperinsulinemic-euglycemic clamp (F) was significantly higher in FGF21-treated mice, with both diets (n = 8/group). Data are presented as means ± SEM.
To determine the mechanism by which FGF21 increased insulin sensitivity even in RC-fed mice, measurements of plasma glucagon, corticosterone, GH, and adiponectin were performed (Table 1). Plasma glucagon measurement was performed in both RC- and HFD-fed mice, and there was no difference between groups. Measurements of plasma corticosterone and GH were performed only in RC-fed mice. Surprisingly, plasma GH was ∼8-fold higher in FGF21-treated mice (Table 1), and plasma corticosterone showed a strong tendency to be higher in FGF21-treated mice (P = .09; Table 1). The increased plasma GH concentrations in the FGF21-treated mice were associated with increased suppressor of cytokine signaling 2 (SOCS2) mRNA expression in liver and reduced IGF-1 mRNA expression (Supplemental Figure 4), suggesting that FGF21 may be inducing a GH-resistant state through up-regulation of SOCS2, resulting in reduced GH induction of IGF-1 in liver. Plasma adiponectin concentrations were also increased by ∼2.6-fold in FGF21-treated mice (Table 1).
FGF21 regulates cellular lipid content and insulin signaling in HFD-fed mice
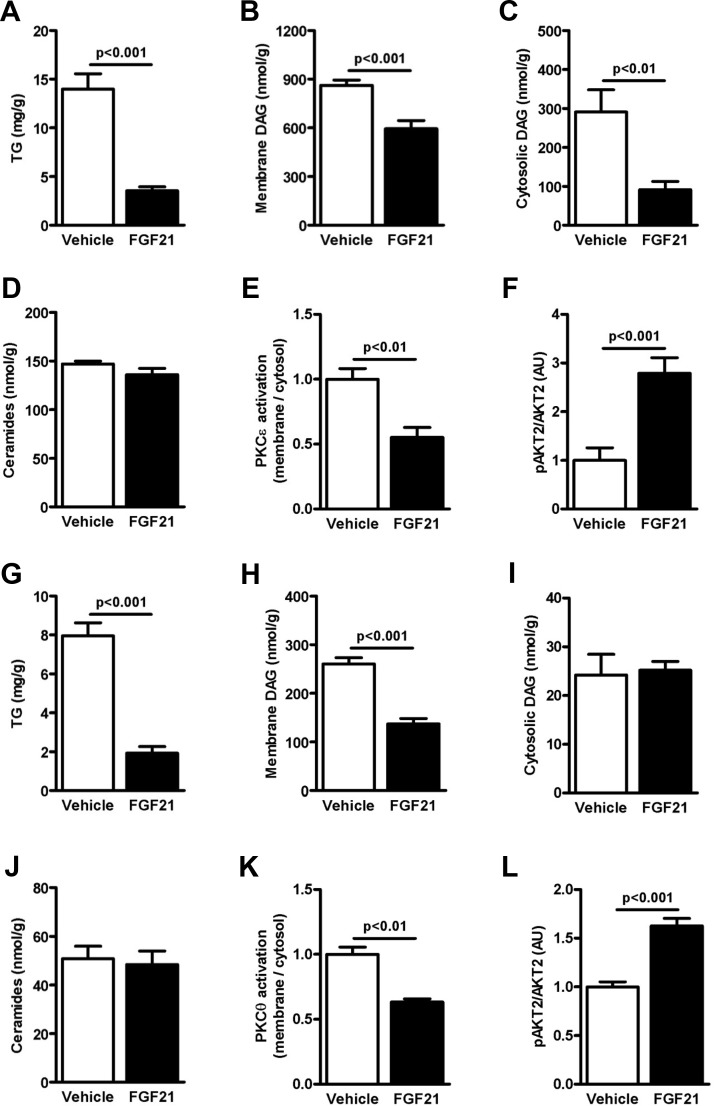
Liver lipid intermediates including triglycerides (Figure 4A), cytosolic DAG (Figure 4B and Supplemental Figure 5A), and membrane DAG (Figure 4C and Supplemental Figure 5B) were decreased in FGF21-treated mice. However, in contrast to hepatic DAG content, there was no difference in liver ceramide content (Figure 4D and Supplemental Figure 5C), thus dissociating changes in liver ceramide content from FGF21-mediated improvements in hepatic insulin sensitivity as well as from increased plasma adiponectin concentrations. These reductions in hepatic DAG content in the FGF21-treated mice were associated with decreased PKCϵ activation (Figure 4E) and a concomitant increase in insulin-stimulated Akt2 phosphorylation (Figure 4F) (postclamp tissues). FGF21 treatment was also associated with an ∼70% reduction in skeletal muscle triglyceride content (Figure 4G). Although cytosolic DAG (Figure 4I and Supplemental Figure 6A) was similar between groups, membrane DAG content (Figure 4H and Supplemental Figure 6B) was significantly decreased in FGF21-treated mice. Similar to what was observed in liver despite the marked effects of FGF21 to increase insulin-stimulated peripheral glucose uptake, there were no effects of FGF21 on muscle ceramide content (Figure 4J and Supplemental Figure 6C), thus dissociating changes in muscle ceramide content from FGF21-mediated improvements in muscle insulin sensitivity. The decrease in membrane DAG content was associated with decreased PKCθ activation (Figure 4K) and increased insulin-stimulated Akt2 phosphorylation (Figure 4L) (postclamp tissues), consistent with increased insulin-stimulated glucose uptake in skeletal muscle during the clamp.
Figure 4.
FGF21 decreases ectopic lipid content in HFD-fed mice. Liver triglycerides (TG) (A), cytosolic DAG (C), and membrane DAG content (C) were significantly decreased in FGF21-treated mice, without differences in liver ceramide content (D) (n = 8/group). Consequently, PKCϵ activation was significantly decreased (E) in FGF21-treated mice and insulin-stimulated Akt2 phosphorylation was increased (F) (n = 6/group). In the skeletal muscle, triglycerides (G) were decreased in FGF21-treated mice, without differences in cytosolic DAG (H). However, muscle membrane DAG (I) was decreased in FGF21-treated mice, without differences in ceramide content (L) (n = 4–8/group). Consequently, activation of PKCθ (J) was significantly decreased, and insulin-stimulated Akt2 phosphorylation (K) was subsequently increased (n = 4–5/group). Data are represented as means ± SEM.
Although the intracellular trafficking of DAG leading to activation of nPKCs is not well understood, recent studies have found that membrane DAG correlates well with activation of nPKC and insulin resistance, whereas DAG and nPKC contents in lipid droplets do not appear to be implicated in mediation of lipid-induced insulin resistance (22). In the present study, we found that FGF21-induced increases in hepatic insulin responsiveness and reduction in PKCϵ translocation were associated with reductions in both membrane and cytosolic fraction DAG content, whereas FGF21-induced increases in muscle insulin responsiveness and reduction in PKCθ translocation were only associated with reductions in membrane DAG content. Although the reason for this difference in DAG localization between liver and skeletal muscle is unclear, these findings are consistent with previous studies (11, 23–25) and may reflect tissue differences in the intracellular kinetics and trafficking of intracellular DAG as it moves from the smooth endoplasmic reticulum to the plasma membrane.
FGF21 increases energy expenditure and improves glucose homeostasis in a BAT-independent fashion
To address the question of whether BAT is necessary for the metabolic effects we observed with FGF21 treatment, we performed BAT lipectomy in mice on HFD and treated them with vehicle or FGF21. Chronic administration of FGF21 in those mice led to a ∼11% increase in O2 consumption (Figure 5A), a trend toward mildly increased CO2 production (Figure 5B), and a ∼11% increase in energy expenditure (Figure 5C). In addition, FGF21-treated mice displayed reduced body weight and body fat, without any change in caloric intake or activity (Table 2). Moreover, glucose tolerance was improved during an intraperitoneal glucose tolerance test in FGF21-treated mice (Figure 5, D and E). Consistent with this observation, plasma insulin concentration and the area under the curve (AUC) during the intraperitoneal glucose tolerance test was reduced in FGF21-treated mice (Figure 5, F and G), suggesting increased insulin sensitivity in these mice, which was further suggested by reduced HOMA-IR in FGF21-treated mice (Table 2).
Figure 5.
FGF21 increases energy expenditure and improves glucose homeostasis in a BAT-independent fashion. Vo2 (A), Vco2 (B), and energy expenditure per body weight (C) were significantly higher in FGF21-treated mice (n = 8/group). Plasma glucose during the intraperitoneal glucose tolerance test (D) and the AUC (E) were significantly lower in FGF21-treated mice. Plasma insulin during the intraperitoneal glucose tolerance test (F) and the AUC (G) were significantly lower in FGF21-treated mice (n = 8/group). These experiments were performed in mice that underwent BAT lipectomy and were fed a HFD. Data are presented as mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Discussion
FGF21 is rapidly gaining interest as a potent metabolic regulator that improves glucose and lipid metabolism but also reduces body weight and fat mass in numerous animal models of insulin resistance and obesity (4, 26–29), although its underlying mechanism is still poorly understood. The tissue-specific action of FGF21 is determined by the expression of FGF21 receptor and the presence of the protein β-klotho, which has been shown to work as a coreceptor for FGF21, besides being required for FGF21 effects on growth and metabolism (30, 31).
The plasma FGF21 concentration is correlated with liver and muscle insulin resistance in humans (32) and appears to be a biomarker of NAFLD (33, 34), suggesting that NAFLD may represent a FGF21-resistant state. Consistent with this possibility, plasma FGF21 concentrations were increased by approximately 10-fold and associated with fatty liver and hepatic insulin resistance in mice fed a ketogenic diet (17). In the current study, we did not observe any changes in plasma FGF21 concentrations after 1 week of high-fat feeding (Table 1). Taken together, these results suggest that plasma FGF21 concentrations and FGF21 resistance may occur only after longer term high-fat feeding.
Administration of FGF21 in HFD–fed mice, a model mimicking a Western diet, resulted in a marked increase in both hepatic and peripheral insulin sensitivity. These improvements in liver and muscle insulin sensitivity could be attributed, in part, to an increase in whole-body energy expenditure, which was associated with a decrease in hepatocellular and myocellular triglyceride and DAG content, reduced activation of PKCϵ and PKCθ in liver and muscle, respectively, and improved insulin signaling in both tissues.
Interestingly, FGF21 administration also improved insulin sensitivity in RC-fed mice. This improvement in insulin responsiveness in FGF21-treated RC-fed mice could be attributed to increased insulin-stimulated glucose uptake in skeletal muscle, BAT and WAT. The increase in insulin-stimulated WAT glucose uptake could be attributed to a marked increase in GLUT-1 mRNA expression, which has been shown to occur with FGF21 treatment (27). The mechanism by which FGF21 increases insulin-stimulated glucose uptake in skeletal muscle and BAT is less clear but may be mediated in part by the increased suppression of lipolysis, resulting in lower plasma fatty acids during the clamp and the ∼2.6-fold increase in plasma adiponectin concentrations observed with FGF21 treatment.
Indeed, Holland et al (35) and Lin et al (36) showed recently that FGF21 stimulates the production and secretion of adiponectin by WAT in mice. In addition, they showed that FGF21-stimulated adiponectin secretion plays a central role on FGF21 effects on whole-body metabolism and insulin sensitivity. Consistent with our study, Lin et al (36) observed that chronic infusion of FGF21 improved hepatic and muscle insulin resistance associated with increased energy expenditure, increased plasma adiponectin, and reduced hepatic and muscle TG content. They also observed that FGF21 effects were almost abolished in adiponectin knockout mice. Accordingly, Holland et al (35) also observed that adiponectin plays an important role in the increased insulin sensitivity induced by FGF21 in ob/ob mice, during both acute and chronic FGF21 infusion. In contrast to our observations, they observed reduction in hepatic ceramides with chronic FGF21 infusion, which they hypothesized may be responsible for the improved hepatic insulin sensitivity, although they did not measure hepatic DAG content under these conditions. This discrepancy between studies may be due to the different mouse models used in each study. We used a model of high-fat diet–induced liver and muscle insulin resistance, whereas they used a genetic model of extreme obesity and insulin resistance (ob/ob mice), which also has increased plasma corticosterone concentrations. Although it has been suggested that reduction of hepatic ceramide content plays a central role in adiponectin-mediated improvements in hepatic insulin sensitivity through activation of ceramidase (37), it was shown recently that adiponectin improved muscle insulin sensitivity by reducing muscle DAG content, whereas liver DAGs were not reported despite improved hepatic insulin sensitivity (38). Therefore, FGF21-induced increased plasma adiponectin concentrations, also observed in our study, may play an important role in mediating improved liver and muscle insulin sensitivity due to reduced ectopic lipid content.
We also examined whether FGF21 had any effects on the hypothalamic-pituitary axis, which in turn might affect insulin responsiveness in muscle and BAT. Surprisingly, plasma GH concentrations were ∼8-fold higher and plasma corticosterone concentrations displayed a strong tendency to be increased in FGF21-treated mice. The latter finding is consistent with previous studies showing that FGF21 induces a state of GH resistance in transgenic mice, leading to increased plasma GH, reduced plasma IGF-1 concentrations, and reduced body size compared with those for WT mice (39). Consistent with these findings, we observed reduced expression of IGF-1 mRNA in livers of FGF21-treated mice and increased mRNA expression of SOCS2.
The finding of improved peripheral insulin sensitivity, with increased insulin-stimulated glucose uptake in skeletal muscle and WAT, specifically, contrasts with previous findings of no changes in peripheral insulin action detected with FGF21 treatment (29). Indeed, in ob/ob mice, FGF21 action was mostly hepatic, without increases in peripheral glucose uptake (29). These findings might be specific to ob/ob mice, in which increased circulating corticosterone levels contribute to their insulin resistance (40), and not to other rodent models of insulin resistance such as HFD-fed mice as used in this study, which more closely mimics insulin resistance observed in obese humans. Increased insulin-stimulated glucose uptake in skeletal muscle of FGF21-treated mice was most likely secondary, due, at least in part, to increased energy expenditure in the liver and WAT, leading to decreased skeletal muscle triglyceride and DAG content. The latter may also be due to improved insulin action in WAT as reflected by increased suppression of plasma fatty acid concentrations during the clamp, which would reduce rates of lipolysis during feeding and decrease delivery of fatty acids to skeletal muscle. Moreover, because DAG content was decreased in skeletal muscle of FGF21-treated mice, PKCθ activation was also decreased, which may account for their protection from lipid-induced muscle insulin resistance (4, 26).
We found that whole-body energy expenditure was increased in FGF21-treated mice, which is consistent with previous observations (4, 26). Our study builds on these previous studies by demonstrating that the effect of FGF21 on energy expenditure can be mediated independently of BAT. Although it has been shown that FGF21 also contributes to BAT thermogenic activation in newborn mice (41), this effect may disappear in older mice. Indeed, Chau et al (42) showed that FGF21 increases energy expenditure in adipocytes, consistent with our observation showing that FGF21 increased O2 consumption in WAT, suggesting that FGF21 can increase energy expenditure in other organs besides BAT, contributing to increased whole-body energy expenditure. Finally, it is also possible that FGF21 may be promoting increased whole-body energy expenditure and insulin sensitivity through its effects on the central nervous system (43).
In conclusion, the present study demonstrates that administration of FGF21 protects mice from lipid-induced liver and muscle insulin resistance. This protection from lipid-induced insulin resistance was associated with reductions in hepatic and skeletal muscle DAG content, leading, respectively, to reduced PKCϵ and PKCθ activation and subsequent improvement in insulin signaling. In contrast, there were no effects of FGF21 on liver or muscle ceramide content, disassociating ceramides from FGF21 protection from lipid-induced liver and muscle insulin resistance. The reduction in ectopic lipid deposition in liver and skeletal muscle could be attributed to increased whole-body energy expenditure mediated in part by an increase in hepatic and WAT energy expenditure. Taken together, these data provide a mechanism by which FGF21 protects mice from lipid-induced liver and muscle insulin resistance. Finally, although there is great interest in developing FGF21 as a novel therapy for treatment of type 2 diabetes due to these salutary effects of FGF21 on hepatic steatosis, energy expenditure, and insulin resistance, the beneficial effects of FGF21 will have to be balanced against the potential deleterious effects of FGF21 on the hypothalamic-pituitary axis.
Acknowledgments
We thank Xiaoxian Ma and Aida Groszman for expert technical assistance and Birgitte Andersen (Novo Nordisk) for providing human recombinant FGF21. We are grateful to OROBOROS INSTRUMENTS for the loan of an Oxygraph-2k.
This work was supported by National Institutes of Health Grants R01 DK-40936, R24 DK-085638, R01 DK-059635, P30 DK-45735, and P30 DK-034989 and a VA Merit Grant (to V.T.S.). F.R.J. was supported by a grant from the Swiss National Science Foundation/Swiss Foundation for Grants in Biology and Medicine (PASMP3_132563), and J.-P.G.C. was supported by a Mentor-Based Postdoctoral Fellowship Award from the American Diabetes Association (G.I.S.).
J.-P.G.C. designed experiments, researched data, and wrote the manuscript. F.R.J. designed experiments, researched data, and wrote the manuscript. M.P. researched data and contributed to discussion. D.P. researched data and contributed to discussion. B.A.G. researched data. J.S. researched data. D.Z. researched data. M.K. researched data. V.T.S. contributed to discussion and reviewed/edited the manuscript. M.J.J. designed experiments, researched data, contributed to discussion, and reviewed/edited the manuscript. G.I.S. designed experiments, reviewed/edited the manuscript, contributed to discussion, and wrote the manuscript. J.-P.G.C. is the guarantor of this work and, as such, has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- area under the curve
- BAT
- brown adipose tissue
- CPT-1
- carnitine palmitoyltransferase 1
- CS
- citrate synthase
- DAG
- diacylglyceride
- EGP
- endogenous glucose production
- FGF21
- fibroblast growth factor 21
- HFD
- high-fat diet
- HOMA-IR
- homeostasis model assessment of insulin resistance
- NAFLD
- nonalcoholic fatty liver disease
- PGC-1
- peroxisome proliferator–activated receptor-γ coactivator 1α
- PKC
- protein kinase C
- RC
- regular chow
- RQ
- respiratory quotient
- SOCS2
- suppressor of cytokine signaling 2
- UCP
- uncoupling protein
- Vo2
- oxygen production
- Vco2
- carbon dioxide production
- WAT
- white adipose tissue
- WT
- wild-type.
References
- 1. Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131 [DOI] [PubMed] [Google Scholar]
- 2. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231 [DOI] [PubMed] [Google Scholar]
- 3. Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51:679–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu J, Lloyd DJ, Hale C, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58:250–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Holland WL, Brozinick JT, Wang LP, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–179 [DOI] [PubMed] [Google Scholar]
- 6. Jornayvaz FR, Shulman GI. Diacylglycerol activation of protein kinase Cϵ and hepatic insulin resistance. Cell Metab. 2012;15:574–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Birkenfeld AL, Lee HY, Guebre-Egziabher F, et al. Deletion of the mammalian INDY homolog mimics aspects of dietary restriction and protects against adiposity and insulin resistance in mice. Cell Metab. 2011;14:184–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 10. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917 [DOI] [PubMed] [Google Scholar]
- 11. Jornayvaz FR, Birkenfeld AL, Jurczak MJ, et al. Hepatic insulin resistance in mice with hepatic overexpression of diacylglycerol acyltransferase 2. Proc Natl Acad Sci USA. 2011;108:5748–5752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yu C, Chen Y, Cline GW, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277:50230–50236 [DOI] [PubMed] [Google Scholar]
- 13. Ayala JE, Samuel VT, Morton GJ, et al. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech. 2010;3:525–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Youn JH, Buchanan TA. Fasting does not impair insulin-stimulated glucose uptake but alters intracellular glucose metabolism in conscious rats. Diabetes. 1993;42:757–763 [DOI] [PubMed] [Google Scholar]
- 15. Lee HY, Birkenfeld AL, Jornayvaz FR, et al. Apolipoprotein CIII overexpressing mice are predisposed to diet-induced hepatic steatosis and hepatic insulin resistance. Hepatology. 2011;54:1650–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Choi CS, Savage DB, Kulkarni A, et al. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J Biol Chem. 2007;282:22678–22688 [DOI] [PubMed] [Google Scholar]
- 17. Jornayvaz FR, Jurczak MJ, Lee HY, et al. A high-fat, ketogenic diet causes hepatic insulin resistance in mice, despite increasing energy expenditure and preventing weight gain. Am J Physiol Endocrinol Metab. 2010;299:E808–E815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Camporez JP, Jornayvaz FR, Lee HY, et al. Cellular mechanism by which estradiol protects female ovariectomized mice from high-fat diet-induced hepatic and muscle insulin resistance. Endocrinology. 2013;154:1021–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pesta D, Gnaiger E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol. 2012;810:25–58 [DOI] [PubMed] [Google Scholar]
- 20. Tschöp MH, Speakman JR, Arch JR, et al. A guide to analysis of mouse energy metabolism. Nat Methods. 2012;9:57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mu J, Pinkstaff J, Li Z, et al. FGF21 analogs of sustained action enabled by orthogonal biosynthesis demonstrate enhanced antidiabetic pharmacology in rodents. Diabetes. 2012;61:505–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cantley JL, Yoshimura T, Camporez JP, et al. CGI-58 knockdown sequesters diacylglycerols in lipid droplets/ER-preventing diacylglycerol-mediated hepatic insulin resistance. Proc Natl Acad Sci USA. 2013;110:1869–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee HY, Choi CS, Birkenfeld AL, et al. Targeted expression of catalase to mitochondria prevents age-associated reductions in mitochondrial function and insulin resistance. Cell Metab. 2010;12:668–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Choi CS, Befroy DE, Codella R, et al. Paradoxical effects of increased expression of PGC-1α on muscle mitochondrial function and insulin-stimulated muscle glucose metabolism. Proc Natl Acad Sci USA. 2008;105:19926–19931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jornayvaz FR, Lee HY, Jurczak MJ, et al. Thyroid hormone receptor-α gene knockout mice are protected from diet-induced hepatic insulin resistance. Endocrinology. 2012;153:583–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coskun T, Bina HA, Schneider MA, et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149:6018–6027 [DOI] [PubMed] [Google Scholar]
- 27. Kharitonenkov A, Shiyanova TL, Koester A, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kharitonenkov A, Wroblewski VJ, Koester A, et al. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007;148:774–781 [DOI] [PubMed] [Google Scholar]
- 29. Berglund ED, Li CY, Bina HA, et al. Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity. Endocrinology. 2009;150:4084–4093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ding X, Boney-Montoya J, Owen BM, et al. βKlotho is required for fibroblast growth factor 21 effects on growth and metabolism. Cell Metab. 2012;16:387–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Adams AC, Cheng CC, Coskun T, Kharitonenkov A. A FGF21 requires βklotho to act in vivo. PloS One. 2012;7:e49977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chavez AO, Molina-Carrion M, Abdul-Ghani MA, Folli F, Defronzo RA, Tripathy D. Circulating fibroblast growth factor-21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care. 2009;32:1542–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li H, Fang Q, Gao F, et al. Fibroblast growth factor 21 levels are increased in nonalcoholic fatty liver disease patients and are correlated with hepatic triglyceride. J Hepatol. 2010;53:934–940 [DOI] [PubMed] [Google Scholar]
- 34. Morris-Stiff G, Feldstein AE. Fibroblast growth factor 21 as a biomarker for NAFLD: integrating pathobiology into clinical practice. J Hepatol. 2010;53:795–796 [DOI] [PubMed] [Google Scholar]
- 35. Holland WL, Adams AC, Brozinick JT, et al. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 2013;17:790–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lin Z, Tian H, Lam KS, et al. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 2013;17:779–789 [DOI] [PubMed] [Google Scholar]
- 37. Holland WL, Miller RA, Wang ZV, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17:55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu Y, Turdi S, Park T, et al. Adiponectin corrects high-fat diet-induced disturbances in muscle metabolomic profile and whole-body glucose homeostasis. Diabetes. 2013;62:743–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Inagaki T, Lin VY, Goetz R, Mohammadi M, Mangelsdorf DJ, Kliewer SA. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab. 2008;8:77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Samuel VT, Beddow SA, Iwasaki T, et al. Fasting hyperglycemia is not associated with increased expression of PEPCK or G6Pc in patients with type 2 diabetes. Proc Natl Acad Sci USA. 2009;106:12121–12126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hondares E, Rosell M, Gonzalez FJ, Giralt M, Iglesias R, Villarroya F. Hepatic FGF21 expression is induced at birth via PPARα in response to milk intake and contributes to thermogenic activation of neonatal brown fat. Cell Metab. 2010;11:206–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chau MD, Gao J, Yang Q, Wu Z, Gromada J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1α pathway. Proc Natl Acad Sci USA. 2010;107:12553–12558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sarruf DA, Thaler JP, Morton GJ, et al. Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes. 2010;59:1817–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]