Abstract
Background:
The aim of our study was to evaluate the prognostic role of immunological microenvironnement in stage II–III CRC patients.
Methods:
We constructed a tissue microarray from 196 consecutive patients with stage II–III CRC and compared CD3, CD4, CD8, CD57, CD68, CXCL9/MIG, CXCL13, and PPARγ immunoreactivity in tumour samples and their matched non-tumour tissue. We assessed their association with relapse-free survival (RFS; primary endpoint) and overall survival (OS) in multivariate Cox models.
Results:
Low densities of CD57+ and CD68+ tumour-infiltrating cells (TIC) independently predicted worse outcomes. A prognostic score combining CD57 (+, > vs −, ⩽2 cells per spot) and CD68 (+, >0 vs −, =0 cells per spot) TIC density discriminated CRC patients at low (CD68+/CD57+), intermediate (CD68+/CD57−), or high (CD68−/CD57−) risk, with hazard ratios for the intermediate-risk and high-risk groups of 2.7 (95% confidence interval (CI): 1.3–5.8) and 9.0 (3.2–25.4) for RFS, and 2.5 (1.2–5.1) and 10.6 (3.8–29.2) for OS, respectively, as compared with the low-risk group. Corresponding 5-year survival rates (95% CI) in the low-, moderate- and high-risk groups were 84% (71–91), 65% (54–74), and 12% (2–47), respectively, for RFS, and 91% (80–96), 76% (66–84), and 25% (7–59), respectively, for OS.
Conclusion:
Tumour CD57+ and CD68+ TIC density assessment independently predicts survival in patients with stage II–III CRC. If validated, our score based on a quick, inexpensive, and well-established method such as point counting on diagnostic tissue sections could be used routinely as a prognostic tool in CRC patients.
Keywords: colorectal cancer, prognosis, biomarker, tumour-infiltrating lymphocyte, tumour-infiltrating macrophage
Despite advances in screening, diagnosis, and treatment, colorectal cancer (CRC) is the third leading cause of cancer-related mortality (Greenlee et al, 2000). Pathological staging is currently the most accurate predictor of CRC prognosis and is used in clinical practice to select patients for adjuvant chemotherapy—more specifically, those with stage II (node-negative) CRC and stage III (node-positive) CRC, patients with stage I disease not deriving benefit from adjuvant chemotherapy due to an excellent prognosis, and those with stage IV disease being already metastatic (AJCC, 2010). However, 10–40% of patients with stage II (node-negative) CRC and 20–80% of those with stage III (node-positive) CRC develop recurrence after curative-intent (R0) surgical resection (AJCC, 2010). Several molecular markers have been shown to predict the likelihood of recurrence in stage II and III CRC, including microsatellite instability (deficient mismatch repair (dMMR)) and allelic imbalances (for example, loss of 8p and 18q chromosomal arms) (Halling et al, 1999; Gryfe et al, 2000; Zhou et al, 2002). However, only the dMMR tumour phenotype remained a significant prognostic factor in early CRC both in a meta-analysis and prospective trials (Popat et al, 2005; Hutchins et al, 2011). Recently, Pages et al (2005) showed that the absence of an immune response within primary colonic cancer (CC) (stage I–IV disease) was associated with early metastatic invasion and worse survival, and that the density of CD3+ CD45RO+ tumour-infiltrating lymphocytes (TIL) was a better predictor of outcome than pathological staging according to the Union Internationale Contre le Cancer (UICC) tumour–node–metastasis (TNM) system (Galon et al, 2006). By contrast, Laghi et al (2009) showed that CD3+ TIL density was prognostic in stage II, but not in stage III CRC. Furthermore, apart from T-cell markers, other tumour immune markers related to macrophages, NK cells, or chemokines have also been found to predict outcome in CRC. However, these putative immune prognostic markers have not been assessed simultaneously in the same patient cohort (Coca et al, 1997; Pages et al, 2005; Galon et al, 2006; Forssell et al, 2007; Hojo et al, 2007; Laghi et al, 2009; Ogino et al, 2009; Ferdinande et al, 2011; Mlecnik et al, 2011a). In the present study, we aimed to evaluate the prognostic value of immune tumour-infiltrating cells (TIC) and chemokines in stage II and III CRC patients. To assess simultaneously on the same patients the prognostic value of multiple immune parameters, we used a tissue microarray (TMA) analysis.
Materials and Methods
Patients
Patients with CRC who underwent primary surgery at our institution between 1990 and 2000 were selected according to the following criteria: curative-intent resection (negative (R0) surgical margin status) for pathologically confirmed colorectal adenocarcinoma, UICC TNM stage II or III tumour (AJCC, 2010), no preoperative chemotherapy, no family history of Lynch syndrome or adenomatous polyposis, and postoperative follow-up of at least 2 years. As increased numbers of TIL represent one of the particular pathological features of CRC with dMMR phenotype (Jass et al, 1998; Laghi et al, 2009), patients with dMMR CRC as defined by molecular analysis or immunohistochemistry (IHC) (Suraweera et al, 2002; Hampel et al, 2005) were excluded.
Tumour stage (TNM) and differentiation, vascular emboli, lymphatic invasion, and perineural invasion (VELIPI) (Pages et al, 2005) were determined from the histopathological reports obtained at the time of the resection.
Construction of TMAs
Paired, paraffin-embedded tumour (TT) and normal (NT) tissues from the whole patient population were used for the construction of TMA (product MTA1; Beecher Instruments, Sun Prairie, WI, USA). Three core biopsy samples measuring 0.6 mm in diameter (0.3 mm2 per spot) from paraffin-embedded TT (centre of the tumour) and the NT counterpart blocks were arrayed into a recipient paraffin block (35 × 22 × 5 mm3). Tissue microarray containing the tissue cores was then cut into 4-μm sections for hematoxylin and IHC staining.
Immunohistochemistry
The sections were affixed to Superfrost Plus slides (CML, Nemours, France), deparaffinised, and rehydrated. Antigen retrieval, consisting of microwave processing at 750 W and 150 W for 15 minutes each and pressure cooking in 0.01 M citrate buffer (pH 7.3), was applied. Dako Cytomation peroxidase blocking reagent (Dako, Les Ulis, France) was used to suppress non-specific staining due to endogenous peroxidase activity.
On the basis of previous published data on tumour immunity, immune cell recruitment and/or inflammation status of tumour environment, and the prognostic role of immune response in CRC, the following markers were selected for IHC analysis on TMA: CD3 (Pages et al, 2005; Galon et al, 2006; Laghi et al, 2009; Pages et al, 2009; Mlecnik et al, 2011b), CD68 (Ohtani et al, 1997; Funada et al, 2003; Forssell et al, 2007; Algars et al, 2011; Zhou et al, 2011), CD57 (Coca et al, 1997; Jonges et al, 2001; Menon et al, 2004), CD8 (Pages et al, 2005, 2009), CD4 (Diederichsen et al, 2003; Hojo et al, 2007) in order to quantify macrophage and lymphocyte cell infiltration as effectors of IFN-γ production. In addition, the following chemokines known to be regulated by IFN-γ pathway were analysed: CXCL9/MIG (Mlecnik et al, 2011b), CXCL10/IP-10 (Mlecnik et al, 2011b), CXCL13/BCA1 (Lin et al, 2007; Agesen et al, 2012), Conversely, PPARγ (Ogino et al, 2009) and IDO (Brandacher et al, 2006; Ferdinande et al, 2011) previously identified as potential prognostic markers in CC and involved in inhibition of Th1 (IFN-γ producer T cells) and Tc1 (cytotoxic T cells) were also selected. The following monoclonal antibodies were used: anti-CXCL9 (clone 49106.11, R&D systems, Lille, France) at 1 : 100 dilution, anti-CXCL13 (clone 53610, R&D systems) at 1 : 20 dilution, anti-PPARγ (clone E-8, Santa Cruz Biotechnology, Heidelberg, Germany) at 1 : 10 dilution, anti-CD4 (clone 4B12, Novocastra, Nanterre, France) at 1 : 20 dilution, anti-CD8 (clone 4B11, Dako) at 1 : 25 dilution, anti-CD57 (clone NK1, Dako) at 1 : 100 dilution, anti-CD68 (clone PGM1, Dako) at 1 : 100 dilution, and polyclonal antibody anti-CD3 (Dako) at 1 : 100 dilution. All incubations were performed at room temperature. Immunoperoxidase staining, using 3,3′-diaminobenzidine as a chromogen, was performed. Nuclei were counterstained with hematoxylin. The number of CD3-, CD4-, CD8- and CD57-positive (intra-epithelial and stromal) cells per spot was quantified by a single pathologist unaware of clinical data. Positivity for CD68 was scored quantitatively according to the number of positive stromal cells per spot: 0, no staining; 1, less or equal to 10-positive cells; 2, more than 10-positive cells. IHC staining of CXCL9, CXCL13/BCA1, and PPARγ was evaluated by semi-quantitative scores. For CXCL9, intact nuclear staining of the colonic crypts of the normal mucosa, lymphocytes, and endothelial cells was used as an internal positive control and was required for adequate evaluation. Normal immunoreactivity of the CXCL9 protein was defined as the presence of nuclear staining. The intensity of nuclear immunostaining was evaluated by the following scoring system: 0, no staining; 1, weak staining; 2, moderate staining; and 3, strong staining. Nuclear immunoreactivity for CXCL13/BCA1 was scored semi-quantitatively according to staining of stromal lymphocytes: 0, no staining; 1, few lymphocytes stained; and 2, numerous lymphocytes stained. Nuclear immunoreactivity for PPARγ was based on staining intensity and graded as 0, no staining; 1, mild-to-moderate staining; and 2, strong staining. The rounded average expression of the three core biopsy samples was used for each patient. No reliable IHC data could be obtained for CXCL10/IP-10 and IDO.
Statistical analyses
The primary endpoint of the prognostic analysis was relapse-free survival (RFS), defined as the time from CRC diagnosis to locoregional relapse, distant relapse, or death related to CRC. Patients without any of these three events were censored at the time of last follow-up or at the death time in case of death from other cause. The secondary endpoint of the prognostic analysis was overall survival (OS), defined as the time from CRC diagnosis to death whatever the cause. Patients alive at the time of last follow-up were censored at that date. RFS and OS probabilities were estimating according to the Kaplan–Meier method. Follow-up duration was estimated by the reverse Kaplan–Meier method.
The prognostic impact of each clinicopathological factor was studied in univariate analysis using logrank test. Clinicopathological factors associated with RFS or OS with a P-value lower than 0.20 were tested in multivariate analysis using Cox model.
The prognostic impact of immune markers measured within the tumour was studied. CD3-, CD4-, and CD8-positive cells were dichotomised at their median value. Despite a median value of one per spot for CD57, we chose a threshold value of >2 per spot that seemed to the pathologist more reliable for the quantification. The number of CD68-positive cells per spot was studied in three categories: no staining; less or equal to 10-positive cells; and more than 10-positive cells. CXCL9/MIG was studied in three categories: no staining; weak staining; moderate to strong staining. PPARγ and CXCL13/BCA1 were studied in three categories as they were graded, that is, for PPARγ, no staining; mild-to-moderate staining; strong staining and for CXCL13/BCA1 no staining, few lymphocytes stained, numerous lymphocytes stained. The prognostic impact of each immune marker was studied using Cox model with adjustment for clinicopathological prognostic factors previously identified. All markers with a P-value lower then 0.20 in the Cox model were analysed all together to determine their independent prognostic value.
Immune markers staining were compared between TT and NT using paired t-test for quantitative variables and Bowker's test of symmetry for qualitative staining variables.
Comparisons of markers staining between patients who received preoperative radiotherapy (preop-RT) and other patients and associations between all markers staining in TT and in NT were analysed using χ2-test or Fisher's exact test. As the number of tests realised for the latter analysis was high, Bonferroni correction was used (P⩽0.0018 as the threshold of significance instead of 0.05). All statistical tests were two-sided. Statistical analyses were performed with the SAS System, version 9.1 (SAS, Grégy-sur-Yerres, France).
Results
Patient characteristics
Characteristics of the 196 eligible patients are shown in Table 1. Adjuvant chemotherapy consisted of intravenous 5-fluorouracil and leucovorin in all the 115 (59%) patients who received adjuvant chemotherapy. The median duration of follow-up was 10 years (Interquartile range 6.8–13.3). Only 11 patients were followed less than 4 years. During follow-up, 73 recurrences and 72 deaths (cancer-related, 54) occurred. Five-year RFS and OS rates were 64% (s.e., 3.5) and 75% (s.e. 3.2), respectively. Prognostic analysis of clinicopathological factors (Cox model) identified tumour location (rectal vs colonic tumour), TNM stage (II vs III), and adjuvant chemotherapy (yes vs no) as independent predictors of RFS, and the same factors plus tumour grade for OS (Table 2).
Table 1. Patient characteristics.
| n (%) | |
|---|---|
|
Age | |
| Mean±s.d. | 61.5±11 |
| Range |
27–86 |
|
Sex | |
| Male | 108 (55) |
| Female |
88 (45) |
|
Tumour site | |
| Colon, right-sided | 51 (26) |
| Colon, left-sided | 98 (50) |
| Rectum |
47 (24) |
|
Nodal status | |
| N0 | 99 (50) |
| N1 | 54 (28) |
| N2 | 31 (16) |
| N positive (not specified) |
12 (6) |
|
Tumour grade | |
| Well to moderately differentiated | 167 (85) |
| Poorly differentiated | 20 (10) |
| Missing |
9 (5) |
|
VELIPI | |
| 0 | 67 (34) |
| 1 | 122 (62) |
| Missing |
7 (4) |
|
Tumour stage | |
| II | 99 (51) |
| III |
97 (49) |
|
Adjuvant chemotherapy | |
| 5-Fluorouracil and folinic acid | 115 (59) |
| No |
81 (41) |
|
Preoperative radiation therapy | |
| Yes | 38 (19) |
| No | 158 (81) |
Abbreviation: VELIPI=vascular emboli, lymphatic invasion and perineural invasion.
Table 2. Five-year relapse-free and overall survival according to clinicopathological factors.
| |
Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|---|
| Relapse-free survival | n | 5-Year rate | P-valuea | HR (95% CI) | P-valueb |
|
Sex | |||||
| Male | 108 | 62 | |||
| Female |
88 |
66 |
0.97 |
|
|
|
Tumour site | |||||
| Colon | 149 | 71 | 1.0 | ||
| Rectum |
47 |
40 |
<0.0001 |
2.4 (1.5–3.9) |
0.0004 |
|
Tumour grade | |||||
| Well-to-moderate differentiation | 167 | 67 | |||
| Poor differentiation |
20 |
42 |
0.012 |
|
|
|
Tumour stage | |||||
| Stage II | 99 | 73 | 1.0 | ||
| Stage III |
97 |
54 |
0.0005 |
3.5 (2.0–6.1) |
<0.0001 |
|
VELIPIc | |||||
| VELIPI no | 67 | 70 | |||
| VELIPI yes |
122 |
61 |
0.11 |
|
|
|
Adjuvant chemotherapy | |||||
| Yes | 115 | 68 | 1.0 | ||
| No |
81 |
59 |
0.37 |
0.5 (0.3–0.9) |
0.013 |
|
Preoperative radiation therapy | |||||
| Yes | 38 | 42 | |||
| No |
158 |
69 |
0.0008 |
|
|
|
Overall survival | |||||
|
Sex | |||||
| Male | 108 | 74 | |||
| Female |
88 |
76 |
0.88 |
|
|
|
Tumour site | |||||
| Colon | 149 | 80 | 1.0 | ||
| Rectum |
47 |
58 |
0.0014 |
2.0 (1.2–3.3) |
0.009 |
|
Tumour grade | |||||
| Well-to-moderate differentiation | 167 | 81 | 1.0 | ||
| Poor differentiation | 20 | 34 | 0.0001 | 2.3 (1.2–4.6) | |
| Unknown |
9 |
|
|
1.5 (0.5–4.1) |
0.045 |
|
Tumour stage | |||||
| Stage II | 99 | 82 | 1.0 | ||
| Stage III |
97 |
68 |
0.016 |
2.8 (1.6–5.0) |
0.0004 |
|
VELIPIc | |||||
| VELIPI no | 67 | 86 | |||
| VELIPI yes |
122 |
69 |
0.30 |
|
|
|
Adjuvant chemotherapy | |||||
| Yes | 115 | 80 | 1.0 | ||
| No |
81 |
68 |
0.068 |
0.5 (0.3–0.9) |
0.02 |
|
Preoperative radiation therapy | |||||
| Yes | 38 | 59 | |||
| No | 158 | 79 | 0.0047 | ||
Abbreviations: CI=confidence interval; HR=hazard ratio; VELIPI=vascular emboli, lymphatic invasion and perineural invasion.
log-rank P-value.
Cox-Wald χ2-test P-value.
Vascular/lymphatic/perineural invasion.
Tissue microarray analysis
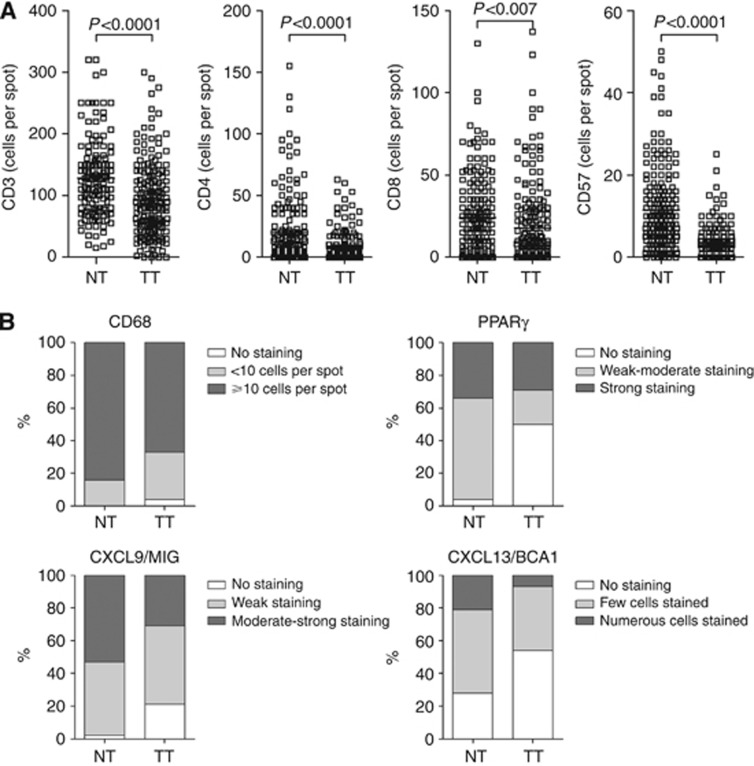
CD3, CD4, CD8, CD57, and CD68 cell density as for PPARγ, CXCL13/BCA1, and CXCL9/MIG staining intensity were significantly decreased in TT as compared with NT (each univariate, P<0.0001, except for CD8, P=0.007) (Figure 1).
Figure 1.
Immune environment differ between tumour tissue and matched normal tissue. (A) Enumeration of positive cells per spot in normal (NT) and tumour tissues (TT) was assessed for CD3, CD4, CD8, and CD57. (B) As in A but CD68-positive cells were studied in three semi-quantitative categories: no staining (white bars); 1 to 10 cells per spot (light grey bars); more than 10 cells per spot (dark grey bars) as for CXCL9/MIG, PPARγ but using three qualitative categories: no staining (white bars); weak to moderate staining (light grey bars); moderate to strong staining (dark grey bars) as indicated in the figure, and for CXCL13/BCA1 no staining (white bars); few cells stained (light grey bars); numerous cells stained (dark grey bars). Paired t-test for quantitative variables and Bowker's test of symmetry for qualitative staining variables, P<0.05 was considered as significant.
Preop-RT impacted on all immune markers but PPARγ expression (Supplementary Table 1). The expression levels of CD57 and CD68 were significant higher in irradiated tumours (n=38) than in non-irradiated tumours (n=158) (P=0.002 and P<0.0001, respectively). The expression levels of CD57, CD68, and CXCL13/BCA1 were significant higher in normal tissue in patients who received preop-RT compared with non- irradiated patients (P=0.009, P=0.004, and P=0.010, respectively), whereas those of CD3, CD4, CD8, and CXCL9/MIG were significant lower (P=0.018, P=0.004, P<0.0001, and P=0.0017 respectively). Therefore, we concluded that preop-RT significantly modified the tumour immune microenvironment, and decided to base further analyses on patient population who did not receive preop-RT.
Among the 158 remaining patients, positive associations were found for TT expression between CD57 and CD68 (P=0.0006), CXCL9/MIG and PPARγ (P=0.0015), and CD8 and CXCL13/BCA1 (P<0.0001), and a negative association for TT expression between CD8 and CXCL9/MIG (P<0.0001). Positive associations were also found for NT expression between CXCL9/MIG and both CD4 (P<0.0001) and PPARγ (P=0.0001), and CD8 and CXCL13/BCA1 (P<0.0001), and a negative association between CD57 and CD4 (P=0.0005) (Supplementary Table 2A and 2B).
Prognostic analyses
Five-year RFS and OS rates in the subset of patients who did not receive preop-RT (n=158) were 69% (s.e.=3.7) and 79% (s.e.=3.4), respectively. The same prognostic clinicopathological factors as in the whole patient cohort were found in this patient subgroup (data not shown).
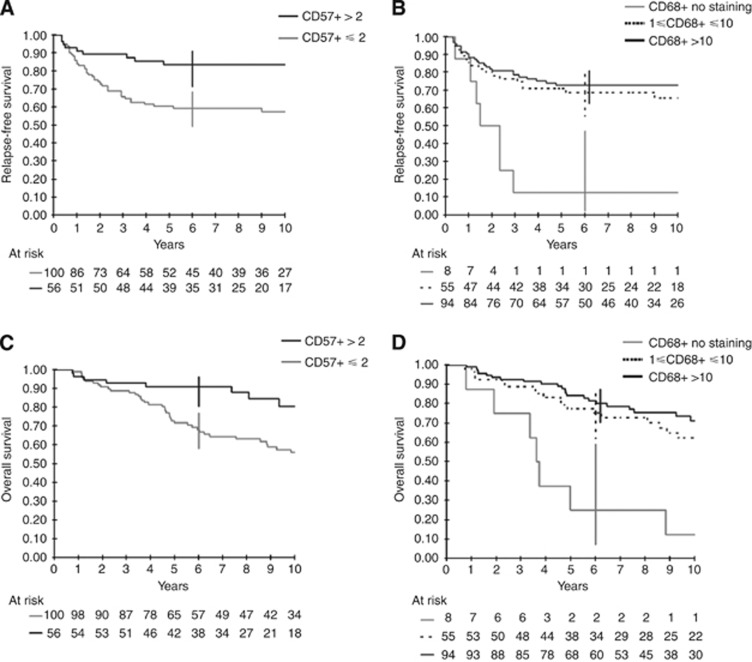
After adjustment for these factors (tumour location, stage, grade, and adjuvant chemotherapy), lower CD3+, CD57+, and CD68+ TIC densities were significantly associated with worse RFS and OS, and lower tumour expression of CXCL9/MIG with worse RFS (Table 3A, Figure 2). In multivariate analysis, only CD57+ and CD68+ TIC densities remained independently associated with RFS and OS (Table 3B). The hazard ratio (HR) for the risk of relapse was 2.7 (95% confidence interval (CI): 1.2–5.7) in patients with ⩽2 CD57+ TIC per spot as compared with those with >2 CD57+ TIC per spot, and 3.5 (1.4–9.1) in patients with no CD68+ TIC as compared with those with >10 CD68+ TIC per spot. However, the risk of relapse was not significantly increased in patients with 1–10 CD68+ TIC per spot as compared with those with >10 CD68+ TIC per spot (HR: 1.2; 95% CI: 0.6–2.3). Similar results were observed regarding OS (Table 3B). Of note, the prognostic effect of CD57+ and CD68+ TIC densities on RFS and OS did not significantly differ according to the disease stage (that is, stage II vs stage III) (Supplementary Table 3).
Table 3A. Prognostic value of CD3, CD8, CD4, CD57, CD68, CXCL9/MIG, PPARγ, and CXCL13/BCA1 in tumour tissue from patients without preop-RT after adjustment for anatomoclinical factors (tumour location, stage, grade, and adjuvant chemotherapy).
| |
|
Relapse-free survival |
Overall survival |
||||
|---|---|---|---|---|---|---|---|
| n | HR | 95% CI | P | HR | 95% CI | P | |
|
CD3 | |||||||
| CD3 ⩽80 cells per spot | 76 | 2.1 | 1.1–4.0 | 2.0 | 1.1–3.8 | ||
| CD3 >80 cells per spot | 73 | 1 | 0.02 | 1 | 0.025 | ||
| Missing |
9 |
|
|
|
|
|
|
|
CD4 | |||||||
| CD4 ⩽2 cells per spot | 88 | 0.9 | 0.5–1.6 | 1.3 | 0.7–2.4 | ||
| CD4 >2 cells per spot | 66 | 1 | 0.72 | 1 | 0.41 | ||
| Missing |
4 |
|
|
|
|
|
|
|
CD8 | |||||||
| CD8 ⩽8 cells per spot | 80 | 1.4 | 0.8–2.6 | 1.5 | 0.8–2.7 | ||
| CD8 >8 cells per spot | 74 | 1 | 0.25 | 1 | 0.22 | ||
| Missing |
4 |
|
|
|
|
|
|
|
CD57 | |||||||
| CD57 ⩽2 cells per spot | 100 | 3.2 | 1.5–6.6 | 2.9 | 1.4–5.9 | ||
| CD57 >2 cells per spot | 56 | 1 | 0.002 | 1 | 0.003 | ||
| Missing |
2 |
|
|
|
|
|
|
|
CD68 | |||||||
| CD68=0 cells per spot | 8 | 5.4 | 2.1–13.4 | 7.6 | 3.0–18.8 | ||
| 1⩽ CD68 ⩽10 cells per spot | 55 | 1.4 | 0.8–2.7 | 1.9 | 1.0–3.5 | ||
| CD68 >10 cells per spot | 94 | 1 | 0.0016 | 1 | <0.0001 | ||
| Missing |
1 |
|
|
|
|
|
|
|
CXCL9/MIG | |||||||
| No staining | 36 | 3.3 | 1.3–8.4 | 3.2 | 1.2–8.4 | ||
| Weak staining | 80 | 2.3 | 1.0–5.3 | 2.6 | 1.0–6.3 | ||
| Moderate to strong | 42 | 1 | 0.041 | 1 | 0.063 | ||
| Missing |
0 |
|
|
|
|
|
|
|
PPARγ | |||||||
| No staining | 73 | 1.3 | 0.7–2.5 | 0.7 | 0.4–1.4 | ||
| Mild-to-moderate | 33 | 0.7 | 0.3–1.8 | 0.7 | 0.3–1.7 | ||
| Strong staining | 51 | 1 | 0.38 | 1 | 0.57 | ||
| Missing |
1 |
|
|
|
|
|
|
|
CXCL13/BCA1 | |||||||
| No staining | 85 | 1.0 | 0.3–3.3 | 3.0 | 0.4–22.2 | ||
| Few lymphocytes stained | 57 | 1.0 | 0.3–3.3 | 3.3 | 0.4–24.5 | ||
| Numerous lymphocytes stained | 12 | 1 | 0.98 | 1 | 0.51 | ||
| Missing | 4 | ||||||
Abbreviations: CI=confidence interval; HR=hazard ratio.
Figure 2.
Relapse-free survival according to CD57 (A) and CD68 (B) tumour-infiltrating cells density and OS according to CD57 (C) and CD68 (D) tumour-infiltrating cells density.
Table 3B. Multivariate analysis adjusted for anatomoclinical parameters (tumour location, stage, grade, and adjuvant chemotherapy) for patients without preop-RT (model based on 156 patients because of missing value for two patients).
| |
|
Relapse-free survival |
Overall survival |
||||
|---|---|---|---|---|---|---|---|
| n | HR | 95% CI | P | HR | 95% CI | P | |
|
CD57 | |||||||
| CD57 ⩽2 cells per spot | 100 | 2.7 | 1.3–5.7 | 2.3 | 1.1–4.8 | ||
| CD57 >2 cells per spot |
56 |
1 |
|
0.011 |
1 |
|
0.026 |
|
CD68 | |||||||
| CD68=0 cells per spot | 8 | 3.6 | 1.4–9.1 | 5.3 | 2.1–13.6 | ||
| 1⩽ CD68 ⩽10 cells per spot | 55 | 1.2 | 0.6–2.3 | 1.6 | 0.9–3.0 | ||
| CD68 >10 cells per spot | 93 | 1 | 0.03 | 1 | 0.002 | ||
Abbreviations: CI=confidence interval; HR=hazard ratio.
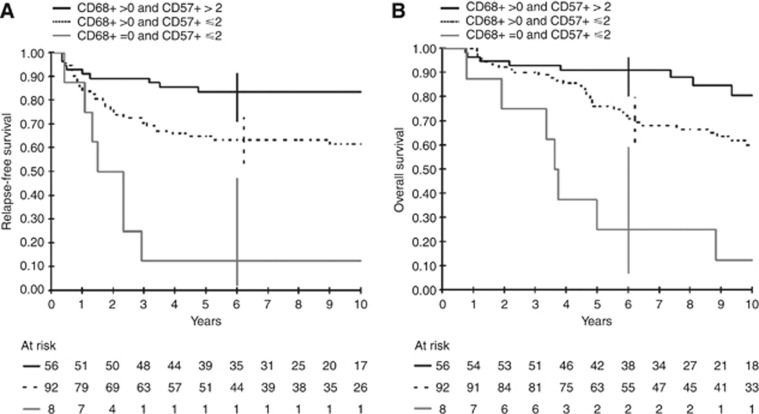
Finally, we generated a prognostic score based on the combination of CD57+ (+, > vs −, ⩽2 cells per spot) and CD68+ (+, >0 vs −, =0 cells per spot) TIC densities. This three-category prognostic score discriminated CRC patients at low (CD68+, CD57+), intermediate (CD68+, CD57−), or high (CD68−, CD57−) risk, with HRs for the intermediate-risk and high-risk groups of 2.7 (95% CI: 1.3–5.8) and 9.0 (3.2–25.4) for RFS, and 2.5 (1.2–5.1) and 10.6 (3.8–29.2) for OS, respectively, as compared with the low-risk group. Corresponding 5-year survival rates in the low-, moderate-, and high-risk groups were 84% (71–91), 65% (54–74), and 12% (2–47), respectively, for RFS, and 91% (80–96), 76% (66–84), and 25% (7–59), respectively, for OS (Figure 3).
Figure 3.
Relapse-free survival (A) and OS (B) according to the prognostic score combining CD57 and CD68 tumour-infiltrating cells density.
Discussion
In the present study, we showed that tumour infiltration by CD57+ and CD68+ cells is an independent prognostic factor for RFS and OS in patients with pMMR CRC, regardless of disease stage (that is, stage II or III, Supplementary Table 3). Although CD3+ TIC density was also prognostic for RFS and OS in univariate analysis in accordance with other reports (Pages et al, 2005), it was correlated with CD57+ TIC density and did not remain an independent prognostic factor after taking into account CD57+ TIC density in multivariate analysis.
Unlike in previous studies (Pages et al, 2005; Galon et al, 2006), we used stringent patient eligibility criteria and methods for this study. First, only patients with stage II or III disease were included. In fact, additional prognostic biomarkers are mainly needed for such patients, as the vast majority of stage I CRC patients will never relapse after curative surgery while most stage IV CRC patients will die from their disease. Second, we excluded patients with dMMR CRC, as an increased number of TIL is a well-known pattern in dMMR CRC (Jass et al, 1998; Laghi et al, 2009). Third, we excluded patients who received preop-RT. As previously suggested by others, we showed here that preop-RT strongly influenced immune microenvironment. Indeed, radiation therapy may enhance tumour-associated antigen and major histocompatibility complex-I expressions, increase CD8+ and CD4+ tumour cell infiltration, and favour the antigen-capture by dendritic cells that may promote immunological recognition of tumour cells (Lugade et al, 2008; Teitz-Tennenbaum et al, 2008; Sharma et al, 2011). Recent works have highlighted the immunological pathways involved in immunogenic cell death after irradiation of tumour in mice models. Irradiation may lead to calreticulin exposure at the cell surface of tumour cells, secretion of HMGB1 and ATP from tumour cells and IL-1β from antigen-presenting cells (APC) resulting in a better uptake of apoptotic bodies by APC and their maturation leading to an induction/amplification of specific tumour antigen IFNγ +CD8+ T cells at the tumour site (Apetoh et al, 2007; Obeid et al, 2007; Ghiringhelli et al, 2009). Therefore, we excluded patients who received preop-RT from the analysis. Fourth, we showed that the stainings of all the immune markers we studied were significantly decreased in the centre of the tumour. This suggests that tumour microenvironment hampers immune cells to infiltrate deeply within the tumour. Furthermore, we only focused the analysis of the immune infiltrate in the centre of the tumour as our TMA did not target the invasive margin of the tumour samples. Therefore, we did not assess the peritumoural nor the crohn-like lymphocytic reactions, which have been shown to be associated with longer survival in CRC (Ogino et al, 2009, 2010). However, the cell counting may be more reproducible in the centre of the tumour, the invasive/peritumoural margin being potentially difficult to delineate reliably and thus difficult to incorporate in the daily practice. In addition, Galon et al (2006) and others (Sinicrope et al, 2009; Ogino et al, 2010) showed that CD3+ TIL density within the tumour was also prognostic of disease-free survival (DFS), although less significantly than in the combined region analysis (centre of the tumour and invasive margin) or than a score based on the overall lymphocytic reaction calculated as the sum of scores of Crohn's like lymphoid reaction, peritumoural lymphocytic reaction, and tumour-infiltrating lymphocytes (Ogino et al, 2009). In the study by Laghi et al (2009) in which patient selection was closer to ours (i.e., Stage II and III without preop-RT), CD3+ TIL density was found to be prognostic only in stage II, but not in stage III CRC. In this study CD3 expression was analysed as a continuous variable at the tumour invasive margin on whole slides and CD57+ tumour cell infiltrate was not studied (Laghi et al, 2009). Finally, Galon et al (2006) explored mainly CD3, CD8, and CD45RO and did not assessed CD57+ and CD68+ tumour cell infiltrate.
We found that the complete absence of CD68+ TIC is characteristic of a small subset of patients with a very poor prognosis. Several reports showed an association between lower CD68+ TIC densities and worse outcome in patients with CRC (Forssell et al, 2007; Algars et al, 2011; Zhou et al, 2011; Zlobec et al, 2011). However, whether CD68 staining corresponds to activated type 1 macrophages or regulatory/suppressive type 2 macrophages, and what are the exact roles of these distinct cell populations in the tumour microenvironment (e.g., induction of regulatory T cells by type 2 macrophages) remain to be elucidated.
We found that CD57 TIC density tended to be associated with that of CD3 (P=0.003, Table 2). Thus, these cells may represent a subpopulation of CD3+ T cells. However, the lack of correlation with CD4+ TIC density and the weak association with CD8+ TIC density (P=0.02, Table 2) suggest that these cells may not belong to the conventional T-cell family. Okada et al (1995) found abundant CD57+ TIC in CRC patients, the majority of which being CD4−, half CD8 +, and one third γδTCR+. Other investigators have reported that CD57+ cells were one of the major TIL, especially in patients with gastric carcinoma (Karimine et al, 1994; Arinaga et al, 1995). However, they did not examine whether or not such CD57+ cells express CD3 (or TCR). In other words, they considered these cells as NK cells rather than CD3+ T cells (Karimine et al, 1994; Arinaga et al, 1995). More recently, CD8+ and CD57+ T cells with cytotoxic functions were found in patients with multiple myeloma (Sze et al, 2001). Higher numbers of CD57+ cells (mostly CD8+ T cells and NK cells) were associated with longer progression-free survival in patients with multiple myeloma treated with thalidomide (Mileshkin et al, 2007). These CD57+ T cells are often associated with chronic antigenic stimulation and denominated as ‘highly antigen experienced CD8+ T cells' in patients with cancer, chronic stimulation by tumour antigens may be responsible for the differentiation of these T cells within the tumour (Strioga et al, 2011). Of note, marked infiltration by CD57+ TIC at the advancing tumour margin was independently associated with longer DFS in one previous series of 93 CRC patients (Menon et al, 2004).
We found a strong association between CD57+ and CD68+ TIC densities. We built a strongly prognostic score based on these two immune markers, which allowed us to discriminate three groups of patients with stage II–III CRC harbouring very different risks of relapse and death. This suggests that CD57+ and CD68+ TIC may interact for their recruitment, reciprocal activation, and survival at the tumour site, favouring tumour clearance. However, our hypothesis warrants further investigations.
Our study has several limitations: its retrospective design, the relative small numbers of CRC patients included before the use of modern chemotherapy, that is, FOLFOX regimen that became the standard of care for patients with stage III colon cancer (Andre et al, 2004) as well as the unavailability of some established prognostic parameters in CRC patients (e.g., the refined TN substage and the number of examined nodes (AJCC, 2010)) that could not be included in our analyse. Considering the adjuvant treatment, we cannot exclude a potential interaction with such treatment and the immune system. However, there is no preclinical/clinical data supporting an immunogenic cell death induced by 5-FU. Conversely, oxaliplatin may influence anti-tumour immune responses as we showed previously (Tesniere et al, 2009). Therefore, such interactions warrant further investigation in future prospective trials. Furthermore, and as discussed earlier, TMA is by itself less indicative than whole slides and can not account for potential heterogeneity in TIC density within the tumour.
In conclusion, CD68+ and CD57+ TIC densities are independent prognostic biomarkers in stage II and III CRC, adding to the growing body of evidence for the influence of the host immune response on CRC outcome. If confirmed in other studies, our results may support the routine use of IHC assessment of both CD68+ and CD57+ TIC densities as a prognostic tool in patients with stage II–III CRC. Whether these markers may also predict the benefit of adjuvant treatment after primary surgery deserves to be assessed. Finally, our results argue for the potential therapeutic interest of immune modulators in CRC patients.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Agesen TH, Sveen A, Merok MA, Lind GE, Nesbakken A, Skotheim RI, Lothe RA. ColoGuideEx: a robust gene classifier specific for stage II colorectal cancer prognosis. Gut. 2012;61:1560–1567. doi: 10.1136/gutjnl-2011-301179. [DOI] [PubMed] [Google Scholar]
- AJCC 2010American Joint Committee on Cancer AJCC Cancer Staging Manual7th ednISBN: 978-0-387-88440-0.
- Algars A, Irjala H, Vaittinen S, Huhtinen H, Sundstrom J, Salmi M, Ristamaki R, Jalkanen S. Type and location of tumour-infiltrating macrophages and lymphatic vessels predict survival of colorectal cancer patients. Int J Cancer. 2011;131:864–873. doi: 10.1002/ijc.26457. [DOI] [PubMed] [Google Scholar]
- Andre T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J, Tabah-Fisch I, de Gramont A. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, Andre F, Delaloge S, Tursz T, Kroemer G, Zitvogel L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- Arinaga S, Karimine N, Nanbara S, Inoue H, Nakashima H, Ueo H, Akiyoshi T. Lymphokine-activated killer cell function of lymphocytes from regional lymph nodes in patients with gastric carcinoma. J Surg Oncol. 1995;58:44–49. doi: 10.1002/jso.2930580110. [DOI] [PubMed] [Google Scholar]
- Brandacher G, Perathoner A, Ladurner R, Schneeberger S, Obrist P, Winkler C, Werner ER, Werner-Felmayer G, Weiss HG, Gobel G, Margreiter R, Konigsrainer A, Fuchs D, Amberger A. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumour-infiltrating T cells. Clin Cancer Res. 2006;12:1144–1151. doi: 10.1158/1078-0432.CCR-05-1966. [DOI] [PubMed] [Google Scholar]
- Coca S, Perez-Piqueras J, Martinez D, Colmenarejo A, Saez MA, Vallejo C, Martos JA, Moreno M. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer. 1997;79:2320–2328. doi: 10.1002/(sici)1097-0142(19970615)79:12<2320::aid-cncr5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Diederichsen AC, Hjelmborg JB, Christensen PB, Zeuthen J, Fenger C. Prognostic value of the CD4+/CD8+ ratio of tumour-infiltrating lymphocytes in colorectal cancer and HLA-DR expression on tumour cells. Cancer Immunol Immunother. 2003;52:423–428. doi: 10.1007/s00262-003-0388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdinande L, Decaestecker C, Verset L, Mathieu A, Moles Lopez X, Negulescu AM, Van Maerken T, Salmon I, Cuvelier CA, Demetter P. Clinicopathological significance of indoleamine 2,3-dioxygenase 1 expression in colorectal cancer. Br J Cancer. 2011;106:141–147. doi: 10.1038/bjc.2011.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forssell J, Oberg A, Henriksson ML, Stenling R, Jung A, Palmqvist R. High macrophage infiltration along the tumour front correlates with improved survival in colon cancer. Clin Cancer Res. 2007;13:1472–1479. doi: 10.1158/1078-0432.CCR-06-2073. [DOI] [PubMed] [Google Scholar]
- Funada Y, Noguchi T, Kikuchi R, Takeno S, Uchida Y, Gabbert HE. Prognostic significance of CD8+ T cell and macrophage peritumoral infiltration in colorectal cancer. Oncol Rep. 2003;10:309–313. [PubMed] [Google Scholar]
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, Perfettini JL, Schlemmer F, Tasdemir E, Uhl M, Genin P, Civas A, Ryffel B, Kanellopoulos J, Tschopp J, Andre F, Lidereau R, McLaughlin NM, Haynes NM, Smyth MJ, Kroemer G, Zitvogel L. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- Gryfe R, Kim H, Hsieh ET, Aronson MD, Holowaty EJ, Bull SB, Redston M, Gallinger S. Tumour microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med. 2000;342:69–77. doi: 10.1056/NEJM200001133420201. [DOI] [PubMed] [Google Scholar]
- Halling KC, French AJ, McDonnell SK, Burgart LJ, Schaid DJ, Peterson BJ, Moon-Tasson L, Mahoney MR, Sargent DJ, O'Connell MJ, Witzig TE, Farr GH, Jr, Goldberg RM, Thibodeau SN. Microsatellite instability and 8p allelic imbalance in stage B2 and C colorectal cancers. J Natl Cancer Inst. 1999;91:1295–1303. doi: 10.1093/jnci/91.15.1295. [DOI] [PubMed] [Google Scholar]
- Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, Nakagawa H, Sotamaa K, Prior TW, Westman J, Panescu J, Fix D, Lockman J, Comeras I, de la Chapelle A. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer) N Engl J Med. 2005;352:1851–1860. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- Hojo S, Koizumi K, Tsuneyama K, Arita Y, Cui Z, Shinohara K, Minami T, Hashimoto I, Nakayama T, Sakurai H, Takano Y, Yoshie O, Tsukada K, Saiki I. High-level expression of chemokine CXCL16 by tumour cells correlates with a good prognosis and increased tumour-infiltrating lymphocytes in colorectal cancer. Cancer Res. 2007;67:4725–4731. doi: 10.1158/0008-5472.CAN-06-3424. [DOI] [PubMed] [Google Scholar]
- Hutchins G, Southward K, Handley K, Magill L, Beaumont C, Stahlschmidt J, Richman S, Chambers P, Seymour M, Kerr D, Gray R, Quirke P. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol. 2011;29:1261–1270. doi: 10.1200/JCO.2010.30.1366. [DOI] [PubMed] [Google Scholar]
- Jass JR, Do KA, Simms LA, Iino H, Wynter C, Pillay SP, Searle J, Radford-Smith G, Young J, Leggett B. Morphology of sporadic colorectal cancer with DNA replication errors. Gut. 1998;42:673–679. doi: 10.1136/gut.42.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonges LE, Nagelkerke JF, Ensink NG, van der Velde EA, Tollenaar RA, Fleuren GJ, van de Velde CJ, Morreau H, Kuppen PJ. Caspase-3 activity as a prognostic factor in colorectal carcinoma. Lab Invest. 2001;81:681–688. doi: 10.1038/labinvest.3780277. [DOI] [PubMed] [Google Scholar]
- Karimine N, Nanbara S, Arinaga S, Asoh T, Ueo H, Akiyoshi T. Lymphokine-activated killer cell activity of peripheral blood, spleen, regional lymph node, and tumour infiltrating lymphocytes in gastric cancer patients. J Surg Oncol. 1994;55:179–185. doi: 10.1002/jso.2930550310. [DOI] [PubMed] [Google Scholar]
- Laghi L, Bianchi P, Miranda E, Balladore E, Pacetti V, Grizzi F, Allavena P, Torri V, Repici A, Santoro A, Mantovani A, Roncalli M, Malesci A. CD3+ cells at the invasive margin of deeply invading (pT3-T4) colorectal cancer and risk of post-surgical metastasis: a longitudinal study. Lancet Oncol. 2009;10:877–884. doi: 10.1016/S1470-2045(09)70186-X. [DOI] [PubMed] [Google Scholar]
- Lin YH, Friederichs J, Black MA, Mages J, Rosenberg R, Guilford PJ, Phillips V, Thompson-Fawcett M, Kasabov N, Toro T, Merrie AE, van Rij A, Yoon HS, McCall JL, Siewert JR, Holzmann B, Reeve AE. Multiple gene expression classifiers from different array platforms predict poor prognosis of colorectal cancer. Clin Cancer Res. 2007;13:498–507. doi: 10.1158/1078-0432.CCR-05-2734. [DOI] [PubMed] [Google Scholar]
- Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM. Radiation-induced IFN-gamma production within the tumour microenvironment influences antitumor immunity. J Immunol. 2008;180:3132–3139. doi: 10.4049/jimmunol.180.5.3132. [DOI] [PubMed] [Google Scholar]
- Menon AG, Janssen-van Rhijn CM, Morreau H, Putter H, Tollenaar RA, van de Velde CJ, Fleuren GJ, Kuppen PJ. Immune system and prognosis in colorectal cancer: a detailed immunohistochemical analysis. Lab Invest. 2004;84:493–501. doi: 10.1038/labinvest.3700055. [DOI] [PubMed] [Google Scholar]
- Mileshkin L, Honemann D, Gambell P, Trivett M, Hayakawa Y, Smyth M, Beshay V, Ritchie D, Simmons P, Milner AD, Zeldis JB, Prince HM. Patients with multiple myeloma treated with thalidomide: evaluation of clinical parameters, cytokines, angiogenic markers, mast cells and marrow CD57+ cytotoxic T cells as predictors of outcome. Haematologica. 2007;92:1075–1082. doi: 10.3324/haematol.11208. [DOI] [PubMed] [Google Scholar]
- Mlecnik B, Tosolini M, Charoentong P, Kirilovsky A, Bindea G, Berger A, Camus M, Gillard M, Bruneval P, Fridman WH, Pages F, Trajanoski Z, Galon J. Biomolecular network reconstruction identifies T-cell homing factors associated with survival in colorectal cancer. Gastroenterology. 2011a;138:1429–1440. doi: 10.1053/j.gastro.2009.10.057. [DOI] [PubMed] [Google Scholar]
- Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman WH, Pages F, Galon J. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011b;29:610–618. doi: 10.1200/JCO.2010.30.5425. [DOI] [PubMed] [Google Scholar]
- Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, Metivier D, Larochette N, van Endert P, Ciccosanti F, Piacentini M, Zitvogel L, Kroemer G. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- Ogino S, Nosho K, Irahara N, Shima K, Baba Y, Kirkner GJ, Mino-Kenudson M, Giovannucci EL, Meyerhardt JA, Fuchs CS. Negative lymph node count is associated with survival of colorectal cancer patients, independent of tumoral molecular alterations and lymphocytic reaction. Am J Gastroenterol. 2010;105:420–433. doi: 10.1038/ajg.2009.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino S, Shima K, Baba Y, Nosho K, Irahara N, Kure S, Chen L, Toyoda S, Kirkner GJ, Wang YL, Giovannucci EL, Fuchs CS. Colorectal cancer expression of peroxisome proliferator-activated receptor gamma (PPARG, PPARgamma) is associated with good prognosis. Gastroenterology. 2009;136:1242–1250. doi: 10.1053/j.gastro.2008.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani H, Naito Y, Saito K, Nagura H. Expression of costimulatory molecules B7-1 and B7-2 by macrophages along invasive margin of colon cancer: a possible antitumor immunity. Lab Invest. 1997;77:231–241. [PubMed] [Google Scholar]
- Okada T, Iiai T, Kawachi Y, Moroda T, Takii Y, Hatakeyama K, Abo T. Origin of CD57+ T cells which increase at tumour sites in patients with colorectal cancer. Clin Exp Immunol. 1995;102:159–166. doi: 10.1111/j.1365-2249.1995.tb06650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- Pages F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P, Zatloukal K, Trajanoski Z, Berger A, Fridman WH, Galon J. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27:5944–5951. doi: 10.1200/JCO.2008.19.6147. [DOI] [PubMed] [Google Scholar]
- Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23:609–618. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- Sharma A, Bode B, Wenger RH, Lehmann K, Sartori AA, Moch H, Knuth A, von Boehmer L, van den Broek M. Gamma-radiation promotes immunological recognition of cancer cells through increased expression of cancer-testis antigens in vitro and in vivo. PLoS One. 2011;6:e28217. doi: 10.1371/journal.pone.0028217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinicrope FA, Rego RL, Ansell SM, Knutson KL, Foster NR, Sargent DJ. Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology. 2009;137:1270–1279. doi: 10.1053/j.gastro.2009.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strioga M, Pasukoniene V, Characiejus D. CD8+ CD28- and CD8+ CD57+ T cells and their role in health and disease. Immunology. 2011;134:17–32. doi: 10.1111/j.1365-2567.2011.03470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suraweera N, Duval A, Reperant M, Vaury C, Furlan D, Leroy K, Seruca R, Iacopetta B, Hamelin R. Evaluation of tumour microsatellite instability using five quasimonomorphic mononucleotide repeats and pentaplex PCR. Gastroenterology. 2002;123:1804–1811. doi: 10.1053/gast.2002.37070. [DOI] [PubMed] [Google Scholar]
- Sze DM, Giesajtis G, Brown RD, Raitakari M, Gibson J, Ho J, Baxter AG, Fazekas de St Groth B, Basten A, Joshua DE. Clonal cytotoxic T cells are expanded in myeloma and reside in the CD8(+)CD57(+)CD28(-) compartment. Blood. 2001;98:2817–2827. doi: 10.1182/blood.v98.9.2817. [DOI] [PubMed] [Google Scholar]
- Teitz-Tennenbaum S, Li Q, Okuyama R, Davis MA, Sun R, Whitfield J, Knibbs RN, Stoolman LM, Chang AE. Mechanisms involved in radiation enhancement of intratumoral dendritic cell therapy. J Immunother. 2008;31:345–358. doi: 10.1097/CJI.0b013e318163628c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault L, Mendiboure J, Pignon JP, Jooste V, van Endert P, Ducreux M, Zitvogel L, Piard F, Kroemer G. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2009;29:482–491. doi: 10.1038/onc.2009.356. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Peng RQ, Wu XJ, Xia Q, Hou JH, Ding Y, Zhou QM, Zhang X, Pang ZZ, Wan DS, Zeng YX, Zhang XS. The density of macrophages in the invasive front is inversely correlated to liver metastasis in colon cancer. J Transl Med. 2011;8:13. doi: 10.1186/1479-5876-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Goodman SN, Galizia G, Lieto E, Ferraraccio F, Pignatelli C, Purdie CA, Piris J, Morris R, Harrison DJ, Paty PB, Culliford A, Romans KE, Montgomery EA, Choti MA, Kinzler KW, Vogelstein B. Counting alleles to predict recurrence of early-stage colorectal cancers. Lancet. 2002;359:219–225. doi: 10.1016/S0140-6736(02)07448-2. [DOI] [PubMed] [Google Scholar]
- Zlobec I, Minoo P, Terracciano L, Baker K, Lugli A. Characterization of the immunological microenvironment of tumour buds and its impact on prognosis in mismatch repair-proficient and -deficient colorectal cancers. Histopathology. 2011;59:482–495. doi: 10.1111/j.1365-2559.2011.03975.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.