Abstract
Autotransporter protein secretion represents one of the simplest forms of secretion across Gram-negative bacterial membranes. Once secreted, autotransporter proteins either remain tethered to the bacterial surface or are released following proteolytic cleavage. Autotransporters possess a diverse array of virulence-associated functions such as motility, cytotoxicity, adherence and autoaggregation. To better understand the role of autotransporters in disease, our research focused on the autotransporters of Yersinia pestis, the aetiological agent of plague. Y. pestis strain CO92 has nine functional conventional autotransporters, referred to as Yaps for Yersinia autotransporter proteins. Three Yaps have been directly implicated in virulence using established mouse models of plague infection (YapE, YapJ and YapK). Whilst previous studies from our laboratory have shown that most of the CO92 Yaps are cell associated, YapE and YapG are processed and released by the omptin protease Pla. In this study, we identified the Pla cleavage sites in YapG that result in many released forms of YapG in Y. pestis, but not in the evolutionarily related gastrointestinal pathogen, Yersinia pseudotuberculosis, which lacks Pla. Furthermore, we showed that YapG does not contribute to Y. pestis virulence in established mouse models of bubonic and pneumonic infection. As Y. pestis has a complex life cycle involving a wide range of mammalian hosts and a flea vector for transmission, it remains to be elucidated whether YapG has a measurable role in any other stage of plague disease.
Introduction
Bacteria of the genus Yersinia that are pathogenic to humans include Yersinia pestis, Yersinia pseudotuberculosis and Yersinia enterocolitica (Bottone, 1999; Naktin & Beavis, 1999; Rollins et al., 2003). Whilst Y. pseudotuberculosis and Y. enterocolitica are enteric pathogens that infect humans through ingestion of contaminated food or water, Y. pestis is primarily a vector-borne pathogen that is the causative agent of plague. The life cycle of Y. pestis primarily involves a rodent host and a flea vector for transmission (Hinnebusch, 1997). However, humans can become accidental hosts, whereby Y. pestis infection results in an acute febrile disease with different clinical presentations depending upon the route of inoculation. These forms of disease include bubonic, pneumonic and septicaemic plague. Bubonic plague occurs when humans are bitten by a flea infected with Y. pestis. Following the flea bite, bacteria disseminate to the proximal lymph nodes, where they proliferate and cause massive inflammation that results in a painful, swollen lymph node, or bubo (Sebbane et al., 2005). Without antibiotic treatment, Y. pestis then disseminates into the bloodstream causing bacteraemia (leading to septicaemic plague) and subsequently colonizes the liver, spleen and in about 5 % of human cases the lungs (Janssen et al., 1958). Colonization of the lungs is known as secondary pneumonic plague, and may be transmitted from human to human by inhalation of respiratory droplets, which can result in primary pneumonic plague (Perry & Fetherston, 1997).
All pathogenic yersiniae share a highly conserved 70 kb low-calcium-response (Lcr) virulence plasmid (named pCD1 in Y. pestis) (Wren, 2003). This plasmid encodes the Ysc type III secretion system (T3SS) and several effector proteins known as Yops, which contribute to Yersinia virulence by modulating host immune defences (Naktin & Beavis, 1999; Perry & Fetherston, 1997; Viboud & Bliska, 2005). In contrast to the enteric yersiniae, Y. pestis possesses two additional virulence plasmids, pPCP1 and pMT1. The multifunctional surface omptin protease/adhesin, Pla, is encoded on pPCP1 and has been demonstrated to cleave host plasminogen and components of the complement pathway and mediate adhesion to and invasion into human endothelial cells (Lähteenmäki et al., 2001; Rakin et al., 1996; Sodeinde & Goguen, 1989; Suomalainen et al., 2007). Because this pathogen has adapted to the varying lifestyles within the flea, rodent and human, and has the capacity to infect by differing routes, it is likely that Y. pestis has evolved a set of ‘host-specific’ and ‘route-specific’ virulence factors that allow it to thrive in each niche. As rodents are a natural host for Y. pestis infection, murine models of bubonic and pneumonic infection have been developed that mimic the progression of human bubonic and pneumonic disease (Cathelyn et al., 2006; Lathem et al., 2005; Sebbane et al., 2005). These models have proven to be invaluable for the identification and characterization of virulence factors in the fully virulent Y. pestis strain CO92.
The simplest mechanism of protein translocation across the inner and outer membranes in Gram-negative bacteria is the type V secretion system of autotransporter proteins (Dautin & Bernstein, 2007). The conventional autotransporter, or type Va, protein consists of three basic domains: an N-terminal signal sequence, a passenger domain (PD) and a C-terminal β-domain (Henderson et al., 2004). The N-terminal signal sequence is required for secretion through the inner membrane via the Sec system. Once in the periplasm, many autotransporters have been shown to interact with periplasmic chaperones and the Bam (Omp85) complex for stabilization of the PD and proper insertion of the β-domain into the outer membrane (Bodelón et al., 2009; Jong et al., 2010; Ruiz-Perez et al., 2009; 2010; Sauri et al., 2009). The PD is then translocated through the periplasm and across the outer membrane via the C-terminal β-domain, which forms a pore in the outer membrane. Once the PD has traversed the outer membrane, it can (i) remain covalently attached to the β-domain, (ii) be proteolytically cleaved from the β-domain but remain non-covalently associated with the β-domain, or (iii) be cleaved from the β-domain and released from the cell. Cleavage of the PD can occur via an autocatalytic mechanism, as with the serine protease autotransporters of the Enterobacteriacae (SPATEs) (Henderson et al., 1998), or by outer-membrane proteases, such as the omptins OmpT and Pla (Lawrenz et al., 2009; Lenz et al., 2011). The sequences of autotransporter PDs vary greatly, and encode a number of different virulence-associated functions involved in adhesion, cytotoxicity, agglutination, motility, proteolytic degradation and complement resistance (Henderson & Nataro, 2001).
Within the Y. pestis CO92 genome, we recently identified genes encoding nine functional conventional autotransporters (referred to here as the Yaps, for Yersinia autotransporter proteins) (Lenz et al., 2011). In examining the contribution of Yaps to plague pathogenesis, our laboratory found unique roles for YapE, YapJ and YapK. In particular, YapE is required for full virulence during bubonic but not pneumonic infection, and promotes adherence to human-derived lung epithelial cells in culture (Lawrenz et al., 2009). The highly similar YapJ and YapK have an additive, non-redundant role in systemic Y. pestis infection (Lenz et al., 2012). YapC is also known to mediate bacterial autoaggregation and adherence to murine-derived macrophage-like cells and human-derived epithelial cells when expressed in Escherichia coli (Felek et al., 2008). Of the Y. pestis Yaps, YapG is unique in that its PD is cleaved at multiple sites by the omptin protease Pla, releasing three major fragments of the YapG PD to the extracellular milieu (Lenz et al., 2011). In analysing expression of each of the yap genes by qRT-PCR, our previous work showed that yapG expression is modestly induced during bubonic and pneumonic plague in mice (Lenz et al., 2011). Additionally, the production of YapG-specific antibodies was demonstrated during a sublethal rabbit model of bubonic plague using in vivo-induced antigen technology (Andrews et al., 2010). Based on these data, and the observation that most characterized autotransporters are either implicated in virulence or have virulence-associated functions, it seems likely that YapG also contributes to Y. pestis pathogenesis. In this report, we further characterized the complex processing of YapG by Pla. In addition, we assessed the contribution of YapG to Y. pestis virulence using the established mouse models of bubonic and pneumonic plague.
Methods
Bacterial strains, growth conditions and animal infections.
All bacterial strains and plasmids used in this study are listed in Table 1. The fully virulent, WT Y. pestis CO92 is a clinical isolate from a pneumonic infection provided by the US Army Medical Research Institute of Infectious Diseases, Fort Detrick, MD, USA (Table 1) (Doll et al., 1994). Y. pestis strains were routinely cultured on brain–heart infusion (BHI) agar (BD Biosciences) at 26 °C for 48 h or in BHI broth (BD Biosciences) with aeration at 26 or 37 °C as indicated. Y. pseudotuberculosis strains were routinely cultured on Luria–Bertani (LB) agar (BD Biosciences) at 26 °C for 48 h or in LB medium at 26 °C with aeration. E. coli DH5α and DH5αPRO were used for plasmid maintenance and propagation. E. coli strains were routinely cultured on LB agar at 37 °C for 16 h or in LB medium at 37 °C with aeration for 16 h. Antibiotic supplementation with carbenicillin (100 µg ml−1) or kanamycin (50 µg ml−1) was provided as necessary.
Table 1. Bacterial strains and plasmids used in this study.
| Strain/plasmid | Description* | Reference/source |
| Y. pestis strains | ||
| CO92 | WT, pMT1+, pPCP1+, pCD1+ and pgm+ | Doll et al. (1994) |
| YP6 | CO92, pCD1− | Cathelyn et al. (2006) |
| YPA45 | CO92 Δpla, pCD1− | Lawrenz et al. (2009) |
| YPA51 | CO92 Δpla-2, pCD1− | P. Price and W. Goldman (University of North Carolina, NC, USA) |
| YP261 | CO92 ΔyapG, pCD1+ | This study |
| YPA53 | CO92 ΔyapG, pCD1− | This study |
| Y. pseudotuberculosis strains | ||
| YPIII | WT | Bölin et al. (1982) |
| IP32953 | WT | R. Isberg (Tufts University, MA, USA); Chain et al. (2004) |
| E. coli strains | ||
| DH5αPRO | deoR endA1 gyrA96 hsdR17(rK− mK+) recA1 relA1 supE44 thi-1 Δ(lacZYA-argF)U169 Φ80dlacZ M15 F− λ− PN25 tetR PlacIq lacI Spr | Clontech |
| DH5α | Φ80d Δ(lacZ)M15 Δ(argF-lac)U169 endA1 recA1 hsdR17(rK− mK+) deoR thi-1 supE44 gyrA96 relA1 | Gibco |
| Plasmids | ||
| pWL204 | Plasmid carrying the λ red recombinase gene and sacB gene for sucrose counterselection; CarbR | Lathem et al. (2007) |
| pKD13 | λ red recombinase system template plasmid; KanR, CarbR | Datsenko and Wanner (2000) |
| pLH29 | Plasmid carrying the FLP recombinase and sacB genes; CarbR | Lawrenz et al. (2009) |
| pMWO-005 | Expression vector containing PLtetO-1 promoter, PN25 tetR repressor, pSC101 ori, and multiple cloning site from pWKS130; KanR | Obrist and Miller (2012) |
| pLP-PROTet-6xHN | Expression vector that contains PLtetO-1 promoter; CarbR | Clontech |
| pLP-PROTet : : yapG | pLP-PROTet-6xHN containing yapG | This study |
| pMWO : : yapG | pMWO-005 containing yapG from pLP-ProTet : : yapG | This study |
| pMWO : : yapG SDM2 | pMWO : : yapG containing KR548–549AG mutations | This study |
| pMWO : : yapG SDM4 | pMWO : : yapG containing KR594–595AG mutations | This study |
| pMWO : : yapG SDM2+4 | pMWO : : yapG containing KR548–549AG and KR594–595AG mutations | This study |
| pMWO : : yapG SDM1 | pMWO : : yapG containing K512A mutation | This study |
| pMWO : : yapG SDM2+3 | pMWO : : yapG containing KR548–549AG and K558A mutations | This study |
| pMWO : : yapG SDM4+5 | pMWO : : yapG containing KR594–595AG and K604A mutations | This study |
| pMWO : : yapG SDM3+5 | pMWO : : yapG containing K558A and K604A mutations | This study |
| pMWO : : yapG SDM2+3+4+5 | pMWO : : yapG containing KR548–549AG, K558A, KR594–595AG and K604A mutations | This study |
| pMWO : : yapG SDM1+4+5 | pMWO : : yapG containing K512A, KR594–595AG and K604A mutations | This study |
| pMWO : : yapG SDM1+2+3 | pMWO : : yapG containing K512A, KR548–549AG and K558A mutations | This study |
| pMWO : : yapG SDM1+2+3+4+5 | pMWO : : yapG containing K512A, KR548–549AG, K558A, KR594–595AG and K604A mutations | This study |
RBS, ribosome-binding site; Kan, kanamycin; Carb, carbenicillin; Sp, spectinomycin.
Strain and plasmid construction.
The ΔyapG in-frame deletion strain (YP261) was constructed using the λ Red recombination system with modifications (Datsenko & Wanner, 2000; Lathem et al., 2007). Briefly, ~500 bp fragments of the 5′ and 3′ regions of yapG were amplified by PCR using the DNA096/DNA097 and DNA098/DNA099 primer sets, respectively (Table 2). After gel purification, the resulting PCR products were mixed with a kanamycin resistance cassette (KanR) flanked by FLP recognition target (FRT) sites, which previously had been amplified from the template plasmid pKD13. In a splicing-by-overhang-extension (SOE)-PCR, the three PCR fragments were joined together using primers DNA096 and DNA099. The resulting SOE-PCR product (yapG5′–FRT–KanR–FRT–yapG3′) was gel purified and transformed into Y. pestis strain CO92 carrying pWL204, which previously had been cultured with 10 mM arabinose to induce expression of the λ Red recombinase gene. Recombinants were initially selected on BHI agar containing kanamycin and then passaged onto BHI agar containing 5 % sucrose (w/v) to cure the λ Red recombinase plasmid, pWL204. The KanR was removed from the mutant strain using the FRT recombinase provided by a version of pLH29 that has been modified by replacing the chloramphenicol resistance gene with an ampicillin resistance gene (Lawrenz et al., 2009). KanS and CarbS recombinants were selected and the in-frame deletion of yapG was verified by PCR. Additionally, the retention of major virulence determinants (pCD1, pMT1, pPCP1 and pgm) was confirmed by PCR.
Table 2. Oligonucleotide primers used in this study.
| Primer name (description) | Primer sequence (5′→3′)* |
| Mutant construction | |
| DNA096 (yapG 5′ region forward) | AGGCTTTGCGTTCCCAGATC |
| DNA097 (yapG 5′ region reverse) | GAAGCAGCTCCAGCCTACACCATGATTTAGAGTTTACCAAT |
| DNA098 (yapG 3′ region forward) | GGTCGACGGATCCCCGGAATAATATCCATTGATAAAACGATACCTGTGCC |
| DNA099 (yapG 3′ region reverse) | CAATCGTGTAGYGATACAGCGTTTTATCC |
| P1Kan (kan of pKD13 forward) | GTGTAGGCTGGAGCTGCTTC |
| P4Kan (kan of pKD13 reverse) | ATTCCGGGGATCCGTCGACC |
| Cloning into pLP-PROTet | |
| JDL52 (yapG forward) | GGGGTACCCATGAAGAATTCAAATAGATCACCCAAAAAC |
| DNA269 (yapG reverse) | CGAAGCTTATAGGCACAGGTATCGTTTTATCAATGGATATTA |
| Site-directed mutagenesis | |
| DC7 (SDM2 and SDM4 forward) | CGTTCCAGACCCAGCGGGCGCCAATGCCG |
| DC6 (SDM2 and SDM4 reverse) | CGGCATTGGCGCCCGCTGGGTCTGGAACG |
| MCL77 (SDM1 forward) | GCAGTCTTTTCAGCTGCATTAGCTGATTACGCGTTGCAAGCC |
| MCL76 (SDM1 reverse) | GGCTTGCAACGCGTAATCAGCTAATGCAGCTGAAAAGACTGC |
| MCL75 (SDM3 forward) | GCAGCCTTTTCAGCCGCATTAGCTGATTACGCGTTGCAAACC |
| MCL74 (SDM3 reverse) | GGTTTGCAACGCGTAATCAGCTAATGCGGCTGAAAAGGCTGC |
| MCL73 (SDM5 forward) | GAAGCCTTTTCAGCCGCATTAGCTGATTACGCGTTGCAAGCT |
| MCL72 (SDM5 reverse) | AGCTTGCAACGCGTAATCAGCTAATGCGGCTGAAAAGGCTTC |
Restriction sites are underlined.
For the YapG induction experiments, Y. pestis strains were cured of the pCD1 virulence plasmid by passage on BHI agar supplemented with magnesium oxalate (20 mM MgCl2 and 20 mM Na2C2O4) at 37 °C (Higuchi & Smith, 1961). Plasmid loss was confirmed by absence of PCR amplification of yopH and yopT. For induction of yapG expression, yapG was amplified from Y. pestis CO92 by PCR with gene-specific primers (Table 2) and initially cloned into the pLP-PROTet-6×HN plasmid (Clontech). Specifically, the yapG PCR product and pLP-PROTet-6×HN were digested with KpnI and HindIII and then ligated and transformed by electroporation into E. coli DH5αPRO (Clontech), which harbours the tetracycline repressor. To allow for the induction of yapG in Y. pestis, the previously constructed pLP-PROTet plasmid was digested with BssSI and HindIII to produce a fragment containing the inducible promoter/operator elements followed by the full-length yapG gene. The fragment was ligated into pMWO-005, a low-copy-number vector carrying origins compatible with Y. pestis and E. coli (Obrist & Miller, 2012), and transformed by electroporation into DH5α. Upon verification of yapG insertion by PCR, the completed plasmid was isolated and transformed into Y. pestis CO92 (strain YP6, YPA45, YPA51 or YPA53).
Site-directed mutagenesis of the putative Pla cleavage sites of YapG was performed using the pMWO : : yapG double-stranded denatured plasmid template and DpnI for selection of mutants (Sambrook & Russell, 2001). Point mutations were introduced into the overlapping regions of oligonucleotide primers DC7 and DC6 to replace either or both of the KR548–549 and KR594–595 codons with AG codons (Table 2). Additional site-directed mutations and combinations of site-directed mutations were similarly introduced using oligonucleotide primers MCL77/MCL76 (K512A), MCL75/MCL74 (K558A) and MCL73/MCL72 (K604A) (Table 2). As each of these codons lies within the direct repeat regions of yapG, the desired site-directed mutations were confirmed by sequencing.
Induction of yapG expression, sample preparation and Western blot analysis.
For induction experiments, Y. pestis cultures were grown overnight, diluted 1 : 25 in fresh BHI medium, and grown for 3 h at 26 °C. yapG expression was induced by the addition of 100 ng anhydrous tetracycline ml−1 (Sigma), and cultures were grown for an additional 2 h. Whole-cell lysates and supernatants from induced and uninduced cultures were prepared for SDS-PAGE as described previously (Lawrenz et al., 2009; Walker & Miller, 2004). Samples were boiled for 10 min, separated by SDS-PAGE and transferred to Immobilon-P membranes (Millipore) for Western blot analysis. Anti-YapG serum was generated in rabbits (Cocalico Biological) against the PD of YapG (aa 50–549) and pre-absorbed against E. coli lysates. Blots were incubated with a 1 : 2000 dilution of anti-YapG serum, followed by incubation with a 1 : 50 000 dilution of horseradish peroxidase-conjugated goat anti-rabbit IgG (Sigma). The blots were developed using chemiluminescence according to the manufacturer’s instructions (Amersham ECL; GE Healthcare Life Sciences).
Animal infections.
All animal experiments were approved by the University of North Carolina Institutional Animal Care and Use Committee (protocol 11-128). Female C57Bl/6J mice and BALBc/J mice (4–6 weeks old; The Jackson Laboratory) were used for all Y. pestis infections. All mice were maintained in a barrier facility and allowed free access to sterilized food and water. For subcutaneous (s.c.) infections, Y. pestis CO92 WT (CO92) and ΔyapG (YP261) were cultured at 26 °C in BHI broth for 16 h with aeration. Subcutaneous inoculations were performed as described by Cathelyn et al. (2006) with doses of ~102 c.f.u. per mouse. For intranasal (i.n.) infections, Y. pestis CO92 WT (CO92) and ΔyapG (YP261) were cultured in BHI broth with aeration at 26 °C for 8–10 h prior to subculture into BHI broth containing 2.5 mM CaCl2 for overnight growth at 37 °C. Cultures were then diluted in sterile PBS to achieve an inoculum of ~104 c.f.u. per mouse, and inoculations were performed as described by Lathem et al. (2005). For all Y. pestis infections, groups of six to eight mice were euthanized by intraperitoneal injection with sodium pentobarbital [150 mg (kg body weight)−1] at the time points indicated. At each time point, tissues were harvested, weighed and homogenized in sterile PBS, and serial dilutions were plated on BHI agar to determine bacterial load. The statistical significance of the data obtained in all independent challenge experiments was assessed using a Mann–Whitney test.
Results and Discussion
Processing of YapG by the omptin protease Pla in Y. pestis
In a previous study conducted by our group, we demonstrated the secretion and localization of the conventional autotransporter proteins in Y. pestis, including YapG (Lenz et al., 2011). Haemagglutinin-tagged YapG was shown by both Western blot analysis and immunohistochemistry to be secreted to the outer membrane and proteolytically released into culture supernatants in a manner that required the omptin protease, Pla (Lenz et al., 2011). The gene encoding Pla is carried on the pPCP1 plasmid, which is not found in the other pathogenic yersiniae (Ferber & Brubaker, 1981). The chromosome of Y. pestis CO92 also contains the pla-2 gene, which is predicted to encode an omptin protease, based on bioinformatic analysis (Parkhill et al., 2001). Although there are no published reports that specifically examine the regulation of pla-2 or Pla-2 function, it is likely that Pla-2 functions differently from Pla, in that the key protein regions within the extracellular loops, L3 and L4, of the Pla β-barrel that are important for plasminogen activation and inactivation of α2-antiplasmin, are not conserved in Pla-2 (Kukkonen et al., 2001). Thus, to examine more comprehensively YapG processing, we wanted to determine whether Pla-2 contributes to the proteolytic release of YapG. Under standard culture conditions of 26 and 37 °C, we were previously unable to detect YapG protein produced from the native chromosomal gene by Western blotting (Lenz et al., 2011). Therefore, to examine YapG translocation and processing, yapG was cloned on a low-copy-number plasmid such that its expression could be induced with anhydrous tetracycline (aTc) from the PLtetO promoter. Expression of yapG was induced in the WT, Δpla and Δpla-2 strains of Y. pestis CO92. Induced and uninduced protein samples isolated from cell pellets (containing mature-length YapG) and concentrated supernatants (containing released YapG) were subjected to Western blot analysis using anti-YapG serum (Fig. 1). In agreement with our previous observations, and under the specific conditions tested, proteolytic release of YapG was observed in the WT and Δpla-2 strains, but not the Δpla strain. Therefore, it appears that Pla is the sole omptin protease responsible for cleavage of YapG in Y. pestis.
Fig. 1.
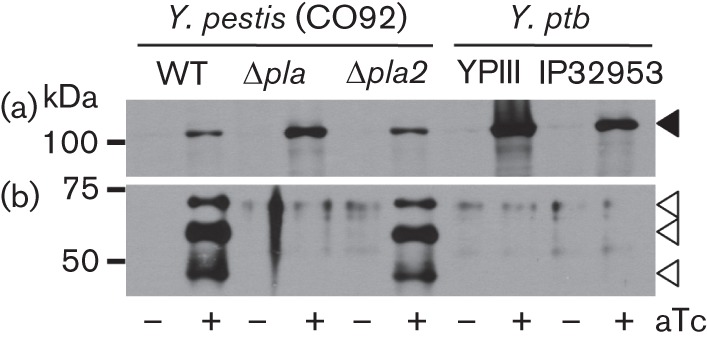
Localization of YapG in Y. pestis and Y. pseudotuberculosis and requirement of Pla for proteolytic cleavage. Western blot demonstrating the localization of YapG in the WT, pla mutant and pla-2 mutant strains of Y. pestis CO92 (pCD1-), and two WT strains, YPIII and IP32953, of Y. pseudotuberculosis (Y. ptb). Protein samples from cell pellets (a) and culture supernatants (b) were probed with anti-YapG serum. −, Uninduced culture; +, culture induced with aTc. Molecular masses (kDa) are indicated on the left. The solid arrowhead indicates mature-length YapG and the open arrowheads denote the Pla-processed forms of YapG.
In addition to being identified as a functional conventional autotransporter in Y. pestis strain CO92, YapG is also conserved in the fully virulent Y. pseudotuberculosis strain IP32953 (Chain et al., 2004; Lenz et al., 2011). Indeed, yapG is present in all four sequenced Y. pseudotuberculosis genomes (IP32953, YPIII, PB1 and IP31758), and the subsequent translated YapG amino acid sequences share 94–99 % pairwise identity with YapG of CO92. One key difference between Y. pestis and Y. pseudotuberculosis is the acquisition of the 9.5 kb pPCP1 plasmid, encoding Pla, in Y. pestis. As Pla is required for proteolytic release of YapG in Y. pestis, we hypothesized that, in the absence of pla in Y. pseudotuberculosis, YapG would remain associated with the bacterial outer membrane. To test our hypothesis, we induced expression of Y. pestis yapG in the WT IP32953 and YPIII strains of Y. pseudotuberculosis (Bölin et al., 1982; Chain et al., 2004). Protein samples isolated from cell pellets and concentrated supernatants were subjected to Western blot analysis using anti-YapG serum. As expected, YapG was observed to remain cell-associated, despite the presence of pla-2 in Y. pseudotuberculosis (Fig. 1). Thus, we determined that, in Y. pseudotuberculosis, YapG remains tethered to the bacterial outer membrane, and the acquisition of Pla in Y. pestis results in proteolytic release of YapG to the extracellular milieu. Because YapG in Y. pestis and Y. pseudotuberculosis share such a high degree of sequence conservation, it is tempting to speculate that the acquisition of Pla may render a different function for YapG between these two pathogenic species.
Identification of YapG cleavage sites
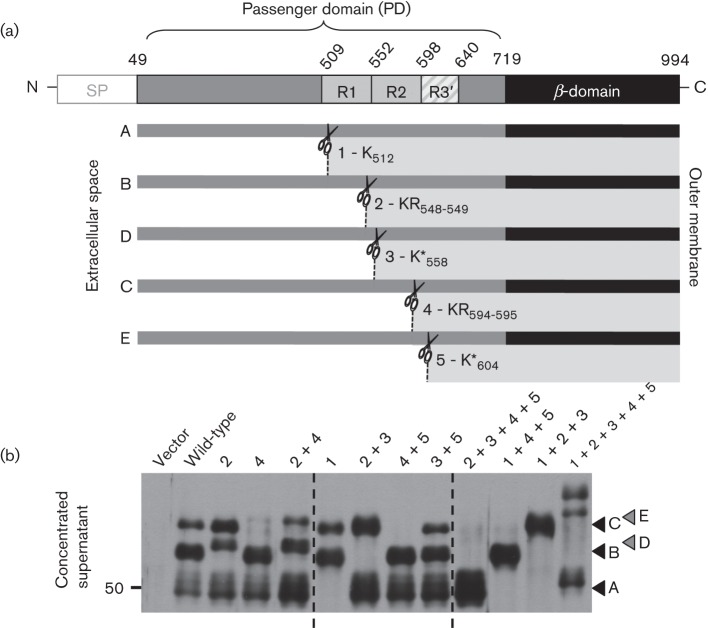
To understand ultimately how Pla cleavage may affect YapG function in Y. pestis, we sought to identify the residues within the YapG PD that are targeted for Pla processing. Upon induction of yapG expression in Y. pestis, we observed the release of three predominant fragments of YapG into the supernatant (Fig. 2a, b, fragments A, B and C). Interestingly, induction of yapG expression in E. coli only resulted in the release of one predominant YapG fragment into the supernatant, which was also dependent upon the activity of the homologous omptin protease, OmpT (Lenz et al., 2011). The appearance of different cleavage products was not surprising, as Pla and OmpT are known to have different substrate specificities (Kukkonen et al., 2001). Despite differences in substrate specificity, both OmpT and Pla are known to cleave substrates at basic amino acids, with a preference of cleavage between two basic amino acids (Agarkov et al., 2008; McCarter et al., 2004). Indeed, YapG contains multiple basic residues that lie within an extended linker region of near-perfect direct repeats in the C terminus of the PD: K512 (site 1), KR548–549 (site 2), K558 (site 3), KR594–595 (site 4) and K604 (site 5). Cleavage of YapG at sites 1–5 would produce fragments of YapG with molecular masses that range from 49.2 to 57.6 kDa (indicated as fragments A–E in Fig. 2a, b), which are consistent with the masses of the three released fragments of YapG observed in Fig. 2(b). Because omptin proteases prefer to cleave between two basic amino acids, we initially decided to address whether the two KR residues are important for Pla cleavage by introducing site-directed mutations into the yapG gene by replacing either or both KR codons with AG codons to make these sites neutral and not basic. To determine whether one or more mutation(s) eliminated cleavage of YapG, the resulting yapG site-directed mutations were expressed in Y. pestis, and samples were collected and subjected to Western blot analysis using anti-YapG serum as described above. Site-directed mutations in site 2 (KR548–549AG), site 4 (KR594–595AG), and sites 2+4 (KR548–549AG/KR594–595AG) predominantly resulted in the release of two new fragments (D and E) of YapG with slightly larger molecular masses (Fig. 2b, left panel). It is important to note that we also observed the same mass shift with a double-alanine (KR548–549AA) mutation, suggesting that the altered cleavage of YapG by Pla is due to altered site specificity rather than a change in conformational flexibility of the protein structure due to the Gly substitution (data not shown). To identify the other residues of YapG that are targeted by Pla, we constructed the following additional site-directed mutations in yapG (individually or in combination with the original mutations): site 1 (K512A), site 3 (K558A), and site 5 (K604A). The site 1 (K512A) mutation resulted in loss of the smallest released fragment (A) of YapG (Fig. 2b, centre), suggesting that, in addition to site 2 (KR548–549) and site 4 (KR594–595), site 1 (K512) is also a primary site of Pla cleavage. Furthermore, the sites 2+3 (KR548–549AG/K558A) and sites 4+5 (KR594–595AG/K604A) mutations resulted in loss of the middle (B and D) and largest (C and E) released fragments of YapG, respectively (Fig. 2b, centre). Mutation of these two additional sites alone [sites 3+5 (K558A/K604A)] did not alter YapG cleavage specificity (Fig. 2b, centre panel), suggesting that site 3 (K558) and site 5 (K604) represent secondary alternate sites for YapG cleavage by Pla. By making combinations of mutations within the primary and secondary cleavage sites, we were able to isolate each of the major released fragments of YapG (Fig. 2b, right panel). Mutation of all primary and secondary sites in yapG did not entirely abolish cleavage of YapG. In fact, mutation of sites 1–5 resulted in three new released fragments of YapG, with slightly different molecular masses (Fig. 2b, right panel). Release of these three new YapG fragments in the site 1–5 mutant was determined to be Pla dependent (data not shown). In summary, through site-directed mutagenesis of yapG, we determined that sites 1 (K512), 2 (KR548–549) and 4 (KR594–595) represent the primary cleavage sites of YapG resulting in fragments A, B and C, respectively, whereas sites 3 (K*558) and 5 (K*604) represent the secondary alternative cleavage sites of YapG resulting in fragments D and E, respectively.
Fig. 2.
Amino acid residues of YapG important for processing by the omptin protease Pla. (a) Schematic of the domain organization of YapG. The signal peptide (SP) is shown in white, the PD in grey and the β-domain in black. The basic Lys and Arg residues within the PD that are demonstrated to be important for Pla cleavage are located within two direct repeats (R1 and R2) and in a third partial direct repeat (R3′). Cleavage of sites 1–5 (K512, KR548–549, K558, KR594–595 and K604) by Pla (illustrated with scissors) results in released fragments A–E. (b) Western blot demonstrating the secretion of WT and mutant YapG in the avirulent (pCD1−) Y. pestis yapG mutant. Bacteria were treated with aTC to induce yapG expression, and culture supernatants were probed with anti-YapG serum. The site-directed mutations (individual or multiple) examined for cleavage are indicated above the blot. The arrows to the right correspond to the predicted fragment sizes illustrated in (a). Sites 1 (K512), 2 (KR548–549) and 4 (KR594–595) represent the major cleavage sites of YapG resulting in fragments A, B and C, respectively, whereas, sites 3 (K*558) and 5 (K*604) represent the secondary alternative cleavage sites of YapG resulting in fragments D and E, respectively. Molecular mass is indicated to the left of the blot in kDa.
Whilst the N terminus of the YapG PD (aa 49–508) has recently been shown to adopt the characteristic β-helical fold of conventional autotransporters, the extended linker of the C-terminal PD (aa 509–719) is predicted to have little to no secondary structure (M. L. Frazier, A. Pokorny, M. C. Lane, J. V. Lomino, W. K. Ballentine III, V. L. Miller, M. R. Redinbo, unpublished data). Indeed, this extended linker region is proline rich, being composed of 10 % (21/210) prolines in comparison with 1.7 % (8/459) prolines in the N-terminal PD region. It is tempting to speculate that this unstructured linker represents a flexible hinge that aids in the extension of YapG on the bacterial surface. This would allow YapG to be more accessible to Pla and may prevent negative interference from other outer-membrane structures, including the capsule, a common sterical barrier to other Gram-negative bacterial autotransporters (Schembri et al., 2004). Despite the lack of a defined secondary structure, the major and minor Pla cleavage sites within this region appeared to be conserved among nearly all (11/12) the predicted Y. pestis protein sequences of YapG available from completed Y. pestis genome sequences in GenBank [with the exception of Angola (GenBank accession no. NC_010159), which appears to be missing a single adenine base in a homopolymeric track of nine adenines, which may be a result of a sequencing error rendering yapG non-functional]. In all, the presence of multiple and alternative Pla cleavage sites in YapG, and their high degree of conservation among YapG sequences of Y. pestis, suggest that processing of YapG by Pla may be evolutionarily significant to the function of YapG in Y. pestis.
Virulence of the yapG mutant in the mouse models of plague infection
Of the Y. pestis Yap autotransporters, YapE has been shown to be required for bubonic infection in mice but has no role in primary pneumonic plague (Lawrenz et al., 2009). YapJ and YapK have both been shown to contribute in an additive manner to systemic infection after intraperitoneal inoculation of mice (Lenz et al., 2012). Additionally, preliminary studies suggested that YapG may contribute to mammalian virulence, in that yapG expression is modestly induced during bubonic and pneumonic plague of mice (Lenz et al., 2011), and that YapG-specific antibodies are produced following a sublethal injection in a rabbit model of bubonic plague (Andrews et al., 2010). To determine whether YapG contributes to plague pathogenesis, we constructed an in-frame deletion of yapG in Y. pestis strain CO92, and compared differences in colonization of the WT and ΔyapG strains using the established mouse models of pneumonic and bubonic infection. Following i.n. inoculation of C57Bl/6 mice with either the WT or ΔyapG strain, no differences in colonization were observed in the lungs or spleen at 12, 24, 48 or 72 h post-infection (p.i.) (Fig. 3a). Because it appeared that the ΔyapG strain had a slight colonization defect in the lungs at 12 h p.i., we also compared the colonization of these strains at 6 h p.i. but were unable to detect any significant differences in either C57Bl/6 or BALB/c mice (data not shown). Because Y. pestis virulence factors vary in their contributions to pneumonic and bubonic plague, we also examined the role of YapG during bubonic infection. Following s.c. injection, no significant differences were observed between WT and ΔyapG colonization in the lymph nodes or disseminated organs (spleen and lungs) (Fig. 3b and data not shown). In addition, no differences in lymph node pathology were observed at 36 or 96 h p.i. in mice injected s.c. with the WT or ΔyapG strain (data not shown). Because Y. pestis most likely enters the dermis (and not subcutaneous space) after the bite of an infected flea (Sebbane et al., 2006), we also determined whether YapG contributed to bubonic infection after intradermal injection. After intradermal injection of either WT or the ΔyapG strain (2×102 c.f.u. into the ear), we did not observe any differences in colonization of the lymph nodes or disseminated organs (spleen and lungs) (data not shown). Thus, using the established models of bubonic and pneumonic infection, no observable contribution of yapG to murine plague was detected.
Fig. 3.
Comparison of WT and yapG mutant colonization and dissemination during pneumonic (a) and bubonic (b) plague. (a) Mice were i.n. inoculated with ~1×104 c.f.u. WT (▪) or yapG mutant (□). (b) Mice were s.c. injected in the neck with ~1×102 c.f.u. WT (▪) or yapG mutant (□). For (a) and (b), data are representative of two independent experiments. Mice were sacrificed at various times p.i. (as indicated below the graphs), and organs were homogenized and plated to determine c.f.u. (g tissue)−1. Each symbol represents bacteria recovered from an individual mouse. Horizontal bars denote the median of each population. Numbers with an ‘×’ below each population indicate the number of mice that were euthanized or found dead at that time point. The dashed lines indicate the limits of detection for each organ.
Whilst autotransporter proteins in general have been shown to mediate a variety of virulence-related functions (Henderson & Nataro, 2001), few of these autotransporters have been shown to contribute directly to virulence in animal models of infection (Alamuri & Mobley, 2008; Alamuri et al., 2010; Allsopp et al., 2010; Dorsey et al., 2005; Elder & Harvill, 2004; Noofeli et al., 2011; Roy et al., 2011). One possible explanation for this phenomenon is functional redundancy, whereby overlapping functions of multiple virulence factors might mask in vitro and in vivo phenotypes of single gene deletions. A prominent example of this includes the redundant contribution of an autotransporter, polysaccharide and fimbrial adhesins of uropathogenic E. coli to biofilm formation (Allsopp et al., 2010, 2012a, b; Kai-Larsen et al., 2010; Melican et al., 2011; Ong et al., 2008; Spurbeck et al., 2011; Ulett et al., 2007a, b; Valle et al., 2008; Wang et al., 2004). Furthermore, Y. pestis is known to infect a wide spectrum of mammalian hosts (Perry & Fetherston, 1997). Whilst YapG does not appear to contribute to Y. pestis virulence in mice, it is possible that YapG might contribute to pathogenesis in another mammalian host or in fleas.
Conclusions
Upon translocation of autotransporter proteins to the outer membrane, PDs can either remain tethered to the bacterial surface or are released following proteolytic cleavage. The mechanisms of PD release include cleavage by inter- or intramolecular autocatalytic mechanisms, or intermolecular cleavage by another outer-membrane protease or autotransporter (Dautin & Bernstein, 2007). Previous work and work presented in this study have demonstrated that the YapG autotransporter of the highly virulent Y. pestis strain CO92 is cleaved and released by the omptin protease Pla. Moreover, we showed that Pla cleaves at multiple primary and secondary sites within the YapG PD, which are conserved among nearly all the predicted protein sequences of YapG obtained from available Y. pestis genome sequences. Altogether, these data suggest that YapG cleavage by Pla may be important for YapG function. In the evolutionarily related pla-negative pathogen Y. pseudotuberculosis, we demonstrated that YapG remained cell associated. Thus, the acquisition of Pla and release of YapG in Y. pestis may render a different function for YapG during the evolutionary process Y. pestis took to become a flea-borne pathogen. In contrast, it is possible that acquisition of Pla may render YapG non-functional. However, this explanation seems less likely, because, if function were to be lost, one would expect to observe an accumulation of inactivating mutations in the yapG gene in the different Y. pestis genomes, which is just not the case.
In an attempt to elucidate the function of YapG, we conducted extensive bioinformatic analyses and numerous in vitro assays by examining the functions of previously characterized autotransporters. In attempting to predict YapG function, we were unable to uncover any conserved domains using the CDD (Marchler-Bauer et al., 2011), Pfam (Finn et al., 2010), SMART (Letunic et al., 2012) or InterPro (Zdobnov & Apweiler, 2001) databases, with the exception of the conserved autotransporter domain and some weak similarity to pertactin within the YapG PD N-terminal to the repeat region that is typically shared by conventional autotransporters. Despite a lack of strong functional predictions, we assayed YapG in both the WT and Δpla backgrounds of Y. pestis for common functions that are possessed by other autotransporters. Among the functions assayed, we examined cytotoxicity, adherence, autoaggregation, proteolytic activity of casein and lipase/esterase activity on emulsified tributyrin. However, under the conditions in which these assays were performed, YapG did not possess any of these functions (M. C. Lane, J. D. Lenz and V. L. Miller, unpublished data). These data do not imply that YapG does not have a defined function; indeed, there are a myriad of functional activities remaining that could be assessed. Without additional clues from homology models, in vivo studies, or a crystal structure, it becomes difficult to narrow down these infinite functional possibilities.
Although the function of YapG remains to be elucidated, we demonstrated here that YapG does not contribute to Y. pestis virulence in the mouse models of bubonic and pneumonic plague. Despite the lack of an in vivo phenotype in mice, we cannot definitively rule out a role for YapG in mammalian disease, as (i) the lack of a phenotype in mice might be the result of functional redundancy with other Y. pestis virulence factors, or (ii) YapG may be important for virulence in other mammalian hosts. Alternatively, YapG may play a role during other stages of the Y. pestis life cycle, including flea colonization or the effective transmission of Y. pestis to the host.
Acknowledgements
The authors would like to thank Matthew Lawrenz for aiding in the design and preparation of the YapG antisera and for assistance with animal infections and yapG cloning; David Cotter for his preliminary analysis leading to the identification of the YapG cleavage sites and assistance in yapG cloning; Eric Weening and Rodrigo Gonzalez for assistance with animal infections and tissue processing; Monica Frazier and Matthew Redinbo for helpful YapG structural discussions; and Kimberly Walker for critical editing and evaluation of this manuscript. Additionally, the Δpla-2 strain was provided as a gift from Paul Price and William Goldman (University of North Carolina, Chapel Hill, NC, USA), and the Y. pseudotuberculosis strain IP32953 was provided as a gift from Ralph Isberg (Tufts University, Medford, MA, USA). This study was supported by funds from National Institutes of Health grants R56AI078930 (V. L. M.) and U54AI057157 (Southeast Regional Center for Biodefense and Emerging Infectious Diseases, subproject 4.1, to V. L. M.), a Morse/Berg Fellowship from the Department of Molecular Microbiology, Washington University (J. D. L.), an Infectious Disease Pathogenesis Research Training Fellowship (T32AI07151-31) (M. C. L.) and a Ruth L. Kirschstein National Research Service Award (F32AI085923) (M. C. L.).
Abbreviations:
- aTc
anhydrous tetracycline
- i.n.
intranasal
- PD
passenger domain
- p.i.
post-infection
- s.c.
subcutaneous
References
- Agarkov A., Chauhan S., Lory P. J., Gilbertson S. R., Motin V. L. (2008). Substrate specificity and screening of the integral membrane protease Pla. Bioorg Med Chem Lett 18, 427–431 10.1016/j.bmcl.2007.09.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alamuri P., Mobley H. L. T. (2008). A novel autotransporter of uropathogenic Proteus mirabilis is both a cytotoxin and an agglutinin. Mol Microbiol 68, 997–1017 10.1111/j.1365-2958.2008.06199.x [DOI] [PubMed] [Google Scholar]
- Alamuri P., Löwer M., Hiss J. A., Himpsl S. D., Schneider G., Mobley H. L. T. (2010). Adhesion, invasion, and agglutination mediated by two trimeric autotransporters in the human uropathogen Proteus mirabilis. Infect Immun 78, 4882–4894 10.1128/IAI.00718-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsopp L. P., Totsika M., Tree J. J., Ulett G. C., Mabbett A. N., Wells T. J., Kobe B., Beatson S. A., Schembri M. A. (2010). UpaH is a newly identified autotransporter protein that contributes to biofilm formation and bladder colonization by uropathogenic Escherichia coli CFT073. Infect Immun 78, 1659–1669 10.1128/IAI.01010-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsopp L. P., Beloin C., Moriel D. G., Totsika M., Ghigo J.-M., Schembri M. A. (2012a). Functional heterogeneity of the UpaH autotransporter protein from uropathogenic Escherichia coli. J Bacteriol 194, 5769–5782 10.1128/JB.01264-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsopp L. P., Beloin C., Ulett G. C., Valle J., Totsika M., Sherlock O., Ghigo J.-M., Schembri M. A. (2012b). Molecular characterization of UpaB and UpaC, two new autotransporter proteins of uropathogenic Escherichia coli CFT073. Infect Immun 80, 321–332 10.1128/IAI.05322-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews G. P., Vernati G., Ulrich R., Rocke T. E., Edwards W. H., Adamovicz J. J. (2010). Identification of in vivo-induced conserved sequences from Yersinia pestis during experimental plague infection in the rabbit. Vector Borne Zoonotic Dis 10, 749–756 10.1089/vbz.2009.0179 [DOI] [PubMed] [Google Scholar]
- Bodelón G., Marín E., Fernández L. A. (2009). Role of periplasmic chaperones and BamA (YaeT/Omp85) in folding and secretion of intimin from enteropathogenic Escherichia coli strains. J Bacteriol 191, 5169–5179 10.1128/JB.00458-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölin I., Norlander L., Wolf-Watz H. (1982). Temperature-inducible outer membrane protein of Yersinia pseudotuberculosis and Yersinia enterocolitica is associated with the virulence plasmid. Infect Immun 37, 506–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottone E. J. (1999). Yersinia enterocolitica: overview and epidemiologic correlates. Microbes Infect 1, 323–333 10.1016/S1286-4579(99)80028-8 [DOI] [PubMed] [Google Scholar]
- Cathelyn J. S., Crosby S. D., Lathem W. W., Goldman W. E., Miller V. L. (2006). RovA, a global regulator of Yersinia pestis, specifically required for bubonic plague. Proc Natl Acad Sci U S A 103, 13514–13519 10.1073/pnas.0603456103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chain P. S. G., Carniel E., Larimer F. W., Lamerdin J., Stoutland P. O., Regala W. M., Georgescu A. M., Vergez L. M., Land M. L. & other authors (2004). Insights into the evolution of Yersinia pestis through whole-genome comparison with Yersinia pseudotuberculosis. Proc Natl Acad Sci U S A 101, 13826–13831 10.1073/pnas.0404012101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko K. A., Wanner B. L. (2000). One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97, 6640–6645 10.1073/pnas.120163297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautin N., Bernstein H. D. (2007). Protein secretion in Gram-negative bacteria via the autotransporter pathway. Annu Rev Microbiol 61, 89–112 10.1146/annurev.micro.61.080706.093233 [DOI] [PubMed] [Google Scholar]
- Doll J. M., Zeitz P. S., Ettestad P., Bucholtz A. L., Davis T., Gage K. (1994). Cat-transmitted fatal pneumonic plague in a person who traveled from Colorado to Arizona. Am J Trop Med Hyg 51, 109–114 [DOI] [PubMed] [Google Scholar]
- Dorsey C. W., Laarakker M. C., Humphries A. D., Weening E. H., Bäumler A. J. (2005). Salmonella enterica serotype Typhimurium MisL is an intestinal colonization factor that binds fibronectin. Mol Microbiol 57, 196–211 10.1111/j.1365-2958.2005.04666.x [DOI] [PubMed] [Google Scholar]
- Elder K. D., Harvill E. T. (2004). Strain-dependent role of BrkA during Bordetella pertussis infection of the murine respiratory tract. Infect Immun 72, 5919–5924 10.1128/IAI.72.10.5919-5924.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felek S., Lawrenz M. B., Krukonis E. S. (2008). The Yersinia pestis autotransporter YapC mediates host cell binding, autoaggregation and biofilm formation. Microbiology 154, 1802–1812 10.1099/mic.0.2007/010918-0 [DOI] [PubMed] [Google Scholar]
- Ferber D. M., Brubaker R. R. (1981). Plasmids in Yersinia pestis. Infect Immun 31, 839–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn R. D., Mistry J., Tate J., Coggill P., Heger A., Pollington J. E., Gavin O. L., Gunasekaran P., Ceric G. & other authors (2010). The Pfam protein families database. Nucleic Acids Res 38 (Database issue), D211–D222 10.1093/nar/gkp985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson I. R., Nataro J. P. (2001). Virulence functions of autotransporter proteins. Infect Immun 69, 1231–1243 10.1128/IAI.69.3.1231-1243.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson I. R., Navarro-Garcia F., Nataro J. P. (1998). The great escape: structure and function of the autotransporter proteins. Trends Microbiol 6, 370–378 10.1016/S0966-842X(98)01318-3 [DOI] [PubMed] [Google Scholar]
- Henderson I. R., Navarro-Garcia F., Desvaux M., Fernandez R. C., Ala’Aldeen D. (2004). Type V protein secretion pathway: the autotransporter story. Microbiol Mol Biol Rev 68, 692–744 10.1128/MMBR.68.4.692-744.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi K., Smith J. L. (1961). Studies on the nutrition and physiology of Pasteurella pestis. VI. A differential plating medium for the estimation of the mutation rate to avirulence. J Bacteriol 81, 605–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch B. J. (1997). Bubonic plague: a molecular genetic case history of the emergence of an infectious disease. J Mol Med (Berl) 75, 645–652 10.1007/s001090050148 [DOI] [PubMed] [Google Scholar]
- Janssen W. A., Fukui G. M., Surgalla M. J. (1958). A study of the fate of Pasteurella pestis following intracardial injection into guinea pigs. J Infect Dis 103, 183–187 10.1093/infdis/103.2.183 [DOI] [PubMed] [Google Scholar]
- Jong W. S. P., ten Hagen-Jongman C. M., Ruijter E., Orru R. V., Genevaux P., Luirink J. (2010). YidC is involved in the biogenesis of the secreted autotransporter hemoglobin protease. J Biol Chem 285, 39682–39690 10.1074/jbc.M110.167650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai-Larsen Y., Lüthje P., Chromek M., Peters V., Wang X., Holm Å., Kádas L., Hedlund K.-O., Johansson J. & other authors (2010). Uropathogenic Escherichia coli modulates immune responses and its curli fimbriae interact with the antimicrobial peptide LL-37. PLoS Pathog 6, e1001010 10.1371/journal.ppat.1001010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukkonen M., Lähteenmäki K., Suomalainen M., Kalkkinen N., Emödy L., Lång H., Korhonen T. K. (2001). Protein regions important for plasminogen activation and inactivation of α2-antiplasmin in the surface protease Pla of Yersinia pestis. Mol Microbiol 40, 1097–1111 10.1046/j.1365-2958.2001.02451.x [DOI] [PubMed] [Google Scholar]
- Lähteenmäki K., Kuusela P., Korhonen T. K. (2001). Bacterial plasminogen activators and receptors. FEMS Microbiol Rev 25, 531–552 [DOI] [PubMed] [Google Scholar]
- Lathem W. W., Crosby S. D., Miller V. L., Goldman W. E. (2005). Progression of primary pneumonic plague: a mouse model of infection, pathology, and bacterial transcriptional activity. Proc Natl Acad Sci U S A 102, 17786–17791 10.1073/pnas.0506840102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathem W. W., Price P. A., Miller V. L., Goldman W. E. (2007). A plasminogen-activating protease specifically controls the development of primary pneumonic plague. Science 315, 509–513 10.1126/science.1137195 [DOI] [PubMed] [Google Scholar]
- Lawrenz M. B., Lenz J. D., Miller V. L. (2009). A novel autotransporter adhesin is required for efficient colonization during bubonic plague. Infect Immun 77, 317–326 10.1128/IAI.01206-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz J. D., Lawrenz M. B., Cotter D. G., Lane M. C., Gonzalez R. J., Palacios M., Miller V. L. (2011). Expression during host infection and localization of Yersinia pestis autotransporter proteins. J Bacteriol 193, 5936–5949 10.1128/JB.05877-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz J. D., Temple B. R. S., Miller V. L. (2012). Evolution and virulence contributions of the autotransporter proteins YapJ and YapK of Yersinia pestis CO92 and their homologs in Y. pseudotuberculosis IP32953. Infect Immun 80, 3693–3705 10.1128/IAI.00529-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I., Doerks T., Bork P. (2012). SMART 7: recent updates to the protein domain annotation resource. Nucleic Acids Res 40 (Database issue), D302–D305 10.1093/nar/gkr931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler-Bauer A., Lu S., Anderson J. B., Chitsaz F., Derbyshire M. K., DeWeese-Scott C., Fong J. H., Geer L. Y., Geer R. C. & other authors (2011). CDD: a Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res 39 (Database issue), D225–D229 10.1093/nar/gkq1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter J. D., Stephens D., Shoemaker K., Rosenberg S., Kirsch J. F., Georgiou G. (2004). Substrate specificity of the Escherichia coli outer membrane protease OmpT. J Bacteriol 186, 5919–5925 10.1128/JB.186.17.5919-5925.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melican K., Sandoval R. M., Kader A., Josefsson L., Tanner G. A., Molitoris B. A., Richter-Dahlfors A. (2011). Uropathogenic Escherichia coli P and Type 1 fimbriae act in synergy in a living host to facilitate renal colonization leading to nephron obstruction. PLoS Pathog 7, e1001298 10.1371/journal.ppat.1001298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naktin J., Beavis K. G. (1999). Yersinia enterocolitica and Yersinia pseudotuberculosis. Clin Lab Med 19, 523–536, vi [PubMed] [Google Scholar]
- Noofeli M., Bokhari H., Blackburn P., Roberts M., Coote J. G., Parton R. (2011). BapC autotransporter protein is a virulence determinant of Bordetella pertussis. Microb Pathog 51, 169–177 10.1016/j.micpath.2011.04.004 [DOI] [PubMed] [Google Scholar]
- Obrist M. W., Miller V. L. (2012). Low copy expression vectors for use in Yersinia sp. and related organisms. Plasmid 68, 33–42 10.1016/j.plasmid.2012.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong C.-L. Y., Ulett G. C., Mabbett A. N., Beatson S. A., Webb R. I., Monaghan W., Nimmo G. R., Looke D. F., McEwan A. G., Schembri M. A. (2008). Identification of type 3 fimbriae in uropathogenic Escherichia coli reveals a role in biofilm formation. J Bacteriol 190, 1054–1063 10.1128/JB.01523-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhill J., Wren B. W., Thomson N. R., Titball R. W., Holden M. T., Prentice M. B., Sebaihia M., James K. D., Churcher C. & other authors (2001). Genome sequence of Yersinia pestis, the causative agent of plague. Nature 413, 523–527 10.1038/35097083 [DOI] [PubMed] [Google Scholar]
- Perry R. D., Fetherston J. D. (1997). Yersinia pestis – etiologic agent of plague. Clin Microbiol Rev 10, 35–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakin A., Boolgakowa E., Heesemann J. (1996). Structural and functional organization of the Yersinia pestis bacteriocin pesticin gene cluster. Microbiology 142, 3415–3424 10.1099/13500872-142-12-3415 [DOI] [PubMed] [Google Scholar]
- Rollins S. E., Rollins S. M., Ryan E. T. (2003). Yersinia pestis and the plague. Am J Clin Pathol 119 (Suppl.), S78–S85 [DOI] [PubMed] [Google Scholar]
- Roy K., Kansal R., Bartels S. R., Hamilton D. J., Shaaban S., Fleckenstein J. M. (2011). Adhesin degradation accelerates delivery of heat-labile toxin by enterotoxigenic Escherichia coli. J Biol Chem 286, 29771–29779 10.1074/jbc.M111.251546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Perez F., Henderson I. R., Leyton D. L., Rossiter A. E., Zhang Y., Nataro J. P. (2009). Roles of periplasmic chaperone proteins in the biogenesis of serine protease autotransporters of Enterobacteriaceae. J Bacteriol 191, 6571–6583 10.1128/JB.00754-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Perez F., Henderson I. R., Nataro J. P. (2010). Interaction of FkpA, a peptidyl-prolyl cis/trans isomerase with EspP autotransporter protein. Gut Microbes 1, 339–344 10.4161/gmic.1.5.13436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Russell D. W. (2001). Protocol 3: In vitro mutagenesis using double stranded DNA templates: selection of mutants with DpnI. In Molecular Cloning: a Laboratory Manual, 3rd edn, pp. 13.19–13.25 Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; [DOI] [PubMed] [Google Scholar]
- Sauri A., Soprova Z., Wickström D., de Gier J.-W., Van der Schors R. C., Smit A. B., Jong W. S. P., Luirink J. (2009). The Bam (Omp85) complex is involved in secretion of the autotransporter haemoglobin protease. Microbiology 155, 3982–3991 10.1099/mic.0.034991-0 [DOI] [PubMed] [Google Scholar]
- Schembri M. A., Dalsgaard D., Klemm P. (2004). Capsule shields the function of short bacterial adhesins. J Bacteriol 186, 1249–1257 10.1128/JB.186.5.1249-1257.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebbane F., Gardner D., Long D., Gowen B. B., Hinnebusch B. J. (2005). Kinetics of disease progression and host response in a rat model of bubonic plague. Am J Pathol 166, 1427–1439 10.1016/S0002-9440(10)62360-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebbane F., Jarrett C. O., Gardner D., Long D., Hinnebusch B. J. (2006). Role of the Yersinia pestis plasminogen activator in the incidence of distinct septicemic and bubonic forms of flea-borne plague. Proc Natl Acad Sci U S A 103, 5526–5530 10.1073/pnas.0509544103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodeinde O. A., Goguen J. D. (1989). Nucleotide sequence of the plasminogen activator gene of Yersinia pestis: relationship to ompT of Escherichia coli and gene E of Salmonella typhimurium. Infect Immun 57, 1517–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurbeck R. R., Stapleton A. E., Johnson J. R., Walk S. T., Hooton T. M., Mobley H. L. T. (2011). Fimbrial profiles predict virulence of uropathogenic Escherichia coli strains: contribution of Ygi and Yad fimbriae. Infect Immun 79, 4753–4763 10.1128/IAI.05621-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomalainen M., Haiko J., Ramu P., Lobo L., Kukkonen M., Westerlund-Wikström B., Virkola R., Lähteenmäki K., Korhonen T. K. (2007). Using every trick in the book: the Pla surface protease of Yersinia pestis. Adv Exp Med Biol 603, 268–278 10.1007/978-0-387-72124-8_24 [DOI] [PubMed] [Google Scholar]
- Ulett G. C., Mabbett A. N., Fung K. C., Webb R. I., Schembri M. A. (2007a). The role of F9 fimbriae of uropathogenic Escherichia coli in biofilm formation. Microbiology 153, 2321–2331 10.1099/mic.0.2006/004648-0 [DOI] [PubMed] [Google Scholar]
- Ulett G. C., Valle J., Beloin C., Sherlock O., Ghigo J.-M., Schembri M. A. (2007b). Functional analysis of antigen 43 in uropathogenic Escherichia coli reveals a role in long-term persistence in the urinary tract. Infect Immun 75, 3233–3244 10.1128/IAI.01952-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle J., Mabbett A. N., Ulett G. C., Toledo-Arana A., Wecker K., Totsika M., Schembri M. A., Ghigo J.-M., Beloin C. (2008). UpaG, a new member of the trimeric autotransporter family of adhesins in uropathogenic Escherichia coli. J Bacteriol 190, 4147–4161 10.1128/JB.00122-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viboud G. I., Bliska J. B. (2005). Yersinia outer proteins: role in modulation of host cell signaling responses and pathogenesis. Annu Rev Microbiol 59, 69–89 10.1146/annurev.micro.59.030804.121320 [DOI] [PubMed] [Google Scholar]
- Walker K. A., Miller V. L. (2004). Regulation of the Ysa type III secretion system of Yersinia enterocolitica by YsaE/SycB and YsrS/YsrR. J Bacteriol 186, 4056–4066 10.1128/JB.186.13.4056-4066.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Preston J. F., III, Romeo T. (2004). The pgaABCD locus of Escherichia coli promotes the synthesis of a polysaccharide adhesin required for biofilm formation. J Bacteriol 186, 2724–2734 10.1128/JB.186.9.2724-2734.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren B. W. (2003). The yersiniae – a model genus to study the rapid evolution of bacterial pathogens. Nat Rev Microbiol 1, 55–64 10.1038/nrmicro730 [DOI] [PubMed] [Google Scholar]
- Zdobnov E. M., Apweiler R. (2001). InterProScan – an integration platform for the signature-recognition methods in InterPro. Bioinformatics 17, 847–848 10.1093/bioinformatics/17.9.847 [DOI] [PubMed] [Google Scholar]