Abstract
Background:
The purpose of this study was to evaluate the expression of Notch-induced transcription factors (NTFs) HEY1, HES1 and SOX9 in colorectal cancer (CRC) patients to determine their clinicopathologic and prognostic significance.
Methods:
Levels of HEY1, HES1 and SOX9 protein were measured by immunohistochemistry in a nonmalignant and malignant tissue microarray of 441 CRC patients, and the findings correlated with pathologic, molecular and clinical variables.
Results:
The NTFs HEY1, HES1 and SOX9 were overexpressed in tumours relative to colonic mucosa (OR=3.44, P<0.0001; OR=7.40, P<0.0001; OR=4.08 P<0.0001, respectively). HEY1 overexpression was a negative prognostic factor for all CRC patients (HR=1.29, P=0.023) and strongly correlated with perineural and vascular invasion and lymph node (LN) metastasis. In 5-fluorouracil (5-FU)-treated patients, the tumour overexpression of SOX9 correlated with markedly poorer survival (HR=8.72, P=0.034), but had no predictive effect in untreated patients (HR=0.70, P=0.29). When HEY1, HES1 and SOX9 expression were combined to predict survival with chemotherapy, in treated patients there was an additive increase in the risk of death with each NTF overexpressed (HR=2.09, P=0.01), but no prognostic import in the untreated patient group (HR=0.74, P=0.19).
Conclusion:
The present study is the first to discover that HEY1 overexpression correlates with poorer outcome in CRC, and NTF expression is predictive of CRC patient survival with 5-FU chemotherapy. If confirmed in future studies, testing of NTF expression has the potential to enter routine pathological practice for the selection of patients to undergo chemotherapy alone or in combination with Notch inhibitors.
Keywords: notch signalling, colon cancer, SOX9, HES1, HEY1
Colorectal cancer (CRC) is the third most common malignancy and the second highest cause of cancer death in USA (Jemal et al, 2011). Colorectal cancer may be categorised on the basis of its site of emergence into proximal colonic, distal colonic or rectal carcinoma, each with distinct demographic, risk factors, molecular and pathological markers, and clinical outcomes (Li and Lai, 2009). Aberrant Notch signalling has been implicated in chemotherapy treatment resistance (Schreck et al, 2010; Miyamoto and Rosenberg, 2011; Izrailit and Reedijk, 2012). In the malignant state, Notch signalling promotes epithelial-to-mesenchymal transition (EMT), chemoresistance, proliferation and metastasis, and activates key pathways, such as cyclooxygenase 2 (COX2), NF-κB and Wnt signalling (Meng et al, 2009; Xie et al, 2011; Zhang et al, 2011; Garcia and Kandel, 2012). Numerous approaches for silencing Notch signalling have been explored, with gamma-secretase inhibitors (GSIs) and siRNAs showing the most promise (Purow et al, 2005; Fan et al, 2010). Many new specific Notch inhibitors are under development, with 23 ongoing phase I/II clinical trials investigating the efficacy of GSIs (www.clinicaltrials.gov). However, enthusiasm regarding the successes with in vitro and xenograft models has been tempered by the lack of efficacy as single-agent therapy in CRC as well as significant mucosal toxicity (Staal and Langerak, 2008; Strosberg et al, 2012). Treatment in concert with chemotherapy shows promise in the preclinical setting, but remains unexplored in patients. Thus, there is a need to better understand the intricacies of Notch signalling effectors to better guide the future development and application of Notch inhibitors.
If predictive or prognostic factors do exist within this pathway, the downstream effectors of Notch activation, the Notch-induced transcription factors (NTFs), in particular HEY1, HES1 and SOX9, are appropriate candidates for further study and the focus of this investigation. Upon Notch activation, there is an increase in the expression of HEY1, HES1 and SOX9, which consequently accumulate in the nucleus, leading to the transcriptional regulation of downstream targets (Maier and Gessler, 2000; Kunnimalaiyaan et al, 2005; Muto et al, 2009). HEY1 has roles in cardiac embryology, osteogenesis and neurogenesis (Sakamoto et al, 2003; Zamurovic et al, 2004; Belandia et al, 2005; Fischer et al, 2007). It is a mediator of Notch signalling in cancer, through regulation of differentiation, tumour suppressor P53, androgen receptor signalling and TGF-β-dependent EMT (Yang and Weinberg, 2008; Villaronga et al, 2009). In neural stem cells, HEY1 promotes proliferation. Furthermore, overexpression in glioblastomas and pancreatic cancer correlated with higher tumour grade and poorer patient survival (Hulleman et al, 2009; Mann et al, 2012). To date, HEY1 tumour expression has not been studied in CRC patients. HES1 acts either as an oncogene or as a tumour suppressor, depending on the tumour context. Implicated mechanisms include regulation of proliferation, EMT, metastasis and chemoresistance (Hughes, 2009; Meng et al, 2009; Kannan et al, 2011; Zage et al, 2011; Ueo et al, 2012). Clinical studies of HES1 overexpression in cancer have shown poorer prognosis in ovarian malignancy, either no impact or a modest trend towards poorer overall survival in two small CRC cohorts and no prognostic association in biliary tract cancer (Reedijk et al, 2008; Wang et al, 2010; Mazur et al, 2012). SOX9 directly promotes HES1 expression, has a critical role in embryogenesis and is required for development, differentiation and lineage commitment in numerous tissues including the intestinal epithelium (Müller et al, 2010; Matheu et al, 2012). In cancer, SOX9 regulates proliferation, differentiation and metastasis (Bastide et al, 2007; Matheu et al, 2012). Clinical studies in breast, gastric biliary tract and CRC indicate poorer prognosis with tumour SOX9 overexpression (Lü et al, 2008; Zhou et al, 2011; Matheu et al, 2012; Mazur et al, 2012). Hence, considering the mechanistic evidence for the roles of HEY1, HES1 and SOX9 in cancer pathogenesis and the preclinical data implicating Notch signalling chemotherapy responsiveness, we set out to characterise the expression of these NTFs in CRC and to explore a potential role in chemotherapy resistance. To this end, this study seeks to determine the prognostic, predictive and clinicopathologic significance of these NTFs and their consequent suitability as biomarkers in determining which patients will benefit most from Notch inhibitor therapy using a CRC tissue microarray (TMA).
Materials and methods
Study populations
Colorectal tissues were collected between 1990 and 1999 from 1056 consenting CRC patients undergoing surgery at Sir Charles Gairdner Hospital (SCGH), Western Australia. Information on patient demographics and tumour features were obtained from SCGH medical records and previous studies of this cohort (Chai et al, 2004; Feng Han et al, 2006; Salama et al, 2009). Tumour site was classified as ‘rectal' when in the rectum, ‘distal' when occurring between the proximal rectal margin and the splenic flexure and ‘proximal' when proximal to and within the splenic flexure. Samples from a total of 280 patients with AJCC stage II (48% male, 52% female) and 161 patients with stage III CRC (49% male, 51% female) were stained for HEY1, HES1 and SOX9 protein expression (n=441). Information on tumour stage, anatomic site, histologic grade, vascular invasion, perineural invasion, 5-flourouracil (5-FU) chemotherapy, microsatellite instability (MSI) determined by BAT-26 marker, β-catenin, COX2, P53, MLH1 and SFRP4 was available. Information on disease-specific survival was obtained from the Cancer Registry of Western Australia. The great majority of cases received 5-FU chemotherapy including folinic acid for 6 cycles, with a minority of older cases receiving 5-FU chemotherapy including levamisole for 12 cycles, each of 4 weeks duration. All rectal cancer patients were treated prior to the introduction of neoadjuvant therapy as the standard of care for local advanced disease. The median follow-up time was 69.7 months for patients with AJCC stage II disease and 52.4 months for patients with stage III disease. At the end of the study period, 31% of patients had died as a result of disease recurrence and 25% had died from unrelated causes. Ethics approval for this study was obtained from the Royal Perth Hospital Human Research Ethics Committee.
Tissue microarray
The TMA was constructed as described previously (Chai et al, 2004). The array comprised 1056 surgically resected cases of which 441 cases were examined for NTF expression in this study. For each case, three cores of 1-mm diameter were taken at random from tumour tissues, and an additional two 1-mm diameter cores were taken from histologically normal colonic mucosa.
Immunohistochemistry
Sections were deparaffinized through three changes of xylene (3 min), rehydrated through graded alcohols to distilled water and subjected to antigen retrieval in EDTA (pH 8.0) under pressure. After blocking endogenous peroxide activity with hydrogen peroxide (HRP), either SOX9 (SC-20095, Santa Cruz, Dallas, TX, USA; 1 : 75), HES1 (AB5702, Millipore, Billerica, MA, USA; 1 : 3000) or HEY1 (AB22614, Abcam, Cambridge, MA, USA; 1 : 75) antibody was applied for 60 min. Dako Envision+Dual link System–HRP was then applied for 30 min incubation. Sections were visualised with diaminobenzidine (Dako, Campbellfield, VIC, Australia) followed by a light counterstain of haematoxylin.
TMA scoring
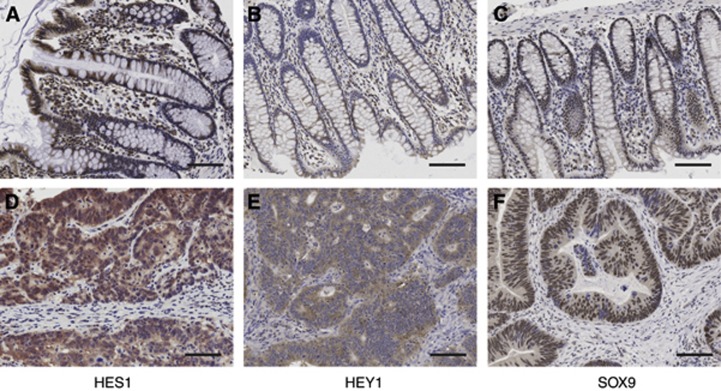
Examination of HEY1, HES1 and SOX9 expressions were blinded to patient clinical data and overseen by a pathologist. At least 150 tumour cells or colonocytes were evaluated per case. Expression was classified based on epithelial cell staining intensity and percent positivity and represented as scores of 0 (absent), 1 (weak), 2 (moderate) and 3 (strong) (Figure 1). For comparison with clinical variables and survival, the full semi-ordinal expression levels of HEY1, HES1 and SOX9 ranging 0–3 in 0.25 increments were used. Dichotomised ‘high' and ‘low' HEY1, HES1 and SOX9 immunoreactivities were used in survival curves and comparisons of staining densities across tissues by classifying as high, tissue sections that had moderate or strong staining in ⩾50% of epithelial cells, with the remainder classified as low.
Figure 1.
Colorectal tissue immunostained for HES1, HEY1 and SOX9. (A, B and C)—staining of HES1, HEY1 and SOX9 in nonmalignant tissue, respectively. (D, E and F)—staining of HES1, HEY1 and SOX9 in tumour tissue, respectively. Bars, 50 μM.
Data analysis
Spearman's ρ was used for the determination of bivariate associations between ordinal NTF expressions and clinical variables. Kaplan–Meier survival curves were produced using the R ‘survival' package. Associations with survival were examined with Cox's proportional hazards models, with ties addressed by Efron's method. Concordance for each independent variable was estimated by Harrell's C statistic (Harrell et al, 1982). To avoid confounding, analyses were age- and gender-adjusted, and stratified by tumour site and stage. The validity of proportional hazards assumptions were assessed by scaled Schoenfeld residuals (Grambsch and Therneau, 1994). All statistical tests were two-tailed and an alpha probability of <0.05 was regarded as statistically significant. Analyses were conducted using Stata Version 12 (Stata-Corp LP, College Station, TX, USA) and the R programming environment (Harrell, 2011).
Results
NTF protein expression
The normal and malignant colorectal tissues from 280 AJCC stage II and 161 AJCC stage III CRC patients were analysed for HEY1, HES1 and SOX9 protein expression. Distributions of protein expression are shown in Figure 1. In normal colonic epithelium, HEY1 and HES1 were highly expressed towards the bowel luminal surface, with expression reducing to none towards the base of the crypt. Conversely, SOX9 was predominantly expressed in basal crypt cells with weaker expression evident towards the bowel luminal surface. SOX9 expression was predominantly nuclear with weak cytoplasmic staining in malignant and nonmalignant tissue. HES1 showed both a nuclear and cytoplasmic localisation, and HEY1 was predominantly expressed in the cytoplasm of patients. Expression data for each NTF had a log-normal distribution by Shapiro–Wilks tests. The expressions of HEY1, HES1 and SOX9 were significantly elevated in malignant relative to normal tissues (OR=3.44, P<0.0001; OR=7.40, P<0.0001, 4.08 P<0.0001, respectively) (Table 1). The total percentage of patients with tumours classed as having overexpressed HEY1, HES1 and SOX9 was 61%, 68% and 60%, respectively.
Table 1. Comparison of the mean densities of HEY1, HES1 and SOX9 in colonic mucosa and colorectal cancer tissues.
| |
Normal |
Tumour |
|
|||
|---|---|---|---|---|---|---|
| Marker | n | Density (cells per mm2) | n | Density (cells per mm2) | T/N | P-value |
| HEY1 |
304 |
108 |
395 |
368 |
3.44 |
<0.0001 |
| SOX9 |
301 |
89 |
392 |
363 |
4.08 |
<0.0001 |
| HES1 | 300 | 55 | 392 | 407 | 7.40 | <0.0001 |
Abbreviations: n=number of patients; T/N=tumour/normal ratio.
Mean density of the Notch transcription factors HEY1, SOX9 and HES1 in normal colonic mucosa and colorectal cancer tissues.
NTF associations
Correlations between NTFs and clinicopathologic and molecular features are listed in Table 2. Notch-induced transcription factors showed significant coexpression across the CRC patients' malignant and nonmalignant tissues. High HEY1 tumour expression correlated significantly with elevated levels of HES1 (ρ=0.382, P<0.005) and SOX9 (ρ=0.190, P<0.005) in malignant tissue and HEY1 (ρ=0.241, P<0.005) and SOX9 (ρ=0.149, P<0.05) in nonmalignant tissue. In addition, HEY1 overexpression strongly correlated with higher stage (ρ=0.221, P<0.005), LN metastasis (ρ=0.255, P<0.005), overexpression of the proinflammatory prostaglandin synthase COX2 (ρ=0.287, P<0.005), DNA mismatch repair protein MLH1 (ρ=0.136, P<0.05), the apoptotic inducer SFRP4 (ρ=0.102, P<0.05), the CRC oncogene β-catenin (ρ=0.166, P<0.005), vascular (ρ=0.134, P<0.05) and perineural (ρ=0.160, P<0.05) invasion, and inversely correlated with MSI (ρ=−0.106, P<0.05). SOX9 overexpression in tumours correlated with vascular invasion (ρ=0.123, P<0.05), and high expression of HES1 (ρ=0.190, P<0.005) and SOX9 (ρ=0.190, P<0.005) in malignant tissue and SOX9 (rho 0.304, P<0.005) in nonmalignant tissue. Elevated HES1 expression in tumours correlated with overexpression of the critical tumour suppressor P53 (ρ=0.101, P<0.05), HEY1 (ρ=0.382, P<0.005), SOX9 (ρ=0.190, P<0.005), SFRP4 (ρ=0.128, P<0.05) and β-catenin (ρ=0.238, P<0.005) in malignant tissue, and HEY1 (ρ=0.235, P<0.005) and HES1 (rho 0.130, P<0.05) in nonmalignant tissue (Table 2). Overexpression of NTFs was not associated with tumour site, patient age or tumour differentiation.
Table 2. Bivariate associations between elevated HEY1, HES1 and SOX9 expression and clinical variables.
| Variable | High tumour HEY1 | High tumour SOX9 | High tumour HES1 | |
|---|---|---|---|---|
| High tumour HEY1 |
ρ |
|
0.190a |
0.382a |
| |
n |
|
372 |
378 |
| High tumour HES1 |
ρ |
0.382a |
0.190a |
|
| |
n |
378 |
389 |
|
| High tumour SOX9 |
ρ |
0.190a |
|
0.190a |
| |
n |
372 |
|
389 |
| High normal HEY1 |
ρ |
0.241a |
0.055 |
0.235a |
| |
n |
290 |
284 |
289 |
| High normal HES1 |
ρ |
0.141 |
−0.008 |
0.130b |
| |
n |
288 |
288 |
293 |
| High normal SOX9 |
ρ |
0.149b |
0.304a |
0.113 |
| |
n |
287 |
294 |
292 |
| AJCC stage III |
ρ |
0.221a |
0.012 |
−0.054 |
| |
n |
383 |
376 |
380 |
| LN metastasisc |
ρ |
0.255a |
0.030 |
−0.025 |
| |
n |
282 |
282 |
285 |
| Vascular invasion |
ρ |
0.134b |
0.123b |
−0.056 |
| |
n |
265 |
264 |
268 |
| Perineural invasion |
ρ |
0.160b |
0.001 |
0.069 |
| |
n |
242 |
243 |
245 |
|
β-catenin |
ρ |
0.166a |
0.072 |
0.238a |
| |
n |
377 |
370 |
374 |
| High COX2 |
ρ |
0.287a |
0.014 |
0.035 |
| |
n |
379 |
371 |
374 |
| High P53 |
ρ |
0.035 |
0.030 |
0.101b |
| |
n |
383 |
376 |
380 |
| High MSI |
ρ |
−0.106b |
−0.071 |
−0.007 |
| |
n |
352 |
345 |
348 |
| High MLH1 |
ρ |
0.136b |
0.058 |
0.020 |
| |
n |
381 |
373 |
378 |
| High SFRP4 |
ρ |
0.102b |
0.043 |
0.128b |
| n | 385 | 377 | 381 |
Abbreviations: AJCC=American Joint Committee on Cancer; LN=lymph node; MSI=microsatellite instability.
Protein data used to determine Spearman's ρ correlation coefficients were collected at the time of colorectal cancer diagnosis.
Correlation is significant at the <0.005 level (two-tailed).
Correlation is significant at the <0.05 level (two-tailed).
LN Metastasis is based on the ratio of positive to negative lymph nodes examined.
Survival analyses
Cox regression of clinicopathologic and molecular features stratified by tumour site is shown in Table 3. Across all patients, tumour overexpression of HEY1 (HR=1.29, P=0.023) or COX2 (HR=2.06, P<0.001) correlated with poorer prognosis. HEY1 overexpression significantly predicted poorer survival in colon (HR=1.31, P=0.037), but not in rectal cancer patients (HR=1.07, P=0.664). In contrast, elevated HES1 and SOX9 expression were not significantly associated with overall survival (HR=1.05, P=0.596; HR=1.14, P=0.195, respectively). High β-catenin predicted better survival in colon, but not in rectal cancer patients, which is consistent with previous studies (Gunther et al, 1998; Morikawa et al, 2011). High MSI was predictive of improved survival in patients with colon, but not rectal tumours, likely due to the very low incidence of high MSI in rectal cancer, as previously observed (Minoo et al, 2010; Table 3).
Table 3. Associations of tumour pathological and molecular features with overall survival.
| Clinical variable | Tumour site | Deaths | HR (adjusted for age and gender) | 95% confidence interval of HR | P-value |
|---|---|---|---|---|---|
| High HEY1 |
All |
208 |
1.29 |
1.0–1.6 |
0.023 |
| |
Colon |
146 |
1.31 |
1.0–1.6 |
0.037 |
| |
Rectum |
62 |
1.07 |
0.8–1.4 |
0.664 |
| High HES1 |
All |
206 |
1.05 |
0.9–1.3 |
0.596 |
| |
Colon |
145 |
1.07 |
0.9–1.2 |
0.763 |
| |
Rectum |
61 |
1.00 |
0.7–1.3 |
0.986 |
| High SOX9 |
All |
208 |
1.14 |
0.9–1.4 |
0.195 |
| |
Colon |
146 |
1.17 |
0.9–1.4 |
0.231 |
| |
Rectum |
62 |
0.97 |
0.7–1.3 |
0.859 |
| AJCC stage III |
All |
541 |
1.88 |
1.6–2.2 |
<0.001 |
| |
Colon |
363 |
2.07 |
1.7–2.5 |
<0.001 |
| |
Rectum |
167 |
1.61 |
1.2–2.1 |
<0.001 |
| High COX2 |
All |
531 |
2.06 |
1.7–2.5 |
<0.001 |
| |
Colon |
356 |
2.12 |
1.7–2.6 |
<0.001 |
| |
Rectum |
164 |
1.97 |
1.4–2.7 |
<0.001 |
| High β-catenin |
All |
528 |
0.78 |
0.6–1.0 |
0.015 |
| |
Colon |
360 |
0.74 |
0.6–0.9 |
0.013 |
| |
Rectum |
157 |
0.94 |
0.7–1.4 |
0.732 |
| High MSI |
All |
509 |
0.74 |
0.5–1.0 |
0.053 |
| |
Colon |
346 |
0.70 |
0.5–1.0 |
0.036 |
| Rectum | 153 | 0.93 | 0.2–3.8 | 0.916 |
Abbreviations: 5-FU=5-fluorouracil chemotherapy; AJCC=American Joint Committee on Cancer; HR=hazard ratio; MSI=microsatellite instability.
Cox regression survival analyses were age and sex adjusted. Tumour expressions were linear based on Cox spline regression.
Patient outcomes were next stratified by AJCC stage and related to HEY1, HES1 and SOX9 protein expression (Table 4). Overexpression of NTF HEY1, HES1 or SOX9 was predictive of reduced survival in stage III (HR=1.34, P=0.007; HR=1.23, P=0.037; HR=1.29, P=0.022 respectively), but not stage II CRC cases (HR=1.05, P=0.73; HR=0.98, P=0.82; HR=1.02, P=0.83 respectively).
Table 4. Associations of HEY1, HES1 and SOX9 expression with overall survival, stratified by AJCC stage.
| Dependent variable | AJCC Stage | Deaths | HR | 95% confidence interval of HR | P-value |
|---|---|---|---|---|---|
|
Tumour HEY1 | |||||
| II | 115 | 1.05 | 0.8–1.4 | 0.728 | |
| |
III |
91 |
1.34 |
1.1–1.7 |
0.007 |
|
Tumour HES1 | |||||
| II | 106 | 0.98 | 0.8–1.2 | 0.816 | |
| |
III |
89 |
1.23 |
1.0–1.5 |
0.037 |
|
Tumour SOX9 | |||||
| II | 107 | 1.02 | 0.8–1.3 | 0.829 | |
| III | 90 | 1.29 | 1.0–1.6 | 0.022 | |
Abbreviations: 5-FU=5-fluorouracil chemotherapy; AJCC=American Joint Committee on Cancer; HR=hazard ratio.
Cox regression survival analyses were age and sex adjusted.
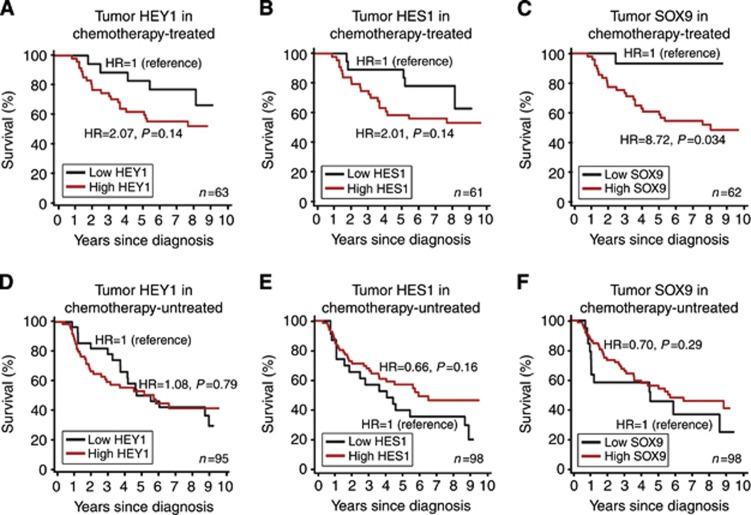
A major factor that affects survival and its prediction by NTF tumour expression, in stage III, but not stage II CRC, is the effectiveness of adjuvant chemotherapy. Consistent with clinical practice at the time, a proportion of stage III CRC cases received chemotherapy, but stage II CRC cases did not. As activation of Notch signalling has been associated with chemotherapy resistance (Schreck et al, 2010; Miyamoto and Rosenberg, 2011; Izrailit and Reedijk, 2012), we investigated whether tumour overexpression of NTFs predicted reduced survival in chemotherapy-treated patients. As shown in Figure 2, stage III CRC patients with tumour overexpression of SOX9 had poorer survival with 5-FU treatment, relative to those with low SOX9 (HR=8.72, P=0.034) over a 10-year period. This was accentuated in patients with rectal cancer (P=0.007) (Supplementary Figure S1). A similar trend towards reduced survival was observed for patients with tumour overexpression of HEY1 or HES1 treated with chemotherapy, but this was not significant (HR=2.07, P=0.14; HR=2.07, P=0.14 respectively). In untreated patients, tumour overexpression of HEY1, HES1 or SOX9 did not correlate with poorer prognosis (HR=1.08, P=0.79; HR=0.66 P=0.16; HR=0.70 P=0.29 respectively) (Figure 2).
Figure 2.
Survival of chemotherapy-treated and -untreated CRC patients by HEY1, HES1 and SOX9 expression. Survival curves for AJCC stage III CRC patients that were chemotherapy-treated based on tumour expression levels of HEY1 (A), HES1 (B) or SOX9 (C), or untreated based on tumour expression levels of HEY1 (D), HES1 (F) or SOX9 (E), grouped as high (⩾1.5) or low (⩽1.5) by immunoreactivity. AJCC=American Joint Committee on Cancer; HR=hazard ratio.
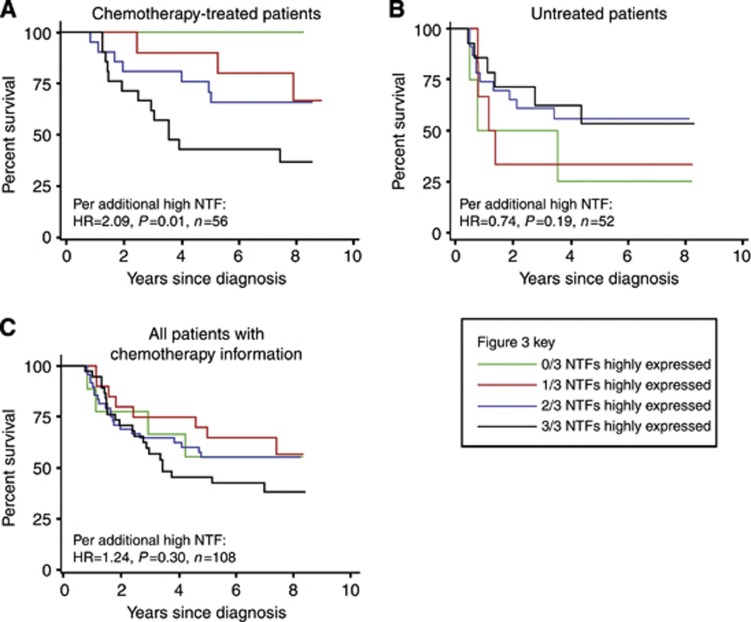
The prognoses of chemotherapy-treated and -untreated stage III CRC patients were then predicted using the collective immunoreactivities of HEY1, HES1 and SOX9 (Figure 3). In chemotherapy-treated patients, there was an additive increase in the risk of death for each NTF highly expressed in tumours (HR=2.09, P=0.01). Untreated patients showed a nonsignificant trend towards reduced risk of death with overexpression of NTFs (HR=0.74, P=0.19).
Figure 3.
Survival curves using a combination of tumour NTF expressions in chemotherapy-treated and untreated patients. Survival curves for stage III CRC patients, treated (A) or not treated (B) with 5-FU, based on a combination of tumour HEY1, HES1 and SOX9 expression levels, grouped as high (⩾1.5) or low (⩽1.5) by immunoreactivity. HR=hazard ratio; NTF=Notch-induced transcription factor.
Discussion
This is the first clinical study to compare the interrelationships of HEY1, HES1 and SOX9 in CRC. We identified significant coexpression of NTFs in CRC patients. Potential causalities for this coexpression of NTFs include their mutual transcriptional activation through Notch signalling, particularly by RBP-Jk, JAG1 and NOTCH1-4, as well as their collective activation by inflammatory IL-1, TNFα and Ikk2 signalling (Maier and Gessler, 2000; Kunnimalaiyaan et al, 2005; Staal and Langerak, 2008; Maniati et al, 2011; Wang et al, 2013). Furthermore, SOX9 is a key mediator of HES1 transcription, and retinoic acid signalling mutually promotes SOX9 and HES1 transcription (Müller et al, 2010). Hence, their clinical coexpression is consistent with our molecular understanding of NTF signalling. All three NTFs were upregulated between 3–7-fold in CRC compared with that in colonic epithelium. The upregulation of HES1 and SOX9 in tumours has been observed previously whereas HEY1 has not been studied (Reedijk et al, 2008; Matheu et al, 2012).
Tumour SOX9 and the combination of HEY1, HES1 and SOX9 protein overexpression were predictive of poorer response to chemotherapy in stage III CRC patients. Previous studies of these NTFs have never explored chemoresponse, and there exist no reports of proteins providing predictive data of this potency for adjuvant chemotherapy in CRC (Clark-Langone et al, 2010; Wang et al, 2010; Jin et al, 2011; Mann et al, 2012; Matheu et al, 2012). At the time of this study, adjuvant chemotherapy was given for stage III but not stage II disease, allowing the reduced effectiveness of chemotherapy observed with NTF overexpression to exert selective survival pressure. More recently, 5-FU and oxaliplatin combinations have become the standard of care in CRC stage III, as well as a proportion of stage II and IV patients, making the prediction of chemotherapy effectiveness by NTFs increasingly relevant to contemporary patients (DeVita et al, 2008). Furthermore, the expression of NTFs may be capable of predicting the survival of patients with other cancers that are treated with 5-FU, and hence could be studied in lung, breast, liver, stomach, oesophageal and head and neck cancer (Shirasaka and Taguchi, 2006). Tumour NTF expression also has the potential to guide the use of Notch inhibitory therapy, alone or in combination with chemotherapy to increase their benefit to patients. Hence, assuming confirmation in a larger study, the uniqueness of this data for the prognostication of adjuvant chemotherapy could see its rapid integration into current pathological practice.
Notch signalling has been implicated in chemoresistance at the preclinical level (Schreck et al, 2010; Miyamoto and Rosenberg, 2011; Izrailit and Reedijk, 2012). In CRC cells, 5-FU-, oxaliplatin- and irinotecan-induced chemoresistance was promoted by NTFs, such as HES1, and abrogated using Notch inhibitory therapy (Meng et al, 2009). Coupled with the functional roles of NTFs in apoptotic resistance and EMT (Bastide et al, 2007; Hughes, 2009; Meng et al, 2009; Kannan et al, 2011; Xie et al, 2011; Zage et al, 2011; Zhang et al, 2011; Garcia and Kandel, 2012; Matheu et al, 2012; Ueo et al, 2012), and the associations of NTF overexpression in this study with markedly poorer survival in chemotherapy-treated patients, suggests further studies that explore the clinical benefit of using combinations of Notch inhibitors and chemotherapy may lead to a reduced incidence of chemoresistance and increased survival times (Purow et al, 2005; Fan et al, 2010). In addition, the clinical limitations caused by the toxicity of existing Notch inhibitors that target upstream of the Notch signalling pathway (Staal and Langerak, 2008; Strosberg et al, 2012), to affect a broad range of targets, may be mitigated by the more targeted therapeutic inhibition of downstream NTFs.
HEY1 overexpression in tumours correlated with greater incidence of LN metastasis, perineural and vascular invasion, and poorer survival accentuated in patients with AJCC stage III CRC or colon cancer. These findings are consistent with previous studies of HEY1 in glioblastoma and pancreatic cancer (Hulleman et al, 2009; Mann et al, 2012). There may be a causal element in overexpressed HEY1's clinical associations with increased risk of LN metastasis and mortality, because HEY1 promotes proliferation and EMT in tumours (Yang and Weinberg, 2008; Hulleman et al, 2009; Villaronga et al, 2009). HEY1 expression was associated with numerous proteins important in CRC. Firstly, overexpression of the Notch signalling target COX2 strongly correlated with HEY1, suggesting this NTF may be involved in COX2's activation, which drives angiogenesis, inflammation and associates with reduced survival (Tseng et al, 2012; Zhou et al, 2012). HEY1 expression also correlated with Wnt pathway members β-catenin and SFRP4, an association attributable to Notch signalling's activation of the Wnt signalling pathway (Zhang et al, 2011). Finally, HEY1 overexpression was associated with high levels of DNA-repair protein MLH1 that reduces the incidence of MSI tumours. HEY1 inversely correlated with tumours that have MSI, which has never been reported previously. This may be due to Notch signalling being more active in tumours with reduced MSI that show high Wnt signalling activity (Morikawa et al, 2011; Zhang et al, 2011). This would also explain in part the association of HEY1 with poorer survival in colon, but not rectal cancer, because MSI, a good prognostic factor, rarely occurs in rectal tumours (Minoo et al, 2010). Taken together, these data suggest HEY1 could be a therapeutic target, and its tumour expression may enhance current CRC prognostication.
HES1 overexpression in tumours correlated significantly with inferior survival in stage III CRC, consistent with trends seen in smaller CRC cohorts (Reedijk et al, 2008; Mazur et al, 2012). This association was confined to 5-FU-treated patients. HES1 overexpression correlated strongly with β-catenin's, a Notch signalling target (Zhang et al, 2011). The association between tumour overexpressions of HES1 and master tumour suppressor P53 may be attributed to the transcriptional activation of P53 by HES1 (Huang et al, 2004). This study indicates that further research into the utility of HES1 tumour expression for the prediction of chemoresponse in CRC is warranted.
SOX9 overexpression in tumours was the strongest predictor of reduced survival in 5-FU-treated patients (HR=8.72, P=0.034) and correlated with vascular invasion. Previously, increased mortality associated with elevated SOX9 has been observed in numerous cancers, including separate cohorts of colon and rectal cancer (Lü et al, 2008; Zhou et al, 2011; Matheu et al, 2012; Mazur et al, 2012). Many studies report mechanistic roles for SOX9 in cancer promotion, specifically in apoptotic resistance, neoplastic transformation and proliferation in glioma, colorectal and prostate cancer (Bastide et al, 2007; Matheu et al, 2012). However, this is the first study to demonstrate value for SOX9 as a biomarker in the prediction of response to 5-FU treatment.
The most important limitation of this study is the lack of randomised chemotherapy, making any comparison between treated and untreated patients confounded by selection bias. In general, patients not receiving treatment were perceived as either at lower risk of relapse or were considered unfit for therapy. Performance status data that might have allowed a degree of compensation for the latter factor was not available. Hence, our analyses of chemoresponse have both been performed within and not between cohorts defined by receipt of therapy.
In conclusion, the present study is the first to discover that tumour HEY1 overexpression correlates with poorer CRC outcome, and NTFs may have value as biomarkers for the prediction of survival with 5-FU chemotherapy treatment. If confirmed, these NTFs, in particular SOX9, have a very real potential to enter routine clinical practice in defining the utility of adjuvant chemotherapy for individual patients. Furthermore, as there are mechanistic roles for these NTFs in CRC chemoresistance and metastasis, studies should be pursued to explore whether patients overexpressing these NTFs may benefit preferentially from Notch-targeted therapies in conjunction with chemotherapy.
Acknowledgments
We thank Barry Iacopetta and Lisa Spalding for their assistance with the tissue microarrays. This work was in part funded by the National Health and Medical Research Council (NHMRC). Patrick Candy was the recipient of a Richard Walter Gibbon Medical Research Scholarship from the University of Western Australia.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Bastide P, Darido C, Pannequin J, Kist R, Robine S, Marty-Double C, Bibeau F, Scherer G, Joubert D, Hollande F, Blache P, Jay P. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J Cell Biol. 2007;178 (4:635–648. doi: 10.1083/jcb.200704152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belandia B, Powell SM, Garcia-Pedrero JM, Walker MM, Bevan CL, Parker MG. Hey1, a mediator of notch signaling, is an androgen receptor corepressor. Mol Cell Biol. 2005;25 (4:1425–1436. doi: 10.1128/MCB.25.4.1425-1436.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai SM, Zeps N, Shearwood A-M, Grieu F, Charles A, Harvey J, Goldblatt J, Joseph D, Iacopetta B. Screening for defective DNA mismatch repair in stage II and III colorectal cancer patients. Clin Gastroenterol Hepatol. 2004;2 (11:1017–1025. doi: 10.1016/s1542-3565(04)00451-3. [DOI] [PubMed] [Google Scholar]
- Clark-Langone KM, Sangli C, Krishnakumar J, Watson D. Translating tumor biology into personalized treatment planning: analytical performance characteristics of the Oncotype DX Colon Cancer Assay. BMC Cancer. 2010;10 (691:691. doi: 10.1186/1471-2407-10-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVita V, Lawrence T, Rosenburg S, DePinho R, Weinberg R. DeVita, Hellman, and Rosenberg's Cancer: Principles & Practice of Oncology. Lippincott Williams & Wilkins; 2008. [Google Scholar]
- Fan X, Khaki L, Zhu TS, Soules ME, Talsma CE, Gul N, Koh C, Zhang J, Li YM, Maciaczyk J, Nikkhah G, DiMeco F, Piccirillo S, Vescovi AL, Eberhart CG. NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells. 2010;28 (1:5–16. doi: 10.1002/stem.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Han Q, Zhao W, Bentel J, Shearwood A-M, Zeps N, Joseph D, Iacopetta B, Dharmarajan A. Expression of sFRP-4 and [beta]-catenin in human colorectal carcinoma. Cancer Letts. 2006;231 (1:129–137. doi: 10.1016/j.canlet.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Fischer A, Steidl C, Wagner TU, Lang E, Jakob PM, Friedl P, Knobeloch K-P, Gessler M. Combined loss of Hey1 and HeyL causes congenital heart defects because of impaired epithelial to mesenchymal transition. Circulation Res. 2007;100 (6:856–863. doi: 10.1161/01.RES.0000260913.95642.3b. [DOI] [PubMed] [Google Scholar]
- Garcia A, Kandel JJ. Notch: A key regulator of tumor angiogenesis and metastasis. Histol Histopathol. 2012;27 (2:151–156. doi: 10.14670/hh-27.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81 (3:515–526. [Google Scholar]
- Gunther K, Brabletz T, Kraus C, Dworak O, Reymond M, Jung A, Hohenberger W, Kirchner T, Kockerling F, Ballhausen W. Predictive value of nuclear beta-catenin expression for the occurrence of distant metastases in rectal cancer. Dis Colon Rectum. 1998;41 (10:1256–1261. doi: 10.1007/BF02258226. [DOI] [PubMed] [Google Scholar]
- Harrell FE. rms: regression modeling strategies. R Package Version. 2011;3:3–3. [Google Scholar]
- Harrell FE, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. J Am Med Assoc. 1982;247:2543–2546. [PubMed] [Google Scholar]
- Huang Q, Raya A, DeJesus P, Chao S-H, Quon KC, Caldwell JS, Chanda SK, Izpisua-Belmonte JC, Schultz PG. Identification of p53 regulators by genome-wide functional analysis. Proc Natl Acad Sci USA. 2004;101 (10:3456–3461. doi: 10.1073/pnas.0308562100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes DP. How the NOTCH pathway contributes to the ability of osteosarcoma cells to metastasize. Cancer Treat Res. 2009;152:479–496. doi: 10.1007/978-1-4419-0284-9_28. [DOI] [PubMed] [Google Scholar]
- Hulleman E, Quarto M, Vernell R, Masserdotti G, Colli E, Kros JM, Levi D, Gaetani P, Tunici P, Finocchiaro G, RRy Baena, Capra M, Helin K. A role for the transcription factor HEY1 in glioblastoma. J Cell Mol Med. 2009;13 (1:136–146. doi: 10.1111/j.1582-4934.2008.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izrailit J, Reedijk M. Developmental pathways in breast cancer and breast tumor-initiating cells: Therapeutic implications. Cancer Letts. 2012;317 (2:115–126. doi: 10.1016/j.canlet.2011.11.028. [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61 (2:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Jin HY, Xu JH, Wang XF, Ding YJ. Expression pattern and clinical significance of hairy enhancer of split 1 in colorectal cancer. Zhonghua Yi Xue Za Zhi. 2011;91 (41:2891–2894. [PubMed] [Google Scholar]
- Kannan S, Fang W, Song G, Mullighan CG, Hammitt R, McMurray J, Zweidler-McKay PA. Notch/HES1-mediated PARP1 activation: a cell type–specific mechanism for tumor suppression. Blood. 2011;117 (10:2891–2900. doi: 10.1182/blood-2009-12-253419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunnimalaiyaan M, Traeger K, Chen H. Conservation of the Notch1 signaling pathway in gastrointestinal carcinoid cells. Am J Physiol Gastrointestinal Liver Physiol. 2005;289 (4:G636–G642. doi: 10.1152/ajpgi.00146.2005. [DOI] [PubMed] [Google Scholar]
- Li FY, Lai MD. Colorectal cancer, one entity or three. J Zhejiang Univ Sci B. 2009;10 (3:219–229. doi: 10.1631/jzus.B0820273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lü B, Fang Y, Xu J, Wang L, Xu F, Xu E, Huang Q, Lai M. Analysis of SOX9 expression in colorectal cancer. Am J Clin Pathol. 2008;130 (6:897–904. doi: 10.1309/AJCPW1W8GJBQGCNI. [DOI] [PubMed] [Google Scholar]
- Maier MM, Gessler M. Comparative analysis of the human and mouse Hey1 promoter: Hey genes are new notch target genes. Biochem Biophys Res Commun. 2000;275 (2:652–660. doi: 10.1006/bbrc.2000.3354. [DOI] [PubMed] [Google Scholar]
- Maniati E, Bossard M, Cook N, Candido J, Emami-Shahri N, Nedospasov S, Balkwill F, Tuveson D, Hagemann T. Crosstalk between the canonical NF-kappaB and Notch signaling pathways inhibits Ppargamma expression and promotes pancreatic cancer progression in mice. J Clin Invest. 2011;121 (12:4685–4699. doi: 10.1172/JCI45797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann CD, Bastianpillai C, Neal CP, Masood MM, Jones DJ, Teichert F, Singh R, Karpova E, Berry DP, Manson MM. Notch3 and hey-1 as prognostic biomarkers in pancreatic adenocarcinoma. PLoS One. 2012;7 (12:e51119. doi: 10.1371/journal.pone.0051119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheu A, Collado M, Wise C, Manterola L, Cekaite L, Tye AJ, Canamero M, Bujanda L, Schedl A, Cheah KSE, Skotheim RI, Lothe RA, Lopez de Munain A, Briscoe J, Serrano M, Lovell-Badge R. Oncogenicity of the developmental transcription factor Sox9. Cancer Res. 2012;72:1301–1315. doi: 10.1158/0008-5472.CAN-11-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur PK, Riener M-O, Jochum W, Kristiansen G, Weber A, Schmid RM, Siveke JT. Expression and clinicopathological significance of Notch signaling and cell-fate genes in biliary tract cancer. Am J Gastroenterol. 2012;107 (1:126–135. doi: 10.1038/ajg.2011.305. [DOI] [PubMed] [Google Scholar]
- Meng RD, Shelton CC, Li Y-M, Qin L-X, Notterman D, Paty PB, Schwartz GK. γ-Secretase inhibitors abrogate oxaliplatin-induced activation of the Notch-1 signaling pathway in colon cancer cells resulting in enhanced chemosensitivity. Cancer Res. 2009;69 (2:573–582. doi: 10.1158/0008-5472.CAN-08-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoo P, Zlobec I, Peterson M, Terracciano L, Lugli A. Characterization of rectal, proximal and distal colon cancers based on clinicopathological, molecular and protein profiles. Int J Oncol. 2010;37 (3:707–718. doi: 10.3892/ijo_00000720. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Rosenberg DW. Role of Notch signaling in colon homeostasis and carcinogenesis. Cancer Sci. 2011;102 (11:1938–1942. doi: 10.1111/j.1349-7006.2011.02049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa T, Kuchiba A, Yamauchi M, Meyerhardt JA, Shima K, Nosho K, Chan AT, Giovannucci E, Fuchs CS, Ogino S. Association of CTNNB1 (beta-catenin) alterations, body mass index, and physical activity with survival in patients with colorectal cancer. JAMA. 2011;305 (16:1685–1694. doi: 10.1001/jama.2011.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A, Iida A, Satoh S, Watanabe S. The group E Sox genes Sox8 and Sox9 are regulated by Notch signaling and are required for Müller glial cell development in mouse retina. Exp Eye Res. 2009;89 (4:549–558. doi: 10.1016/j.exer.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Müller P, Crofts J, Newman B, Bridgewater L, Lin C-Y, Gustafsson J-Å, Ström A. SOX9 mediates the retinoic acid-induced HES-1 gene expression in human breast cancer cells. Breast Cancer Res Treat. 2010;120 (2:317–326. doi: 10.1007/s10549-009-0381-6. [DOI] [PubMed] [Google Scholar]
- Purow BW, Haque RM, Noel MW, Su Q, Burdick MJ, Lee J, Sundaresan T, Pastorino S, Park JK, Mikolaenko I, Maric D, Eberhart CG, Fine HA. Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1, is critical for glioma cell survival and proliferation. Cancer Res. 2005;65 (6:2353–2363. doi: 10.1158/0008-5472.CAN-04-1890. [DOI] [PubMed] [Google Scholar]
- Reedijk MOS, Zhang H, Chetty R, Tennert C, Dickson BC, Lockwood G, Gallinger SES. Activation of Notch signaling in human colon adenocarcinoma. Int J Oncol. 2008;33 (6:1223–1229. doi: 10.3892/ijo_00000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto M, Hirata H, Ohtsuka T, Bessho Y, Kageyama R. The basic helix-loop-helix genes Hesr1/Hey1 and Hesr2/Hey2 regulate maintenance of neural precursor cells in the brain. J Biol Chem. 2003;278 (45:44808–44815. doi: 10.1074/jbc.M300448200. [DOI] [PubMed] [Google Scholar]
- Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27 (2:186–192. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- Schreck KC, Taylor P, Marchionni L, Gopalakrishnan V, Bar EE, Gaiano N, Eberhart CG. The Notch target Hes1 directly modulates Gli1 expression and Hedgehog signaling: a potential mechanism of therapeutic resistance. Clin Cancer Res. 2010;16 (24:6060–6070. doi: 10.1158/1078-0432.CCR-10-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaka T, Taguchi T. Timeline from discovery of 5-FU to development of an oral anticancer agent S-1 and its drug concept. Gan To Kagaku Ryoho. 2006;33 (Suppl 1:4–18. [PubMed] [Google Scholar]
- Staal FJT, Langerak AW. Signaling pathways involved in the development of T-cell acute lymphoblastic leukemia. Haematologica. 2008;93 (4:493–497. doi: 10.3324/haematol.12917. [DOI] [PubMed] [Google Scholar]
- Strosberg JR, Yeatman T, Weber J, Coppola D, Schell MJ, Han G, Almhanna K, Kim R, Valone T, Jump H, Sullivan D. A phase II study of RO4929097 in metastatic colorectal cancer. Eur J Cancer. 2012;48 (7:997–1003. doi: 10.1016/j.ejca.2012.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng YC, Tsai YH, Tseng MJ, Hsu KW, Yang MC, Huang KH, Li AF, Chi CW, Hsieh RH, Ku HH, Yeh TS. Notch2-induced COX-2 expression enhancing gastric cancer progression. Mol Carcinog. 2012;51 (12:939–951. doi: 10.1002/mc.20865. [DOI] [PubMed] [Google Scholar]
- Ueo T, Imayoshi I, Kobayashi T, Ohtsuka T, Seno H, Nakase H, Chiba T, Kageyama R. The role of Hes genes in intestinal development, homeostasis and tumor formation. Development. 2012;139:1071–1082. doi: 10.1242/dev.069070. [DOI] [PubMed] [Google Scholar]
- Villaronga MA, Lavery DN, Bevan CL, Llanos S, Belandia B. HEY1 Leu94Met gene polymorphism dramatically modifies its biological functions. Oncogene. 2009;29 (3:411–420. doi: 10.1038/onc.2009.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Tian Y, Wang J, Phillips K, Binch A, Dunn S, Cross A, Chiverton N, Zheng Z, Shapiro I, Le Maitre C, Risbud M. Inflammatory cytokines induce NOTCH signaling in nucleus pulposus cells: implications in intervertebral disc degeneration. J Biol Chem. 2013;288 (23:16761–16774. doi: 10.1074/jbc.M112.446633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Fu Y, Chen X, Ye J, Lü B, Ye F, Lü W, Xie X. The expressions of bHLH gene HES1 and HES5 in advanced ovarian serous adenocarcinomas and their prognostic significance: a retrospective clinical study. J Cancer Res Clin Oncol. 2010;136 (7:989–996. doi: 10.1007/s00432-009-0744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie M, Zhang L, He C-S, Xu F, Liu J-L, Hu Z-H, Zhao L-P, Tian Y. Activation of Notch-1 enhances epithelial-mesenchymal transition in gefitinib-acquired resistant lung cancer cells. J Cell Biochem. 2011;113:1501–1513. doi: 10.1002/jcb.24019. [DOI] [PubMed] [Google Scholar]
- Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14 (6:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Zage PE, Nolo R, Fang W, Stewart J, Garcia-Manero G, Zweidler-McKay PA. Notch pathway activation induces neuroblastoma tumor cell growth arrest. Pediatr Blood Cancer. 2011;58:682–689. doi: 10.1002/pbc.23202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamurovic N, Cappellen D, Rohner D, Susa M. Coordinated activation of Notch, Wnt, and transforming growth factor-β signaling pathways in Bone Morphogenic Protein 2-induced osteogenesis. J Biol Chem. 2004;279 (36:37704–37715. doi: 10.1074/jbc.M403813200. [DOI] [PubMed] [Google Scholar]
- Zhang X, Chen T, Zhang J, Mao Q, Li S, Xiong W, Qiu Y, Xie Q, Ge J. Notch1 promotes glioma cell migration and invasion by stimulating β-catenin and NF-κB signaling via AKT activation. Cancer Sci. 2011;103:181–190. doi: 10.1111/j.1349-7006.2011.02154.x. [DOI] [PubMed] [Google Scholar]
- Zhou C-J, Guo J-Q, Zhu K-X, Zhang Q-H, Pan C-R, Xu W-H, Wang H-J, Liu B. Elevated expression of SOX9 is related with the progression of gastric carcinoma. Diagnost Cytopathol. 2011;39 (2:105–109. doi: 10.1002/dc.21348. [DOI] [PubMed] [Google Scholar]
- Zhou L, Wang DS, Li QJ, Sun W, Zhang Y, Dou KF. The down-regulation of Notch1 inhibits the invasion and migration of hepatocellular carcinoma cells by inactivating the Cyclooxygenase-2/Snail/E-cadherin pathway in vitro. Dig Dis Sci. 2012;7:7. doi: 10.1007/s10620-012-2434-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.