Abstract
Shewanella species are a group of facultative Gram-negative microorganisms with remarkable respiration abilities that allow the use of a diverse array of terminal electron acceptors (EA). Like most bacteria, S. oneidensis possesses multiple terminal oxidases, including two heme-copper oxidases (caa3- and cbb3-type) and a bd-type quinol oxidase. As aerobic respiration is energetically favored, mechanisms underlying the fact that these microorganisms thrive in redox-stratified environments remain vastly unexplored. In this work, we discovered that the cbb3-type oxidase is the predominant system for respiration of oxygen (O2), especially when O2 is abundant. Under microaerobic conditions, the bd-type quinol oxidase has a significant role in addition to the cbb3-type oxidase. In contrast, multiple lines of evidence suggest that under test conditions the caa3-type oxidase, an analog to the mitochondrial enzyme, has no physiological significance, likely because of its extremely low expression. In addition, expression of both cbb3- and bd-type oxidases is under direct control of Crp (cAMP receptor protein) but not the well-established redox regulator Fnr (fumarate nitrate regulator) of canonical systems typified in Escherichia coli. These data, collectively, suggest that adaptation of S. oneidensis to redox-stratified environments is likely due to functional loss of the caa3-type oxidase and switch of the regulatory system for respiration.
Keywords: oxidase, respiration, Shewanella
Introduction
To respire on oxygen (O2), all aerobic organisms utilize terminal oxidases to catalyze the oxidation of a respiratory substrate such as c-type cytochrome and quinol, and the reduction of O2 to water (Borisov et al., 2011). In prokaryotes, there are two major groups of terminal oxidases: the universal heme-copper oxidases (HCO) and the bd-type quinol oxidases (Pereira et al., 2001; Borisov et al., 2011). The HCO is further divided into three families: A, B and C (Wikström and Verkhovsky, 2007; Borisov et al., 2011; Lee et al., 2012). The A-family includes the aa3-type cytochrome c oxidase such as that in Paracoccus denitrificans (caa3-type in some cases, the aa3-type enzymes with a c heme-containing domain, as observed in Bacillus stearothermophilus) and the bo3-type quinol oxidase as in Escherichia coli (Puustinen et al., 1991; Giuffrè et al., 1996; Baker et al., 1998). The B-family includes a number of oxidases from extremophilic prokaryotes, such as the ba3-type enzyme of Thermus thermophilus (Chang et al., 2009). The enzymes of the C-family are all cbb3-type cytochrome c oxidases (Ekici et al., 2012).
Unlike eukaryotes carrying the single cytochrome c oxidase, most bacteria characterized so far host multiple respiratory oxidases. It has been suggested that differences in O2 affinity, proton-pumping efficiency and availability of electron donors are critical in determining expression of individual terminal oxidases in widely varying environmental conditions (Han et al., 2011). Given that the proton-pumping stoichiometry in bo3- and cbb3-HCOs (0.5H+/e−) is half of that in aa3-HCOs (1H+/e−), aa3-HCO is energetically advantageous when O2 is abundant. As a consequence, in organisms carrying an aa3-HCO it is the predominant enzyme under O2-rich growth conditions whereas cbb3-HCO is expressed only under low O2 or microaerobic conditions (Baker et al., 1998). In bacteria lacking aa3-HCO, either bo3- or cbb3-HCO is able to become the major terminal oxidase supporting aerobic growth. For instance, E. coli and Rhodobacter capsulatus utilize bo3- and cbb3-HCOs as the dominating driving force for aerobic respiration, respectively (Puustinen et al., 1991; Ekici et al., 2012).
Facultative anaerobes such as E. coli adopt different metabolic modes in response to the availability of electron donors and acceptors: aerobic respiration, anaerobic respiration and fermentation (Perrenoud and Sauer, 2005; Vemuri and Aristidou, 2005). Owing to the amount of energy released by each process, aerobic respiration is preferred over anaerobic respiration, which in turn is preferred over fermentation (Green and Paget, 2004). This hierarchy is maintained by monitoring environmental O2 and cellular redox state, predominantly by Fnr (fumarate nitrate regulator) as well as the Arc (aerobic respiration control) two-component system. Under microaerobic and/or anaerobic conditions, these regulators activate the expression of genes encoding components of alternative electron transport chains, and simultaneously repress the expression of some aerobic functions.
Shewanella are Gram-negative facultative anaerobes predominantly residing in redox-stratified environments, which compel this group of microorganisms to accommodate different O2 concentrations and use a variety of electron acceptors (EA) such as trimethylamine-N-oxide, dimethyl sulfoxide, NO3−, Fe3+, Mn4+, and so on, when O2 is depleted (Fredrikson et al., 2008). To facilitate the adaptation, Shewanella have evolved a large number of the c-type cytochromes as well as some b- and d-type cytochromes to respire these EAs, as exemplified in the model species S. oneidensis (Heidelberg et al., 2002; Meyer et al., 2004; Bretschger et al., 2007; Gao et al., 2010a). The genome of S. oneidensis encodes two cytochrome c terminal oxidases: SO4606-4609 (caa3-HCO) and SO2364-2361 (CcoN-O-Q-P, cbb3-HCO), and a quinol oxidase SO3286-3285 (CydA-B, bd-type) (Heidelberg et al., 2002). It is natural to assume that the caa3-HCO is largely responsible for respiration when O2 is abundant whereas the cbb3-HCO is of importance under O2 limitation (Marritt et al., 2012). However, our previous study on c-type cytochromes revealed that mutants missing either ccoP or ccoO displayed a defect in growth under O2-rich conditions much more severe than that missing SO4606 (an essential subunit II of caa3-HCO), suggesting that cbb3-HCO rather than caa3-HCO dominates in aerobiosis of S. oneidensis (Gao et al., 2010a).
In an attempt to decipher why Shewanella have resided in redox-stratified niches throughout evolution, we take on to assess the function of oxidases in S. oneidensis. We present evidence suggesting that cbb3-HCO, rather than caa3-HCO, is indeed the major oxidase functioning under both aerobic and microaerobic conditions. The bd-type enzyme, although dispensable under aerobic conditions, confers a significant contribution to respiration of O2 under microaerobic conditions. Further exploration has revealed that Crp (cAMP receptor protein), but not Fnr or Arc, is the global regulator directly controlling expression of these oxidases. Our report therefore demonstrates that adaptation of S. oneidensis to redox-stratified environments is likely due to a combined effect of both Crp regulation and loss of the caa3-HCO terminal oxidase.
Materials and methods
Bacterial strains, plasmids and culture conditions
A list of all bacterial strains and plasmids used in this study is given in Table 1 and a list of primers used in this study is provided in Supplementary Table S1. For genetic manipulations, E. coli and S. oneidensis strains were grown under aerobic conditions in Luria-Bertani (LB, Difco, Detroit, MI, USA) medium at 37 °C and room temperature, respectively. When needed, the growth medium was supplemented with chemicals at the following concentrations: 2, 6-diaminopimelic acid, 0.3 mM; ampicillin, 50 μg ml−1; gentamycin, 15 μg ml−1; kanamycin, 50 μg ml−1; and tetracycline, 15 μg ml−1.
Table 1. Strains and plasmids used in this studya.
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| E. coli | ||
| DH5α | Host for regular cloning | Lab stock |
| WM3064 | Host for pir-dependent plasmids and donor strain for conjugation; ΔdapA | W Metcalf, UIUC |
| BL21(DE3) | Expression host for pTP247 | Lab stock |
| S. oneidensis | ||
| MR-1 | Wild type | Lab stock |
| HG0610 | petC deletion mutant derived from MR-1; ΔpetC | Gao et al., 2010a |
| HG0624 | crp deletion mutant derived from MR-1; Δcrp | Gao et al., 2010b |
| HG0624-2356 | crp and fnr double deletion mutant derived from MR-1; ΔcrpΔfnr | Gao et al., 2010b |
| HG2356 | fnr deletion mutant derived from MR-1; Δfnr | Gao et al., 2010b |
| HG2361 | ccoP deletion mutant derived from MR-1; ΔccoP | Gao et al., 2010a |
| HG2363 | ccoO deletion mutant derived from MR-1; ΔccoO | Gao et al., 2010a |
| HG2364 | ccoN deletion mutant derived from MR-1; ΔccoN | This study |
| HG2364-3285 | ΔccoNΔcydB | This study |
| HG2364-4606 | ΔccoNΔcoxB | This study |
| HG3285 | cydB deletion mutant derived from MR-1; ΔcydB | This study |
| HG3285-4606 | ΔcydBΔcoxB | This study |
| HG3988-0624 | ΔarcAΔcrp | Gao et al., 2008a |
| HG3988-2356 | ΔarcAΔfnr | Gao et al., 2008a |
| HG3988-0624-2356 | ΔarcAΔcrpΔfnr | Gao et al., 2008a |
| HG4606 | coxB deletion mutant derived from MR-1; ΔcoxB | Gao et al., 2010a |
| HG4607 | coxA deletion mutant derived from MR-1; ΔcoxA | This study |
| HGTRIOX | ΔccoNΔcydBΔcoxB | This study |
| Plasmids | ||
| pDS3.0 | Apr, Gmr, derivative from suicide vector pCVD442 | Lab stock |
| pHG101 | Promoterless broad-host Kmr vector | Wu et al., 2011 |
| pHG102 | pHG101 containing the S. oneidensis arcA promoter | Wu et al., 2011 |
| pTP247 | Gateway destination His-tag expression vector | Gao et al., 2008b |
| pTP247-Crp | pTP247 containing crp | This study |
| pTP327 | Apr, Tetr, lacZ reporter vector | Gao et al., 2010b |
| pTP327-Pcco-lacZ | pTP327 containing ∼400 bp upstream sequence of cco | This study |
| pTP327-Pcyd-lacZ | pTP327 containing ∼400 bp upstream sequence of cyd | This study |
| pTP327-Pcox-lacZ | pTP327 containing ∼400 bp upstream sequence of cox | This study |
plasmids containing mutational structures were constructed, as described in the text and not included in the table.
In-frame deletion mutagenesis, complementation and physiological characterization
In-frame deletion mutagenesis, complementation and physiological characterization were carried out in essentially the same manner as described previously (Gao et al., 2008a; Wu et al., 2011). M1-defined medium containing 0.02% (w/v) of vitamin-free casamino acids was used in all physiological experiments (Gao et al., 2008a). Aerobic cultures were grown with rigorous shaking (250 r.p.m.) in 500 ml Erlenmeyer flasks containing 20 ml of medium. Microaerobic cultures of 20 ml were grown in 500 ml rubber-stoppered serum bottles, with a gas atmosphere of 1% O2 and 99% N2. Anaerobic media and cultures were prepared as reported earlier (Gao et al., 2009). For the viable assays, cells of S. oneidensis grown in LB at 30 °C to an OD600 of ∼0.6 were adjusted to ∼107 CFU ml−1 with fresh LB, followed by three 10-fold serial dilutions. Ten microlitres of each diluted sample (from 104 to 107 CFU ml−1) was spotted onto LB plates. All plates were incubated at 30 °C before being read. The assay was repeated at least for three times with similar results.
Promoter activity assay
To locate the promoters of the cco, cox and cyd operons, upstream sequences of these operons were analyzed using the promoter prediction program Neutral Network (Reese, 2001). To construct the Pcco-lacZ, Pcox-lacZ and Pcyd-lacZ reporters, ∼400 bp DNA fragments upstream of the cco, cox and cyd operons were amplified by PCR with primers listed in Supplementary Table S1 and cloned into pTP327 (Gao et al., 2010b). After verification by DNA sequencing, the reporter plasmids were transferred into each S. oneidensis strain by conjugation. Cells grown to an OD600 of ∼0.1 (early exponential phase), ∼0.3 (mid-exponential phase) and ∼0.8 of OD600 (early stationary phase) under aerobic conditions and grown to ∼0.1 of OD600 under microaerobic conditions were harvested by centrifugation. Cell pellets were washed once with phosphate-buffered saline, and resuspended in phosphate-buffered saline to an optical density of 1.0 (OD600) for sonication. The total protein concentration of the cell lysates was determined by the bicinchoninic acid assay (Pierce Chemical, Dallas, Tx, USA). β-galactosidase activity assay was performed using an assay kit (Beyotime, Dalian, China), as described previously (Wu et al., 2011).
Some samples were subjected to quantitative real-time reverse transcription-PCR (qRT-PCR) analysis for verification, which was performed on an ABI7300 96-well qRT-PCR system (Applied Biosystems, Foster City, CA, USA), as described previously (Yuan et al., 2011; Dong et al., 2012).
Nadi assay
The Nadi test was used for visual assessment of cytochrome c oxidase-dependent respiration (Marrs and Gest, 1973). A solution of 1% ∂-naphthol in 95% ethanol and 1% N,N-dimethyl-p-phenylenediamine monohydrochloride was applied to cover colonies grown on LB agar plates. Formation of indophenol blue was timed as an indicator of cytochrome c oxidase activity.
Expression and purification of S. oneidensis Crp protein and EMSA
The entire clone set of S. oneidensis open reading frames has been constructed, as reported previously (Gao et al., 2008b). The crp gene within pDONR221 (the entry vector) was transferred to pTP247 (the destination His-tag expression vector). Protein expression and purification was performed, as previously described (Gao et al., 2008b). The probes used for electrophoretic motility shift assay (EMSA) were prepared by PCR with 33P end-labeled primers. The binding reaction was performed with ∼25–50 fmol (∼2–5 nM) of labeled probes and various amounts of protein with or without 10 μM cAMP in 12 μl binding buffer containing 100 mM Tris/HCl (pH 7.4), 20 mM KCl, 10 mM MgCl2, 2 mM dithiothreitol, 0.2 μg μl−1 poly (dI·dC), and 10% glycerol at 15 °C for 60 min and resolved on pre-run 4.8% polyacrylamide native gels (Gao et al., 2008a). The band shifts were visualized by autoradiography.
Results
c-type cytochromes that are important for growth with abundant O 2
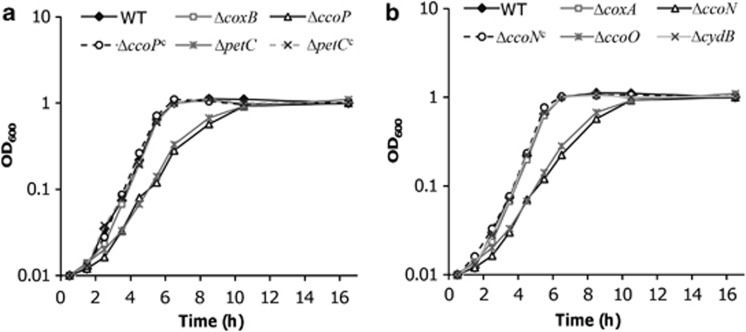
Impacts of c-type cytochromes on aerobic growth of S. oneidensis have been investigated previously with 36 single knockout strains (Gao et al., 2010a). Although removal of most of these c-type cytochromes resulted in growth that was comparable to that of the parental wild-type strain, 10 mutants displayed distinguishable growth defect, of which ΔpetC, ΔccoP and ΔccoO were most significant. PetC is an essential component of the cytochrome bc1 complex, which transfers electrons to all c-type cytochrome oxidases (Londer et al., 2008; Gao et al., 2010b). Thus, the impaired growth observed in the petC mutants is not unexpected. However, the similar observation from the ccoP and ccoO mutants was surprising, given that the genome encodes a caa3-HCO (SO4606-4609), which is supposed to operate under O2-replete conditions. To further confirm that ΔccoP and ΔccoO, rather than ΔSO4606 (ΔcoxB), are defective in aerobic growth, we created mutants lacking one of the other essential subunits in these oxidases, ΔccoN (encoding the reductase subunit) and ΔcoxA (SO4607, encoding subunit I), and assayed their growth. Results in Figure 1 show that ΔccoN and ΔcoxA are indistinguishable from ΔccoO or ΔccoP and ΔcoxB, respectively.
Figure 1.
Growth of S. oneidensis c-type cytochrome mutants compared with their parental wild-type strain. Superscript ‘c' represents the mutant strain containing a copy of the corresponding gene on the complementation vector. All strains were cultured under vigorously agitated conditions. The data are averages from at least three independent cultures. For clarity, error bars (s.d.<5% of presented data) are omitted.
To rule out polarity issues introduced by the mutations, genetic complementation for mutants with growth defects was carried out using pHG101 or pHG102, as described previously (Wu et al., 2011). In all cases, phenotypic differences were insignificant between the mutant strains containing the corresponding plasmid-borne gene and the wild type containing the empty vector, indicating that the observed phenotype of the mutants was due to the introduced mutation (Table 2). Collectively, these data suggest that the complexes within the electron transfer pathway from cytochrome bc1 to cbb3-HCO are required for optimal growth under aerobic conditions.
Table 2. S. oneidensis strains subjected to genetic complementation.
| Strain | Plasmida | Gene(s) on plasmidb | Generation time (M/C)c |
|---|---|---|---|
| WT | 1 | ||
| WT | pHG101 | 1.03±0.06 | |
| WT | pHG102 | 0.98±0.04 | |
| ΔccoO | pHG101 | ccoNO | 0.79±0.04/0.99±0.05 |
| ΔccoP | pHG102 | ccoP | 0.82±0.06/1.04±0.04 |
| ΔccoN | pHG101 | ccoNO | 0.77±0.05/1.00±0.04 |
| ΔcydB | pHG101 | cydAB | 0.47±0.06/1.06±0.08d |
| ΔcoxB | — | — | 0.97±0.03 |
| ΔcoxA | — | — | 1.02±0.05 |
| ΔcydBΔcoxB | pHG101 | cydAB | 0.44±0.05/1.01±0.06d |
| ΔccoNΔcoxB | pHG101 | ccoNO | 0.79±0.04/1.04±0.07 |
| ΔccoNΔcydB | pHG101 | coxB | No growth/no growth |
| ΔccoNΔcydB | pHG101 | cydAB | No growth/0.74±0.07 |
| ΔccoNΔcydB | pHG101 | ccoNO | No growth/0.47±0.06d |
Abbreviations: M/C, generation time of the mutant/generation time of the mutant complemented; WT, wild type.
The designated vectors for complementation.
Genes on the designated vector for complementation.
Generation time of each strain grown under aerobic conditions is normalized to that of the wild-type strain.
The maximum cell densities were compared as the generation times differed insignificantly.
The cytochrome cbb 3 oxidase is crucial for growth with abundant O 2
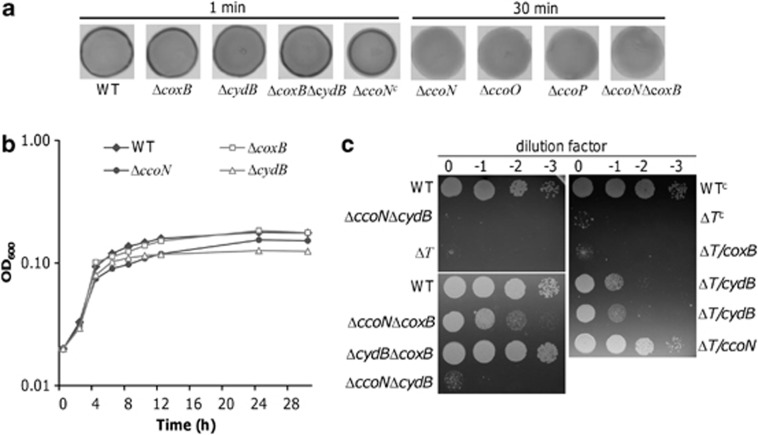
To determine whether caa3-HCO retains some capacity of aerobic respiration, we performed the Nadi assay, which can specifically detect cytochrome c oxidase-dependent respiration. Using N, N-dimethyl-p-phenylenediamine monohydrochloride as an exogenous electron donor, cytochrome c oxidase catalyzes the rapid formation of indophenol blue from colorless α-naphthol. Although formation of indophenol can occur spontaneously, the process is extremely slow, resulting in a significant delay in strains devoid of a functional cytochrome c oxidase. In S. oneidensis, both caa3- and cbb3-HCOs are supposed to be able to carry out the Nadi reaction. Compared with the wild-type strain, ΔcoxB exhibited a similar reaction rate, forming indophenol blue visibly in <1 min and developing maximum coloration within 5 min (Figure 2a). In contrast, formation of indophenol blue in ΔccoN did not occur before colonies became completely blue through spontaneous indophenol formation (results at 30 min are shown). Given that the Nadi reaction only requires a terminal oxidase and a c-type cytochrome, it is possible that removal of either CcoP or CcoO may not annul the reaction. To test this hypothesis, we performed the experiment with ΔccoP and ΔccoO, and found that both of these two mutants behaved exactly like ΔccoN, indicating that the integrity of the Cco complex is essential for the reaction. In addition, the defective phenotype in the Nadi assay that resulted from the ccoN deletion was corrected by its expression in trans. All together, these data suggest that cbb3-HCO dominates aerobic respiration under test conditions.
Figure 2.
Physiological characteristics of S. oneidensis oxidase-deficient mutants. (a) Nadi assay. The method is based on the rapid formation of indophenol blue from colorless α-naphtol catalyzed by cytochrome c oxidase, using N,N-dimethyl-p-phenylenediamine monohydrochloride as an exogenous electron donor. Nadi-positive and -negative strains were photographed 1 and 30 min after the reaction, respectively. ΔccoNc represents ΔccoN containing a copy of ccoN on the complementation vector. (b) Growth under microaerobic conditions. All strains were grown in the defined medium with 1% O2 in the gas atmosphere and growth was monitored at OD600. For clarity, error bars (s.d.<5% of presented data, n⩾3) are omitted. (c) Drop-plate assay. Cultures at the mid-log phase were adjusted to ∼107 CFU ml−1, 10 μl of which were dropped on the LB agar plates in the absence (left panel) or presence of kanamycin (right panel). Cells were incubated at 30 °C for 18 h except those on the lower left panel, which were incubated for additional 6 h for further confirmation of growth of ΔccoNΔcydB. ΔT represents the triple mutant, ΔccoNΔcydBΔcoxB. WTc and ΔTc represent these strains containing the empty vector. Experiments were performed at least three times and consistent results were obtained.
Both cytochrome cbb 3 and bd oxidase are important under microaerobic conditions
The observation that S. oneidensis is able to carry out aerobic respiration without cbb3-HCO indicates that at least one of other oxidases is functional. In addition to caa3- and cbb3-HCO, S. oneidensis also carries a bd-type quinol oxidase encoded by cydAB. To explore the role of the bd-type quinol oxidase in O2 respiration, we constructed ΔcydB, in which the essential subunit II was removed, and characterized the mutant under aerobic conditions. Consistent with the lack of a cytochrome c component, cells without the bd-type quinol oxidase were positive in the Nadi assay (Figure 2a). Moreover, compared with its parental strain, ΔcydB did not elicit any noticeable difference in either growth rate or maximum cell density, indicating that the enzyme has a negligible impact on growth when O2 is abundant (Figure 1).
We then examined growth of mutants devoid of one of the oxidases with O2 of microaerobic levels, as both cbb3-HCO and the bd-type oxidase are proposed to function preferentially under these conditions (Borisov et al., 2011). The mutation in coxB did not elicit any noticeable difference compared with the wild-type strain, reinforcing the idea that caa3-HCO is dispensable under test conditions (Figure 2b). On the contrary, loss of either cbb3- or bd-type oxidase caused significant reduction in maximum cell density, indicating that both enzymes contribute to O2 respiration under microaerobic conditions (data at a linear scale are shown in Supplementary Figure S2A). Notably, the biomass of ΔcydB cells was much lower than that of ΔccoN, which is likely due to the low efficiency of the bd-type oxidase.
To further test whether the caa3-type enzyme is completely dispensable for aerobic respiration, we made attempts to construct double mutants devoid of two oxidases under aerobic conditions, including ΔccoNΔcoxB, ΔccoNΔcydB and ΔcoxBΔcydB. Although construction of both ΔccoNΔcoxB and ΔcoxBΔcydB went smoothly, with numerous tries no ΔccoNΔcydB colonies were obtained after the resolution (the last step of the mutagenesis procedure), which was supposed to theoretically produce a population of a 50:50 mixture of the mutant and wild-type cells. When the resolution was performed under anaerobic conditions as reported earlier (Kouzuma et al., 2012), ΔccoNΔcydB and ΔccoNΔcydBΔcoxB were obtained, suggesting that cbb3- and bd-type oxidases are synthetic lethal under aerobic conditions. To confirm this, we performed the drop-plate assay of the triple mutant ΔccoNΔcydBΔcoxB strains complemented with each of the deleted genes. The cells were prepared from cultures grown on fumarate under anaerobic conditions. As shown in Figure 2c, under aerobic conditions both ΔccoNΔcydB and ΔccoNΔcydBΔcoxB were deficient in growth, whereas the double mutants lacking coxB were able to grow. In addition, expression of coxB in trans was unable to restore its growth. In contrast, the synthetic lethal phenotypes resulting from the ccoN or cydB deletions were corrected by their expression in trans. These data, collectively, indicate that aerobic growth of S. oneidensis requires either cbb3- or bd-type oxidase.
Expression levels of cco, cox and cyd operons likely account for their roles in respiration
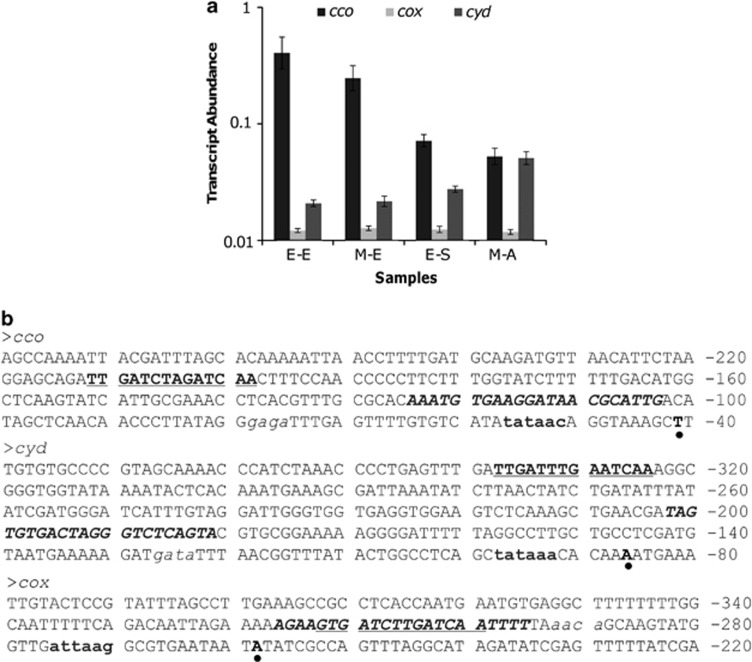
Data presented thus far establish that in S. oneidensis the cbb3- and bd-type oxidases are involved in aerobic respiration and the caa3-type is negligible. Given that transcription is the primary level of regulation, we reasoned that the operons encoding these oxidases may be transcribed differently. The messenger RNA abundance of the cco, cox and cyd operons in samples of various growth stages was therefore measured using qRT-PCR (Figure 3a). Transcription of the cox operon was extremely low regardless of growth conditions. On the contrary, both cco and cyd operons responded to growth conditions at the transcriptional level. The abundance of the cco messenger RNA, lowest under microaerobic conditions, was inversely proportional to cell densities under aerobic conditions, suggesting that expression of cco is favored in O2-rich environments. Expression of the cyd operon, at a limited level under aerobic conditions, was enhanced substantially under microaerobic conditions.
Figure 3.
Expression of the cco, cox and cyd operons. (a). Expression of the cco, cox and cyd operons by qRT-PCR. Samples were collected at the early exponential phase (E-E), the mid-exponential phase (M-E) and the early stationary phase (E-S) of aerobic cultures and the M-E phase of microaerobic cultures (M-A). Experiments were performed independently at least three times and error bars represent s.d. (b) Upstream sequences of the cco, cox and cyd operons (numbered relative to translation start sites). Transcription starting sites are pointed by dots. Predicted −35 and −10 boxes are in italics and bold lower case, respectively. Predicted Crp- and Fnr-binding sites are in italics and underlined, respectively.
To confirm these results, we then employed a lacZ-based reporter system to assess the promoter activity of cco, cox and cyd operons in vivo, represented as Pcco, Pcox and Pcyd, respectively. Analysis of upstream sequences of these operons by the promoter prediction program Neutral Network (Reese, 2001) revealed that the most confident transcription initiation sites of cco and cyd are located much closer to the translation starting sites (−42 and −87, respectively) than that of cox (−259) (Figure 3b). Accordingly, the ∼400 bp upstream sequences of cco, cox and cyd operons were amplified and placed in front of the full-length E. coli lacZ gene on plasmid pTP327. The resulting vectors, verified by sequencing, were introduced into S. oneidensis strains cultured under aerobic or microaerobic conditions. Results obtained from these samples using the lacZ-based reporter system were comparable to those from qRT-PCR. These data, consistent with their significance in aerobic respiration, indicate that cbb3-HCO is the predominant driving force for aerobic respiration. whereas the bd-type oxidase facilitates the process when O2 becomes limited.
Compensatory expression of the cyd operon
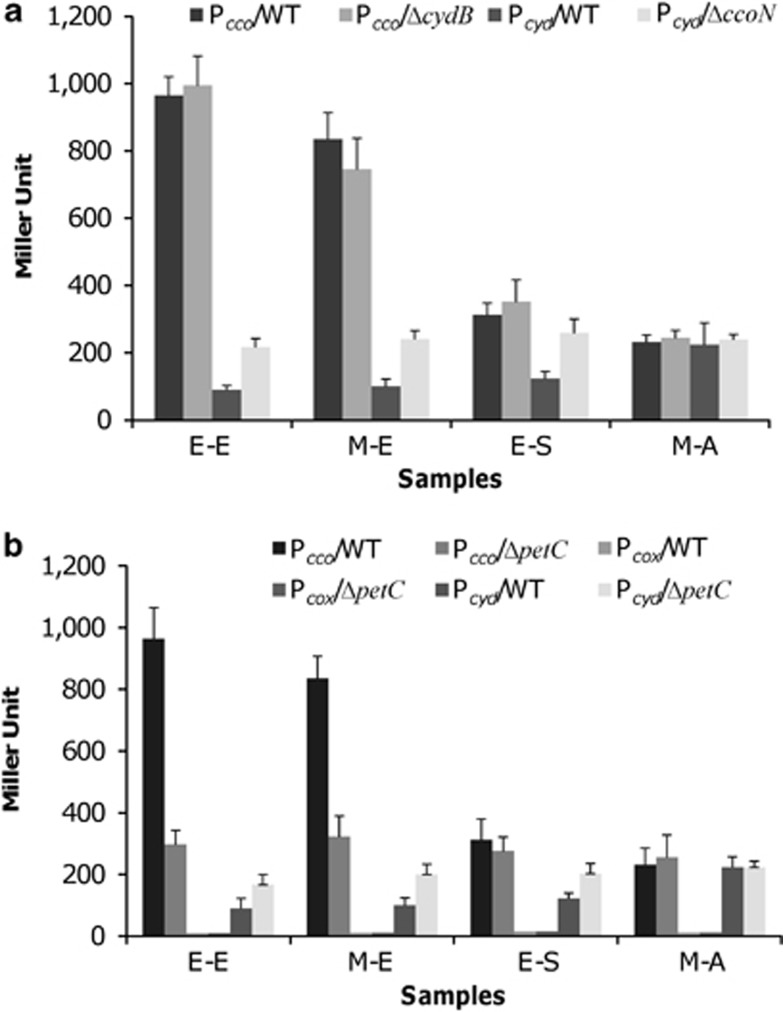
In combination, the cbb3- and bd-type oxidases appear to be synthetic lethal, conferring a possible regulatory interplay between these two systems. To test this hypothesis, we examined the activity of Pcco and Pcyd in cyd− and cco− backgrounds, respectively. Results showed that the absence of Cyd had no effect on cco transcription (Figure 4a). However, when cco was removed transcription of cyd increased substantially when O2 was abundant, reaching a level close to that observed under microaerobic conditions, under which the activity of Pcyd was hardly affected. This result implies that expression of cyd is possibly maximized under microaerobic conditions.
Figure 4.
Promoter activities of the cco, cox and cyd operons. Approximate, 400 bp upstream sequences were transcriptionally fused to full-length lacZ for β-galactosidase activity assay. Data are given in Miller units. (a) Activities of the cco promoter in ΔcydB and the cyd promoter in ΔccoN, grown to phases the same as in Figure 3a. (b) Activities of the cco, cox and cyd promoters in ΔpetC grown to phases the same as in Figure 3a.
The compensatory expression of the cyd operon under aerobic conditions suggests that cbb3-HCO, when abundant, represses production of the bd-type oxidase. To confirm this, expression of cyd in ΔpetC was examined. We expected that expression of cyd would increase as in ΔccoN, as cbb3-HCO is unable to function in the petC− background. Strain ΔpetC was cultured under the same conditions as described above and β-galactosidase activity was measured (Figure 4b). In the absence of PetC, expression of cco was significantly reduced, with the largest difference (∼25% remaining) observed in the early exponential phase samples. In contrast, expression of cyd in ΔpetC was increased to a level comparable to that observed in ΔccoN, thus confirming that cyd is indeed subjected to compensatory induction once cco is missing. As expected, expression of cox remained at the extremely low level, regardless of PetC.
Impacts of global regulators ArcA, Crp and Fnr on expression of cco, cox and cyd operon
In S. oneidensis, global regulators mediating adaptation of metabolic modes in response to the availability of O2 include the Arc system, Crp and Fnr (Gao et al., 2010b). On one hand, S. oneidensis Fnr, unlike its E. coli analog, which is the primary factor controlling the switch between aerobic and anaerobic metabolism, has no significant role in the process (Maier and Myers, 2001; Cruz-Garcia et al., 2011). On the other hand, both the Arc system and Crp have roles in respiration, with the former primarily functioning under aerobic conditions and the latter being predominant under anaerobiosis (Saffarini et al., 2003; Gao et al., 2010b).
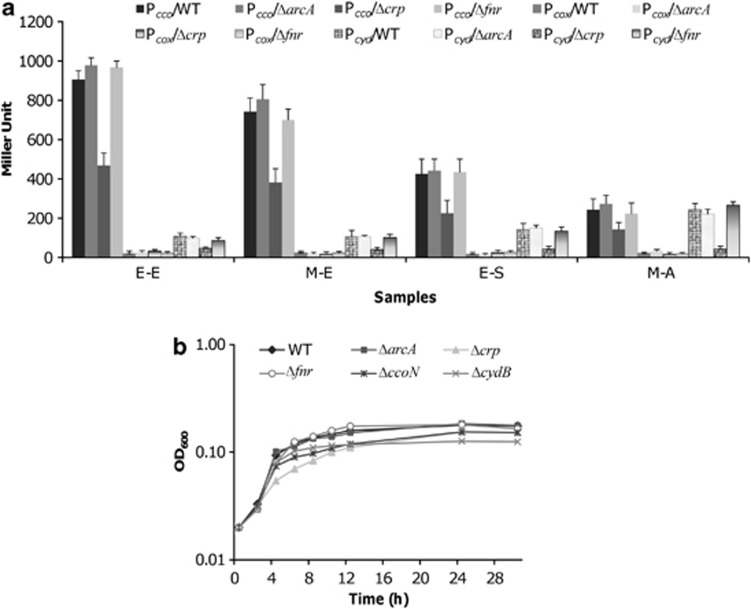
Regions upstream of all cco, cox and cyd operons are predicted to contain Crp- and Fnr- but not ArcA-binding motifs, implicating that these terminal oxidases may be subjected to direct regulation by Crp and Fnr (Gao et al., 2010b) (Figure 3a). To gain insight into effects of such control in vivo, we measured the activity of Pcco, Pcox and Pcyd in the arcA−, crp− or fnr− background, respectively. As shown in Figure 5a, the promoter activity of the cox operon was too low to be meaningfully compared between the wild type and any mutant strains. Consistent with the lack of ArcA-binding motifs, the expression levels of cco and cyd operons were hardly altered in the arcA− background compared with the wild-type strain. Interestingly, removal of Crp and Fnr elicited different impacts on the activity of Pcco and Pcyd, respectively, despite the coexistence of well-conserved Crp- and Fnr-binding sites. Under all tested conditions, response of neither Pcco nor Pcyd to the loss of Fnr was statistically significant, reinforcing the idea that Fnr has an extremely limited role in regulation. By contrast, Crp was essential for expression of the cyd operon but exerted a relatively moderate impact on Pcco under all tested conditions.
Figure 5.
Impacts of ArcA, Fnr and Crp on oxidases in S. oneidensis. (a) Promoter activities of the cco, cox and cyd operons in strains lacking ArcA, Fnr or Crp, respectively. Cells were prepared the same as in Figures 3b and 4. (b) Growth of the S. oneidensis wild-type and mutant strains under microaerobic conditions. All strains were grown in the defined medium with 1% O2 in the gas atmosphere and growth was monitored at OD600. Strains with indistinguishable phenotypes: WT=ΔarcA=Δfnr=ΔarcAΔfnr; Δcrp=ΔarcAΔcrp=ΔcrpΔfnr=ΔarcAΔcrpΔfnr. For clarity, only wild-type and single mutants ΔarcA, Δcrp and Δfnr are presented in the figure and error bars (s.d.<5% of presented data, n⩾3) are omitted.
We have previously shown that both ΔarcA and Δcrp grow significantly slower, whereas Δfnr is not distinguishable relative to the wild-type strain under vigorously agitated conditions (Gao et al., 2010b). Given that the promoter activities of cco, cox and cyd are affected by Crp only, we reasoned that Δcrp would be defective more severely in growth under microaerobic conditions where both the cytochrome cbb3- and bd-type oxidases are involved. To test this, growth of ΔarcA, Δcrp, Δfnr, ΔarcAΔcrp, ΔcrpΔfnr, ΔarcAΔfnr and ΔarcAΔcrpΔfnr under microaerobic conditions was assayed (Figure 5b). As expected, the crp mutant displayed a significant defect in growth. On the contrary, both ΔarcA and Δfnr grew similarly in comparison with the wild-type strain, and so did ΔarcAΔfnr (data at a linear scale shown in Supplementary Figure S2B). Moreover, other strains carrying multiple mutations were not distinct from Δcrp, implicating that loss of Crp in these strains was accountable for their growth defect. Collectively, these data converge on the idea that Crp is the regulator controlling respiration of not only a number of EAs anaerobically, but also O2.
DNA-binding characteristics of Crp
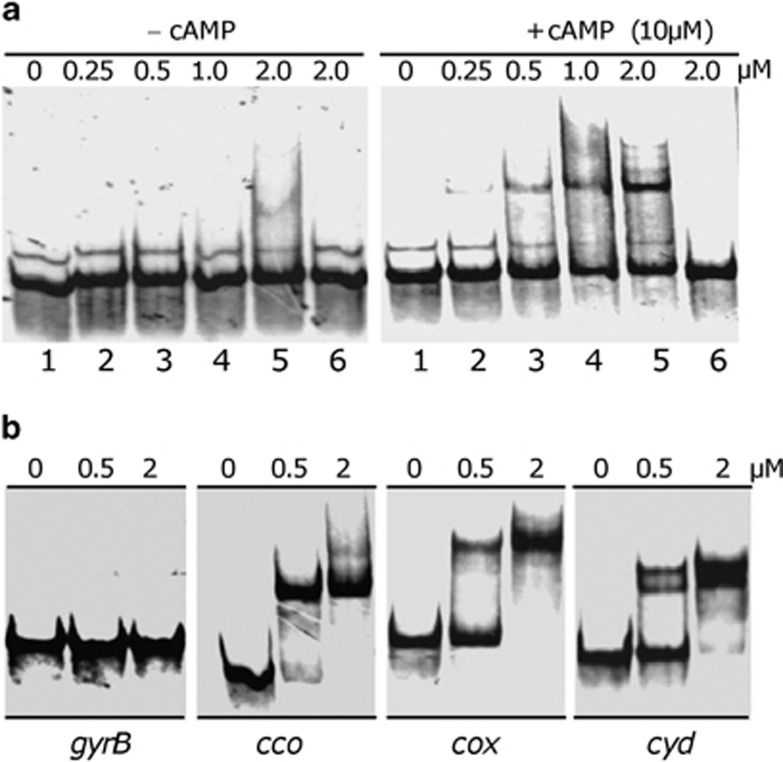
Although predicted Crp-binding motifs are identified in upstream regions of both cco and cyd operons, different effects of Crp on Pcco and Pcyd warrants an EMSA assay to determine whether Crp binds directly to the cco and cyd promoter regions. The His-tagged Crp protein was produced in E. coli and purified from inclusion bodies (Gao et al., 2008b). It has been previously shown that expression of the dms operon (encoding dimethyl sulfoxide reductase) is dependent on Crp. We therefore chose its upstream sequence for calibration of the Crp binding in a preliminary experiment (Saffarini et al., 2003; Gralnick et al., 2005). A DNA fragment of ∼200 bp covering the predicted Crp-binding site was amplified with 33P end-labeled primers, and assayed with the purified His-tagged Crp with or without cAMP in EMSA. Significant binding to the DNA probe occurred at a protein concentration of 0.25 μM for Crp in the presence of 10 μM cAMP (Figure 6a). In contrast, Crp did not bind in the absence of cAMP, even when the protein concentration was increased to 4 μM. The binding of Crp-cAMP to the target promoter was not reduced by addition of the nonspecific competitor poly(dI·dC) DNA, but was outcompeted by adding 100-fold excess unlabeled probe. These results demonstrate that Crp binds the dms promoter in a sequence-specific manner and such a capacity is dependent on cAMP.
Figure 6.
Crp binding to selected promoters by EMSA. (a) Interaction of the dms promoter DNA with S. oneidensis His-tagged Crp. The probe was prepared by PCR with 33P end-labeled primers. The EMSA assay was performed with 2 nM 33P end-labeled probes and various amounts of Crp (left panel) or Crp and cAMP (right panel). The protein concentrations for lanes 1–5 are 0, 0.25, 0.5, 1.0, 2.0 μM, respectively. Non-specific competitor DNA (0.2 μg poly dI·dC) was added to all lanes and specific competitor (10 μM unlabeled dms probe) was added (lane 6). (b) The binding assay was performed in the presence of 0, 0.5 or 2 μM Crp, 10 μM cAMP, and 2–5 nM radiolabeled promoter DNA. 0.2 μg μl−1 poly(dI·dC) was used in all these binding reactions to block nonspecific interactions. Promoter region of gyrB was used as negative control.
We then applied EMSA to upstream fragments of the cco, cox and cyd operons covering predicted Crp-binding sites with Crp and 10 μM cAMP. A similar length upstream fragment of gyrB (encoding DNA gyrase subunit B) was included in the assay as a negative control, according to the method established previously (Gao et al., 2008a). A gel shift band was observed with all three of targeted upstream sequences when 0.5 μM of Crp was added to the reaction mixture and the intensity of the shifted band became stronger with 2 μM of Crp. In contrast, the gyrB fragment was unable to cause a visible motility shift (Figure 6b). These results provide evidence for the direct binding of Crp to the cco, cox and cyd promoter regions, although regulatory effects of these interactions differ.
Discussion
Once regarded to be present only in proteobacteria (Pereira et al., 2001), cbb3-HCOs have been suggested to exist in all bacteria with exception of Thermotogales, Deinococcales and Firmicutes on the basis of the occurrence of CcoN and CcoO (Ducluzeau et al., 2008). In a number of species, cbb3-HCO is the sole HCO, and more rarely, as in Helicobacter pylori, serves as the only terminal oxidase (Pitcher and Watmough, 2004; Ekici et al., 2012). In these cases, it is not surprising that cbb3-HCO have been found to be highly expressed under O2-saturating conditions (Swem and Bauer, 2002). However, in most bacteria carrying cbb3-HCO, it coexists with other HCO(s), which are preferentially expressed over cbb3-HCO under O2-rich conditions (Pereira et al., 2001; Han et al., 2011). Here, we report on a new twist on the utilization of terminal oxidases during aerobic growth. S. oneidensis predominantly applies cbb3-HCO for respiration of O2, whereas caa3-HCO is dispensable, likely due to the substantial difference in their expression.
We present evidence suggesting that transcription of cbb3-HCO is directly proportional to the O2 level and the bd-type terminal oxidase is preferentially expressed under microaerobic conditions. This is not surprising because cbb3-HCO is thought to have a lower affinity for O2 than the bd-type terminal oxidase, as evidenced in R. capsulatus, which contains these two enzymatic complexes only (Swem and Bauer, 2002). In S. oneidensis, the bd-type terminal oxidase alone, although expressed at an elevated level in the absence of cbb3-HCO, supports impaired growth under O2-saturating conditions. Along with findings that the bd-type oxidases are found to account for nitric oxide resistance, we believe that the oxidase primarily has alternative functions relevant to physiology, such as adaptation to a wide variety of stress conditions (Giuffrè et al., 2012; Fu et al., 2013).
Why does caa3-HCO lose its primary position in aerobic respiration of S. oneidensis? By using O2 as an electron acceptor, facultative anaerobic bacteria like S. oneidensis conserve larger amount of energy in comparison with other EAs, thereby supporting much better growth. As a result, respiration of EAs other than O2 requires low O2 environments, as evidenced by findings that the expression of some terminal reductases is not allowed or limited under O2-rich conditions (Baraquet et al., 2009; Dong et al., 2012). To survive and proliferate at submicromolar O2 levels, S. oneidensis utilizes the C-family heme-copper oxidase that can likely either tolerate or adapt to low O2 environments. In this regard, we propose two evolutionary mechanisms underlying the loss of caa3-HCO from S. oneidensis. The enzyme may not be advantageous in its O2-limited natural habitat, given its low affinity for O2, thereby relieving any selective pressure to retain it. Alternatively, the loss of caa3-HCO may have decreased the competitiveness of S. oneidensis in O2-rich environments, forcing it to occupy redox-stratified niches.
In S. oneidensis, multiple lines of evidence suggest that reduced O2 concentration, and the lowered internal energetic status that results, are being sensed such that expression of different terminal oxidases can be regulated. Intriguingly, neither Fnr nor ArcA has a significant role in regulation of these oxidases. In the case of Fnr, although this can be readily explained by the inactivation of the protein in the presence of O2, previous studies suggest that the regulator is not of significance in physiology in general (Maier and Myers, 2001; Cruz-Garcia et al., 2011). Unlike its E. coli counterpart, ArcA of S. oneidensis shows profound impacts on aerobic growth without directly mediating expression of any of the terminal oxidases (Gao et al., 2008a, 2010b). The growth defect of an arcA-null mutant has been suggested to result from a reduced rate of protein synthesis (Yuan et al., 2012). By contrast, Crp has a predominant role in mediating the expression of different terminal oxidases. Unlike its E. coli counterpart, which is mainly responsible for the activation of genes involved in the catabolism of organic carbon substrates, S. oneidensis Crp appears to control genes that are functionally more diverse (Saffarini et al., 2003; Görke and Stülke, 2008; Murphy et al., 2009; Murphy and Saltikov, 2009). Nevertheless, the EMSA results presented here are consistent with previously reported in vivo data (Charania et al., 2009), and argue for an identical mechanism for the activation of Crp. It appears that the low internal energetic status favors the production of cAMP, as evidenced by a twofold increase in Crp under O2-limited conditions, and Crp primarily functions under anaerobic conditions (Gao et al., 2010b). Regulation by cAMP-Crp may be particularly critical in adaptation of S. oneidensis to redox-stratified environments, as the expression of different electron transport chains can fluctuate with the levels of intracellular cAMP. We speculate that metabolic fine-tuning offered by this differential regulatory mechanism is advantageous over on-off switching by Fnr and/or Arc, especially for microorganisms that are competitively inferior to those with an aa3-type oxidase.
Acknowledgments
We thank the reviewers for taking the time to carefully read and edit this paper. This research was supported by Major State Basic Research Development Program (973 Program: 2010CB833803), Natural Science Foundation of Zhejiang province (R3110096), and Major Program of Science and Technology Department of Zhejiang province (2009C12061), and National Natural Science Foundation of China (31270097) to HG.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Baker SC, Ferguson SJ, Ludwig B, Page MD, Richter OMH, van Spanning RJM. Molecular genetics of the genus Paracoccus: metabolically versatile bacteria with bioenergetic flexibility. Microbiol Mol Biol Rev. 1998;62:1046–1078. doi: 10.1128/mmbr.62.4.1046-1078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraquet C, Théraulaz L, Iobbi-Nivol C, Méjean V, Jourlin-Castelli C. Unexpected chemoreceptors mediate energy taxis towards electron acceptors in Shewanella oneidensis. Mol Microbiol. 2009;73:278–290. doi: 10.1111/j.1365-2958.2009.06770.x. [DOI] [PubMed] [Google Scholar]
- Borisov VB, Gennis RB, Hemp J, Verkhovsky MI. The cytochrome bd respiratory oxygen reductases. Biochim Biophys Acta. 2011;1807:1398–1413. doi: 10.1016/j.bbabio.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretschger O, Obraztsova A, Sturm CA, Chang IS, Gorby YA, Reed SB, et al. Current production and metal oxide reduction by Shewanella oneidensis MR-1 wild type and mutants. Appl Environ Microbiol. 2007;73:7003–7012. doi: 10.1128/AEM.01087-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Hemp J, Chen Y, Fee JA, Gennis RB. The cytochrome ba3 oxygen reductase from Thermus thermophilus uses a single input channel for proton delivery to the active site and for proton pumping. Proc Natl Acad Sci USA. 2009;106:16169–16173. doi: 10.1073/pnas.0905264106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charania MA, Brockman KL, Zhang Y, Banerjee A, Pinchuk GE, Fredrickson JK, et al. Involvement of a membrane-bound class III adenylate cyclase in regulation of anaerobic respiration in Shewanella oneidensis MR-1. J Bacteriol. 2009;191:4298–4306. doi: 10.1128/JB.01829-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Garcia C, Murray AE, Rodrigues JLM, Gralnick JA, McCue LA, Romine MF, et al. Fnr (EtrA) acts as a fine-tuning regulator of anaerobic metabolism in Shewanella oneidensis MR-1. BMC Microbiol. 2011;11:64. doi: 10.1186/1471-2180-11-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Wang J, Fu H, Zhou G, Shi M, Gao H. A Crp-dependent two-component system regulates nitrate and nitrite respiration in Shewanella oneidensis. PLoS ONE. 2012;7:e51643. doi: 10.1371/journal.pone.0051643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducluzeau AL, Ouchane S, Nitschke W. The cbb3 oxidases are an ancient innovation of the domain bacteria. Mol Biol Evol. 2008;25:1158–1166. doi: 10.1093/molbev/msn062. [DOI] [PubMed] [Google Scholar]
- Ekici S, Pawlik G, Lohmeyer E, Koch HG, Daldal F. Biogenesis of cbb3-type cytochrome c oxidase in Rhodobacter capsulatus. Biochim Biophys Acta. 2012;1817:898–910. doi: 10.1016/j.bbabio.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson JK, Romine MF, Beliaev AS, Auchtung JM, Driscoll ME, Gardner TS, et al. Towards environmental systems biology of Shewanella. Nat Rev Micro. 2008;6:592–603. doi: 10.1038/nrmicro1947. [DOI] [PubMed] [Google Scholar]
- Fu H, Chen H, Wang J, Zhou G, Zhang H, Zhang L, et al. 2013Crp-dependent cytochrome bd oxidase confers nitrite resistance to Shewanella oneidensis Environ Microbioldoi: 10.1111/1462-2920.12091 [DOI] [PubMed]
- Gao H, Barua S, Liang Y, Wu L, Dong Y, Reed S, et al. Impacts of Shewanella oneidensis c-ype cytochromes on aerobic and anaerobic respiration. Microb Biotech. 2010a;3:455–466. doi: 10.1111/j.1751-7915.2010.00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Pattison D, Yan T, Klingeman DM, Wang X, Petrosino J, et al. Generation and validation of a Shewanella oneidensis MR-1 clone set for protein expression and phage display. PLoS ONE. 2008b;3:e2983. doi: 10.1371/journal.pone.0002983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Wang X, Yang Z, Palzkill T, Zhou J. Probing regulon of ArcA in Shewanella oneidensis MR-1 by integrated genomic analyses. BMC Genomics. 2008a;9:42. doi: 10.1186/1471-2164-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Wang X, Yang ZK, Chen J, Liang Y, Chen H, et al. Physiological roles of ArcA, Crp, and EtrA and their interactive control on aerobic and anaerobic respiration in Shewanella oneidensis. PLoS ONE. 2010b;5:e15295. doi: 10.1371/journal.pone.0015295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Yang ZK, Barua S, Reed SB, Romine MF, Nealson KH, et al. Reduction of nitrate in Shewanella oneidensis depends on atypical NAP and NRF systems with NapB as a preferred electron transport protein from CymA to NapA. ISME J. 2009;3:966–976. doi: 10.1038/ismej.2009.40. [DOI] [PubMed] [Google Scholar]
- Giuffrè A, Borisov VB, Mastronicola D, Sarti P, Forte E. Cytochrome bd oxidase and nitric oxide: from reaction mechanisms to bacterial physiology. FEBS Lett. 2012;586:622–629. doi: 10.1016/j.febslet.2011.07.035. [DOI] [PubMed] [Google Scholar]
- Giuffrè A, D'Itri E, Giannini S, Brunori M, Ubbink-Kok T, Konings WN, et al. The caa3 terminal oxidase of Bacillus stearothermophilus. J Biol Chem. 1996;271:13987–13992. doi: 10.1074/jbc.271.24.13987. [DOI] [PubMed] [Google Scholar]
- Görke B, Stülke J. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Microbiol. 2008;6:613–624. doi: 10.1038/nrmicro1932. [DOI] [PubMed] [Google Scholar]
- Gralnick JA, Brown CT, Newman DK. Anaerobic regulation by an atypical Arc system in Shewanella oneidensis. Mol Microbiol. 2005;56:1347–1357. doi: 10.1111/j.1365-2958.2005.04628.x. [DOI] [PubMed] [Google Scholar]
- Green J, Paget MS. Bacterial redox sensors. Nat Rev Micro. 2004;2:954–966. doi: 10.1038/nrmicro1022. [DOI] [PubMed] [Google Scholar]
- Han H, Hemp J, Pace LA, Ouyang H, Ganesan K, Roh JH, et al. Adaptation of aerobic respiration to low O2 environments. Proc Natl Acad Sci USA. 2011;108:14109–14114. doi: 10.1073/pnas.1018958108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberg JF, Paulsen IT, Nelson KE, Gaidos EJ, Nelson WC, Read TD, et al. Genome sequence of the dissimilatory metal ion-reducing bacterium Shewanella oneidensis. Nat Biotech. 2002;20:1118–1123. doi: 10.1038/nbt749. [DOI] [PubMed] [Google Scholar]
- Kouzuma A, Hashimoto K, Watanabe K. Influences of aerobic respiration on current generation by Shewanella oneidensis MR-1 in single-chamber microbial fuel cells. Biosci Biotechnol Biochem. 2012;76:270–275. doi: 10.1271/bbb.110633. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Reimann J, Huang Y, Ädelroth P. Functional proton transfer pathways in the heme–copper oxidase superfamily. Biochim Biophys Acta. 2012;1817:537–544. doi: 10.1016/j.bbabio.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Londer YY, Giuliani SE, Peppler T, Collart FR. Addressing Shewanella oneidensis “cytochromome”: the first step towards high-throughput expression of cytochromes c. Protein Exp Purif. 2008;62:128–137. doi: 10.1016/j.pep.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Maier TM, Myers CR. Isolation and characterization of a Shewanella putrefaciens MR-1 electron transport regulator etrA mutant: reassessment of the role of EtrA. J Bacteriol. 2001;183:4918–4926. doi: 10.1128/JB.183.16.4918-4926.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marritt SJ, McMillan DG, Shi L, Fredrickson JK, Zachara JM, Richardson DJ, et al. The roles of CymA in support of the respiratory flexibility of Shewanella oneidensis MR-1. Biochem Soc Trans. 2012;40:1217–1221. doi: 10.1042/BST20120150. [DOI] [PubMed] [Google Scholar]
- Marrs B, Gest H. Genetic mutations affecting the respiratory electron-transport system of the photosynthetic bacterium Rhodopseudomonas capsulata. J Bacteriol. 1973;114:1045–1051. doi: 10.1128/jb.114.3.1045-1051.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TE, Tsapin AI, Vandenberghe I, De Smet L, Frishman D, Nealson KH, et al. Identification of 42 possible cytochrome c genes in the Shewanella oneidensis genome and characterization of six soluble cytochromes. Omics. 2004;8:57–77. doi: 10.1089/153623104773547499. [DOI] [PubMed] [Google Scholar]
- Murphy JN, Durbin KJ, Saltikov CW. Functional roles of arcA, etrA, cyclic AMP (cAMP)-cAMP receptor protein, and cya in the arsenate respiration pathway in Shewanella sp. strain ANA-3. J Bacteriol. 2009;191:1035–1043. doi: 10.1128/JB.01293-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JN, Saltikov CW. The ArsR repressor mediates arsenite-dependent regulation of arsenate respiration and detoxification operons of Shewanella sp. strain ANA-3. J Bacteriol. 2009;191:6722–6731. doi: 10.1128/JB.00801-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira MM, Santana M, Teixeira M. A novel scenario for the evolution of haem-copper oxygen reductases. Biochim Biophys Acta. 2001;1505:185–208. doi: 10.1016/s0005-2728(01)00169-4. [DOI] [PubMed] [Google Scholar]
- Perrenoud A, Sauer U. Impact of global transcriptional regulation by ArcA, ArcB, Cra, Crp, Cya, Fnr, and Mlc on glucose catabolism in Escherichia coli. J Bacteriol. 2005;187:3171–3179. doi: 10.1128/JB.187.9.3171-3179.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher RS, Watmough NJ. The bacterial cytochrome cbb3 oxidases. Biochim Biophys Acta. 2004;1655:388–399. doi: 10.1016/j.bbabio.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Puustinen A, Finel M, Haltia T, Gennis RB, Wikstrom M. Properties of the two terminal oxidases of Escherichia coli. Biochemistry. 1991;30:3936–3942. doi: 10.1021/bi00230a019. [DOI] [PubMed] [Google Scholar]
- Reese MG. Application of a time-delay neural network to promoter annotation in the Drosophila melanogaster genome. Comput Chem. 2001;26:51–56. doi: 10.1016/s0097-8485(01)00099-7. [DOI] [PubMed] [Google Scholar]
- Saffarini DA, Schultz R, Beliaev A. Involvement of cyclic AMP (cAMP) and cAMP receptor protein in anaerobic respiration of Shewanella oneidensis. J Bacteriol. 2003;185:3668–3671. doi: 10.1128/JB.185.12.3668-3671.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swem DL, Bauer CE. Coordination of ubiquinol oxidase and cytochrome cbb3 oxidase expression by multiple regulators in Rhodobacter capsulatus. J Bacteriol. 2002;184:2815–2820. doi: 10.1128/JB.184.10.2815-2820.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri GN, Aristidou AA. Metabolic engineering in the -omics era: elucidating and modulating regulatory networks. Microbiol Mol Biol Rev. 2005;69:197–216. doi: 10.1128/MMBR.69.2.197-216.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikström M, Verkhovsky MI. Mechanism and energetics of proton translocation by the respiratory heme-copper oxidases. Biochim Biophys Acta. 2007;1767:1200–1214. doi: 10.1016/j.bbabio.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Wu L, Wang J, Tang P, Chen H, Gao H. Genetic and molecular characterization of flagellar assembly in Shewanella oneidensis. PLoS ONE. 2011;6:e21479. doi: 10.1371/journal.pone.0021479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Wei B, Lipton MS, Gao H. Impact of ArcA loss in Shewanella oneidensis revealed by comparative proteomics under aerobic and anaerobic conditions. Proteomics. 2012;12:1957–1969. doi: 10.1002/pmic.201100651. [DOI] [PubMed] [Google Scholar]
- Yuan J, Wei B, Shi M, Gao H. Functional assessment of EnvZ/OmpR two-component system in Shewanella oneidensis. PLoS ONE. 2011;6:e23701. doi: 10.1371/journal.pone.0023701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.