Abstract
Laminarialean species (so-called kelps) are the largest photosynthetic organisms in aquatic environments, constituting significant ecological components of coastal ecosystems. The largest kelps such as Macrocystis exhibit differentiation between stipe and blade, as well as buoyancy to maintain the distal portion at the water's surface for photosynthesis, while bearing reproductive structures only near the base on special blades (sporophylls). There is a considerable gap between basic kelps such as Chorda and derived kelps, and the evolution of kelp specialization remains unclear. Here we report novel reproductive adaptations in the recently discovered species Aureophycus aleuticus; unlike any known kelps, A. aleuticus forms zoidangia only on the expanded, disc-shaped holdfast. Molecular phylogeny suggests that A. aleuticus is most basal among derived kelps. Because Aureophycus lacks any of the elaborate anatomical structures found in other derived kelps, we suggest that it exhibits some of the most ancestral morphological features of kelps.
Laminarialean species (so-called kelps) are the largest photosynthetic organisms in aquatic environments, sometimes exceeding 50 m in length, and constitute a significant ecological element of coastal ecosystems in temperate and colder seas, providing habitat for diverse plants and animals1,2,3. They are the most structurally complex seaweeds, and the largest kelps such as Macrocystis exhibit differentiation between stipe and blade, as well as buoyancy to maintain the distal portion at the water's surface for photosynthesis, while bearing reproductive structures only near the base on special blades (sporophylls)1,3. There is a considerable gap between basic (so-called ‘primitive') kelps such as Chorda and derived (‘advanced') kelps such as Laminaria and Macrocystis (Laminariaceae, etc.), but due to the lack of any significant fossil record and incomplete molecular phylogeny, the evolution of kelp specialization remains unclear4,5.
The distinctive new laminarialean species Aureophycus aleuticus has been described from Kagamil Island in the Aleutian Islands6. The discovery of such a large, unique kelp species was surprising because it was believed that the genus or family level diversity of kelps was well-understood. Consequently, the discovery of this species was a highlight of the Census of Marine Life project7. However, because of the limited resolution of the molecular phylogeny by rbcL, rDNA ITS, and nad6 sequences, and because reproductive structures remained unknown, the phylogenetic position of Aureophycus in the derived Laminariales (Alariaceae, Costariaceae, Laminariaceae and Lessoniaceae) has remained unclear6.
Fortunately, a second locality of the species has been found at St. George Island in the Bering Sea, ca. 500 km from the only previously known locality at Kagamil Is. (Supplementary Information 1). Therefore, we carried out additional molecular phylogenetic analyses using 8 chloroplast and mitochondrial gene sequences to elucidate the phylogenetic relationship of the species with other kelp families, and made seasonal collections of the species to discover the reproductive structures.
Results
Molecular phylogeny
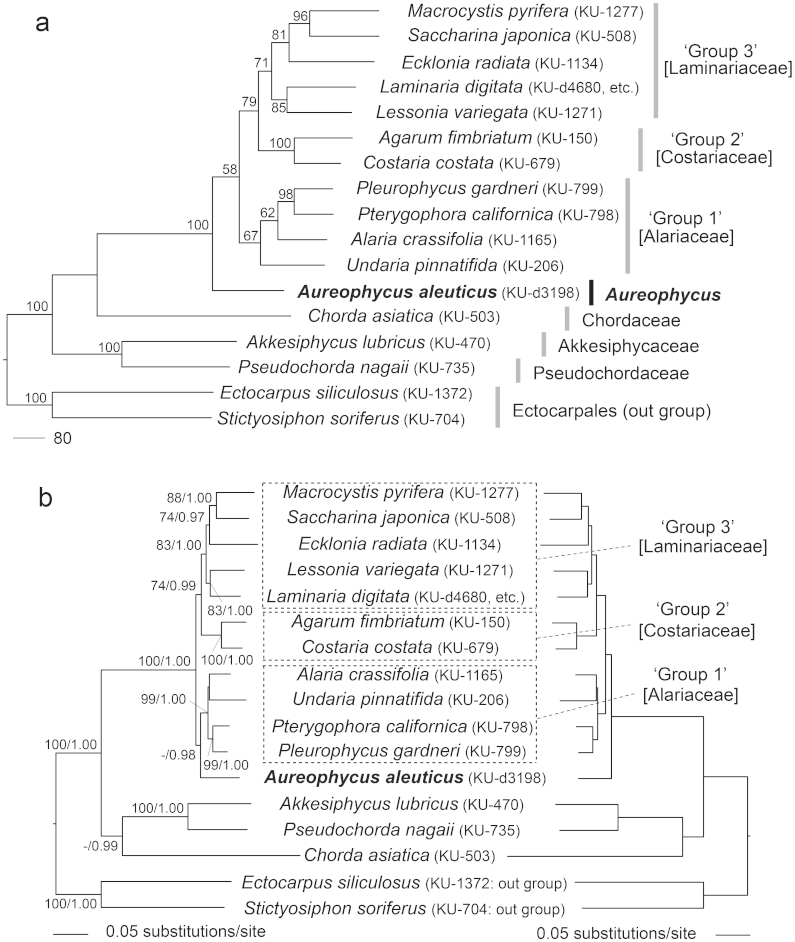
In the MP (Maximum Parsimony) molecular phylogeny using concatenated DNA sequences of chloroplast rbcL, atpB, psaA, psaB, psbA, psbC and mitochondrial cox1 and cox3 genes (Fig. 1a), derived laminarialean taxa including A. aleuticus having differentiated blade and stipe were sister to Chordaceae, basic members of Laminariales having simple terete thalli. Within derived Laminariales (excluding Akkesiphycaceae, Pseudochordaceae and Chordaceae), A. aleuticus branched first, supported by a high bootstrap value. The other derived Laminariales formed two or three groups corresponding to ‘Group 1 to 3' in Lane et al. (2006)4. In ML (Maximum Likelihood) analyses, two slightly different topologies were equally supported (Fig. 1b): A. aleuticus was basal to all other derived Laminariales as in the MP tree, or A. aleuticus was basal to one of the two large clades (‘Group 1' corresponding to Alariaceae) of derived Laminariales, and the clade including Aureophycus and ‘Group 1' was sister to the clade of ‘Group 2' and ‘Group 3'. In Bayesian analyses, the tree topology was the same as the one in ML analyses in which Aureophycus was basal to the clade of ‘Group 1' (data not shown).
Figure 1. Molecular phylogeny of representative laminarialean species including Aureophycus aleuticus based on the concatenated DNA sequences of chloroplast rbcL, atpB, psaA, psaB, psbA, psbC and mitochondrial cox1 and cox3 genes.
(a), Maximum Parsimony (MP) tree. Only bootstrap values > 50% and posterior probabilities > 0.90 are shown. (b), Maximum Likelihood (ML) trees without constraint (left) and with constraint (right). Likelihood score: -40964.19 (left), -40969.69 (right). Topology test results (P-values): 0.23301 (SH test), 0.19381 (AU test). ‘-' means < 50 and ‘*' means full statistical support (i.e. 100% or 1).
Molecular phylogenetic trees based on the eight independent genes (i.e., chloroplast rbcL, atpB, psaA, psaB, psbA, psbC and mitochondrial cox1 and cox3 genes) mostly supported monophyly of the derived Laminariales including Aureophycus both in MP and ML analyses (Supplementary Information 4). Exceptionally in the rbcL data set, Aureophycus grouped with Pterygophora and Pleurophycus, but the statistical support for the node was low. There were some differences in the topologies of the branches connecting the derived taxa. The robustness of the trees was somewhat different depending on the genes, perhaps because of the difference in the gene resolutions for elucidating the genus level relationships due to different evolutionary rate (Supplementary Information 5). Among the genes in ML analyses, psaB and psbC trees included 7 and 6 nodes with strong bootstrap supports (>90%), whereas fewer robust nodes were included in the data sets of the other genes. The tree topologies in the analyses using concatenated sequences of 7 genes (excluding psbA gene which showed lowest pairwise p-distance (Supplementary Information 5) and suggested the phylogenetic position of Aureophycus basal to basic Laminariales (Supplementary Information 4) were essentially the same as those based on 8 genes (Supplementary Information 6).
Morphological studies
The thalli of A. aleuticus are composed of a basal system (discoidal, semi-cushion-shaped holdfast), flattened stipe, and simple blade (Fig. 2a). In young thalli the basal system first expands unilaterally and then circumferentially to eventually encircle the base of the stipe, which is unique in Laminariales (Fig. 2b). The entire bottom surface of the basal system is at first tightly attached to the substrate. But as the basal system expands and competes with the basal systems of neighboring individuals, it becomes partly free from the substrate at its margin, and several basal systems often overlap (Fig. 2c). The stipe is 0.5–1.0 m in length, solid and very strong. The blade is lanceolate, smooth and flat, up to ca. 2 m in length, 0.15–0.50 m in width in the broadest part, medium to dark brown, and relatively thin. It lacks a midrib, but towards the base of the blade the edges are thickened and appear as a conspicuous golden yellow V-shape in submerged blades (Fig. 2a). No hairs or mucilaginous structures (ducts or gland cells), common in derived Laminariales, were found within the blade, even in old thalli. Trumpet-shaped hyphae, functionally comparable to phloem in land plants, are found throughout the blade. No reproductive cells or sporophylls were observed on the blades or stipes, even on fertile thalli forming sori on the basal system.
Figure 2. Habit and morphology of Aureophycus aleuticus (St. George Is., 24 October 2012).
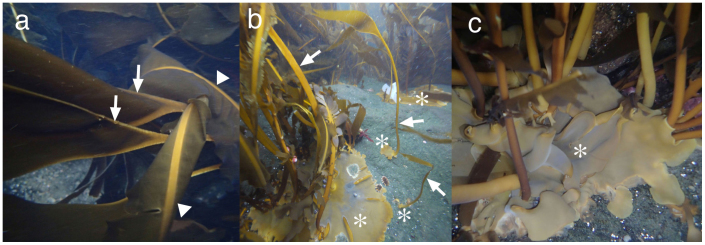
(a), erect part of sporophyte with characteristic marginal thickening at the transitional zone between blade and stipe (arrows). Arrowheads show midribs of Alaria sp., growing mixed with Aureophycus. (b), Sporophytes of different developmental stages. Arrows show flattened stipes and asterisks show basal systems (discoidal holdfast forming sorus). Note that the basal systems show unilateral development in the early stages, and the iridescent color of the basal systems including those of rather young thalli showing signs of sorus formation. (c), developed basal systems overlapping each other, with iridescent color on the surface (asterisk) showing sorus formation. [Photographs by H. Kawai].
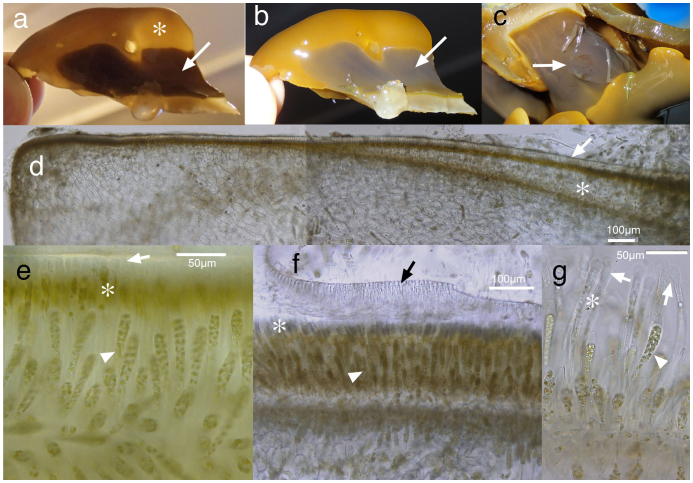
The basal system is golden yellow when vegetative, but appears milky, purplish, and iridescent underwater in fertile portions where zoidangial (sporangial) sori develop (Fig. 2c). The iridescent portions appear darker when emersed (Fig. 3a,b). The surface of the basal system is covered with a cuticle 3–4 μm in thickness (Fig. 3c,d). When mature, paraphyses (protective cells of zoidangia) develop from the peripheral cells and push the cuticle upward (Fig. 3d) as the sorus develops. As paraphyses elongate, the slender, sparsely-pigmented lower parts appear as a clear zone, and unilocular zoidangia develop here among the paraphyses (Fig. 3e). At the tip of each paraphysis a mucilaginous cap expands and pushes the cuticle upward, detaching it from the paraphyses, and it eventually peels away (Fig. 3f,g). Unilocular zoidangia are lanceolate, up to ca. 100 μm long, provided with a releasing structure (thickening of the wall) at the tip, and contain 32 zoids without eyespots (Fig. 3g). Released zoids showed some tendency to swim upwards, but no marked taxis was noticed.
Figure 3. Morphology and anatomy of the basal system of Aureophycus aleuticus (St. George Is., 22–24 October 2012).
(a, b), sorus under transmitted (a) and strong epi-illumination showing iridescence of sorus (b). Note the difference between vegetative portion (asterisk) and sorus (arrow). (c), fertile sorus showing partly detached cuticle (arrow). (d), cross section of basal system (discoid holdfast) showing the development of sorus. Note that the space between cuticle (arrow) and original peripheral layer becomes thicker toward the right side of the section (asterisk) by the development of paraphyses; (e, f), detachment of cuticle (arrow) by the expansion of mucilaginous caps (appendages) at the tips of paraphyses (asterisk). Arrowhead shows unilocular zoidangia. (g), unilocular zoidangium (arrowhead), paraphysis (asterisk) and mucilaginous cap (arrows). [Photographs by H. Kawai].
In culture, zoids developed into sexually dimorphic dioecious gametophytes (Fig. 4a), and fertilized eggs (zygotes) developed into young sporophytes (Fig. 4b,c), in a similar manner as other derived Laminariales.
Figure 4. Gametophytes and young sporophytes of Aureophycus aleuticus.

(a), vegetative female gametophyte (left, asterisk) and fertile male gametophyte (right). Arrow shows antheridium. (b), Fertile female gametophyte (asterisk) and young sporophyte (embryos). Arrow shows zygote. (c), Fertile gametophyte (asterisk) with young sporophyte (arrow). [Photographs by H. Kawai].
Discussion
Since its discovery in 2006 and its description as the novel kelp species Aureophycus aleuticus6, the reproductive structures of this species have remained unknown. No sign of sporophytic reproductive structures was found on the well-developed thalli of the summer collections at Kagamil Island, Aleutian Islands. Although it was assumed that sori of unilocular zoidangia were likely to form somewhere on the thalli, as in all other derived kelp species, because of the unique thallus morphology it was difficult to guess the locus of sori formation, including the presence or absence of sporophylls6. Since the species was considered to be annual, and its juvenile thalli were also found near the well-developed thalli, it was expected that the sporophytes form reproductive structures in later seasons (autumn to winter), with gametophytes developed from them becoming fertile during winter to spring, to form the next sporophytic generation. However, because of the difficulty in accessing the remote habitat, the question remained unanswered.
Fortunately, a second locality was found at St. George Is. and it became possible to collect specimens in later seasons. In the seasonal collections, no reproductive structures were found from July to September, but finally in the October collections unique reproductive structures formed on the basal system were found. No sori were found on the blades, and no separate sporophylls have been found on the stipes even on the thalli with zoidangia on the basal system, and we concluded that the sori on the basal system were the sole reproductive structures in Aureophycus sporophyte. The anatomy of the sori was similar to those found in advanced kelps on the blades or on sporophylls, but the locus of sorus formation is unique in Laminariales and any other brown algae.
In contrast to the well-demarcated taxonomy at the species level, the phylogeny of kelps remains confused. Traditionally six families have been recognized in the order; three for basic members (Akkesiphycaceae8, Pseudochordaceae9 and Chordaceae) and three for derived members (Alariaceae, Laminariaceae and Lessoniaceae). Chordaceae, sister of derived families, have terete thalli (Fig. 5a). Derived families have differentiation between stipe and flattened blades (Fig. 5c,d). Members of the Laminariaceae generally have the simplest external morphology, with an unbranched stipe and tough blade (Fig. 5c), whereas members of the Alariaceae have a relatively thin blade supported by a midrib (Fig. 5d), and the Lessoniaceae have more elaborate thalli with branched stipes and buoyant gas-bladders. However, molecular phylogeny has not supported the traditional familial taxonomy, and a new family Costariaceae was suggested4. As to phylogeography, Laminariales are thought to have originated in the Northwestern Pacific and spread eastward10,11, diverging into the more elaborate morphologies of the Alariaceae and Lessoniaceae during this expansion. The finding that Aureophycus is one of the most basic members of derived Laminariales provides a significant clue for elucidating the phylogeography of Laminariales, especially the evolution and eastward spread of derived kelps from the NW Pacific to the NE Pacific through the Aleutian Islands.
Figure 5. Schematic presentation of basic morphology of different kelp species focusing the basic thallus structure and formation of reproductive cells.
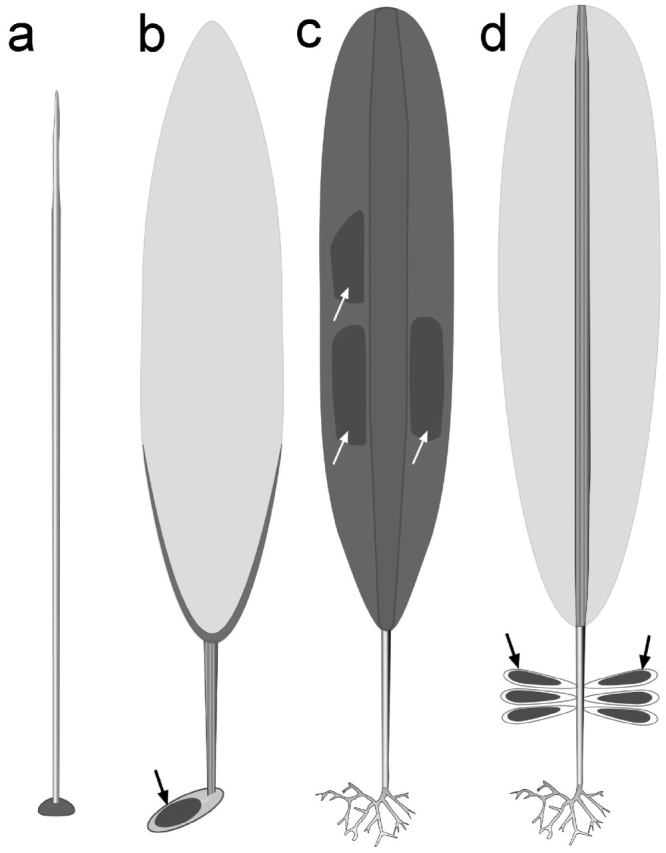
(a), Pseudochordaceae and Chordaceae having terete erect thallus and small discoidal holdfast. Unilocular zoidangia are formed on the entire surface of the erect thallus. (b), Aureophycus aleuticus. Erect thallus has characteristic flat stipe and thickened portion at the transitional zone to the blade. Sorus of unilocular zoidangia (arrow) is formed on the well-developed holdfast (basal system) of unilateral growth at an early stage of development. (c), Laminariaceae, erect thallus has thick blade and forms sorus on the blades (arrows). Most members, especially those with large erect thallus, have an epidermal rhizoidal holdfast. (d), Alariaceae, erect thallus with thin blade supported by thick midrib. Sori (arrows) are formed on the blade or on specialized blades near the base (sporophylls). [Illustration by H. Kawai].
Derived kelps share several anatomical features of the reproductive structures of the sporophytes: The unilocular zoidangia, each developing with an elongated protective cell (paraphysis), are aggregated into sori, whereas they are formed on the entire surface of the thalli in basic members8,9 (Fig. 5a). In the morphologically simple derived kelp species the sori are formed on the surface of the single blade (Fig. 4c, e.g., Laminaria, Costaria, etc.), but in more complex forms sori develop on special blades called sporophylls (Figure 4d, e.g., Alaria). For example, Macrocystis can form very large sporophytes exceeding 60 m in length and grows as deep as 20–25 m. At such depths, where the microscopic filamentous gametophytes grow and basal parts of the sporophytes are attached, the light intensity can be very low2. Although zoids of most marine macroalgae have photo-orientation mechanisms, the zoids of derived kelps are unique among brown algae in lacking phototaxis12; it is therefore significant that many of the largest kelp species, especially those with buoyant thalli such as Macrocystis and Alaria, have special mechanisms to reduce the long-distance dispersal of their zoids by forming sporophylls lacking buoyancy at the basal part of the stipe13. These features clearly contrast with those in basic members, which form zoidangia on the entire erect thalli and have eyespot and phototaxis: they have relatively small thalli and their habitats are restricted to shallower and clearer waters. Furthermore, in derived kelps, development of an epidermal rhizoidal holdfast, buoyancy (pneumatocysts, inflation of stipe), midrib of the erect thalli, and branching of stipes have evolved (Supplementary Information 7, 8). It is noteworthy that accompanying such evolutionary trends, forming larger sporophytes adapted to deeper habitats, sexually dimorphic gametophytes, oogamy, and loss of phototaxis in male gametes have occurred (Supplementary Information 7, 8).
In speculating about the morphology of ancestral derived kelps, the unique sporophyte morphology of Aureophycus, especially the unilateral development of the basal system, exclusively basal formation of sori, and the simple blade supported by a flattened stipe, may provide significant clues. Traditionally, two types of thallus structures have been distinguished in derived kelps: Species like Laminaria spp. have tough blades throughout their length composed of extensively-developed cortical cells, and species like Alaria spp. have thin membranous blades supported by midribs. Evolutionarily, the first type corresponds to the lineages of the families Laminariaceae/Lessoniaceae (‘Group 3' in Lane et al. (2006)) and Costariaceae (‘Group 2'), and the latter corresponds to Alariaceae (‘Group 1')4. Aureophycus exhibits a third type of thallus structure, a thin membranous blade supported by thickened margins at the transition between stipe and blade. As to the phylogenetic position of Aureophycus, it is still not totally clear whether it is basal to all three clades (‘Group' 1–3) as suggested in MP and ML with constraint, or basal to ‘Group 1' and sister to the clade of ‘Group 1, 2' as suggested in ML without constraint and BI. However, the results of character mapping of representative taxonomic features on to the trees (Supplementary Information 7, 8) were favorable to the former scenario suggested in the trees of MP and one of the ML (with constraint) analyses, because the unique evolution of the distinctive features of derived Laminariales such as trumpet-shaped hyphae and intercalary (localized) meristem were better supported in the latter scenario.
In either case of the phylogenetic position of Aureophycus in Laminariales, there is a considerable morphological gap (difference) between the derived kelps including Aureophycus and their sister group Chordaceae, which retains more basic features, and it is difficult to speculate about the ancient morphology of their common ancestors. Chordaceae grow in relatively protected habitats on unstable substrates, whereas most derived kelps grow on more exposed rocky shores, and have well-developed holdfasts to resist detachment by wave action and tougher thalli that withstand strong wave action and desiccation.
Small discoidal holdfasts are apparently ancestral to rhizoidal holdfasts formed by epidermal elaboration, because all basic taxa (i.e., Akkesiphycaceae, Pseudochordaceae and Chordaceae) have small discoidal holdfasts. On the other hand, derived taxa basically have epidermal rhizoids. Some taxa in Laminaria (e.g., L. yezonesis, L. ephemera) and Lessonia (e.g., L. nigrescens) also have discoidal holdfasts (Supplementary Information 9), however, they are considered to have secondarily evolved from rhizoidal holdfasts, because their sister taxa have rhizoidal holdfasts or they form rhizoidal structures in their early developmental stages. The molecular phylogeny of Aureophycus at the most basal position of the derived kelps is consistent with this notion. It is noteworthy that all very large, buoyant kelps (e.g., Macrocystis, Alaria and Nereocystis) have epidermal rhizoidal holdfasts. This may be because they are more effective than discoidal holdfasts in competition with neighbors, and physical resistance to pulling forces by buoyancy and wave action, permitting longevity of perennial species. Therefore, ancient derived kelps are considered to have had discoidal holdfast as in Aureophycus, but in the evolution of larger sporophytes and adaptation to deeper habitats, rhizoidal holdfasts have evolved, and it became necessary to form reproductive sori on the erect thallus or on sporophylls, instead of the discoidal holdfast.
The finding that the basal system forming sori shows unilateral development may suggest that the ancestral kelps connecting Aureophycus and Chordaceae originally had a prostrate type of sporophyte, and have secondarily evolved foliose erect thalli as seen in Aureophycus. Then the erect thalli might have further developed elaborate thalli in two different manners, one by supporting the membranous blade by a midrib as in Alaria, and the other by developing tougher thalli supported by denser inner cortical filaments as in Laminaria.
As to the family-level taxonomy, Aureophycus is distinct from other laminarialean families by the following features: 1) sporophyte morphology composed of membranous blade supported by thickened margins at the transitional zone, flat stipe and large discoidal basal system; 2) formation of reproductive sori only on the basal system; 3) lack of any mucilaginous structures. Molecular phylogeny supports the independence of Aureophycus from any other families of Laminariales. Therefore, we suggest the establishment of the new family Aureophycaceae to accommodate the monotypic genus Aureophycus.
Aureophycaceae fam. nov. H. Kawai et L.M. Ridgway.
Type: Aureophycus aleuticus H. Kawai, T. Hanyuda, M. Lindeberg et S.C. Lindstrom.
Description and diagnosis
Sporophytum e lamina lanceolata, stipite complanato, et haptero discoideo constans; lamina tenuis membranacea, ad zonam transitionalem limbo incrassato litteram V elevatam formante instructa, sine undulationibus, filis tenuibus laxis necnon hyphis tubaeformibus impleta; stipes rectangularis solidus parum translucens; hapteron hemidiscoideum, cum sporangiis unilocularibus. Nec organa mucosa nec pili praesentes.
Sporophyte composed of a lanceolate blade, flattened stipe and discoidal holdfast; the blade thin, membranous, with obvious thickened rim at the transition zone forming a raised “V”, without undulations, filled with fine, loose filaments including trumpet-shaped hyphae, without mucilage organs or hairs; the stipe rectangular, solid; the holdfast semi-discoidal, forming unilocular sporangia.
The family is distinguished from related laminarialean families in having characteristic sporophyte morphology of thin blade with V-shaped thickened transition zone, flattened stipe and wide discoid holdfast forming sori with unicellular paraphyses and unilocular zoidangia.
Holotype. UBC A85831, Kagamil Island (52° 57′ 07″ N, 169° 43′ 02″ W), Island of Four Mountains, Aleutian Islands, Alaska (USA), July 15, 2006. Isotype: UBC A85832.
Known distribution. Kagamil Island, Aleutian Islands and St. George Island, Bering Sea, Alaska, U.S.A.
Methods
Sporophytes were collected at several localities on St. George Island, Pribilof Islands, Alaska, U.S.A. by snorkeling (Supplementary Information 1). Specimens were pressed on herbarium sheets on site. Portions of the sporophytes were quickly dried in silicagel and used for molecular analyses. For anatomical studies, fresh specimens were hand-sectioned and examined using a BX-50 compound microscope (Olympus, Tokyo, Japan) with Nomarski optics, and photographed using a VB-7000 digital camera (Keyence, Tokyo, Japan).
For molecular phylogenetic study, genomic DNA was extracted from the silicagel-dried algal tissue using a DNeasy Plant Mini Kit (Qiagen, Hilden, Germany), following the manufacturer's instructions. Polymerase chain reaction (PCR) amplifications of the chloroplast atpB, psaA, psaB, psbA, psbC, rbcL, mitochondrial cox1 and cox3 genes were carried out with the TaKaRa PCR Thermal Cycler Dice (Takara Shuzo, Shiga, Japan) using the KOD FX (ToYoBo, Osaka, Japan). Primers used for PCR and/or sequencing are listed in Supplementary Information 2. The profiles of PCR reactions were identical to those described previously14. After PEG purification15, PCR products were sequenced using the CE DTCS Quick Start Kit (Beckman Coulter, Fullerton, CA, USA) and the CEQ8000 DNA analysis system (Beckman Coulter) according to the manufacturer's instructions. For the molecular phylogenetic analyses, published and newly determined sequence data of the Laminariales were used (Supplementary Information 3). Ectocarpus siliculosus and Stictyosiphon soriferus (Ectocarpales, which is sister of Laminariales) were chosen as the outgroups. Alignments were prepared using the Clustal X computer program16 and then manually adjusted prior to phylogenetic analyses.
Concatenated DNA sequences (8 genes, total 10,583 bp) as well as independent sequences of the 8 genes were subjected to maximum parsimony (MP), maximum likelihood (ML). The MP tree was constructed in MEGA 517 using Closest Neighbor Interchange (CNI) with a search level of 1, and initial trees by random addition (10,000 reps). With the aid of the Kakusan4 program18, the best-fit evolutionary model for each codon position of each gene was determined by comparing different evolutionary models via the corrected Akaike Information Criterion19 for ML analysis, and the Bayesian Information Criterion20 for the Bayesian analysis. The ML analysis was performed by the likelihood-ratchet method21, implemented in Phylogears 2.0.2010.08.3122. Two hundred sets of 25% site-upweighted data were created using the ‘pgresampleseq' command in Phylogears22, and trees based on the upweighted data were constructed using Treefinder23 under the maximum likelihood criterion. After eliminating redundant trees from the calculation, the ML tree of the original data was inferred by Treefinder with the best-fit substitution model and by using the trees of upweighted data mentioned above as starting trees. The robustness of the resulting phylogenies was tested by bootstrap analyses with 1,000 (MP) and 200 (ML) resamplings24. Bayesian analysis with the selected evolutionary models was done using MrBayes v3.2.125. The Bayesian analysis was initiated with a random starting tree and ran four chains of Markov chain Monte Carlo iterations simultaneously for 8,000,000 generations, keeping one tree every 100 generations. The first 8,000 trees sampled were discarded as ‘burn-in', based on the stationarity of ln L as assessed using Tracer version 1.4.126; a consensus topology and posterior probability values were calculated from the remaining trees.
Shimodaira–Hasegawa tests27 (SH test) and approximately unbiased tests28 (AU; Shimodaira 2002) were performed with Treefinder to test the tree topologies. The ML tree with constraint (Aureophycus aleuticus is sister taxa to the clade including three groups, Alariaceae, Costariaceae, and Laminariaceae) were obtained as mentioned above, and statistically compared with the ML tree without constraint.
Cultures were started from the fertile basal system collected on 28 October 2012. Released zoids were cultured in plastic Petri-dishes containing 100 mL PESI medium29. The sets of culture conditions used were 5°C short day (SD; 8:16 h light : dark), 5°C long day (LD; 16:8 h light:dark), and 10°C LD, under cool-white-type fluorescent illumination of approximately 30 μmol·m−2 ·s−1. Unialgal clonal culture strains of A. aleuticus are deposited in KU-MACC (Kobe University Macroalgal Culture Collection; male gametophyte, KU-3187; female gametophyte, KU-3186).
Author Contributions
L.M.R. found new distribution of the species, K.H. studied the phenology by seasonal observations, T.H. did molecular phylogeny, H.K. found new reproductive structures with L.M.R. and did morphological observations, molecular phylogeny, culture experiments and wrote the paper.
Supplementary Material
Supplementary Information 1-9
Acknowledgments
We thank Dr. E. C. Henry for critical discussions and reading the manuscript; the local government of St. George Island, Alaska, USA for support of field collections; NSF grant DEB-0212138 (PI: R.A. Andersen), and JSPS Grants-in-Aid for Scientific Research (No.22370034) to H. K.
References
- Bold H. C. & Wynne M. J. Introduction to the Algae: Structure and reproduction. 2nd ed. Prentice-Hall, Englewood Cliffs, NJ, USA, 720 pp. (1985). [Google Scholar]
- Dayton P. K. Ecology of kelp communities. Ann. Rev. Ecol. Syst. 16, 215–245 (1985). [Google Scholar]
- Graham L. E. & Wilcox L. W. Algae. Prentice-Hall, London 640 pp. (2000). [Google Scholar]
- Lane C. E., Mayes C., Druehl L. D. & Saunders G. W. A multi-gene molecular investigation of the kelp (Laminariales, Phaeophyceae) supports substantial taxonomic re-organization. J. Phycol. 42, 493–512 (2006). [Google Scholar]
- Silberfeld T. et al. A multi-locus time-calibrated phylogeny of the brown algae (Heterokonta, Ochrophyta, Phaeophyceae): Investigating the evolutionary nature of the “brown algal crown radiation”. Mol. Phylogenet. Evol. 56, 659–674 (2010). [DOI] [PubMed] [Google Scholar]
- Kawai H., Hanyuda T., Lindeberg M. & Lindstrom S. C. Morphology and molecular phylogeny of Aureophycus aleuticus gen. et sp. nov. (Laminariales, Phaeophyceae) from the Aleutian Islands. J. Phycol. 44, 1013–1021 (2008). [DOI] [PubMed] [Google Scholar]
- Census of Marine Life. First Census of Marine Life 2010. Highlights of a decade of discovery. 64 pp. Eds. Ausubel, J. H., Crist, D. T. & Waggoner, P. E. http://www.coml.org/pressreleases/census2010/PDF/Highlights-2010-Report-Low-Res.pdf (2010) [Last accessed on 24 June 2013].
- Kawai H. Life history and systematic position of Akkesiphycus lubricus (Phaeophyceae). J. Phycol. 22, 286–291 (1986). [Google Scholar]
- Kawai H. & Kurogi M. On the life history of Pseudochorda nagaii (Pseudochordaceae fam. nov.) and its transfer from Chordariales to Laminariales (Phaeophyta). Phycologia 24, 289–296 (1985). [Google Scholar]
- Lüning K. & Tom Dieck I. The distribution and evolution of the Laminariales: North Pacific–Atrantic relationships. In: NATO ASI Series G22. Evolutionary biogeography of the marine algae of the North Atlantic (Ed. by Garbary, D. J. & South, G. R.), pp. 187–204. Springer Verlag, Berlin (1990). [Google Scholar]
- Sasaki H. & Kawai H. Taxonomic revision of the genus Chorda (Chordaceae, Laminariales) based on sporophyte anatomy and molecular phylogeny. Phycologia 46, 10–21 (2007). [Google Scholar]
- Kawai H. Green flagellar autofluorescence in brown algal swarmers and their phototactic responses. Bot. Mag. Tokyo. 105, 171–184 (1992). [Google Scholar]
- Abbott I. A. & Hollenberg G. J. Marine Algae of California. Stanford University Press, California, xii + 872 pp (1976). [Google Scholar]
- Kawai H., Hanyuda T., Draisma S. G. A. & Müller D. G. Molecular phylogeny of Discosporangium mesarthrocarpum (Phaeophyceae) with a reassessment of the Discosporangiales. J. Phycol. 43, 186–94 (2007). [Google Scholar]
- Lis J. T. Fractionation of DNA Fragments by Polyethylene Glycol Induced Precipitation. Methods in Enzymology 65, 347–353 (1980). [DOI] [PubMed] [Google Scholar]
- Thompson J. D., Gibson T. J., Plewniak F., Jeanmougin F. & Higgins D. G. The Clustal X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucl. Acids Res. 24, 4876–4882 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K. et al. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 28, 2731–2739 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe A. S. Kakusan4 and Aminosan: two programs for comparing nonpartitioned, proportional and separate models for combined molecular phylogenetic analyses of multilocus sequence data. Mol. Ecol. Res. 11, 914–921 (2011). [DOI] [PubMed] [Google Scholar]
- Akaike H. A new look at the statistical model identification. IEEE Trans. Autom. Control 19, 716–723 (1974). [Google Scholar]
- Schwarz G. Estimating the dimension of a model. Ann. Stat. 6, 461–464 (1978). [Google Scholar]
- Vos R. A. Accelerated likelihood surface exploration: the likelihood ratchet. Syst. Biol. 52, 368–373 (2003). [DOI] [PubMed] [Google Scholar]
- Tanabe A. S. Phylogears Version 2.0.2010.08.31. Software distributed by the author. Available from URL: http://www.fifthdimension.jp/(2010). [Last accessed on 24 June 2013].
- Jobb G., von Haeseler A. & Strimmer K. TREEFINDER: a powerful graphical analysis environment for molecular phylogenetics. BMC Evol. Biol. 4, 18 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39, 783–791 (1985). [DOI] [PubMed] [Google Scholar]
- Ronquist F. & Huelsenbeck J. P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574 (2003). [DOI] [PubMed] [Google Scholar]
- Rambaut A. & Drummond A. J. Tracer v.1.5. Available from URL: http://tree.bio.ed.ac.uk/software/tracer/(2009). [Last accessed on 24 June 2013].
- Shimodaira H. & Hasegawa M. Multiple comparisons of loglikelihoods with applications to phylogenetic inference. Mol. Biol. Evol. 16, 1114–1116 (1999). [Google Scholar]
- Shimodaira H. An approximately unbiased test of phylogenetic tree selection. Syst. Biol. 51, 492–508 (2002). [DOI] [PubMed] [Google Scholar]
- Tatewaki M. Formation of crustaceous sporophyte with unilocular sporangia in Scytosiphon lomentaria. Phycologia 6, 62–66 (1966). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information 1-9