Summary
Reactive oxygen species (ROS) play key roles in mucosal defense, yet how they are induced and the consequences for pathogens are unclear. We report that ROS generated by epithelial NADPH oxidases (Nox1/Duox2) during Campylobacter jejuni infection impair bacterial capsule formation and virulence by altering bacterial signal transduction. Upon C. jejuni invasion, ROS released from the intestinal mucosa inhibit the bacterial phosphotyrosine network that is regulated by the outer membrane tyrosine kinase Cjtk (Cj1170/OMP50). ROS-mediated Cjtk inactivation results in an overall decrease in the phosphorylation of C. jejuni outer membrane / periplasmic proteins including UDP-GlcNAc/Glc 4-epimerase (Gne), an enzyme required for N-glycosylation and capsule formation. Cjtk positively regulates Gne by phosphorylating an active site tyrosine, while loss of Cjtk or ROS treatment inhibits Gne activity, causing altered polysaccharide synthesis. Thus, epithelial NADPH oxidases are an early antibacterial defense system in the intestinal mucosa that modifies virulence by disrupting bacterial signaling.
Introduction
Accounting for more than 20% of all deaths under the age of 5 years, diarrhoeal disease is a major cause of mortality (Bulletin WHO 2003). Campylobacter jejuni is the predominant enteric bacterial pathogen in humans worldwide and C. jejuni infection is associated with a variety of serious sequelae including Guillain-Barre syndrome, reactive arthritis and irritable bowel syndrome (Janssen et al., 2008). Despite its importance as a pathogen little is understood of how C. jejuni initiates disease in the human intestine or how the intestinal mucosa responds to infection. The gastrointestinal tract and mucosal surfaces in general harbor a number of innate defense strategies to maintain epithelial integrity during challenge from pathogenic organisms (Hooper and Macpherson, 2010). The physical organization of the mucosal barrier and secretion of anti-microbial mediators constitute the first line of defense. A common microbicidal process in innate immunity is the NADPH oxidase (Nox2)-mediated release of ROS into the neutrophil phagosome for pathogen killing (Nauseef, 2007). In this sealed membrane compartment highly reactive ROS are generated that lead to DNA and protein damage, thereby contributing to inactivation of autoinducers and protein export pathways (Rosen et al., 2009; Rothfork et al., 2004).
ROS are also generated by mucosal epithelial barrier tissues through the related oxidase complexes Nox1-p22phox and Duox2-DuoxA2. While Nox1 expression is restricted to colon, Duox2 can be found in all segments of the intestinal tract (Bae et al., 2010; Rokutan et al., 2008). Pathogen-associated molecules can lead to upregulation of Nox1 or Nox1-associated regulatory proteins (Rada and Leto, 2008), implicating this oxidase in immune cell signaling. In addition, anti-bacterial properties of Duox in the intestine of Drosophila or zebrafish larvae have been reported (Flores et al., 2010; Ha et al., 2009). The availability of an oxygenation zone close to the epithelial surface provides a suitable milieu for production of superoxide and H2O2 by epithelial oxidases in the intestine (Marteyn et al., 2011). For mucosal surfaces in the lung, a role for a Duox-lactoperoxidase (LPO) system producing microbicidal hypothiocyanite has been proposed (Fischer et al., 2011; Xu et al., 2009). The relevance of this mechanism to mucosal host defense in vivo has not been confirmed and relies on sufficient generation of halides. However, diffusion and decomposition of ROS in the extracellular environment of the intestine will likely diminish the local concentration of ROS and therefore, if and how intestinal pathogens might be affected by ROS is not known.
Innate immune mechanisms elicited by C. jejuni invasion of the intestinal epithelium are essential for limiting the duration and severity of infection. C. jejuni appears to have evolved a repertoire of virulence mechanisms for immune avoidance. In addition to protection from environmental stress, the bacterial capsule is one of the established pathogenicity factors in C. jejuni. Capsule-deficient organisms show impaired colonization in chicks, decreased epithelial invasion, reduced virulence in ferrets, and are highly sensitive to complement-mediated killing (Bacon et al., 2001; Keo et al., 2011). Our earlier observations that capsule expression and virulence of C. jejuni are modulated by host cell factors in the extracellular milieu points to the likely involvement of an infection-dependent, diffusible signaling process between host and pathogen (Corcionivoschi et al., 2009).
Here, we examined the possibility that H2O2 represents the soluble host factor released from the human intestinal epithelium following infection by C. jejuni. Upon contact with the bacterium NADPH oxidases were redistributed to the bacterial entry site resulting in release of H2O2 into the extracellular environment at the mucosal surface. Continuous exposure to low concentrations of H2O2, as encountered by bacteria in the intestine, was sufficient to alter bacterial signal transduction, i.e. disruption of the C. jejuni phosphotyrosine network located in the outer membrane and periplasmic space. An unconventional C. jejuni bacterial tyrosine kinase (BY-kinase) was identified that controlled capsule formation by phosphorylating an epimerase involved in polysaccharide biosynthesis. Thus, epithelial NADPH oxidases function as an innate mucosal defense system by targeting bacterial phosphotyrosine signaling to modulate pathogenicity.
RESULTS
Campylobacter infection triggers Nox1/Duox2-mediated ROS generation
We reported that host epithelial cells modulate C. jejuni virulence by an unidentified mechanism (Corcionivoschi et al., 2009). With immune defense systems in mind, we hypothesized that the release of ROS from intestinal epithelium infected by C. jejuni was responsible for this effect. Indeed, here we show that co-culture of C. jejuni 81-176 with HCT-8 intestinal epithelial cells triggered the release of H2O2 into the medium (Figure 1A). H2O2 levels rose progressively within 30 minutes following infection and stabilized after 3 hours (Figure 1B). Addition of catalase (CAT) or pretreatment of cells with the flavoenzyme inhibitor diphenylene iodonium (DPI) confirmed the presence of H2O2 in the medium and the likely involvement of NADPH oxidase as a ROS-generating enzyme system (Figure 1C). In vivo the intestinal epithelium expresses predominantly either Nox1 (colon) or Duox2 (small bowel) (Bedard and Krause, 2007). ROS were also induced following co-culture of C. jejuni with cells that harbor Nox1 and Duox2, respectively (Figures 1D, E). RT-PCR experiments indicated expression of the Nox1 complex including NoxA1 and NoxO1, but not of any other NADPH oxidase isoforms in HCT-8 cells (Figure 1F). Transcriptional upregulation of the Nox1 complex or of other Nox/Duox family members was not detected, as predicted by the rapid onset of ROS generation. Supporting these observations knockdown of Nox1 attenuated H2O2 production by HCT-8 cells when co-cultured with C. jejuni (Figure 1G). Confocal images of uninfected versus C. jejuni-infected HCT-8 cells revealed infection-dependent redistribution of the Nox1-p22phox complex to areas of lamellipodia formation and to points of entry of C. jejuni organisms (Figure 1H, Figures S1A-F).
Figure 1. Exposure of intestinal cells to C. jejuni 81-176 leads to NADPH oxidase-mediated release of hydrogen peroxide.
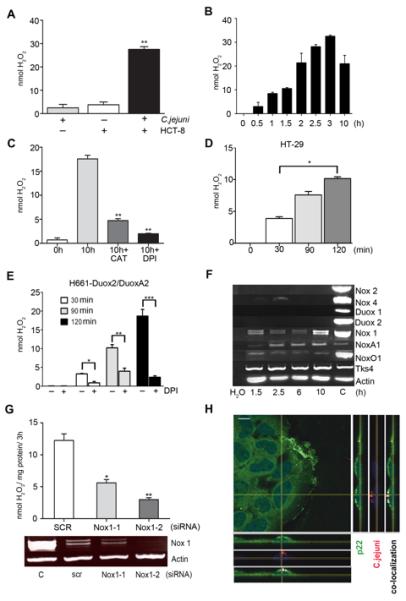
(A) H2O2 production after co-culture of C. jejuni with HCT-8 cells for 10 h. Statistical significance relative to non-inhibited H2O2 production is indicated, **P<0.0001, n=3, ±S.E.M. (B) Time course of H2O2 release from HCT-8 cells during co-culture with C. jejuni. (C) H2O2 production after co-culture of C. jejuni with HCT-8 cells for 10 h in the presence of inhibitors. Catalase (CAT) and DPI were added prior to exposure to bacteria, **P<0.0001, n=3, ±S.E.M. (D) H2O2 release from HT-29 cells during co-culture with C. jejuni at various time points, *P=0.0005, n=3, ±S.E.M. (E) H2O2 release from H661-Duox2/DuoxA2 cells during co-culture with C. jejuni at various time points, *P=0.0005, ** P=0.0001, *** P=0.00005, n=3, ±S.E.M. Preincubation with DPI (15 μM, 30 min) was used as control. (F) Semi-quantitative RT-PCR for expression of Nox/Duox and functionally associated genes in HCT-8 cells. Actin served as loading control and cDNA (C) or plasmid cDNA was used as gene-specific PCR control. (G) Knockdown of Nox1 in HCT-8 cells using Nox1-1, Nox1-2 and scrambled siRNA followed by H2O2 measurement. Actin PCR and independent control PCR (C) are shown. Statistical significance is relative to SCR siRNA. *P=0.0005 and ** P=0.0001, n=3, ±S.E.M. (H) Confocal analysis of the Nox1-p22phox complex and TAMRA-labeled C. jejuni (red) in HCT-8 cells after 3 h of exposure (co-localization in white). Anti-p22phox staining (green), nuclei (DAPI, blue), scale bar 10 μm. See also Figure S1.
We wished to confirm the in vivo relevance of these findings and established a polarized ex vivo organ culture model created from human biopsy tissues taken from normal large and small intestine at the time of routine endoscopy. Nox1 was highly expressed in colon tissue (Ct 28), while Duox2 expression in healthy, non-inflamed duodenum and colon tissues was variable (Ct 32-36, Figure S2A). Phagocyte-specific Nox2 expression was low (Ct 34-35), confirming that biopsies originated from healthy, non-inflamed tissue. Using the pIVOC methodology (Figure S2B), we observed time-dependent ROS production following C. jejuni challenge of the apical surface of duodenum and colon tissue (Figure 2A). This confirms that H2O2 is continuously released from healthy human intestinal tissue following C. jejuni infection. Both NADPH oxidases, Nox1 and Duox2, respond to Campylobacter infection in a similar manner. The activity of both oxidases was inhibited by preincubation of pIVOC organ culture with the flavoenzyme inhibitor diphenyleneiodonium (DPI). Duodenal biopsies incubated with either C. jejuni or with thapsigargin, which leads to Duox activation, showed maximal H2O2 release from these tissues, while exposure to soluble stimuli such as TNFα was less effective (Figure 2B). Confocal images of infected pIVOC tissue confirmed Duox and Nox1-p22phox recruitment at sites of intimate contact of C. jejuni with intestinal epithelium (Figure 2C (duodenum), Figure 2D (colon)). Thus, Campylobacter-induced release of ROS can be detected in a human ex vivo model constructed to mimic conditions in vivo by restricting bacterial challenge to the epithelial surface of intact mucosal tissue.
Figure 2. Release of NADPH oxidase-mediated hydrogen peroxide in an ex vivo model of gastrointestinal epithelium after exposure to C. jejuni.
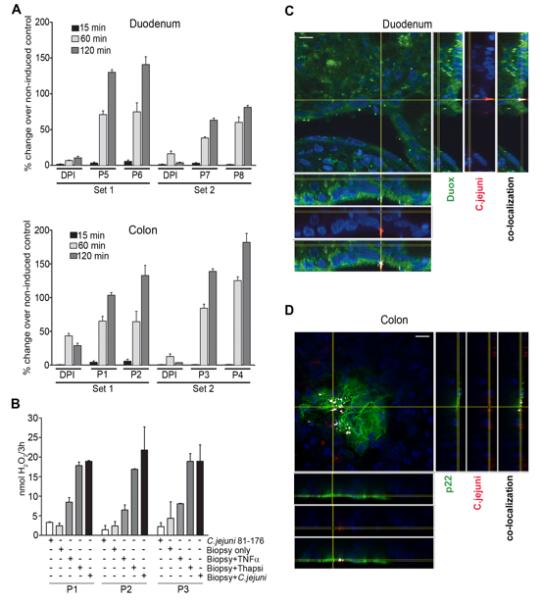
(A) H2O2 generation in colonic and duodenal biopsies mounted as pIVOC and incubated with C. jejuni. The percentage change of H2O2 over time with or without DPI is shown. Non-infected control biopsies were taken as base line. Data are representative of four sets from eight patients (P 1-8), error bars represent ±S.D. of 3 independent measurements per time point. (B) H2O2 release from duodenum biopsies of 3 different patients (P) either without stimulation, stimulated with TNFα (40 ng/ml), thapsigargin (25 μM), or co-cultured with C. jejuni for 3 h. Error bars represent ±S.D. of 3 independent measurements per treatment. (C) Immunofluorescence of duodenal pIVOC biopsy infected with C. jejuni for 3 h and stained for Duox2 (green) and C. jejuni (red). Scale bar 10 μm. (D) Immunofluorescence of colonic pIVOC biopsy infected with C. jejuni for 3 h and stained for p22phox (green) and C. jejuni (red). Co-localization is indicated in white, scale bar 10 μm. See also Figure S2.
Oxidase activation requires direct contact between C. jejuni and the host cell
In order to explore further the nature of the interaction between bacteria and eukaryotic cells that underpinned ROS production, we co-cultured C. jejuni and HCT-8 cells in conditions that included a filter separating C. jejuni from the mammalian cells. The absence of ROS production and lamellipodia formation by host cells in this setting confirmed that direct contact between C. jejuni and host cells is necessary for redistribution and activation of the Nox1-p22phox complex, and that stimulation of cells by secreted bacterial compounds is unlikely (Figure 3A and Figure S3A). Although C. jejuni cannot activate human TLR5 or TLR9, and does not lead to sustained TLR4 activation in many intestinal cells, TLR2 stimulation could occur (Al-Sayeqh et al., 2010; Galkin et al., 2008; Young et al., 2007). HCT-8 cells responded to stimulation with the TLR2 ligand Malp-2 by producing ROS, but heat-inactivated C. jejuni failed to activate Nox1 (Figure 3B), indicating that passive host - C. jejuni contact is not sufficient to induce ROS generation. We confirmed the notion that active processes such as adhesion and/or invasion are required by analyzing ROS generation triggered by a C. jejuni cadF deletion mutant, known to have impaired ability to adhere to and invade epithelial cells (Monteville et al., 2003). Reduced invasion by absence of CadF paralleled less efficient ROS generation (Figures 3C, D). Entry of C. jejuni into host cells is dependent on the actin/microtubule network (Watson and Galan, 2008; Young et al., 2007). Nocodazole or cytochalasin D treatment markedly attenuated entry of C. jejuni 81-176 into HCT-8 cells, membrane recruitment of Nox1 (Figure 3E, Figure S3B), and abolished ROS production (Figure 3F). Pretreatment of HCT-8 cells with the Src-family kinase inhibitor PP2 profoundly inhibited C. jejuni invasiveness and down-regulated ROS production (Figures 3G, H). In addition, inhibition of the GTPase Rac1 or of PI-3 kinase, both implicated in host cell entry by C. jejuni (Hu et al., 2006; Krause-Gruszczynska et al., 2007), caused significant reduction in ROS generation (Figure 3I). A caveat of using these inhibitors is their potential for disrupting multiple signaling pathways, as Src and Rac1 have been connected to Nox1-induced ROS generation (Gianni et al., 2010; Ueyama et al., 2006). Nevertheless, our multi-facetted approach suggests strongly that bacterial invasion serves as a trigger for mucosal ROS generation.
Figure 3. Active contact of C. jejuni 81-176 with HCT-8 cells is required for H2O2 production.
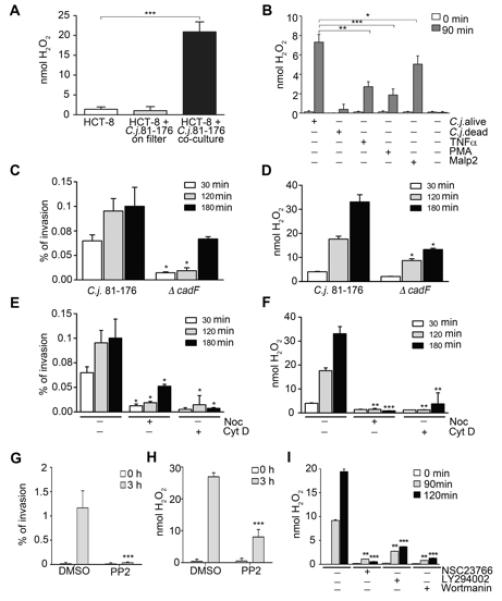
(A) H2O2 release by HCT-8 cells in the absence of C. jejuni, in the presence of C. jejuni separated from cells using a 0.2 μm filter, or when C. jejuni is co-cultured in contact with cells, **P<0.0001, n=3, ±S.E.M. (B) H2O2 release by HCT-8 cells stimulated with TNFα (40 ng/ml), PMA (0.1 ng/ml), Malp2 (10 ng/ml), live C. jejuni or heat-killed C. jejuni (MOI 100). One-way ANOVA (P<0.0001) and a two-tailed non-parametric t test were used, n=3, * P=0.005, ** P=0.0001, *** P=0.00005. (C-I) Invasion of C. jejuni correlates with H2O2 release. Invasion is expressed as % of inoculum. (C, D) Comparison of wildtype C. jejuni with invasion-deficient C. jejuni Δ cadF in HCT-8 cells. (E – I) Pretreatment of HCT-8 cells with indicated inhibitors (nocodazole, cytochalasin D, PP2, NSC23766, LY294002, wortmannin) for 45 min followed by incubation with C. jejuni, Two-tailed non-parametric t test in C-I, *** P<0.0001, ** 0.001<P≤0.0001, * 0.005≤P≤0.001 n=3, ±S.E.M. When not otherwise specified, the non treated sample was compared to the treated sample. See also Figure S3.
Hydrogen peroxide exposure impairs extracellular C. jejuni capsule formation and virulence
Epithelium-derived H2O2 present in the lumen during the course of infections will likely not irreversibly damage pathogens, but may attenuate the pathogenicity of extracellular organisms. To test if the presence of host-derived ROS alone can mediate the loss of the C. jejuni capsule, we exposed bacteria to H2O2 (Figure 4A). Capsule loss was almost complete between 50μM and 5mM H2O2, concentrations which did not alter C. jejuni viability or its ability to grow (Figure 4B, Figure S4A). As observed earlier with co-culture CPS depletion was detected after 6-8h of H2O2 exposure (Figure S4B). C. jejuni continuously exposed to, but separated from, direct contact with COS cells expressing Nox4 lost its capsule in a similar manner, an effect that was partially rescued by short pretreatment of cells with DPI (Figure 4C). Exogenously expressed Nox4 was used here due to its unique ability to release constitutively H2O2 at low rates (30-40 nmol H2O2 /h/mg protein) (von Lohneysen et al., 2010), while Nox1 or Duox enzymes remain dormant without stimulation. In co-culture conditions either with cells (HCT-8 cells as previously published, H661-Duox2/DuoxA2 cells Figure S4C) or with biopsies (Figure 4D) C. jejuni infection will trigger Nox1 or Duox2 activation, leading to ROS generation and CPS depletion. Electron microscopy of Alcian blue-stained C. jejuni confirmed the disappearance of the capsule polysaccharide layer upon H2O2 treatment, although overall morphology of C. jejuni was not altered (Figure 4E). For comparison, a C. jejuni kpsM mutant, which is deficient in high molecular weight glycan required for CPS synthesis and cannot produce a capsule, is shown. In line with CPS depletion following co-culture or genetic manipulation (Bacon et al., 2001; Corcionivoschi et al., 2009), C. jejuni organisms exhibited reduced adhesion (Figure 4F) and invasiveness (Figure 4G) after exposure to H2O2. Motility of H2O2 treated C. jejuni or C. jejuni ΔkpsM mutant in soft agar was attenuated (Figure 4H), presumably due to a change in bacterial surface properties following CPS depletion. In contrast, bacterial motility in liquid media was not altered (data not shown). Thus, exogenous H2O2 treatment of bacteria mimics the effect of intestinal co-culture on C. jejuni CPS production and reduces bacterial virulence.
Figure 4. Exposure of C. jejuni 81-176 to H2O2 leads to loss of bacterial capsule and decreased motility and invasion.
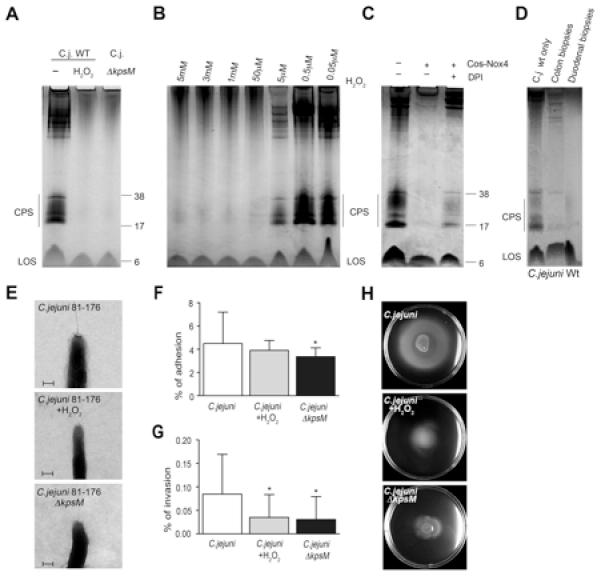
(A) Capsule polysaccharide (CPS) staining using lysates derived from C. jejuni with or without exposure to 5 mM H2O2 (10 h). For comparison capsule-deficient C. jejuni ΔkpsM was used. Lipooligosaccharide (LOS) is indicated. (B) H2O2 concentration-dependent loss of CPS staining in C. jejuni lysates (10h exposure). (C) C. jejuni was exposed to H2O2 released from constitutively active Nox4 (Cos-Nox4 cells), followed by CPS staining. Cells and bacteria were physically separated using a 0.3 μm filter. DPI preincubation of Cos-Nox4 cells for 30 min and inhibitor wash-out was used as control. (D) CPS staining of lysates derived from extracellular C. jejuni after exposure to colon or duodenal biopsies (8h). (E) Electron microscopy images of C. jejuni with and without exposure to 5 mM H2O2. C. jejuni Δ kpsM was used for comparison. Scale bar 200 nm. (F-H) Adhesion, invasion and motility of C. jejuni, H2O2-exposed C. jejuni and C. jejuni ΔkpsM in HCT-8 cells. Adhesion and invasion are expressed as % of inoculum. *P=0.01, n=3, ±S.E.M. See also Figure S4.
Hydrogen peroxide shuts down phosphotyrosine signaling by a C. jejuni outer membrane tyrosine kinase
In eukaryotic cells ROS play a key role in modulating tyrosine phosphorylation, mainly by inhibiting protein tyrosine phosphatases. Although a much less common phenomenon in bacteria, signaling via tyrosine phosphorylation has been implicated in capsular exopolysaccharide synthesis in prokaryotes (Bechet et al., 2009b). The H2O2-mediated loss of CPS prompted us to analyze C. jejuni tyrosine phosphorylation patterns. Tyrosine phosphorylated proteins were detected in outer membrane fractions, but not in the cytosol or inner membrane fractions (Figure S5A). Co-culture with Nox1-expressing HCT-8 cells, which is associated with capsule depletion, decreased significantly outer membrane protein (OMP) tyrosine phosphorylation in C. jejuni (Figure 5A). Downregulation of protein tyrosine phosphorylation was achieved within 8 hours of co-culture and was rescued by inhibition of ROS production using DPI or by decomposition of H2O2 using catalase (Figure 5B). A similar loss of tyrosine phosphorylation was observed in C. jejuni co-cultured with duodenal or colon biopsies, or with Duox2-expressing cells (Figure 5C and Figure S5B). Furthermore, exposure of bacteria to exogenous H2O2 (Figure 5D and Figure S5C) or to H2O2 produced by Cos-Nox4 cells (Figure 5E), both of which alter CPS (see Figures 4A-D), caused the disappearance of all but one tyrosine phosphorylated bands. Tryptic digest of OMPs followed by 2-D gel electrophoresis and anti-phosphotyrosine blotting revealed several tyrosine phosphorylated spots (Figure 5F I), which were lost in OMPs derived from C. jejuni co-cultured with cells (Figure 5F II). Thus, exposure of C. jejuni to H2O2, regardless of whether derived from epithelial cells or administered exogenously, results in greatly reduced tyrosine phosphorylation of bacterial OMPs. This finding appears to exclude ROS-mediated inhibition of a bacterial tyrosine phosphatase as mechanism causing the drop in phosphotyrosine content.
Figure 5. ROS-mediated disruption of phosphotyrosine signaling by Cj1170 (OMP50), a bacterial tyrosine kinase controlling capsule synthesis.
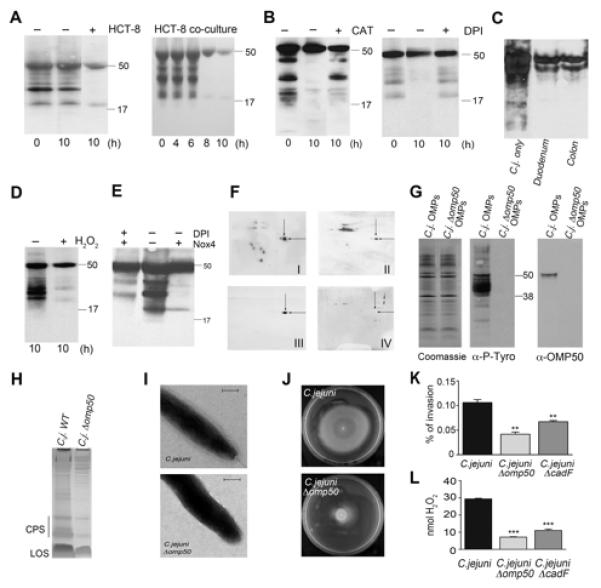
(A – E) Exposure of C. jejuni to H2O2 alters bacterial tyrosine phosphorylation. (A) Time course of co-culture of C. jejuni with HCT-8 cells. Tyrosine phosphorylation of outer membrane proteins (OMPs) is shown. (B) Phosphotyrosine incorporation in C. jejuni OMPs derived from HCT-8 co-cultures in the absence or presence of catalase or DPI pretreatment. (C) Phosphotyrosine content of C. jejuni exposed to medium (C. j. only) or of extracellular C. jejuni collected after 6h of infection of duodenal or colon biopsies. (D-E) Exposure of C. jejuni to 5 mM H2O2 (D) or to Cos-Nox4 cells separated by a filter (E) for 10 h followed by OMP extraction and phosphotyrosine detection with or without DPI pretreatment. (F) Phosphotyrosine 2D immunoblots of C. jejuni OMPs without treatment (I) and after co-culture with HCT-8 cells (II). Blot (I) was stripped and re-probed with OMP50 antibody (III). The indicated spot was also analyzed by MS-MS. C. jejuni Δomp50 OMPs were run on 2D gels and blotted with OMP50 antibody (IV). (G) Coomassie, anti-phosphotyrosine and anti-OMP50 immunoblots of OMPs derived from C. jejuni wt and C. jejuni Δomp50. (H) CPS staining of C. jejuni and C. jejuni Δomp50 lysates. (I) Electron microscopy images of C. jejuni and C. jejuni Δomp50. Scale bar 200 nm. (J, K) Comparison of wildtype C. jejuni with C. jejuni Δomp50 regarding motility in soft agar and HCT-8 invasion. (L) ROS generation after co-culture of indicated C. jejuni strains with HCT-8 cells. Adhesion- and invasion-deficient C. jejuni ΔcadF served as control. Invasion is expressed as % of inoculum. **P=0.0003, ***P<0.0001 n=3, ±S.E.M. See also Figure S5.
Bacterial tyrosine kinases (BY-kinases) constitute a family of recently discovered enzymes that control key regulatory prokaryotic networks (Cuthbertson et al., 2009; Lee and Jia, 2009). Previous analysis of the C. jejuni genome failed to identify a putative BY-kinase (Voisin et al., 2007). Using a bioinformatics approach based on homology to particular sequence motifs, we identified Cj1170c, an outer membrane protein, previously designated OMP50 (Bolla et al., 2000), as candidate BY-kinase. Based on its molecular weight we hypothesized that autophosphorylated OMP50 was represented by the remaining, tyrosine phosphorylated band at 50kDa in H2O2-treated or co-cultured OMP fractions. MS-MS analysis of the remaining tyrosine phosphorylated spot (Figure 5F II, indicated by arrows) followed by data base search revealed multiple OMP50-derived sequences. An antibody raised to OMP50 protein recognized a band at 50 kDa in the OMP fraction (Figure 5G) and the indicated spot in 2-D separation gels (Figure 5F III). The identity of OMP50 as the predominant tyrosine phosphorylated protein remaining in H2O2- or cell-exposed C. jejuni extracts was confirmed by analyzing a C. jejuni Δomp50 site-specific insertional mutant. The C. jejuni Δomp50 strain showed no apparent signs of major morphological changes or of growth inhibition. In OMP fractions prepared from this mutant, the band at 50kDa could not be detected by anti-OMP50 or anti-phosphotyrosine blotting (Figures 5F IV, 5G). In fact, deletion of OMP50 abolished tyrosine phosphorylation in general, indicating that this protein constitutes most likely the only BY-kinase in C. jejuni (Figure 5G). Next, we investigated if OMP50-deficiency would recapitulate the C. jejuni phenotypes observed after exposure to ROS. CPS depletion and the absence of the capsule structure indicated that OMP50 is required for polysaccharide biosynthesis or export (Figures 5H, I). Furthermore, OMP50 deficiency substantially reduced motility and invasiveness of the bacterium, coupled with significantly decreased Nox1-dependent ROS generation (Figures 5J-L), indicating that OMP50 is a major virulence factor of C. jejuni. To alleviate concerns regarding phase variation, we reconstituted omp50 in the C. jejuni Δ omp50 mutant, which reversed the motility and invasion phenotype to wildtype C. jejuni (Figures S5D-E). The motility phenotype could be linked to polysaccharide depletion, or might be result of the overall elimination of phosphotyrosine signaling by OMP50 deletion.
The BY-kinase OMP50 contains an essential regulatory tyrosine residue
Sequence and structural alignment of OMP50 with other known BY-kinases indicates that OMP50 represents a non-canonical prototype in this family. OMP50 is a genuine OMP, possibly forming a monomeric pore (Bolla et al., 2000), instead of following the typical BY-kinase topology in gram-negative bacteria that is characterized by two inner membrane spanning α-helices and a cytosolic catalytic domain. The putative kinase domain in the C-terminus of OMP50 contains Walker A and Walker A’ motifs, but lacks the consensus Walker B sequence (hhhhD) (Figure 6A and Figure S6). Important elements of the autoactivation switch seem to be conserved such as the combination of a putative internal regulatory tyrosine residue (Y338 OMP50, Y574 Etk) and the interacting arginine residue (R377 OMP50, R614 Etk) (Lee et al., 2008). A potential tyrosine (Y) cluster is also present, although the number and spacing of tyrosine residues is less distinctive. Minimal differences in OMP50 sequences derived from C. jejuni or C. lari exist, none of which would affect the conserved kinase motifs.
Figure 6. Cjtk (OMP50) represents a unique outer membrane BY-kinase.
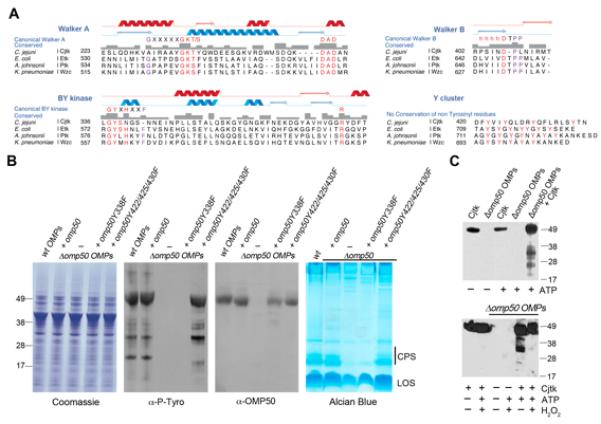
(A) Alignment of typical BY-kinase motifs in E.coli Etk, A. johnsonii Ptk, K. pneumoniae Wzc with C. jejuni OMP50 (Cjtk). Etk secondary structure is in blue and prediction for Cjtk in red (see also Figure S6). (B) Identification of the active site tyrosine in Cjtk. C. jejuni Δomp50 strain was complemented with omp50 wildtype or indicated omp50 mutants, followed by Coomassie blue staining, anti-phosphotyrosine and anti-OMP50 immunoblotting of OMP fractions, and by Alcian blue staining of capsular polysaccharides. (C) OMP phosphorylation by purified Cjtk is redox-sensitive. In vitro phosphorylation of C. jejuni Δomp50 OMPs by purified Cjtk (anti-phosphotyrosine blot) in the presence (bottom panel) or absence (top panel) of 10 mM H2O2. See also Figure S6.
Bacterial in vivo tyrosine phosphorylation was examined by comparing tyrosine phosphorylation patterns of OMP fractions derived from C. jejuni wildtype, C. jejuni Δomp50 mutant and a C. jejuni Δomp50 strain complemented with OMP50. Deletion of OMP50 was accompanied by loss of tyrosine phosphorylation, which was recovered by OMP50 reintroduction (Figure 6B). In some, but not in all BY-kinases a regulatory tyrosine is critically involved in kinase activity (Bechet et al., 2009a). Mutation of this tyrosine to phenylalanine leads to diminished BY-kinase activity. A similar mutation in OMP50 (OMP50 Y338F) abolished in vivo kinase activity of OMP50 when introduced in the Δomp50 mutant background. In line with earlier observations, complementation with OMP50 Y338F reduced CPS significantly without altering OMP50 or general protein expression (Figure 6B). An OMP50 triple tyrosine mutant in the Y cluster (OMP50 Y422/425/430F) was similarly introduced into the Δomp50 strain, but did not cause any discernible phenotype, suggesting that in contrast to other BY-kinases, phosphorylation of three of the four tyrosine residues in the Y cluster is not essential for OMP50 activity towards substrates (Figure 6B). As these results indicate that OMP50 represents not only an outer membrane protein but also a BY-kinase, we propose renaming the protein Cjtk (C. jejuni tyrosine kinase).
In vitro phosphorylation of OMPs by Cjtk is ROS sensitive
To confirm tyrosine kinase activity of Cjtk in vitro, we purified Cjtk from micelles prepared from C. jejuni wildtype OMP fractions (Figures S7A, S7B) and subjected OMP fractions derived from the Δomp50 (cjtk) mutant, which lack Cjtk and overall tyrosine phosphorylation, to kinase assays. The purified Cjtk was constitutively active and phosphorylated several OMP proteins on tyrosine (Figure 6C, upper panel). We then asked if addition of H2O2 would disrupt in vitro phosphorylation of OMP proteins. While autophosphorylation of Cjtk persisted, as observed earlier (Figure 5), tyrosine phosphorylation of Cjtk substrates in the Cjtk-deficient OMP fraction was substantially reduced (Figure 6C, lower panel). Thus, in vitro and in vivo tyrosine phosphorylation of proteins contained in the OMP fraction is disrupted by oxidation.
Cjtk modulates UDP-GlcNAc/Glc 4-epimerase (Gne) activity by active site tyrosine phosphorylation
How BY-kinases may regulate bacterial signaling networks becomes more apparent when the limited number of previously identified bacterial substrates is considered. One of the targets is UDP-glucose dehydrogenase (ugd), an enzyme involved in polysaccharide and colonic acid biosynthesis and export (Lacour et al., 2008). Tyrosine phosphorylation of bacterial proteins can lead to activation (Ugd, Cap5O) or inactivation (phage integrases), or can affect general house-keeping functions such as DNA replication or heat-shock response (Bechet et al., 2009b). Loss of CPS in the C. jejuni Δomp50 strain pointed to modification of at least one enzyme involved in polysaccharide biosynthesis. We extracted and sequenced the five tyrosine phosphorylated spots present after 2-D separation of OMPs (see Figures 5F I, 7A). One of the proteins was identified as Cj1131c, a bifunctional UDP-GlcNAc/Glc 4-epimerase (GalE, renamed Gne) (Bernatchez et al., 2005). The extracted tyrosine phosphorylated protein migrated at the expected molecular weight of 36 kDa (Figure 7B) and was absent when OMPs isolated from a C. jejuni ΔgalE (gne) mutant were probed with anti-phosphotyrosine antibody after 2-D gel electrophoresis (Figure 7C).
Figure 7. Activity of UDP-GlcNAc/Glc 4-epimerase (Gne) is positively regulated by Cjtk-mediated phosphorylation of the active site tyrosine 146.
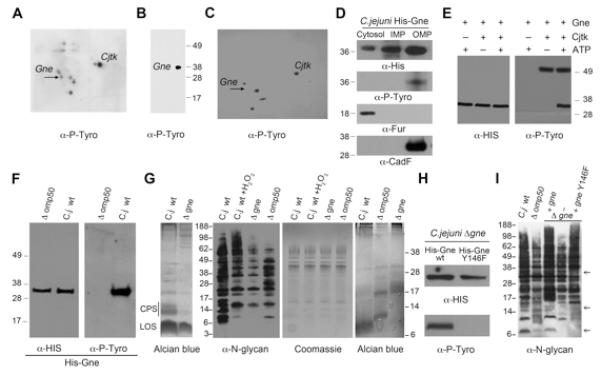
(A, B) Phosphotyrosine detection after 2D separation of C. jejuni OMP lysates. Arrow indicates spot recovered for MS-MS analysis and identified as Cj1131c (Gne). The extracted spot was run in 1D SDS-PAGE and blotted with anti-phosphotyrosine antibody (B). (C) 2D separation and phosphotyrosine detection of OMPs derived from deletion mutant C. jejuni ΔgalE/gne. Arrow indicates the missing spot corresponding to Gne protein. (D) Cytoplasm, IMP and OMP preparations of C. jejuni expressing histidine-tagged Gne were probed with anti-His and anti-phosphotyrosine antibodies. An anti-Fur and anti-CadF antibodies were used as control to assess the purity of the fractions. (E) Purified Cjtk and E.coli purified His-Gne were subjected to in vitro kinase assays and probed with anti-phosphotyrosine and anti-His antibody. (F) OMPs were prepared from wildtype C. jejuni and C. jejuni Δomp50, both expressing histidine-tagged Gne. His-Gne was affinity purified and visualized with anti-phosphotyrosine and anti-His antibody (see also Figure S7). (G) Comparative analysis of carbohydrate structures in C. jejuni wildtype, C. jejuni wt exposed to H2O2, and C. jejuni mutants Δomp50 and Δgne. Left panel: Alcian blue gel of CPS profile; middle panels: N-glycosylation blot profile and corresponding Coomassie gel; right panel: Alcian blue gel of LOS profile. (H) In vivo phosphorylation of Gne was assessed by immunoblotting using C. jejuni Δgne reconstituted with either His-Gne wt or His-Gne Y146F and subsequent affinity purification of wt or mutant His-Gne. (I) N-glycosylation profile of C. jejuni wt or Δomp50, compared to C. jejuni Δgne and to Δgne reconstituted with His-Gne wt or with His-Gne Y146F. Arrows indicate variations of glycosylation on Δomp50 and Δgne Y146F when compared to the wt and Gne reconstituted C. jejuni. See also Figure S7.
Enzymes involved in carbohydrate biosynthesis are commonly expressed in the bacterial cytosol and are thought to be phosphorylated by the cytosolic kinase domain of inner membrane-associated BY-kinases. As Gne constitutes a phosphorylation target of the outer membrane kinase Cjtk, the localization of Gne in C. jejuni was explored. In the absence of a readily available anti-Gne antibody, we expressed histidine-tagged Gne in wildtype C. jejuni and probed C. jejuni fractions by immunoblotting with anti-histidine antibodies. Gne was present in all three fractions, although the majority of the enzyme was in membrane-associated fractions. Tyrosine-phosphorylated Gne was only present in the OMP fraction, which was expected due to the localization of Cjtk and the absence of tyrosine phosphorylation in cytosol and IMP fractions (Figure 7D and Figure S5A). Fractionations were validated by using an antibody recognizing the cytosolic ferric uptake regulator Fur and the outer membrane protein CadF.
To ascertain that Gne constitutes a genuine substrate of Cjtk, His-Gne was expressed in E. coli, affinity purified (Figure S7C) and subjected to kinase assays. Gne was efficiently phosphorylated by Cjtk in vitro, confirming Gne as Cjtk substrate (Figure 7E). Kinase assays in the presence of H2O2 revealed that Cjtk activity is not directly affected by H2O2 in vitro (Figure S7D). Thus, the OMP fraction contains either yet unidentified factors that permit generation of ROS with higher oxidation efficiency than H2O2 or provides endogenous Cjtk substrates in a structural context that is ROS sensitive. Bacterial in vivo phosphorylation of Gne was tested by introduction of His-Gne into C. jejuni wildtype and Δomp50 mutant, followed by OMP isolation and affinity purification of His-Gne. His-Gne was detected in both C. jejuni strains, but was only tyrosine phosphorylated in wildtype C. jejuni (Figure 7F). In vitro exposure of phosphorylated His-Gne to H2O2 did not remove phosphotyrosine content, indicating the structural integrity of the protein (Figure S7E). As previously described (Bernatchez et al., 2005) the ΔgalE (gne) mutant showed pronounced loss of CPS and distinct changes in the N-glycan and lipooligosaccharide profiles (Figure 7G). The N-glycan patterns of C. jejuni exposed to H2O2 or of Cjtk-deficient (Δomp50) C. jejuni were similar, but revealed apparent differences when compared to the C. jejuni wildtype strain and the ΔgalE (gne) strain. These results suggest that ROS exposure phenocopies the influence of Cjtk on endogenous substrates, while complete Gne deficiency leads to more pronounced changes in carbohydrate biosynthesis.
Our observations suggest that Cjtk kinase activity plays a positive regulatory role in Gne’s epimerase activity. A predicted structural model of the active site of Gne places a tyrosine residue (Gne Y146) at a prominent position in the saccharide binding pocket (Bernatchez et al., 2005). This tyrosine residue is conserved throughout bacterial UDP-GlcNAc and UDP-Glc 4-epimerases. Conservative mutation of Y146 to F146 in His-Gne and expression of the wildtype or mutant enzyme in a Gne-deficient C. jejuni strain (C. jejuni 81-176 ΔgalE (gne)) revealed that mutation of Y146 abolishes tyrosine phosphorylation of Gne (Figure 7H). Gne Y146 was also verified as Cjtk phosphorylation site in vitro (Figure S7F). C. jejuni Δgne Y146 displayed reduced CPS similar to the deletion of complete gne (Figure S7G). The N-glycan pattern of C. jejuni Δ gne reconstituted with gne Y146F was similar to the pattern observed when analyzing the C. jejuni Δomp50 (cjtk) mutant or N-glycan patterns of C. jejuni exposed to host cell co-culture (Figure 7I and Figure S7H). The pattern of N-glycans changed more severely when Gne was deleted, suggesting that loss of Gne tyrosine phosphorylation by exposure to ROS or by absence of Cjtk acts as a modifier of epimerase activity. Taken together these data provide evidence for the mechanism triggering ROS-dependent downregulation of C. jejuni capsular polysaccharide. In the presence of ROS, Cjtk-mediated tyrosine phosphorylation of the Gne active site (and likely those of other substrates) is defective, thereby disrupting epimerase activity necessary for efficient carbohydrate production.
DISCUSSION
We describe here a previously unappreciated role for mucosal ROS in antibacterial defense. ROS released from the intestinal epithelium in the course of C. jejuni infection attenuate the pathogenicity of extracellular bacteria by disrupting tyrosine phosphorylation-mediated bacterial capsule production. C. jejuni phosphotyrosine signaling is regulated by Cjtk, a unique member of the expanding BY-kinase family, which modifies several periplasmic and/or outer membrane proteins including an epimerase required for polysaccharide synthesis.
Given the expected rapid dispersion of ROS in an extracellular environment, the mechanism by which ROS impart host defense and exert bacterial control must differ from that of innate immune cells. Moreover, C. jejuni can mount a transcriptional response upon ROS exposure via the oxidative stress regulator PerR. This leads to de-repression of target genes including KatA (catalase) and AhpC (alkylhydroxyperoxidase), which will assist in detoxifying H2O2 (Palyada et al., 2009). An attractive strategy that permits mucosal ROS to achieve bacterial control by a non-microbicidal mechanism is the interference with virulence factors, thus weakening pathogenicity. A decrease in invasion of epithelial cells after exposure of Salmonella to H2O2 was described as a repellent effect of unspecified nature (Botteaux et al., 2009). As we show here, in C. jejuni infection this host-protective effect is afforded by disabling regulatory control of the bacterial phosphotyrosine network. Details of the mechanism for redox-mediated inactivation of C. jejuni phosphotyrosine signaling will require more in-depth studies, as not only Cjtk, but also its endogenous substrates and interacting protein complexes might be affected. Regulation of a bacterial enzyme by a combination of redox- and phosphorylation-dependent mechanisms has been described recently (Gruszczyk et al., 2011). An additional caveat of translating complex events triggered by exposure of bacteria to ROS to the minimalist in vitro phosphorylation approach using purified proteins is the loss of overall context regarding in vivo modifications of proteins and of the oxidant itself. Exposure of C. jejuni to H2O2 may induce the release of ferrous iron from iron-containing proteins, which will promote generation of short-lived ROS with highly increased oxidative capacity such as hydroxyl radicals (Palyada et al., 2009). The in vivo setting was successfully mimicked in vitro when utilizing purified Cjtk and the complete OMP fraction, but not when using recombinant Gne purified from E. coli.
We speculate that extracellular ROS could affect other invasive bacteria in a similar fashion. However, the manner in which BY-kinase networks are altered by oxidants will likely differ among bacteria, depending on the structure and location of the BY-kinase. The current model for regulating the activity of inner membrane localized BY-kinases is based on a cyclic phosphorylation-dephosphorylation process by engaging specific cytosolic low molecular weight protein tyrosine phosphatases (LMW-PTP) (ie Wzc/Wzb or Etk/Etp). Although a single LMW-PTP (Cj1258) has been identified in C. jejuni (Tolkatchev et al., 2006), the redox-sensitive decrease in tyrosine phosphorylation and the outer membrane location of the identified kinase Cjtk (OMP50) suggests a distinct mechanism for Campylobacter BY-kinase regulation. The C. jejuni LMW-PTP displays high overall homology to mammalian LMW-PTPs and is likely a target of oxidative inactivation, which would lead to increased phosphotyrosine content in the OMP fraction of ROS-exposed C. jejuni rather than to the decrease that we observe. In addition, as Cjtk-mediated phosphorylation appears to exclude cytoplasmic proteins, the identity or localization of tyrosine phosphorylated substrates in C. jejuni and of the available ATP source will likely differ from other organisms. The epimerase Gne, the here identified Cjtk substrate, partitions into several bacterial compartments. It is possible that each Gne subpopulation fulfills different tasks, and certain steps require tyrosine phosphorylation.
Phosphotyrosine signaling has been linked with capsule expression in several prokaryotes (Cuthbertson et al., 2009; Grangeasse et al., 2010). Similarly, phosphoproteomic studies of Klebsiella pneumonia revealed a tight connection between tyrosine phosphorylation, exopolysaccharide biosynthesis and pathogenicity of the bacterium (Lin et al., 2009). While unencapsulated/capsule-depleted pathogenic bacteria sometimes show enhanced adherence and invasion, capsule loss is predominantly associated with attenuated virulence in colonization and sepsis models of infection. Thus, ROS-induced inhibition of BY-kinase-mediated CPS expression could function as a generalized mucosal defense against encapsulated bacteria. By shutting down BY-kinase activity, a network hub, one can predict disruption of several nodes and wide-ranging effects on many bacterial species. Considering the multifunctional nature of many bacterial nodes, altering the activity or location of just one bacterial protein may impinge on several virulence determinants. A case in point is the here identified Cjtk target Gne, a UDP-GlcNAc/Glc 4-epimerase, which is required for the synthesis of three major bacterial cell surface carbohydrate structures (CPS, LOS, Pgl heptasaccharide). The pleiotropic effect on key bacterial functions by interference with BY-kinase signaling underlines their potential as promising targets for antimicrobial therapy.
The concept that ROS act as a virulence modifier in the mucosa rather than as microbicidal agent opens up new avenues of research. As much as epithelium-derived H2O2 is beneficial for the host during Campylobacter infection and likely in other bacterial infections of mucosal surfaces, it can be easily envisioned that this host response can be subverted by some pathogens to their advantage (Winter et al., 2010). In addition, although the commensal intestinal microbiota is confined to the outer loose mucus layer and thus cannot trigger mucosal ROS generation in vivo, changes in mucin expression in injury, inflammatory disease or cancer may provide access to the epithelium. In these circumstances, the microbiota itself may stimulate H2O2 release (Swanson et al., 2011), raising the possibility that microbiota-induced changes in redox balance in the lumen could modulate the pathogenicity of intestinal pathogens, or could result in aberrant microbiota. In this context, analysis of the reciprocal effects of the commensal flora and Campylobacter would be informative. A more complete understanding of the inter-relationships between infecting organism, resident microbiota and host mucosal Nox/Duox activation will await the development of more tractable animal models mimicking human Campylobacter infection.
EXPERIMENTAL PROCEDURES
Co-culture of C. jejuni 81-176 and C. jejuni mutants with cells
Agar grown bacteria were resuspended in RPMI 1640 media and cells at 60-70% confluence were incubated with 1ml of bacterial suspension (OD600=0.4; 107 bacteria). Bacteria and cells were co-cultured at 37°C using microaerobic conditions (5% O2, 5% CO2 and 90% N2) for various time periods. Non-adherent bacteria were removed by centrifugation (3300g, for 5 min), and were used for isolation of CPS and the outer membrane fractions. Viable counts were performed for inocula to ensure that comparable numbers of live bacteria were present for each bacterial strain.
Polarized In Vitro Organ Culture (pIVOC)
pIVOC was performed using biopsy material obtained from children during routine endoscopy undertaken for clinical purposes. Between 3 and 5 biopsies were taken from normal appearing mucosa in either the third part of the duodenum or in the colon. Fully informed consent was obtained from parents and, where appropriate, children. Ethical permission was provided for this study from the Ethics Committee of Our Ladys Children Hospital Crumlin, Ireland. The pIVOC was adapted to monitor stimulated H2O2 production and confocal microscopy of C. jejuni infected mucosal epithelium. Details are described in the online Supplemental Information.
Immunofluorescence
C. jejuni 81-176 was TAMRA-labelled before a 3h infection of cells plated in Ibidi chamber μ-slides VI0.4 (Ibidi GmbH, Martinsried, Germany) in microaerophilic conditions. Samples were fixed with 2% formaldehyde for 30 min at 37°C. After washing, cells were permeabilized with 0.1% Triton X-100, blocked with 5% BSA and incubated for 3 h with primary antibodies. Mouse anti-p22phox, affinity purified anti-Nox1 or anti-Duox1/2 rabbit antibodies were used for detection. Secondary antibody was Alexa Fluor 488-conjugated goat anti-rabbit IgG or Alexa Fluor 647-conjugated goat anti-mouse IgG (Invitrogen). Nuclei were stained with DAPI (Invitrogen). Further details are described in the online Supplemental Information.
Electron Microscopy
C. jejuni were fixed in ice-cold 2.5% glutaraldehyde, 1% paraformaldehyde in 0.1 M cacodylate buffer and left overnight at room temperature with gentle inversion. A freshly prepared saturated solution of Alcian Blue (Sigma-Aldrich) in de-ionized water was filtered through a 0.22 μm pore size filter (Millipore) and was used as a positive stain for capsular polysaccharides. Samples were spread on carbon- and formvar-coated copper grids and washed with water. Samples were analyzed on a Tecnai12 Biotwin (FEI company) operating at 120 kV and images were acquired on a 4k × 4k CCD Eagle camera (FEI company).
Statistical Analysis
Data are expressed as mean ± SEM, n=3. Statistical differences between means were determined by a two-tailed Student’s t test. Differences between multiple groups were tested using analysis of variance (ANOVA) for repeated measures. p values are indicated in figure legends.
Supplementary Material
SUPPLEMENTAL INFORMATION Supplemental information includes seven figures, Supplemental Experimental Procedures, and Supplemental References and can be found with this article online.
ACKNOWLEDGMENTS
We thank B. Wren, J.M. Bolla, P. Guerry, A. Stinzi, N. Dorrell, C. Szymanski, S. Backert, and D. Roos for kindly providing reagents, and S. Schuller, G. Rautureau and K. O’Neill for advice and technical assistance. The work was supported by Science Foundation Ireland (SFI PI Grant 04/IN3/B646) and the National Children Research Centre (both to B.B.), and an SFI Stokes award (to U.G.K.). T.H.S was funded by the EPSRC (Grant SB1738), J.M. was supported by Wellcome University Award. We would like to acknowledge staff and participating patients at the National Referral Center for Pediatric Gastroenterology, Our Lady’s Children’s Hospital Crumlin.
REFERENCES
- Al-Sayeqh AF, Loughlin MF, Dillon E, Mellits KH, Connerton IF. Campylobacter jejuni activates NF-kappaB independently of TLR2, TLR4, Nod1 and Nod2 receptors. Microb Pathog. 2010;49:294–304. doi: 10.1016/j.micpath.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Bacon DJ, Szymanski CM, Burr DH, Silver RP, Alm RA, Guerry P. A phase-variable capsule is involved in virulence of Campylobacter jejuni 81-176. Mol Microbiol. 2001;40:769–777. doi: 10.1046/j.1365-2958.2001.02431.x. [DOI] [PubMed] [Google Scholar]
- Bae YS, Choi MK, Lee WJ. Dual oxidase in mucosal immunity and host-microbe homeostasis. Trends Immunol. 2010;31:278–287. doi: 10.1016/j.it.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Bechet E, Gruszczyk J, Terreux R, Gueguen-Chaignon V, Vigouroux A, Obadia B, Cozzone AJ, Nessler S, Grangeasse C. Identification of structural and molecular determinants of the tyrosine-kinase Wzc and implications in capsular polysaccharide export. Mol Microbiol. 2009a;77:1315–1325. doi: 10.1111/j.1365-2958.2010.07291.x. [DOI] [PubMed] [Google Scholar]
- Bechet E, Guiral S, Torres S, Mijakovic I, Cozzone AJ, Grangeasse C. Tyrosine-kinases in bacteria: from a matter of controversy to the status of key regulatory enzymes. Amino Acids. 2009b;37:499–507. doi: 10.1007/s00726-009-0237-8. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Bernatchez S, Szymanski CM, Ishiyama N, Li J, Jarrell HC, Lau PC, Berghuis AM, Young NM, Wakarchuk WW. A single bifunctional UDP-GlcNAc/Glc 4-epimerase supports the synthesis of three cell surface glycoconjugates in Campylobacter jejuni. J Biol Chem. 2005;280:4792–4802. doi: 10.1074/jbc.M407767200. [DOI] [PubMed] [Google Scholar]
- Bolla JM, De E, Dorez A, Pages JM. Purification, characterization and sequence analysis of Omp50,a new porin isolated from Campylobacter jejuni. Biochem J. 2000;352(Pt 3):637–643. [PMC free article] [PubMed] [Google Scholar]
- Botteaux A, Hoste C, Dumont JE, Van Sande J, Allaoui A. Potential role of Noxes in the protection of mucosae: H(2)O(2) as a bacterial repellent. Microbes Infect. 2009;11:537–544. doi: 10.1016/j.micinf.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Corcionivoschi N, Clyne M, Lyons A, Elmi A, Gundogdu O, Wren BW, Dorrell N, Karlyshev AV, Bourke B. Campylobacter jejuni cocultured with epithelial cells reduces surface capsular polysaccharide expression. Infect Immun. 2009;77:1959–1967. doi: 10.1128/IAI.01239-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbertson L, Mainprize IL, Naismith JH, Whitfield C. Pivotal roles of the outer membrane polysaccharide export and polysaccharide copolymerase protein families in export of extracellular polysaccharides in gram-negative bacteria. Microbiol Mol Biol Rev. 2009;73:155–177. doi: 10.1128/MMBR.00024-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Lennemann NJ, Krishnamurthy S, Pocza P, Durairaj L, Launspach JL, Rhein BA, Wohlford-Lenane C, Lorentzen D, Banfi B, et al. Enhancement of Respiratory Mucosal Anti-viral Defenses by Iodide Oxidation. Am J Respir Cell Mol Biol. 2011;45:874–881. doi: 10.1165/rcmb.2010-0329OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores MV, Crawford KC, Pullin LM, Hall CJ, Crosier KE, Crosier PS. Dual oxidase in the intestinal epithelium of zebrafish larvae has anti-bacterial properties. Biochem Biophys Res Commun. 2010;400:164–168. doi: 10.1016/j.bbrc.2010.08.037. [DOI] [PubMed] [Google Scholar]
- Galkin VE, Yu X, Bielnicki J, Heuser J, Ewing CP, Guerry P, Egelman EH. Divergence of quaternary structures among bacterial flagellar filaments. Science. 2008;320:382–385. doi: 10.1126/science.1155307. [DOI] [PubMed] [Google Scholar]
- Gianni D, Taulet N, DerMardirossian C, Bokoch GM. c-Src-mediated phosphorylation of NoxA1 and Tks4 induces the reactive oxygen species (ROS)-dependent formation of functional invadopodia in human colon cancer cells. Mol Biol Cell. 2010;21:4287–4298. doi: 10.1091/mbc.E10-08-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grangeasse C, Terreux R, Nessler S. Bacterial tyrosine-kinases: structure-function analysis and therapeutic potential. Biochim Biophys Acta. 2010;1804:628–634. doi: 10.1016/j.bbapap.2009.08.018. [DOI] [PubMed] [Google Scholar]
- Gruszczyk J, Fleurie A, Olivares-Illana V, Bechet E, Zanella-Cleon I, Morera S, Meyer P, Pompidor G, Kahn R, Grangeasse C, Nessler S. Structure analysis of the Staphylococcus aurues UDP-N-acetyl-mannosamine dehydrogenase Cap5O involved in capsular polysaccharide biosynthesis. J Biol Chem. 2011;286:17112–17121. doi: 10.1074/jbc.M110.216002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha EM, Lee KA, Seo YY, Kim SH, Lim JH, Oh BH, Kim J, Lee WJ. Coordination of multiple dual oxidase-regulatory pathways in responses to commensal and infectious microbes in drosophila gut. Nat Immunol. 2009;10:949–957. doi: 10.1038/ni.1765. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- Hu L, McDaniel JP, Kopecko DJ. Signal transduction events involved in human epithelial cell invasion by Campylobacter jejuni 81-176. Microb Pathog. 2006;40:91–100. doi: 10.1016/j.micpath.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Janssen R, Krogfelt KA, Cawthraw SA, van Pelt W, Wagenaar JA, Owen RJ. Host-pathogen interactions in Campylobacter infections: the host perspective. Clin Microbiol Rev. 2008;21:505–518. doi: 10.1128/CMR.00055-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keo T, Collins J, Kunwar P, Blaser MJ, Iovine NM. Campylobacter capsule and lipooligosaccharide confer resistance to serum and cationic antimicrobials. Virulence. 2011;2:30–40. doi: 10.4161/viru.2.1.14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause-Gruszczynska M, Rohde M, Hartig R, Genth H, Schmidt G, Keo T, Konig W, Miller WG, Konkel ME, Backert S. Role of the small Rho GTPases Rac1 and Cdc42 in host cell invasion of Campylobacter jejuni. Cell Microbiol. 2007;9:2431–2444. doi: 10.1111/j.1462-5822.2007.00971.x. [DOI] [PubMed] [Google Scholar]
- Lacour S, Bechet E, Cozzone AJ, Mijakovic I, Grangeasse C. Tyrosine phosphorylation of the UDP-glucose dehydrogenase of Escherichia coli is at the crossroads of colanic acid synthesis and polymyxin resistance. PLoS ONE. 2008;3:e3053. doi: 10.1371/journal.pone.0003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DC, Jia Z. Emerging structural insights into bacterial tyrosine kinases. Trends Biochem Sci. 2009;34:351–357. doi: 10.1016/j.tibs.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Lee DC, Zheng J, She YM, Jia Z. Structure of Escherichia coli tyrosine kinase Etk reveals a novel activation mechanism. EMBO J. 2008;27:1758–1766. doi: 10.1038/emboj.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MH, Hsu TL, Lin SY, Pan YJ, Jan JT, Wang JT, Khoo KH, Wu SH. Phosphoproteomics of Klebsiella pneumoniae NTUH-K2044 reveals a tight link between tyrosine phosphorylation and virulence. Mol Cell Proteomics. 2009;8:2613–2623. doi: 10.1074/mcp.M900276-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteyn B, Scorza FB, Sansonetti PJ, Tang C. Breathing life into pathogens: the influence of oxygen on bacterial virulence and host responses in the gastrointestinal tract. Cell Microbiol. 2011;13:171–176. doi: 10.1111/j.1462-5822.2010.01549.x. [DOI] [PubMed] [Google Scholar]
- Monteville MR, Yoon JE, Konkel ME. Maximal adherence and invasion of INT 407 cells by Campylobacter jejuni requires the CadF outer-membrane protein and microfilament reorganization. Microbiology. 2003;149:153–165. doi: 10.1099/mic.0.25820-0. [DOI] [PubMed] [Google Scholar]
- Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev. 2007;219:88–102. doi: 10.1111/j.1600-065X.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- Palyada K, Sun YQ, Flint A, Butcher J, Naikare H, Stintzi A. Characterization of the oxidative stress stimulon and PerR regulon of Campylobacter jejuni. BMC Genomics. 2009;10:481. doi: 10.1186/1471-2164-10-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada B, Leto TL. Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contrib Microbiol. 2008;15:164–187. doi: 10.1159/000136357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokutan K, Kawahara T, Kuwano Y, Tominaga K, Nishida K, Teshima-Kondo S. Nox enzymes and oxidative stress in the immunopathology of the gastrointestinal tract. Semin Immunopathol. 2008;30:315–327. doi: 10.1007/s00281-008-0124-5. [DOI] [PubMed] [Google Scholar]
- Rosen H, Klebanoff SJ, Wang Y, Brot N, Heinecke JW, Fu X. Methionine oxidation contributes to bacterial killing by the myeloperoxidase system of neutrophils. Proc Natl Acad Sci USA. 2009;106:18686–18691. doi: 10.1073/pnas.0909464106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothfork JM, Timmins GS, Harris MN, Chen X, Lusis AJ, Otto M, Cheung AL, Gresham HD. Inactivation of a bacterial virulence pheromone by phagocyte-derived oxidants: new role for the NADPH oxidase in host defense. Proc Natl Acad Sci USA. 2004;101:13867–13872. doi: 10.1073/pnas.0402996101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson PA, 2nd, Kumar A, Samarin S, Vijay-Kumar M, Kundu K, Murthy N, Hansen J, Nusrat A, Neish AS. Enteric commensal bacteria potentiate epithelial restitution via reactive oxygen species-mediated inactivation of focal adhesion kinase phosphatases. Proc Natl Acad Sci USA. 2011;108:8803–8808. doi: 10.1073/pnas.1010042108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolkatchev D, Shaykhutdinov R, Xu P, Plamondon J, Watson DC, Young NM, Ni F. Three-dimensional structure and ligand interactions of the low molecular weight protein tyrosine phosphatase from Campylobacter jejuni. Protein Sci. 2006;15:2381–2394. doi: 10.1110/ps.062279806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueyama T, Geiszt M, Leto TL. Involvement of Rac1 in activation of multicomponent Nox1- and Nox3-based NADPH oxidases. Mol Cell Biol. 2006;26:2160–2174. doi: 10.1128/MCB.26.6.2160-2174.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voisin S, Watson DC, Tessier L, Ding W, Foote S, Bhatia S, Kelly JF, Young NM. The cytoplasmic phosphoproteome of the Gram-negative bacterium Campylobacter jejuni: evidence for modification by unidentified protein kinases. Proteomics. 2007;7:4338–4348. doi: 10.1002/pmic.200700483. [DOI] [PubMed] [Google Scholar]
- von Lohneysen K, Noack D, Wood MR, Friedman JS, Knaus UG. Structural insights into Nox4 and Nox2: motifs involved in function and cellular localization. Mol Cell Biol. 2010;30:961–975. doi: 10.1128/MCB.01393-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RO, Galan JE. Campylobacter jejuni survives within epithelial cells by avoiding delivery to lysosomes. PLoS Pathog. 2008;4:e14. doi: 10.1371/journal.ppat.0040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter SE, Thiennimitr P, Winter MG, Butler BP, Huseby DL, Crawford RW, Russell JM, Bevins CL, Adams LG, Tsolis RM, et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature. 2010;467:426–429. doi: 10.1038/nature09415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Szep S, Lu Z. The antioxidant role of thiocyanate in the pathogenesis of cystic fibrosis and other inflammation-related diseases. Proc Natl Acad Sci USA. 2009;106:20515–20519. doi: 10.1073/pnas.0911412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KT, Davis LM, Dirita VJ. Campylobacter jejuni: molecular biology and pathogenesis. Nat Rev Microbiol. 2007;5:665–679. doi: 10.1038/nrmicro1718. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTAL INFORMATION Supplemental information includes seven figures, Supplemental Experimental Procedures, and Supplemental References and can be found with this article online.


