Abstract
This study investigates the effects of water solutions on the antioxidant content of green tea leaf extracts. Green teas prepared with tap water and distilled water were compared with respect to four antioxidant assays: total phenol content, reducing power, DMPD assay, and trolox equivalent antioxidant capacity assay. The results indicate that green tea prepared with distilled water exhibits higher antioxidant activity than that made with tap water. The high performance liquid chromatography showed that major constituents of green tea were found in higher concentrations in tea made with distilled water than in that made with tap water. This could be due to less calcium fixation in leaves and small water clusters. Water solutions composed of less mineralisation are more effective in promoting the quality of green tea leaf extracts.
1. Introduction
Green tea is an infusion of the leaves of the Camellia sinensis plant, a member of the Theaceae family [1, 2]. As a popular drink favoured by Asians, in particular Chinese, green tea has received much global attention for its promotion of human health. A number of studies have shown that green tea possesses a wide range of biological activities [3], including antioxidant activity [4–9], anti-inflammatory activity [10], antimutagenic as well as anticarcinogenic activities [11–14], and neuroprotective effects [15]. The major functional constituents of green tea, catechins, account for 8–15% of the dry leaf weight [16].
A variety of catechins, which are members of the polyphenol family, are present in green tea leaves. The four most abundant green tea epicatechins are (–)-epigallocatechin gallate (EGCG), (–)-epicatechin gallate (ECG), (–)-epigallocatechin (EGC), and (–)-epicatechin (EC). In addition, four minor catechins are found in green tea leaves, including catechin (C), catechin gallate (CG), gallocatechin (GC), and gallocatechin gallate (GCG) [16, 17]. Catechins have been reported to possess strong antioxidant activity and are widely accepted as important antioxidants, which eliminate free radicals [6].
It is presumed that different tea-processing procedures and water components will result in different qualities of green tea [18–21]. Previous studies have demonstrated that the temperature [22–24], pH [25], organic solvent [26], pressure [27], soaking time [28], and elements of water [29, 30] affect the antioxidant activity of green tea. However, how the physics-based mechanism of the water solution affects tea extracts has rarely been discussed.
The aim of this study is to compare the effects of making green tea extracts with tap water versus distilled water on the antioxidant activities of the extracts. Comparisons are made using the results of four antioxidant activity assays and high performance liquid chromatography component analyses. Following the literature review, a possible physics-based mechanism to describe our observations is discussed.
2. Materials and Methods
2.1. Chemicals and Reagents
The green tea leaves were from TenRen Tea Co., Ltd., Taipei, Taiwan. Gallic acid (GA), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), GC, EC, EGC, and EGCG were obtained from Sigma (USA). N,N-Dimethyl-p-phenylenediamine dihydrochloride (DMPD) and iron (III) chloride were purchased from Riedel-de Haen (Germany). Folin-Ciocalteu reagent was from Fluka (Germany). Methanol, acetonitrile, and acetic acid were purchased from Merck (Darmstadt, Germany).
2.2. Processing of Green Tea
Instead of a brewing process [7, 31], green tea solutions were prepared using tap water and distilled water at 25°C. The former was taken from the suburban area of the City of Greater Taichung, Taiwan. Distilled water (<1 μS/cm) was purified using a Millipore Alpha-Q Luton ultrapure water system such that both the organic and inorganic impurities were removed. Two water samples were divided into 25 mL aliquots in 50 mL polypropylene centrifuge tubes. Aqueous extracts were prepared by soaking a 31.25 mg infusion of ground up and homogenized dry green tea leaves in 25 mL water at 25°C for 20 min. After centrifugation (1250 rpm), the green tea supernatant was collected for further examination; five replicates were made for statistical analysis.
2.3. Total Phenol Content
The total phenol content of green tea was measured according to Gutfinger's method [32]. Each green tea solution was mixed with 250 μL Folin-Ciocalteu reagent for 1 min and 50 μL 2% Na2CO3 for 5 min. The absorbance at 655 nm (Sunrise ELISA Plate Reader, Tecan, Austria) denoted the total phenol content, and it increased with the content of phenols.
2.4. Reducing Power
As described in a previous report [33], the reducing power of green tea was determined using a mixture of 250 μL phosphate buffer (200 mM, pH 6.6), 250 μL potassium ferricyanide (1% by weight), and 500 μL green tea. The mixture was then incubated at 50°C for 20 min. Trichloroacetic acid (250 μL, 10% by weight) was mixed with distilled water (250 μL) and FeCl3 (750 μL, 0.1% by weight), allowed to react for 30 min, and the absorbance at 700 nm was measured using a spectrophotometer. Higher absorbance indicated greater reducing power.
2.5. DMPD Assay
Antioxidant activities of green tea were measured according to Schlesier's method [34]. In principle, colorless DMPD will form a stable purple free radical cation, DMPD+•, in the presence of a suitable oxidant, such as FeCl3. Purple DMPD+• is scavenged and decolorized upon addition of an antioxidant. Equal volumes of 24 mM DMPD, 2.4 mM FeCl3, and 0.6 M acetic acid were mixed together for 5 min to generate DMPD+•, and the absorbance at 495 nm was measured. The antioxidant activity was inferred as the ability to scavenge free radicals, and a decrease in absorbance was inferred as increasing scavenging power, defined as follows:
| (1) |
where A1 denotes the absorbance of total free DMPD+• radicals and A2 denotes the absorbance following addition of the antioxidant.
2.6. Trolox Equivalent Antioxidant Capacity Assay
The trolox equivalent antioxidant capacity (TEAC) assay was carried out using the procedure described by Erkan's method [35]. In brief, ABTS+• was produced by reacting 7 mM ABTS with 2.45 mM potassium persulfate in the dark at 4°C for 12 h. A 30 μL antioxidant aliquot was added to 2 mL ABTS+• radical solution, allowed to react for 10 min, and the absorbance measured at 734 nm.
As stated previously, the antioxidant capacity was inferred as the ability to scavenge free radicals, expressed as follows:
| (2) |
where A3 represents the absorbance of total free ABTS+• radicals, and A4 represents the absorbance following addition of the antioxidant.
2.7. High Performance Liquid Chromatography
The extract of green tea was mixed with an internal standard solution (0.5 mg gallic acid diluted to 25 mL with 70% methanol) at a ratio of 1 : 1. The samples were spiked with various concentrations of stock solutions and then analyzed.
A stock solution was prepared by dissolving four marker substances (GC, 1.0 mg; EGC, 5.0 mg; EGCG, 2.5 mg; and EC, 10.0 mg) in 1 mL of 70% methanol; the solution was then stored in a refrigerator. Stock solutions were diluted with 70% methanol into a series of standard solutions (GC: 0.78, 1.04, 1.25, 1.56, 2.08, and 3.13 μg/mL; EGC: 2.50, 6.25, 7.81, 10.41, 15.63, and 31.25 μg/mL; EGCG: 2.60, 3.13, 3.91, 5.21, and 7.81 μg/mL; EC: 2.08, 4.17, 4.63, 5, 6.25, and 8.33 μg/mL). A 20 μL aliquot of each solution was injected and analyzed two times using HPLC, and the standard curves were plotted as peak areas versus concentrations. Recovery was determined by comparing the amount of marker substances added to the marker substances found. The limits of detection were based on a signal to noise (S/N) ratio of 3 : 1 as a minimum.
HPLC was performed on an Agilent 1220 series system. Satisfactory separation of the markers was obtained using a reversed-phase column (Cosmosil 5C18-AR II, 5 μm, 25 cm × 4.6 mm I.D., Nacalai Tesque, Kyoto, Japan) at 280 nm, an elution flow rate of 0.8 mL/min, and a linear solvent gradient of A-B (A, 10 mM KH2PO4 (pH 4.0); B, CH3CN, CH3OH, and H2O at a ratio of 1.5: 2.5: 1, respectively, [v/v/v]) as follows: 0 min, 20% B; 5 min, 20% B; 15 min, 30% B; 45 min, 60% B.
2.8. Statistical Analysis
The paired t-test method was used to evaluate differences between groups. A value of P < 0.05 was considered statistically significant (*), and P < 0.01 was highly significant (**).
3. Results and Discussion
3.1. Total Phenol Content
Figure 1 shows the differences in A655 between the tea solutions. Indicating the total amount of phenol, the A655 for the control (no green tea extract), green tea made with distilled water, and green tea made with tap water were 0.06 ± 0.02, 0.81 ± 0.03, and 0.69 ± 0.03, respectively. Phenols are regarded as significant constituents of green tea because of their free-radical scavenging ability.
Figure 1.
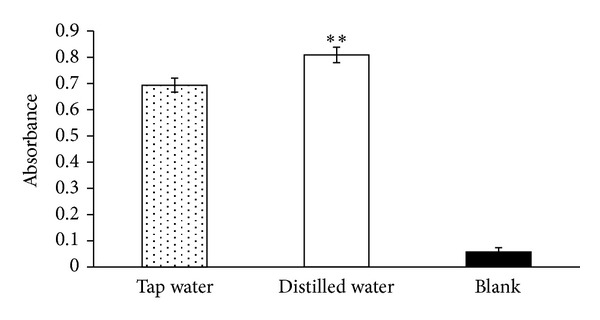
Effects of water solution on the total phenol content of green tea.
3.2. Reducing Power
The reducing power of a compound serves as an indicator of its antioxidant activity [33]. Figure 2 shows the reducing power (absorbance at 700 nm) of the 3 groups tested. The A700 for the control, green tea made with distilled water, and green tea made with tap water were 0.11 ± 0.02, 0.53 ± 0.03, and 0.47 ± 0.06, respectively.
Figure 2.
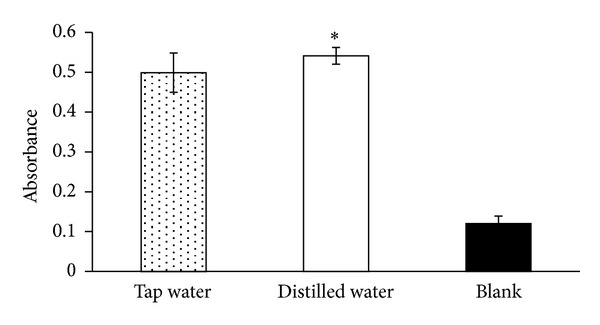
Effects of water solution on the reducing power of green tea.
3.3. DMPD Assay
Figure 3 shows that the inhibition of DMPD+• radical cations by green tea was 84.04 ± 3.10% when made with distilled water and 82.15 ± 2.46% when made with tap water. Compared to tea made with tap water, there was a 1.89% improvement in DMPD+• scavenging activity in the tea made with distilled water. Thus, the distilled water was able to extract more antioxidant substances from the green tea leaves as determined by DMPD+• scavenging activity.
Figure 3.
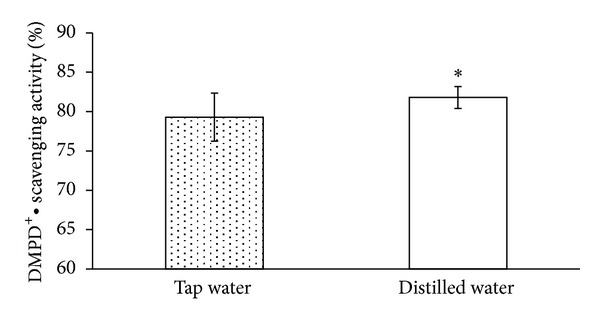
Effects of water solution on DMPD+• scavenging activity in green tea.
3.4. Trolox Equivalent Antioxidant Capacity Assay
Figure 4 indicates that ABTS+• radicals were inhibited by 78.75 ± 1.81% and 71.01 ± 1.41% in green tea made using distilled water and tap water, respectively. Results of the TEAC assay demonstrate that green tea made with distilled water has superior ABTS+• scavenging activity (7.74% increase) compared to green tea made with tap water. These results suggest that distilled water has a clear advantage over tap water in terms of its ability to extract more antioxidants into the tea solution.
Figure 4.
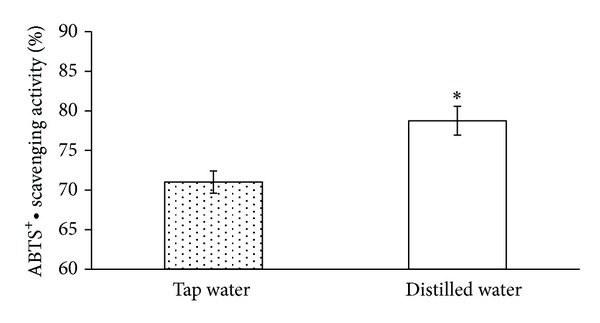
Effects of water solutions on ABTS+• scavenging activity in green tea.
3.5. High Performance Liquid Chromatography
Calibration curves were prepared by plotting the peak-area ratios (using gallic acid as an internal standard) against the corresponding concentrations. Using linear regression to analyze data in the concentration range of interest, the detection limits were between 0.078 and 1.25 μg/mL (S/N = 3) for the components.
By substituting the peak-area ratios of individual peaks acquired using HPLC, the contents of individual components in the green tea were determined. The average amounts of 4 constituents in teas made using tap water and distilled water, respectively, were (mg/g ± SD): GC, 4.43 ± 0.94 and 3.35 ± 0.53; EGC, 5.86 ± 1.78 and 21.03 ± 0.47; EGCG, 1.43 ± 0.07 and 12.36 ± 0.03; EC, 5.52 ± 0.21 and 10.26 ± 0.18. Of these 4 constituents, EGC, EGCG, and EC show higher amounts in the distilled water sample than its tap water counterpart, as shown in Figure 5.
Figure 5.
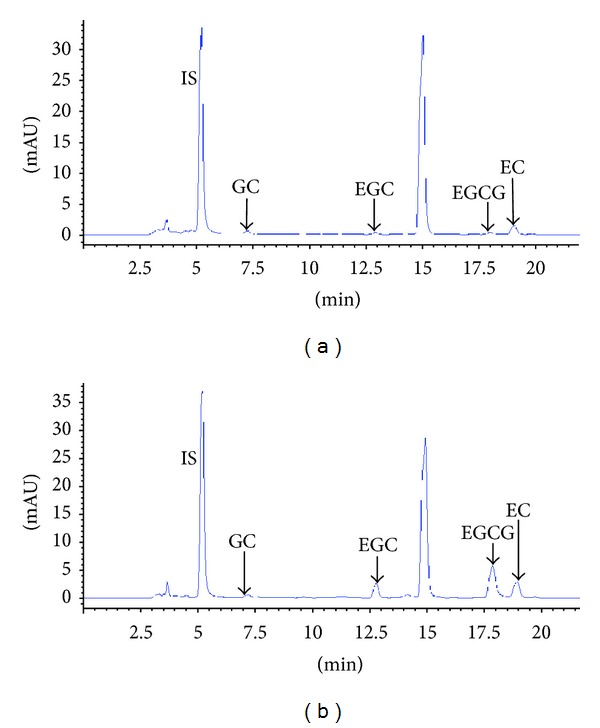
High performance liquid chromatography spectra of green tea prepared with different water solutions. (a) Tap water and (b) distilled water (IS = gallic acid, GC = gallocatechin, EGC = (–)-epigallocatechin, EGCG = (–)-epigallocatechin gallate, and EC = (–)-epicatechin).
3.6. Ion Concentration
The extraction efficiency of green tea leaves depends on the presence of electrolytes. Therefore, the ions present in water were further determined by the inductively coupled plasma mass spectrometry (SCIEX Elan 5000, Perkin Elmer, Überlingen, Germany). Table 1 shows the cation contents in two waters of this study. There is a higher cation concentration in tap water than distilled water. Corresponding to previous reports [36], the higher the mineralisation, the lower the green tea leaves extraction yields of organic matter. The reason may be also attributed to that calcium is taken up by leaves from highly mineralised water and assumed to be complexed with pectins in cell walls. The formation of complex will retard extraction of green tea leaves thus explaining the decrease in extraction yield. Besides, tea polyphenols which are the leading functional component may combine with ions such as Ca2+ and Mg2+ to be partially retained in tea residue [37].
Table 1.
Cation compositions of tap water and distilled water.
| Water | Cation concentration (ppb) | ||||
|---|---|---|---|---|---|
| Ca2+ | Al3+ | Na+ | Mg2+ | K+ | |
| Tap water | 21690 | 13.33 | 10120 | 6698 | 1361 |
| Distilled water | 115.2 | 0.465 | 9.035 | 2.159 | 0.941 |
Except calcium fixation in leaves, the water structure may also affect extraction efficiency. Water structures have been studied for over 100 years in various research fields, including physics, chemistry, and physical chemistry [38]. Many theoretical and experimental approaches for studying the structure of water have been well developed (e.g., statistical thermodynamics, molecular dynamics simulation, infrared spectrum, Raman spectrum, X-ray diffraction, neutron diffraction, and NMR) [39–41]. Electrolytes have been shown to affect the median number of water clusters and the cluster size [39, 42]. This observation supports the idea that water organized into smaller clusters can extract more functional constituents of green tea leaves and hence enhances the antioxidant activity of green tea. The rate-determining step in tea leaf infusion was determined to be the diffusion of solutes through the leaf matrix to the surface [43]. Smaller water clusters provide larger surface areas, thus increasing contact opportunities between water molecules and green tea leaves and allowing the extraction of more solutes from the leaves. In addition, the interaction between the sample and the extracting solvent is enhanced due to the increased number of water clusters, resulting in a highly efficient extraction. In this study, cations increase the median water cluster size [39] and decrease extraction of green tea leaves.
4. Conclusions
This study investigated the effects of water conditions on the efficiency of constituent extraction from green tea leaves. The increase in polyphenol extraction in green tea prepared with distilled water increases its antioxidant activity. Our results revealed that distilled water may be a good choice for extracting compounds probably due to its less calcium fixation in leaves and smaller water cluster size, thus enhancing the antioxidant activity of green tea made with distilled water. These results provide an alternative application of distilled water in the processing of certain foods.
Conflict of Interests
The authors declare that they have no competing interests. All authors have no financial relation with the commercial identities mentioned in the paper.
Acknowledgment
The authors feel deeply indebted to the National Science Council, Taiwan, for all the resources gained under Grant no. NSC101-2622-E-241-002-CC3.
References
- 1.Brown MD. Green tea (Camellia sinensis) extract and its possible role in the prevention of cancer. Alternative Medicine Review. 1999;4(5):360–370. [PubMed] [Google Scholar]
- 2.Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Critical Reviews in Food Science and Nutrition. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- 3.Sharangi AB. Medicinal and therapeutic potentialities of tea (Camellia sinensis L.)—a review. Food Research International. 2009;42(5-6):529–535. [Google Scholar]
- 4.Henning SM, Niu YT, Lee NH, et al. Bioavailability and antioxidant activity of tea flavanols after consumption of green tea, black tea, or a green tea extract supplement. American Journal of Clinical Nutrition. 2004;80(6):1558–1564. doi: 10.1093/ajcn/80.6.1558. [DOI] [PubMed] [Google Scholar]
- 5.Beltz LA, Bayer DK, Moss AL, Simet IM. Mechanisms of cancer prevention by green and black tea polyphenols. Anti-Cancer Agents in Medicinal Chemistry. 2006;6(5):389–406. doi: 10.2174/187152006778226468. [DOI] [PubMed] [Google Scholar]
- 6.Seeram NP, Henning SM, Niu Y, Lee R, Scheuller HS, Heber D. Catechin and caffeine content of green tea dietary supplements and correlation with antioxidant capacity. Journal of Agricultural and Food Chemistry. 2006;54(5):1599–1603. doi: 10.1021/jf052857r. [DOI] [PubMed] [Google Scholar]
- 7.Lin S-D, Liu E-H, Mau J-L. Effect of different brewing methods on antioxidant properties of steaming green tea. LWT—Food Science and Technology. 2008;41(9):1616–1623. [Google Scholar]
- 8.Bancirova M. Comparison of the antioxidant capacity and the antimicrobial activity of black and green tea. Food Research International. 2010;43(5):1379–1382. [Google Scholar]
- 9.Costa ASG, Nunes MA, Almeida IMC, et al. Teas, dietary supplements and fruit juices: a comparative study regarding antioxidant activity and bioactive compounds. LWT—Food Science and Technology. 2012;49:324–328. [Google Scholar]
- 10.Cao H, Kelly MA, Kari F, et al. Green tea increases anti-inflammatory tristetraprolin and decreases pro-inflammatory tumor necrosis factor mRNA levels in rats. Journal of Inflammation. 2007;4, article 1 doi: 10.1186/1476-9255-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khatiwada J, Verghese M, Walker LT, Shackelford L, Chawan CB, Sunkara R. Combination of green tea, phytic acid, and inositol reduced the incidence of azoxymethane-induced colon tumors in Fisher 344 male rats. LWT—Food Science and Technology. 2006;39(10):1080–1086. doi: 10.1089/jmf.2010.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Merwe JD, Joubert E, Richards ES, et al. A comparative study on the antimutagenic properties of aqueous extracts of Aspalathus linearis (rooibos), different Cyclopia spp. (honeybush) and Camellia sinensis teas. Mutation Research. 2006;611(1-2):42–53. doi: 10.1016/j.mrgentox.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 13.Yang CS, Sang S, Lambert JD, Hou Z, Ju J, Lu G. Possible mechanisms of the cancer-preventive activities of green tea. Molecular Nutrition & Food Research. 2006;50:170–175. doi: 10.1002/mnfr.200500105. [DOI] [PubMed] [Google Scholar]
- 14.Jeong H-S, Jang S, Jang M-J, et al. Effects of (–)-epigallocatechin-3-gallate on the activity of substantia nigra dopaminergic neurons. Brain Research. 2007;1130(1):114–118. doi: 10.1016/j.brainres.2006.10.078. [DOI] [PubMed] [Google Scholar]
- 15.Mandel S, Weinreb O, Amit T, Youdim MBH. Cell signaling pathways in the neuroprotective actions of the green tea polyphenol (–)-epigallocatechin-3-gallate: implications for neurodegenerative diseases. Journal of Neurochemistry. 2004;88(6):1555–1569. doi: 10.1046/j.1471-4159.2003.02291.x. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida Y, Kiso M, Goto T. Efficiency of the extraction of catechins from green tea. Food Chemistry. 1999;67(4):429–433. [Google Scholar]
- 17.Lee SC, Kim SY, Jeong SM, Park JH. Effect of far-infrared irradiation on catechins and nitrite scavenging activity of green tea. Journal of Agricultural and Food Chemistry. 2006;54:399–403. doi: 10.1021/jf051866x. [DOI] [PubMed] [Google Scholar]
- 18.Huang Y, Sheng J, Yang F, Hu Q. Effect of enzyme inactivation by microwave and oven heating on preservation quality of green tea. Journal of Food Engineering. 2007;78(2):687–692. [Google Scholar]
- 19.Sinija VR, Mishra HN, Bal S. Process technology for production of soluble tea powder. Journal of Food Engineering. 2007;82(3):276–283. [Google Scholar]
- 20.Komes D, Horžić D, Belščak A, Ganić KK, Vulić I. Green tea preparation and its influence on the content of bioactive compounds. Food Research International. 2010;43:167–176. [Google Scholar]
- 21.Hu J, Chen Y, Ni D. Effect of superfine grinding on quality and antioxidant property of fine green tea powders. LWT—Food Science and Technology. 2012;45:8–12. [Google Scholar]
- 22.Xi J, Shen D, Zhao S, Lu B, Li Y, Zhang R. Characterization of polyphenols from green tea leaves using a high hydrostatic pressure extraction. International Journal of Pharmaceutics. 2009;382:139–143. doi: 10.1016/j.ijpharm.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 23.von Staszewski M, Pilosof AMR, Jagus RJ. Antioxidant and antimicrobial performance of different Argentinean green tea varieties as affected by whey proteins. Food Chemistry. 2011;125(1):186–192. [Google Scholar]
- 24.Jun X, Deji S, Ye L, Rui Z. Comparison of in vitro antioxidant activities and bioactive components of green tea extracts by different extraction methods. International Journal of Pharmaceutics. 2011;408:97–101. doi: 10.1016/j.ijpharm.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Zimmermann BF, Gleichenhagen M. The effect of ascorbic acid, citric acid and low pH on the extraction of green tea: how to get most out of it. Food Chemistry. 2011;124(4):1543–1548. [Google Scholar]
- 26.Li F, Wang F, Yu F, et al. In vitro antioxidant and anticancer activities of ethanolic extract of selenium-enriched green tea. Food Chemistry. 2008;111(1):165–170. [Google Scholar]
- 27.Xi J, Shen D, Li Y, Zhang R. Ultrahigh pressure extraction as a tool to improve the antioxidant activities of green tea extracts. Food Research International. 2011;44:2783–2787. [Google Scholar]
- 28.Vuong QV, Stathopoulos CE, Golding JB, Nguyen MH, Roach PD. Optimum conditions for the water extraction of L -theanine from green tea. Journal of Separation Science. 2011;34(18):2468–2474. doi: 10.1002/jssc.201100401. [DOI] [PubMed] [Google Scholar]
- 29.Zhou DR, Chen YQ, Ni DJ. Effect of water quality on the nutritional components and antioxidant activity of green tea extracts. Food Chemistry. 2009;113:110–114. [Google Scholar]
- 30.Bae MS, Lee SC. Effect of deep sea water on the antioxidant activity and catechin content of green tea. Journal of Medicinal Plants Research. 2010;4(16):1662–1667. [Google Scholar]
- 31.Lin S-D, Liang C-H, Liu E-H, Mau J-L. Antioxidant properties of water extracts from parching green tea. Journal of Food Biochemistry. 2010;34(3):477–500. [Google Scholar]
- 32.Gutfinger T. Polyphenols in olive oils. Journal of the American Oil Chemists Society. 1981;58(11):966–968. [Google Scholar]
- 33.Shyu Y-S, Lin J-T, Chang Y-T, Chiang C-J, Yang D-J. Evaluation of antioxidant ability of ethanolic extract from dill (Anethum graveolens L.) flower. Food Chemistry. 2009;115(2):515–521. [Google Scholar]
- 34.Schlesier K, Harwat M, Böhm V, Bitsch R. Assessment of antioxidant activity by using different in vitro methods. Free Radical Research. 2002;36(2):177–187. doi: 10.1080/10715760290006411. [DOI] [PubMed] [Google Scholar]
- 35.Erkan N, Ayranci G, Ayranci E. Antioxidant activities of rosemary (Rosmarinus Officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chemistry. 2008;110(1):76–82. doi: 10.1016/j.foodchem.2008.01.058. [DOI] [PubMed] [Google Scholar]
- 36.Mossion A, Potin-Gautier M, Delerue S, Le Hécho I, Behra P. Effect of water composition on aluminium, calcium and organic carbon extraction in tea infusions. Food Chemistry. 2008;106(4):1467–1475. [Google Scholar]
- 37.Zhou D, Chen Y, Ni D. Effect of water quality on the nutritional components and antioxidant activity of green tea extracts. Food Chemistry. 2009;113:110–114. [Google Scholar]
- 38.Stillinger FH. Water revisited. Science. 1980;209(4455):451–457. doi: 10.1126/science.209.4455.451. [DOI] [PubMed] [Google Scholar]
- 39.Li R, Jiang Z, Yang H, Guan YJ. Effects of ions in natural water on the 17O NMR chemical shift of water and their relationship to water cluster. Journal of Molecular Liquids. 2006;126:14–18. [Google Scholar]
- 40.Suresh SJ, Kapoor K, Talwar S, Rastogi A. Internal structure of water around cations. Journal of Molecular Liquids. 2012;174:135–142. [Google Scholar]
- 41.Shimokawa S, Yokono T, Mizuno T, Tamura H, Erata T, Araiso T. Effect of far-infrared light irradiation on water as observed by X-ray diffraction measurements. Japanese Journal of Applied Physics 2. 2004;43(4):L545–L547. [Google Scholar]
- 42.Markovich G, Pollack S, Giniger R, Cheshnovsky O. Photoelectron spectroscopy of Cl-, Br-, and I —solvated in water clusters. The Journal of Chemical Physics. 1994;101(11):9344–9353. [Google Scholar]
- 43.Astill C, Birch MR, Dacombe C, Humphrey PG, Martin PT. Factors affecting the caffeine and polyphenol contents of black and green tea infusions. Journal of Agricultural and Food Chemistry. 2001;49(11):5340–5347. doi: 10.1021/jf010759+. [DOI] [PubMed] [Google Scholar]


