Abstract
Significance: The main objective of this review was to provide an exposition of investigations, conducted in Drosophila melanogaster, on the role of reactive oxygen species and redox state in the aging process. While early transgenic studies did not clearly support the validity of the oxidative stress hypothesis of aging, predicated on the accumulation of structural damage, they spawned a broader search for redox-related effects that might impact the aging process. Recent Advances: Initial evidence implicating the thiol redox state as a possible causative factor in aging has been obtained in Drosophila. Overexpression of genes, such as GCL, G6PD, Prx2, and Prx5, which are involved in the maintenance of thiol redox homeostasis, has strong positive effects on longevity. Further, the depletion of peroxiredoxin activity in the mitochondria through the double knockdown of Prx5 and Prx3 not only results in a redox crisis but also elicits a rapid aging phenotype. Critical Issues: Herein, we summarize the present status of knowledge about the main components of the machinery controlling thiol redox homeostasis and describe how age-related redox fluctuations might impact aging more acutely through disruption of the redox-sensitive signaling mechanisms rather than via the simple accumulation of structural damage. Future Directions: Based on these initial insights into the plausible impact of redox fluctuations on redox signaling, future studies should focus on the pathways that have been explicitly implicated in aging, such as insulin signaling, TOR, and JNK/FOXO, with particular attention to elements that are redox sensitive. Antioxid. Redox Signal. 19, 788–803.
Historical Background
Linkage between energy utilization and life span
Studies in the fruit fly, Drosophila melanogaster, have historically made important contributions toward the development of basic concepts in many areas of biology, including the aging process, especially in defining the role of oxygen metabolism in the determination of life span. In 1917, Loeb and Northrop showed that the life span of D. melanogaster is inversely associated with the ambient temperature (61), which, in turn, was later demonstrated to be linked to the rate of oxygen consumption within the physiological range, whose upper limit for long-term survival and optimal fecundity is ∼27°C (67). A major advance in the field of aging that can be largely credited to the Drosophila model was Pearl's (77) interpretation of the effects of temperature on longevity, namely, shorter life spans at elevated temperatures are ascribable to a relatively higher physical activity or the rate of living (ROL). As originally proposed, the length of life of an individual organism is governed by two distinct factors: (i) a genetically determined metabolic potential, that is, the total amount of energy consumed during life, which varies in different genotypes, and (ii) the rate at which this metabolic potential is expended by the individual or the rate of its metabolism. It is a widely recognized phenomenon that within the range of viability, flies and other poikilotherms are physically more active and consume more oxygen at higher than at lower ambient temperatures (103). Similarly, decreases in flying and walking activity, achieved by reducing the size of housing containers and population density, result in longer life spans and vice versa. Indeed, variations in the rate of oxygen consumption induced by virtually any nontoxic regimes have been found to have a corresponding effect on the life spans of Drosophila and other insects (64, 83, 84).
The existence of a close association between ambient temperature, oxygen consumption, and aging in Drosophila was first clearly established by Miquel et al. in 1976 (67). They showed that while longevity of flies was greater at 21°C than at 27°C, flies switched from high to a low temperature, and vice versa, for a part of their existence, had life spans that were intermediate between the two. Flies maintained at a higher temperature also exhibited a relatively more accelerated age-related decline in the speed of walking and a more pronounced deterioration in the fine structure of cells. While the rates of oxygen consumption were relatively lower and life spans were longer at cooler temperatures, the total amount of oxygen consumed by flies kept at different ambient temperatures, or metabolic potential, was quite constant (4–5 ml oxygen/mg body weight). Similar inferences about the existence of an inverse relationship between rate of metabolism/oxygen consumption and length of survival were drawn in other insect species, such as the milkweed bug (66) and the housefly (103). Indeed, no evidence has as yet been presented to contradict the notion that a decrease in metabolic rate/oxygen consumption within the physiological range extends the life span of poikilotherms. Nevertheless, the concept, encapsulated by the ROL theory, has been criticized by some authors, mainly because the life spans of species such as the rat and the pigeon, which have similar metabolic rates, are quite different [discussed in Refs. (72, 104)]. Such a criticism seems to emanate from a misinterpretation of the ROL hypothesis, namely, the total lifetime energy consumption (metabolic potential) of different individuals/species with a comparable metabolic rate is virtually identical. This erroneous notion derives from the conflation of Pearl's ROL theory with Rubner's (98) finding that metabolic energy expended per gram body weight during lifetime in five mammalian species, which varied sixfold in longevity, was relatively constant (∼200 kcal), insinuating that different genotypes have the same metabolic potential. Nevertheless, as originally laid out, the ROL theory implied that longevity of a given individual is an inverse function of the rate at which the metabolic potential is expended, and different individuals have different metabolic potentials, determined by their genetic makeup. Increasing or decreasing the rate at which this potential is used up will shorten or lengthen the life span accordingly. Because the ROL hypothesis is mainly focused on the individual organism, a rigorous test of its validity still remains a daunting proposition [reviewed in Ref. (104)].
Nevertheless, these early studies in Drosophila led to the recognition that the rate of energy consumption, and by extension oxygen utilization, directly affects the length of survival. This relationship is particularly noticeable in poikilotherms, because their metabolic rate, unlike in mammals, is highly variable. The ROL theory, largely based on studies in Drosophila, accorded with the long-established recognition that oxygen is a potentially toxic, although vital, substance for the survival of aerobes [reviewed in Ref. (35)]. A subsequent search for a biochemical explanation of oxygen toxicity and its role in the aging process culminated in the enunciation of the free radical hypothesis of aging (34), which proposed that senescence-related functional losses are caused by the gradual accumulation of macromolecular structural damage, inflicted by the oxygen-derived free radicals, especially the hydroxyl free radical. Based on additional information about the mechanisms of reactive oxygen species (ROS) production, the modes of action of antioxidant defenses, and the nature of macromolecular oxidative modifications, the concept embodying the free radical hypothesis was broadened and retermed as the oxidative stress (OS) hypothesis. In this view, the intracellular rates of ROS production and the antioxidant defenses that eliminate them are chronically imbalanced, implying that the cells exist under a state of OS, which acts as the primary driving force underlying the aging process.
Structural damage-based OS hypothesis of aging
The main features and predictions of the OS hypothesis of aging, also discussed elsewhere (104), are as follows: (i) the gap between antioxidant defenses and ROS production widens during aging, especially during the postreproductive period, due to a decline in antioxidant defenses and/or an elevation in the rates of ROS (H2O2) generation. (ii) The progressive accrual of structural damage with age is presumed to cause the corresponding losses in cellular functions. (iii) Enhancements of antioxidant defenses and/or decreases in ROS generation are predicted to depress the amounts of accrued structural damage and thereby result in delayed physiological manifestations of senescence and an extension of longevity. (iv) Variations in life spans among closely related species should correlate with the levels of structural damage and the rates of ROS production and/or overall efficiency of antioxidant defenses.
The Drosophila model has been extensively employed to test the predictions of the OS hypothesis. Studies on the effects of age on the rates of mitochondrial generation of H2O2, the levels of antioxidant defenses, and the amounts of macromolecular oxidative damage have, in general, accorded with some, but not all, of the main predictions of the hypothesis. For instance, the rate of mitochondrial H2O2 generation, measured in the flight muscle, was found to increase 1.3–2-fold with age (104, 105). Mitochondrial H2O2 is produced by the auto-oxidation of the components of the electron transport chain, and consequently any retardation of the electron relay may cause an elevation in O2−/H2O2 generation. Mitochondrial state 3 or ADP-stimulated rates of oxygen consumption, respiratory control ratios, rates of uncoupled respiration and activity of complex IV, or cytochrome c oxidase (CcO) were observed to decrease significantly as a function of age in the flight muscle mitochondria of D. melanogaster (27). Exposure of mitochondria to KCN, which inhibits CcO activity, elevated the production of H2O2. These findings collectively suggested that enhancement of mitochondrial H2O2 production during aging may be caused by a decline in CcO activity. Further studies indicated that CcO holoprotein progressively loses structural integrity during aging due to differential losses in the levels of constituent polypeptides (91, 106). Another study, involving a comparison among five different species of Dipteran flies, which varied more than twofold in their life spans, showed that the rates of mitochondrial O2−/H2O2 generation were inversely correlated, and the levels of protein carbonyls were directly related to the average life span of the species (105).
Studies in Drosophila and other species have indicated that there is no generalized age-related trend in activities of various antioxidant enzymes, with some showing a decline while others exhibiting an elevation or no change. In Drosophila, activities of catalase and thioredoxin reductase (TrxR) and the ratios of GSH:GSSG and NADPH:NADP+ were found to decrease, whereas superoxide dismutase (SOD) activity tended to increase, with age (102). Since there is no broad improvement in antioxidant defenses, while the rates of O2−/H2O2 production tend to rise, it can be surmised that the level of OS, that is, the imbalance between pro- and anti-oxidants, widens during the aging process. The inference that there is a progressive age-related increase in the intracellular generation of H2O2 has provided an important clue for further explorations into the mechanisms by which ROS may play a role in the aging process, specifically, how increasing the steady-state concentrations of H2O2 affect cellular constituents/functions.
Age-related accrual of macromolecular oxidative damage has indeed been well documented in Drosophila and many other species (104, 116), but whether such damage is in fact the main causative factor or a relatively minor contributor to the concomitant age-related losses in physiological fitness remains controversial. One reason for skepticism is that the age-related increases in the steady-state amount of oxidative damage are neither universal nor proportional to the severity of concurrent losses in physiological functions. For instance, (i) the basal levels of oxidized DNA nucleotides in the cells of young organisms are ∼10−6, while the levels in older animals exhibit relatively small increases, usually in the range of 25%–100% (2, 10, 33, 109). That such a meager increase in absolute damage is functionally consequential seems implausible, based on the available evidence. For instance, oxoguanine DNA glycosylase 1-null mice, deficient in the intramitochondrial removal of 8-oxodeoxyguanine (8-oxodG), a product of DNA oxidation, accumulated several-fold greater 8-oxodG content during aging than the wild-type controls, but showed no discernable deleterious effects on the mitochondrial respiratory function; further, the null mice were phenotypically normal and did not age prematurely (109). Similarly, MnSOD heterozygous mice accumulated 30%–80% greater 8-oxodG content than the controls, but showed no shortening of life span (120). (ii) In rat, mitochondrial protein carbonyl content was found to decrease with age in the liver, remain unchanged in the heart, and to increase in the brain (21). In mice, mitochondrial protein carbonylation increased with age in the skeletal muscle mitochondria of C57BL/6, but not DBA/2 mice (56). Together, such data suggest that oxidative damage may not necessarily increase during aging. (iii) In some cases, significant macromolecular damage is not associated with adverse functional effects; for example, mitochondria in the cerebral cortex of Fischer 344 rats displayed an age-associated increase in the amounts of markers of oxidative damage, such as 3-nitrotyosine, 4-hydroxynonenal, and protein carbonyls, but there was no accompanying loss in mitochondrial respiratory functions (31). (iv) Another set of findings militating against the concept that accrual of the structural damage is the main causative factor in aging is that the oxidized macromolecules are preferentially degraded and would thus tend not to accumulate with age (56, 104). Nonetheless, structural damage could still play a causative role in aging if such damaged molecules are toxic, or not fully replaced by de novo biosynthesis. In the latter case, the net amount of the native undamaged macromolecules would be diminished, and the overall functional capacity of the tissue would be attenuated accordingly. Unfortunately, studies quantifying age-related alterations in the molar amounts of various macromolecules have not as yet been performed extensively. Without such information, any opinions about the role of oxidative damage in the causation of aging remain perforce speculative.
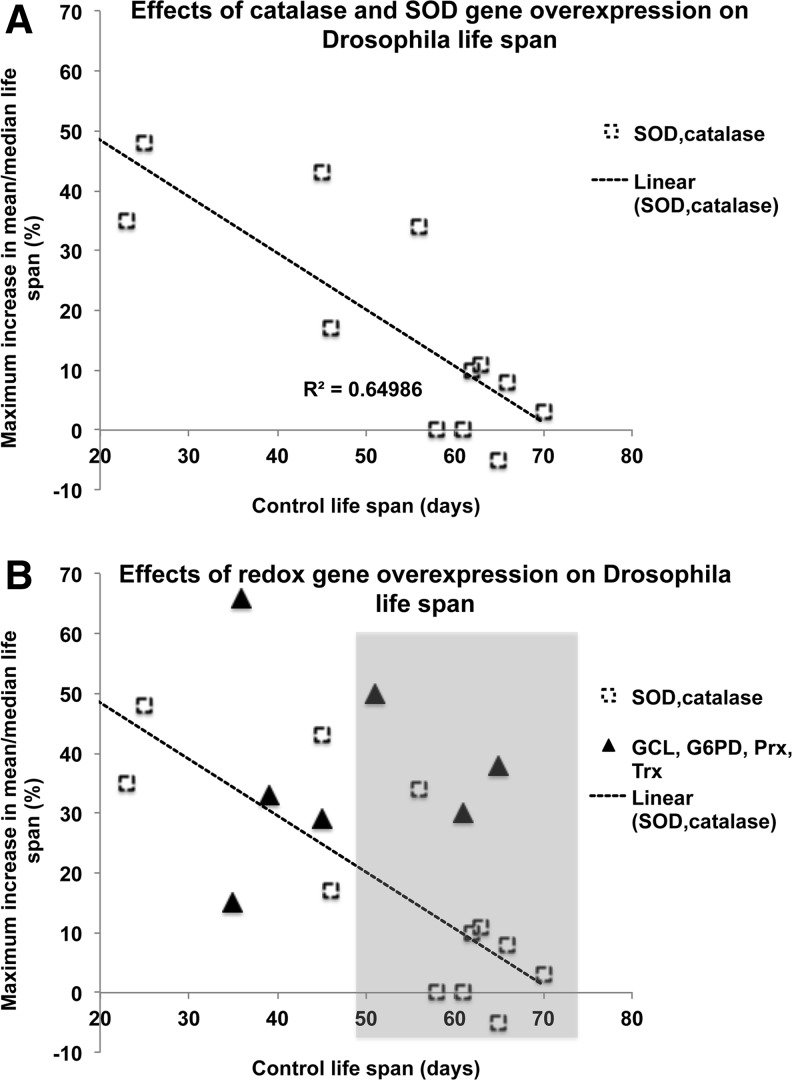
The notion that aging is associated with a shift toward a pro-oxidant state due to an elevation in ROS production, while the antioxidant defenses remain relatively static, spawned a wide array of transgenic investigations in the Drosophila model (72, 99, 102, 117, 130). The main focus of these initial experimental attempts was to augment the levels of enzymes, such as SOD and catalase, given their role in the catabolism of O2− and H2O2. It was reasoned that overexpression of these enzymes would diminish the steady-state levels of ROS/OS and attenuate the age-related accrual of various types of oxidative damage, resulting in extension of the life span. While space limitations here preclude a full treatment, the findings may be summarized as follows: Introduction of extra copies of CuZnSOD, MnSOD, or catalase bearing their endogenous regulatory domains had no beneficial effects on longevity. Co-overexpression of CuZnSOD and catalase was found to have a rather substantial life span extension effect, but proved to be sensitive to background effects, whereby co-overexpression in long-lived flies had only marginal beneficial effects. In the second wave of transgenic studies, these genes were overexpressed either ectopically in a relatively broad manner using constitutive regulatory elements or alternatively in a more targeted tissue- or temporal-specific manner (75). Notably, significant life span extensions were reported in one study involving targeted expression of CuZnSOD to neuronal tissue and in a second series of studies involving the induction of broad expression of either CuZnSOD or MnSOD in adults. These results were encouraging, but again longevity effects displayed a significant sensitivity to background, becoming considerably reduced in longer-lived controls. This relationship can be discerned clearly in the graph presented in Figure 1A, in which maximum reported life-span extensions for all transgenic studies involving SOD and/or catalase overexpression are plotted against their relevant control life spans.
FIG. 1.
Relationship between life span extensions of Drosophila overexpressing antioxidant genes and their respective controls. (A) Life span extensions, reported from various transgenic studies in which levels of SOD and/or catalase were increased, are plotted against their respective control life spans, revealing an inverse relationship (75). (B) Life span extensions reported from transgenic studies reviewed here (51, 54, 69, 74, 81, 100, 119) in which levels of GCL, G6PD, Prxs, Trxs, or TrxRs were increased are plotted against their respective control life spans and are depicted as filled triangles, overlaying the SOD/CAT data from above. Background effects are considerably diminished in this group such that longevity effects in relatively long-lived backgrounds are particularly robust (see shaded area). GCL, glutamylcysteine ligase; G6PD, glucose-6-phosphate dehydrogenase; Prx, peroxiredoxin; SOD, superoxide dismutase; Trx, thioredoxin; TrxR, thioredoxin reductase.
These relatively disappointing results, coupled with the appearance of a series of reports, discussed above, suggesting that age-related increases in oxidative damage were relatively minor, have undermined the long-held presumption of a causative link between aging and accumulation of oxidative damage, and have prompted a re-evaluation of some of the core assumptions of the OS Hypothesis of Aging. Accordingly, a substitute hypothesis, termed the redox state hypothesis of aging, has been proposed (104).
The Redox Stress Hypothesis of Aging
During the past two decades, our understanding of the nature of the mechanisms causing OS and the biological role/significance of ROS has been fundamentally altered. Previously, OS/damage was believed to arise from one-electron oxidations of macromolecules by free radicals. However, this notion has been replaced by the view that OS in vivo is primarily caused by nonradical two-electron oxidants, such as H2O2, rather than by one-electron oxidants. Another departure from the previous concept that held that ROS are invariably toxic in nature was modified to accommodate the evidence that protein activity/function in cell signaling can be regulated by oxidation/reduction of specific protein cysteinyl thiols. H2O2 along with certain other stable products of macromolecular oxidation may act as second-messenger signaling molecules by modifying specific cysteine thiols of signal-transducing proteins, thereby altering their function. Typically, under physiological conditions, generation of H2O2 or an alteration in the cellular redox state is believed to cause modifications of specific protein thiols that include, among others, the formation of sulfenic acids, glutathionylation, or intra-/interprotein disulfide formation, which result in a reversible gain/loss of protein function. Such modifications are reversed by the thioredoxin (Trx) and glutaredoxin (Grx) systems. Thus, the thiol/disulfide redox states of oxidation-prone cysteine thiols act as functional switches for a large set of proteins in cells. Under in vivo conditions of OS, such as those during aging, where H2O2 production is elevated, the thiol/disulfide signaling mechanisms may consequently be disrupted. Sustained increases in the H2O2 concentration would cause enhancement in the levels of both reversible thiol oxidation, such as sulfenic acids, intra-/interprotein disulfides, and mixed protein disulfides, and also in the amounts of irreversibly oxidized thiols, such as sulfonic acid, thereby increasing the disulfide load/state of the redox sensitive proteins [reviewed in Ref. (41)].
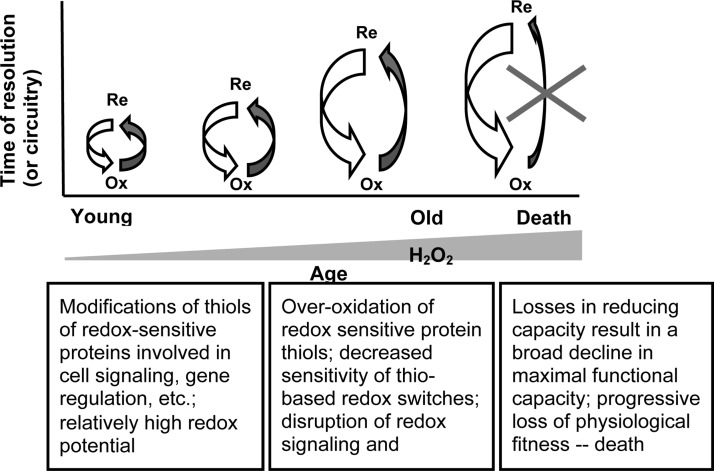
Accordingly, to reflect the state of current evidence, as detailed below, we proposed that the OS hypothesis of aging should be replaced by the redox stress hypothesis, which stipulates that the age-associated pro-oxidizing shift in the cellular redox state promotes oxidation of the cysteinyl thiols of redox-sensitive proteins, and thus compromises the efficiency of cell signaling networks (104). In this scenario, the post-translational modifications of the redox-sensitive proteins alter protein function and represent the primary factor in the causation of deleterious senescent alterations. It is perhaps instructive to envision this process from a kinetic vantage point, specifically the rate of interconversion between protein thiols in their reduced and oxidized states, as depicted in Figure 2. Thus, in younger animals, the thiol/disufide ratio is relatively high, and oxidation events resulting in modification of redox-sensitive protein thiols are efficiently reversed, often by means of TRX- and GSH-related enzymatic machinery. With age, the cellular milieu becomes more pro-oxidizing, and the thiol/disulfide ratio declines. Both the degree and level of protein thiol oxidation are enhanced, resulting in a slower reversion of the post-translational modifications, dictated by either the need to replace modified protein through turnover, or, as in the case of sulfinic acid reduction, reliance on the kinetically slower reactions of the sulfiredoxin (Srx) enzymes (39, 92). As members of the enzymatic resolution machinery (TrxR, peroxiredoxin [Prx], Trx, and Grx) are themselves redox-sensitive, the thiol/disulfide switching mechanisms would be further compromised, and it is imagined that in older animals, this would result in the loss of efficiency in the maintenance of redox homeostasis.
FIG. 2.
Schematic changes in the resolution of redox switches with age. Increases in O2−•/H2O2 production in postreproductive adults cause both reversible and irreversible modifications in protein thiols. Reaction kinetics of thiol reduction will vary as a function of the degree of oxidation of both the target protein thiols as well as the Trxs/Grxs/Srxs that reduce the oxidized thiols belonging to the target proteins. Thus, the reduction of Prx S-S or Prx SOH by Trx or GSH+GST is considerably faster than the reduction of Prx SO2H by Srx (39, 92, 94). The heights of the curved arrows represent the relative rate of resolution of these post-translational modifications as a function of the increasing pro-oxidizing state that accompanies age. Components of the redox system are themselves subject to inactivation via oxidation of functional thiols, further enhancing the disruption of these redox switches. Grx, glutaredoxin; GST, glutathione-S-transferase; Srxs, sulfiredoxins.
Redox-Sensitive Factors/Reactions and Aging in Drosophila
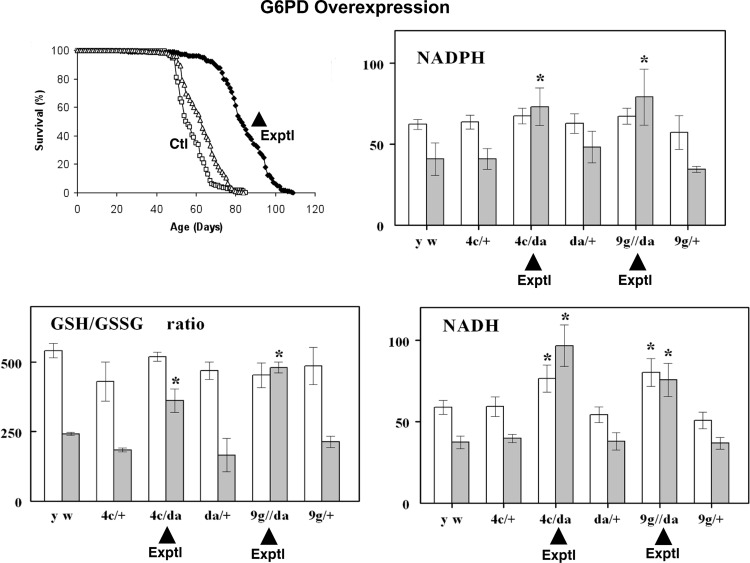
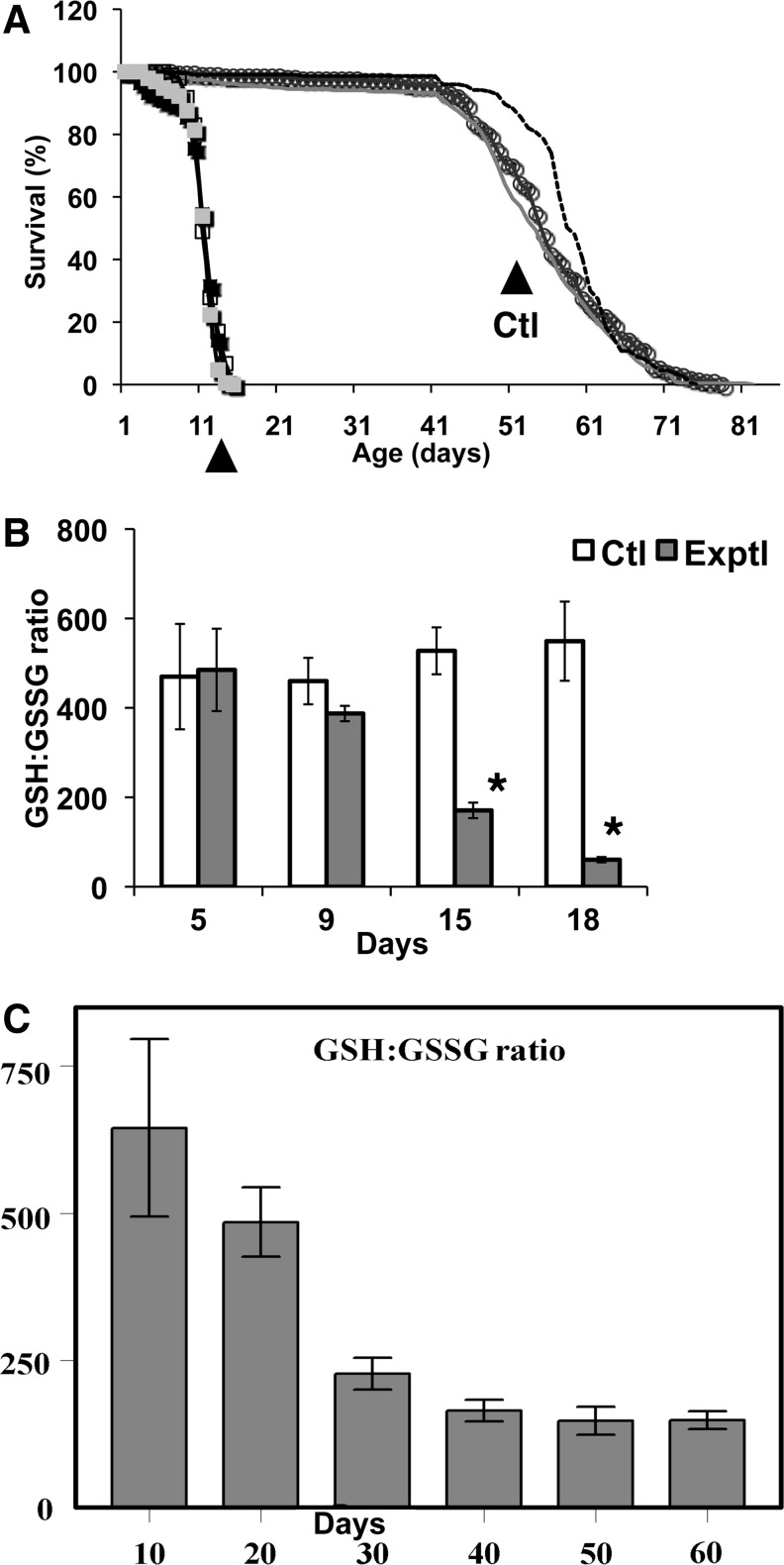
Characterization of age-related biochemical alterations has shown that perhaps the most ubiquitous changes involve factors that are responsible for maintaining the redox state of the cellular milieu. Indeed, changes in the ratios of the primary redox couple, reduced/oxidized glutathione (GSH), and the levels of mixed protein disulfides have been found to consistently display a pro-oxidative shift in aging fruit flies (86, 89, 90, 102) as well as rodents (87, 88). These observations led us to investigate further whether transgenic enhancement of the GSH–NADPH redox system would slow down the aging process and extend the life span. Increased production of these reducing species by overexpression of the enzymes involved in their synthesis, namely, either glutamylcysteine ligase (GCL) (GSH synthesis) or glucose-6-phosphate dehydrogenase (G6PD), a component of the pentose phosphate pathway whose activity is the predominant source of NADPH, was found to confer extension of the Drosophila life span by up to 50% (54, 74). The strongest effects on longevity were observed upon targeted overexpression of a catalytic subunit of GCL in neuronal tissues, while global overexpression of GCLc exhibited beneficial longevity effects at low levels (∼2–3-fold), but deleterious effects at higher levels (3–10-fold) (62, 74). The longevity effects were not accompanied by any evident health span tradeoffs, based on the measures of reproduction, physical activity, and oxygen consumption. Similarly, G6PD overexpressed in neuronal tissues and when expressed globally also exhibited beneficial longevity effects (up to 40%, Fig. 3). Extensive biochemical analysis of the G6PD overexpressors permitted us to document a significant increase in the redox potential of tissues, as reflected by elevation in the levels of NADPH, NADH, and GSH:GSSG ratios (Fig. 3) (54). In contrast, genetic manipulations that caused a severe pro-oxidant shift in redox state, or a redox crisis, appeared to result in an accelerated senescence phenotype. Such manipulations included suppression of GSH biosynthesis by underexpressions of GCL or of mitochondrial Prxs, enzymes known for their major role in the maintenance of the cellular redox state. A decrease in GCLc expression by ∼95% resulted in an ∼30% shortening of life span and an enhanced susceptibility to oxidative stressors. The underexpression of Prxs caused a several-fold decrease in the GSH:GSSG ratio, a depletion in protein sulfhydryl content, an increase in apoptosis and a dramatic shortening (∼80%) of life span (62, 82) (Fig. 4). Notably, similar, although less-severe, biochemical alterations also occurred in flies undergoing normal aging (86, 128).
FIG. 3.
Increased longevity and restoration of redox balance in flies overexpressing G6PD. Figures are adapted from Legan et al. (54) with overexpressor flies denoted as Exptl and control flies as Ctl. Life span of flies overexpressing G6PD was increased by up to 40%. Significant elevations in the NADH and NADPH levels as well as the GSH:GSSG ratio were observed in older fly lines ( filled bars) overexpressing G6PD (denoted by a black triangle) relative to controls. The reversion to a younger redox potential in older flies overexpressing G6PD correlated with extended longevity. GSSG, glutathione disulfide. *, denotes significant differences between experimental and age-matched controls (p<0.001).
FIG. 4.
Shortened life span and rapid onset of GSH:GSSG reduction in mitochondrial Prx mutants. (A) Under-expression of mitochondrial-localized Prxs causes significant shortening of lifespan (up to 20% of control) (B) Changes in the redox state as measured by the GSH:GSSG ratios display early onset and are significant by day 15 relative to controls (open bars=control, filled bars=mitochondrial Prx double mutant). At 15 days, * represents p=0.021 and at 18 days, * represents p=0.016. (C) A similar, although delayed, reduction in the GSH:GSSG ratio is a characteristic of normal aging. Panels (A) and (B) are drawn from Radyuk et al. (82), and panel (C) comes from Rebrin et al. (86).
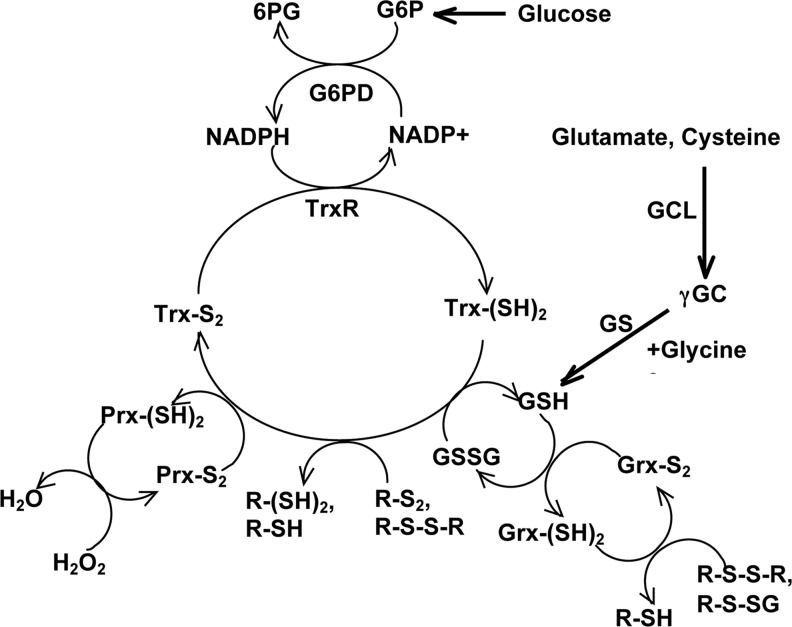
GSH and NADPH serve as the two most preeminent suppliers of reducing equivalents: they act as electron-donating coenzymes/cofactors in the catalyzed eliminations of ROS and play a central role in the maintenance of redox homeostasis. As alluded to above and as discussed in a recent review (104), the present incarnation of the OS paradigm favors the notion that rather than the accumulation of the structural damage, it is the breakdown in redox-based signaling due to age-associated changes in redox homeostasis that may serve as the more proximate causative agent in senescence. From this perspective, the maintenance of redox homeostasis becomes a critical factor in the determination of life span. The main enzymatic components maintaining this system are depicted in Figure 5.
FIG. 5.
Interactions among the redox maintenance system in Drosophila. Cellular reducing equivalents NADPH and GSH are generated through the reactions catalyzed by G6PD, GCL, and GS (see text for discussion). R, an alkyl group; 6GP, 6-phosphogluconate; γGC, gamma-glutamylcysteine; GS, glutathione synthase.
Central to this scheme are the Prxs, which act as reductants of peroxides, including H2O2, and are in turn reduced by Trx. Oxidized thioredoxin (Trx-S2) is reduced by TrxR, which then receives reducing equivalents from NADPH. In addition, Trx catalyzes the reduction of protein disulfide linkages as well as GSSG. GSH is required for the reduction of Grx, which catalyzes the deglutathionylation of protein mixed disulfides (R-S-SG and R-S-S-R). This redox maintenance system relies on the availability of functional thiol groups in the active centers of Trx, Grx, Prx, and TrxR. Numerous studies support the view that overoxidation of these thiol groups would perturb redox signaling. One experimental strategy to counteract redox stress could be the augmentation of the levels of enzymes involved in the redox balance. We describe here the results of studies designed to test this notion, particularly focusing on the Drosophila model.
Peroxiredoxins
The Prxs comprise a family of peroxidases that can modulate the redox state due to their ability to eliminate H2O2 and organic peroxides via a thiol-dependent mechanism. The Prx family in Drosophila consists of 7 members, distinguishable by their structure, number of functional cysteines, subcellular localization, and mode of elimination of the reactive intermediates. The effects of the variations in the levels of these proteins on survival of flies under normal and stress conditions were demonstrated in studies involving over- or underexpression of Prxs. As of yet, two Prxs were found to affect aging in flies, Jafrac1 (also known as Dpx4783 and cTPx) and dPrx5. Jafrac1 was identified as a homolog of the mammalian Prx2, based on the features of the amino acid sequence (53) as well as the ability of the human Prx2 gene to rescue the jafrac1 mutant effects, such as mitochondrial dysfunction characterized by reduction in the ATP levels and depletion of mitochondria, and greater susceptibility to the OS, induced by paraquat (24, 51). Targeted overexpression of Jafrac1 in neuronal tissue not only conferred resistance to paraquat but also extended the life span of flies under normoxia by up to 29% (in males) and 26% (in females) (51). The stress resistance, conferred by Jafrac1 overexpression, correlated with a decrease in ROS levels in the fly brains, underscoring its role in neuronal tissue. Localization studies revealed that Jafrac1 was expressed in a global manner, although the levels were particularly elevated in the brain, imaginal discs, and malpighian tubules (51, 95). In mammals, Prx II was also found to be expressed in the brain (59) and a wide variety of other tissues (58). In a series of epistatic studies, Jafrac1 expression was shown to be linked to FOXO/JNK signaling, exhibiting upregulation in response to the oxidative stressor, paraquat, in a manner that was both FOXO- and JNK-dependent. Further, Jafrac1 seemed to function in a feedback loop, that is, inhibiting phosphorylation of the JNK kinase, Basket, and thus attenuation of the JNK signal (51).
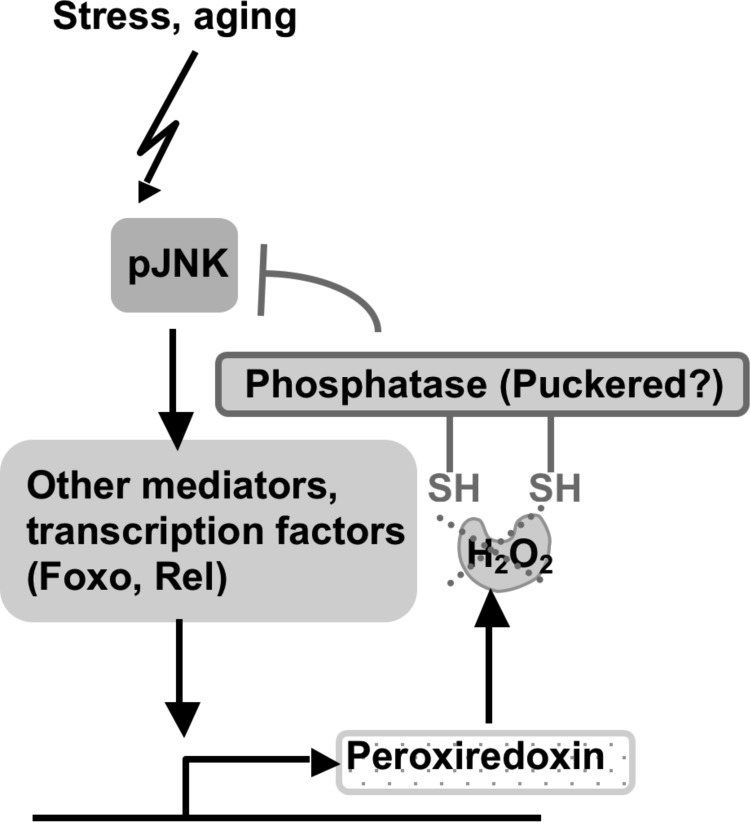
Another Prx that has strong effects on the life span in Drosophila is the Prx subtype 5, dPrx5. This Prx was identified as a strong antioxidant, based on in vivo studies where absence of dPrx5 resulted in enhanced susceptibility to H2O2 and paraquat- and tissue-specific apoptotic changes normally observed in older flies (81, 128). Conversely, broad overexpression of dPrx5 conferred marked resistance to OS and a life span extension of >30% (81, 128). The response to immune stressors, such as bacterial infection, was also attenuated in the transgenic flies (80). This effect is notable in that constitutive overactivation of the immune response is a known signature feature of aging (49), and its experimental overactivation has been shown to shorten the life span significantly in the fly (57). In response to a microbial challenge, Jafrac1 was found to act as a negative regulator of JNK activation (80). Studies performed by Ahn et al. also revealed the association of dPrx5 with JNK/FOXO signaling in fly gut epithelial cells during the immune response (4), suggesting that dPrx5 modulates activation of JNK signaling by affecting phosphorylation of the JNK kinases (Fig. 6). In turn, induction of Prx5 in gut epithelial cells is dependent on both JNK kinase (Basket in Drosophila) and Foxo (4). Altogether, data suggest that the JNK/FOXO module is a primary target of Prx-mediated signaling that affects resistance to stresses and the life span in Drosophila. The data also suggest a circuitry in the Prx/JNK/FOXO-mediated responses. Thus, a stressor such as OS may initially activate the JNK/FOXO signaling pathway, which in turn induces expression of various protective effectors, including Prxs. Subsequently, the Prxs are thought to attenuate the JNK signaling pathway by reducing phosphorylation of the JNK kinases in what constitutes a feedback loop.
FIG. 6.
Prxs as regulators attenuating signaling pathways in a feedback loop. The activity of JNK kinase is regulated by phosphorylation/dephosporylation through upstream kinases and coupling phosphatases. Prxs may modulate JNK signaling by protecting from oxidation those functional cysteines associated with the coupling phosphatase (Puckered) (4, 51, 80).
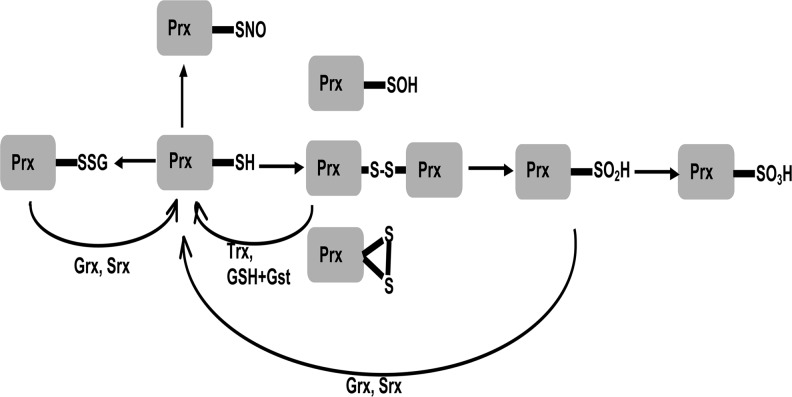
Current evidence suggests that Prxs are largely regulated post-translationally through functional cysteines, which can be reversibly inactivated by various modifications, such as glutathionylation (13, 76) or oxidation beyond the reversible sulfenic (SOH) and sulfinic acid (SO2H) states to the irreversible sulfonic form, SO3H, as well as other modifications, rendering the Prxs nonfunctional [reviewed in Refs. (93, 94)] (Fig. 7). Different Prxs are differentially susceptible to overoxidation; for example, Prx2 is more easily overoxidized compared to Prx1 or Prx3 (108), while Prx5 is relatively resistant (48). Replenishment of Prx at the post-translational level is mediated by Trx, Grx, or Srx (93, 94), or by GSH and glutathione-S-transferase (GST) (85). It is unclear whether JNK/FOXO-mediated responses include activation of these proteins. However, accumulation of the overoxidized Prx2 in the mutant for JNKK Hemipterous (51) would suggest that compromised JNK signaling results in a failure to promote Prx recycling.
FIG. 7.
Post-translational modifications of Prx functional cysteines. Thiols are oxidized to sulfenic acid and disulfides (reversible by Trxs or GSH and GST), or further oxidized to sulfinic acid or inactivated by glutathionylation (reversible by Grxs and Srxs). Inactivation of Prxs by oxidation to sulfonic acid or S-nitrosylation is considered irreversible.
Sulfiredoxins
Besides Trxs and GSH, a group of proteins known as the Srxs participates in Prx recycling by reducing cysteine residues that have been overoxidized to sulfinic acid or glutathionylated (14, 76). Analysis of these enzymes is in the early stages, and as such, their significance has yet to be clarified. While studies as of yet have been largely restricted to the mammalian models, at least one putative Srx gene, designated as CG6762, has been identified in Drosophila, but it currently lacks validation.
Thioredoxins
Trxs are a family of low-molecular-weight (∼12 kDa) dithiol–disulfide oxidoreductases, which catalyze the reduction of disulfides. They contain a highly conserved active center with two reactive cysteine residues that undergo oxidation to the corresponding disulfide by transferring two reducing equivalents from Trx to a disulfide-containing substrate, such as protein disulfides or GSSG. The resulting disulfide at the Trx active site is in turn reduced by TrxR, which catalyzes the transfer of two reducing equivalents from NADPH to Trx. In Drosophila, Trxs play an important role in maintaining redox homeostasis, as they function as the principle reductants of GSH (15, 45).
The role of Trxs in the aging process was initially investigated by Mitsui et al. (70) by expressing human Trx in mice. The recipient mice exhibited relatively higher levels of Trx activity as well as extensions in both median and maximum life spans. However, the significance of this finding remained controversial because of the relatively short life spans of the controls. A replication of this finding in a different background did not confirm the extension of a maximum life span, but did show delayed death in males during the initial part of life span (78). In Drosophila, out of 11 predicted Trxs, 3 classical Trx genes have been identified (deadhead [dhd], Trx-2, and TrxT), and mutants for all 3 have been characterized. The dhd and trxT genes form a cluster on the X-chromosome, and both appear to be germ-line specific, dhd being associated with the nurse cell nuclei and karyosomes of the ovary, and trxT with the spermatocytes (111). While Trx-2 has been colocalized with Trx-T, it is also expressed at significant levels in somatic tissues, and thus represents an attractive target for the evaluation of longevity effects. Trx-2 (synonym CG3864) was detected during a search for the longevity-associated genes (100). The absence of Trx-2 shortens longevity significantly relative to the wild-type flies or flies lacking either of the other main Trxs. Moreover, the trx-2;dhd and trx-2;trxT double mutants were similar in longevity to the trx-2 single-mutant flies. Using a P-element Trx-2 transgene, it was possible to not only rescue the longevity deficits for all of the mutants, but also to slightly extend the life span relative to the wild-type control under both normal conditions and under OS (exposure to paraquat) (112). Because only one transgenic line was used and because the randomness of transgene insertion in the genome can have a confounding effect, the results of these experiments should be regarded as tentative. In a second study with trx-2 mutants, the loss of this Trx not only caused hypersensitivity to paraquat treatment but also elicited a large increase in protein carbonyl levels, particularly in the older flies. While the mutant flies tended to mount an adaptive response in the form of increased levels of antioxidant defenses, including SOD1, catalase, and GSH synthetase, the GSH levels remained depressed (118). Compensatory upregulation of the other Trxs (dhd and TrxT) was not observed.
A series of studies by Umeda-Kameyama et al. (119) have indicated that targeting of Trxs to neuronal tissue results in ameliorative effects (ca 50% or more in old flies) in the Drosophila models of neurodegeneration. In a Parkinson's disease model, created by misexpression of Pael-R, a substrate of the Parkin gene, pan-neuronal overexpression of any of the three Trxs (Trx-2, dhd, or TrxT) caused an attenuation of damage to dopaminergic neurons and improvement in locomotory deficits, characteristic of age-dependent Parkinson's disease. The rescue effects were particularly evident in aged flies. Similarly, overexpression of Trxs suppressed neurotoxicity caused by expression of polyglutamine in a Drosophila model of Machado-Joseph disease (119). In one instance, overexpression of TrxT by the elav-GAL4 driver, which permits broad expression in neuronal tissues, resulted in life span extension. While tantalizing, these results need to be more rigorously controlled for position effects, given the use of only a single TrxT transgenic line and one control (119).
In recent studies, potential Trx targets and regulators that might be involved in regulating survivorship under normal and stress conditions have been identified. The Trx, Trx-2, was found to genetically interact with an extramitochondrial variant of the apoptosis-inducing factor (AIF) to mediate cytotoxicity, and thus participate in the regulation of cell death (42). AIF carries domains that classify it as a redox-active oxidoreductase, and was originally identified as a mitochondrion-localized protein, which translocates to the nucleus upon proapoptotic stimuli, where it triggers fragmentation of DNA (110). However, it is not yet established whether similar mechanisms exist in Drosophila, as the capacity of the Drosophila ortholog to trigger apoptosis and interact with Trx-2 was demonstrated only with its truncated form, which lacks a mitochondrial-targeting signal, and is thereby misdirected to extramitochondrial compartments (42). On the other hand, both Trx-2 and AIF were among 16 identified genes, required for survival from fungal infection (40), suggesting that the two genes are inter-related and involved in mediating common signal transduction pathways that modulate responses and adaptation to environmental challenges.
Another Trx, TrxT, was induced in response to MnSOD overexpression, which had previously been reported to extend the life span (18). From this, the authors surmised that MnSOD overexpression might elicit increases in H2O2 production that would activate various stress pathways, such as NF-kappa B and JNK, which in turn could activate downstream effectors such as TrxT. While attractive, there is as yet no experimental validation of this hypothesis.
Thioredoxin Reductases
Unlike the mammals, where GSSG is reconverted to GSH by the activity of GSH reductase, in Drosophila this function is largely assumed by TrxR, which functions to reduce both Trx and GSSG (45, 68). According to the latest FlyBase report, there are two genes encoding TrxR in Drosophila, trxr-1 and trxr-2. trx-1 specifies three distinct isoforms, produced by alternative splicing. All three include a large common domain, comprising the catalytic C-terminus and an FAD-binding site, both of which are necessary for catalytic activity, but differ with respect to their N-terminal sequence, which apparently determines their differential subcellular localization. One of the isoforms, isoform B, is localized to the mitochondria, while the TrxR1-A isoform is cytosolic (69). Mutations in the trxr-1 gene that affect expression of either the cytosolic or mitochondrial forms, or both, were lethal (69). The third isoform, alternatively transcribed from the trx-1 gene, as well as the products of a second trxr-2 gene, remains to be characterized experimentally.
The functional impact of TrxR-1 A (cyt) and TrxR-1 B (mt) in vivo has been inferred from a series of mutant/transgenic studies (69). Hemizygous mutants affecting expression of either the cytosolic or the mitochondrial TrxR-1 isoform, or both, had a significant life span-shortening effect, which was rescued by ectopic expression. Mutants lacking the cytosolic isoform could be rescued by ectopic expression of the mitochondrial form, but the cytosolic isoform could not compensate for the absence of the mitochondrial isoform. Although the differential rescue effects of TrxRcyto and TrxRmito ectopic expression have been demonstrated in the trxr-1 mutants using the UAS/GAL4 system, their effects on aging have yet to be investigated in a wild-type background. In an earlier study, the role of Drosophila TrxR-1 in the aging process was tested by transgenic overexpression of TrxR1 from a large (∼8 kb) genomic fragment, containing the entire coding domain as well as the endogenous promoter (71). While no effects on longevity were revealed under normal conditions, transgenic lines overexpressing TrxR-1 were more resistant to exposure to 100% oxygen. Consistent with this finding was the observation that TrxR-1 expression was induced in response to the administration of OS (11).
While the absence of longevity effects, shown in a single study, supports the notion that overexpression of TrxR in tissues where it is normally found at significant levels confers beneficial effects only in the presence of acute OS, a more rigorous tissue and temporal-specific investigation is needed. This is underscored by our finding that enhanced GSH production has positive effects on aging when GCLc, a rate-limiting enzyme in the GSH synthesis pathway, is overexpressed in neuronal tissues, but not when overexpressed constitutively at high levels (74). If maintenance of the GSH redox ratio in neuronal tissue is indeed critical, appropriate targeting of TrxR might also have positive longevity effects.
Glutaredoxins
Like Trxs, Grxs are oxidoreductases, which specifically catalyze the deglutathionylation of proteins, utilizing cysteine residues in their active sites. Grxs have been studied in a variety of contexts, including sulfhydryl homeostasis and regulation of redox signal transduction. The ability of Grxs to regulate protein S-glutathionylation suggests that it could play a vital role in controlling redox-responsive protein activity and, by extension, may critically impact disease progression [see Ref. (124) for a comprehensive review on these issues].
As of yet, the role of Grxs in the aging process has received scant attention; however, a study conducted by Gallogly et al. (30) suggested a connection between age-related Grx-1 loss and apoptosis. Specifically, cardiomyocytes derived from old rats were found to exhibit an ∼40% decrease in Grx activity compared to cells from young adults. The Grx-1 loss was correlated with a reduction in NF-kappaB transcriptional activity and a decrease in production of the anti-apoptotic NF-kB target gene Bcl-xL. A comparable (∼50%) decrease in Grx activity in H9c2 cardiomyocytes, induced by siRNAi was also found to cause a drop in NF-kappaB transcriptional activity and a marked increase in apoptosis (30).
To the best of our knowledge, the role of the Grx genes in the aging of Drosophila has not been investigated. One Grx is encoded by the clot gene (CG11024), whose product has been shown to act in the biosynthetic pathway of Drosophila eye pigments (drosopterins) (32). Based on the deduced amino acid sequence, it contains the Cys-Pro-Tyr-Cys motif, a signature of Grx, but it cannot yet be clearly distinguished from a possible Trx-like protein. There are two additional Drosophila candidate homologs corresponding to the mammalian Grx-1 (CG7975) and Grx-2 (CG6852) genes. However, in the absence of functional analysis, both of these genes remain virtual.
Perspectives and Future Directions
While the currently available information is relatively meager, it strongly suggests a likely connection between the thiol redox state and the aging process. Initial studies on the genes involved in the maintenance of thiol homeostasis have shown that their overexpression has strong positive effects on longevity. In Figure 1B, transgenics overexpressing the redox-related genes (GCL, G6PD, Prx, and Trx) are compared to SOD and/or catalase overexpressors, based on life-span extension relative to control life span. It appears that the positive effects of these redox-related genes are less sensitive to background, such that strong positive effects are observed in relatively long-lived flies (shaded area). In addition to these encouraging life span effects, it is notable that, at least in some cases, the level of their products has been documented to falloff with age. Such preliminary evidence supports the need for additional studies elucidating the mechanisms by which the redox-sensitive targets/pathways affect the aging process. Insights garnered in such studies may then serve as the basis for the development of specific redox-based intervention strategies to slow aging and enhance health span.
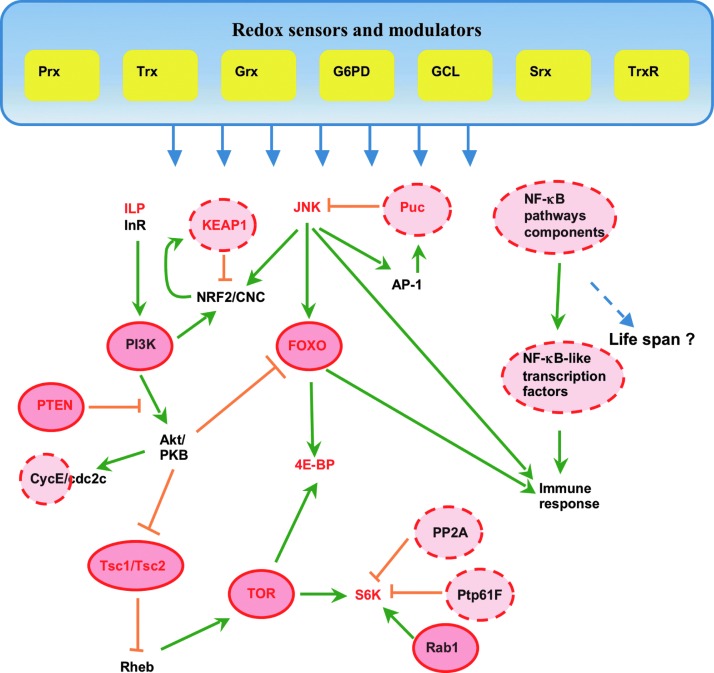
Among pathways worthy of further exploration are some of the now iconic aging pathways such as insulin signaling (InS) and TOR, as well as the various interacting pathways that include JNK, NRF2, and NFKB (among others). Both InS and TOR are nutrient-sensing pathways, conserved in many organisms from yeast to humans, and are well established for their association with longevity. Mutations resulting in the reduction of InS in worms were found to increase the life span and confer greater resistance to OS, and these effects were shown to be dependent on DAF16 (FOXO) mobilization to the nucleus (47, 52, 60). Shortly thereafter, similar longevity effects were reproduced in the Drosophila model (16, 114). Longevity-associated effects of the TOR pathway were identified in Drosophila in a series of screens for mutants or overexpressors that extend life span, where the first identified component was S6 kinase, a downstream effector of TOR [reviewed in Ref. (46)]. The effects of reduced activity of both the InS and TOR pathways largely overlap with those elicited by dietary restriction [reviewed in Ref. (28)], and like DR, they appear to induce a shift from growth and reproduction toward somatic maintenance. Some of the interactions involving these two pathways and others are depicted in Figure 8.
FIG. 8.
Cumulative scheme adapted from the data published in (1, 7–9, 43, 46, 63, 113, 115) showing components of pathways implicated in regulating the life span in Drosophila and their interactions. Potential redox-sensitive targets are circled in dashed red, and established redox modifications are circled in red. Components with life span-extension effects are indicated by red letters. Genes involved in redox homeostasis may act as redox sensors and modulators of signaling pathways implicated in longevity. InS, TOR, JNK, and NRF2 pathways are experimentally determined to extend the life span in Drosophila and other organisms. TOR signaling is activated by the upstream kinases PIP3 and Akt, which respond to different signals mediated via InS. Two TOR effector targets are kinase S6 and 4E-BP, an initiation factor eIF4E-binding protein. GTPase Rab1 and the phosphatases Prtp61F and PP2A are modulators of the TOR pathway and affect phosphorylation of kinase S6. JNK activity is modulated by the phosphatase Puckered. TOR, InS, and JNK signaling integrate at FOXO, and both FOXO and JNK signaling affects the Drosophila immune response. PTEN modulates both InS and TOR signaling and thus constitutes another key regulatory site sensitive to redox. NRF2 activity (CNC in Drosophila) is regulated by PI3K and JNK kinases among others and through its interaction with redox-sensitive protein KEAP1. InS, insulin signaling. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.
Within the InS and TOR pathways, there exist a number of candidate redox-sensitive targets, which include phosphatases (PTEN and PTP1B), kinases (PI3K, ASK1, PKB, MEKK1, and TOR), members of the FOXO family [reviewed in Refs. (19, 23)] and the TSC1/TSC2 complex (125). As may be observed in the scheme, a number of these targets are held in common, and unwinding the potential crosstalk between these pathways and others promises to be a fruitful area of investigation in the future. The phosphatase, PTEN, seems to be particularly interesting, as it contains a cysteine sulfhydryl group that in the presence of H2O2 is readily converted to a sulfenic residue, which in turn can undergo various reversible modifications. Should such modifications diminish enzyme activity, a possibility in older animals, it would result in both suppression of FOXO and activation of TOR, events associated with more rapid aging. The redox-sensitive nature of this modification is underscored by studies in mice, where it was found to be sensitive to Prx levels that presumably prevent oxidation of PTEN catalytic cysteines by controlling local H2O2 fluxes (12). Moreover, increasing the levels of Srx, known to protect against over oxidation in Prxs, resulted in an increase in the PTEN levels (55).
The positioning of FOXO in the scheme, depicted in Figure 8, would suggest that it plays a key role in the integration of multiple signals. While the redox properties of the Drosophila FOXO have yet to be determined, evidence for redox-sensitive regulation of Foxo4 in mouse cells was recently obtained in studies by Dansen et al. (20). Upon exposure to H2O2, Foxo4 forms a heterodimer with p300 acetyltransferase via formation of a disulfide bridge. Once the heterodimer is formed, p300 acetylates Foxo at a lysine residue and thus inhibits its transcriptional activity. The dynamic resolution of the heterodimer complex is in turn regulated by Trx. This thiol-dependent regulation of FOXO was supported by both substitution of critical cysteine residues and treatment with NAC, which abrogated the p300–lysine interaction (20).
A connection between JNK signaling and FOXO has been amply demonstrated in the fly model (51, 121, 122). In response to OS, JNK signaling fosters nuclear localization of FOXO, and the upregulation of key redox effectors (Prxs, Trx-interacting proteins [TXNIP]) as well as various detoxification agents such as GSTs and cytochrome p450's (Cyp450) (5, 6, 22, 51). Since similar GST activation was also observed in long-lived GCL overexpressor flies (79), it would be of interest to determine whether the longevity effects might be connected to the redox-sensitive signaling orchestrated through the JNK/FOXO pathway. There exist multiple redox-sensitive targets in the JNK pathway both upstream (MEKK1 and ASKI) and downstream. Of particular note is the phosphatase puckered, which is upregulated in response to JNK signaling, and whose principle target is JNK kinase Basket (8, 121). The presence of a redox-sensitive cysteine renders this species sensitive to redox fluctuations that would contribute to the upregulation of the innate immune response observed in older animals. Indeed, the other major component known to regulate the immune response in Drosophila and mammals is the NF-kappaB pathway, and it too has been shown to be subject to redox regulation [reviewed in Ref. (44)]. Finally, studies on NRF2/KEAP1 signaling in Drosophila have revealed a role for this redox stress-response pathway in aging (113), perhaps by its upregulation of genes that affect redox, including Prxs, Srxs, and GCL (37, 107). By analogy with its mammalian counterpart, it is likely to be itself a subject of redox regulation. Indeed, activity of human or murine KEAP1 depends on disulfide formation or S-nitrosylation of cysteines, caused by changes in redox (29, 36), and the redox environment has been shown to impact formation of the intramolecular disulfides and glutathionylated cysteines in vitro (38). However, it is yet to be experimentally determined whether the function of Drosophila KEAP1 is also dependent on such cysteine modifications.
Epigenetics, Redox State, and Aging
The word inexorable is often used to characterize the aging process, and it has been a subject of pressing importance to determine what might underlie this apparent one-way progressive deterioration. What are the changes that become irreversibly imprinted in memory of cells, whereby aging like development becomes a unidirectional irreversible process, and how might fluctuations in redox state impact this dynamic phenomenon? A flurry of recent studies has identified a wide array of so-called epigenetic changes that accompany the aging process, and these are thought to have significant modulatory effects on aging. Epigenetic factors that are considered to drive changes in gene expression, including age-related fluctuations in noncoding RNAs, comprise modifications of histones (methylation, acetylation, ADP-ribosylation, etc.) as well as DNA (primarily methylation). In one scenario, the accumulation of such modifications might slowly restrict the cell's ability to maintain homeostasis through its resident gene expression programs and thus serve to dictate what one might term an aging program. How such age-associated changes come into being is of course a matter of great interest, and here the poise of the redox state may not be inconsequential.
At the present juncture, there is ample evidence that changes in epigenetic modeling cause alterations in gene expression, including direct effects on the redox-related genes. For instance, chemical or genetic inhibition of histone methylation has been found to upregulate the expression of TXNIP, resulting in inhibition of Trx activity (129). Hypermethylation of the CpG sites in the Nrf2 promoter resulted in reduced levels of Nrf2 transcription, as well as a set of downstream GST genes (65, 126).
While investigations into the possible impact of redox state on epigenetic states have been minimal, recent studies have implicated the redox effects on histone acetylation through the oxidative modulation of histone deacetylase (HDAC) activity (73). The function of HDAC and nucleocytoplasmic translocation depend on the formation of a disulfide bridge between conserved cysteine residues during oxidation, a process controlled by Trx1. Trx1 was also found to modulate the intramolecular disulfide bonds between cysteines in the histone deacetylases, DnaJb5 and HDAC4, formed in response to ROS-generating stimuli (3). The further involvement of HDAC Cys residues in the regulation of activity was demonstrated in studies where carbonylation of these Cys residues resulted in modulation of H3 and H4 histone acetylation, followed by liberation of latent transcription factors and derepression/activation of downstream response genes (25).
Other data, while tantalizing, are at this stage indirect. Thus, in a recent microarray study on the long-lived GCLc transgenic fly model, in which levels of GSH were enhanced, it was shown that the expression of multiple histones was significantly increased (79). How this might affect chromatin modeling has not yet been explored, but the connection is an intriguing one.
While the possible role of redox poise on epigenetic states remains underinvestigated, important inroads have been made into the plausible role of epigenetic states on aging. Thus, dramatic changes in the chromatin structure, determined by mapping histone modifications (increased levels of di- or trimethylation of lysine 9 of the H3) or heterochromatin-binding protein 1 (HP1) were found to occur with age in Drosophila (123). Altering the heterochromatin levels by genetically manipulating HP1 and JAK/STAT signaling resulted in derepression of silenced genes and aberrant gene expression patterns associated with old age (50). In a similar vein genetic manipulations of histone deacetylases Sir2 and Rpd3 (96, 97) as well as the Polycomb Repressive Complex 2 (PRC2), responsible for histone 3 trimethylation (101), and PHO (a DNA-binding PcG protein involved in recruiting PRC2 to chromatin) (17) had significant longevity effects. Drug inhibitors of histone deacetylases, such as trichostatin A and BuA, exhibited similar longevity effects (127). Moreover, effects on longevity in flies and other organisms were achieved by supplementation with the polyamine spermidine, a treatment known to trigger epigenetic deacetylation of histone H3 through inhibition of histone acetyltransferases, thus suppressing OS and necrosis (26). It is evident that understanding the possible causal role of the redox state in modulating fluctuations of the chromatin status with age represents an important avenue of investigation.
Abbreviations Used
- 6GP
6-phosphogluconate
- 8-oxodG
8-oxodeoxyguanine
- AIF
apoptosis-inducing factor
- CcO
cytochrome C oxidase
- G6PD
glucose-6-phosphate dehydrogenase
- GCL
glutamylcysteine ligase
- Grx
glutaredoxin
- GS
glutathione synthase
- GSH
glutathione
- GSSG
glutathione disulfide
- GST
glutathione-S-transferase
- HDAC
histone deacetylase
- HP1
heterochromatin-binding protein 1
- InS
insulin signaling
- OS
oxidative stress
- PRC2
Polycomb Repressive Complex 2
- Prx
peroxiredoxin
- ROL
rate of living
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- Srx
sulfiredoxin
- Trx
thioredoxin
- TrxR
thioredoxin reductase
- TXNIP
thioredoxin-interacting proteins
- γGC
gamma-glutamylcysteine
Acknowledgments
The research of the authors during the past decade has been supported by the grants RO1 AG7657, RO1 AG13563, RO1 AG17077 and RO1 AG17526 to R.S.S. and RO1 AG15122 and RO1 AG20715 to W.C.O., from the National Institute on Aging–National Institutes of Health.
References
- 1.Abraham RT. TOR signaling: an odyssey from cellular stress to the cell growth machinery. Curr Biol. 2005;15:R139–R141. doi: 10.1016/j.cub.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal S. Sohal RS. DNA oxidative damage and life expectancy in houseflies. Proc Natl Acad Sci U S A. 1994;91:12332–12335. doi: 10.1073/pnas.91.25.12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ago T. Liu T. Zhai P. Chen W. Li H. Molkentin JD. Vatner SF. Sadoshima J. A redox-dependent pathway for regulating class II HDACs and cardiac hypertrophy. Cell. 2008;133:978–993. doi: 10.1016/j.cell.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 4.Ahn HM. Lee KS. Lee DS. Yu K. JNK/FOXO mediated PeroxiredoxinV expression regulates redox homeostasis during Drosophila melanogaster gut infection. Dev Comp Immunol. 2012;38:466–473. doi: 10.1016/j.dci.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Alic N. Andrews TD. Giannakou ME. Papatheodorou I. Slack C. Hoddinott MP. Cocheme HM. Schuster EF. Thornton JM. Partridge L. Genome-wide dFOXO targets and topology of the transcriptomic response to stress and insulin signalling. Mol Syst Biol. 2011;7:502. doi: 10.1038/msb.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barthel A. Ostrakhovitch EA. Walter PL. Kampkotter A. Klotz LO. Stimulation of phosphoinositide 3-kinase/Akt signaling by copper and zinc ions: mechanisms and consequences. Arch Biochem Biophys. 2007;463:175–182. doi: 10.1016/j.abb.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 7.Becker T. Loch G. Beyer M. Zinke I. Aschenbrenner AC. Carrera P. Inhester T. Schultze JL. Hoch M. FOXO-dependent regulation of innate immune homeostasis. Nature. 2010;463:369–373. doi: 10.1038/nature08698. [DOI] [PubMed] [Google Scholar]
- 8.Biteau B. Karpac J. Hwangbo D. Jasper H. Regulation of Drosophila lifespan by JNK signaling. Exp Gerontol. 2011;46:349–354. doi: 10.1016/j.exger.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryan H. Olayanju A. Goldring C. Park K. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem Pharmacol. 2012;85:705–717. doi: 10.1016/j.bcp.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Cadet J. Douki T. Ravanat JL. Measurement of oxidatively generated base damage in cellular DNA. Mutat Res. 2011;711:3–12. doi: 10.1016/j.mrfmmm.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Candas M. Sohal RS. Radyuk SN. Klichko VI. Orr WC. Molecular organization of the glutathione reductase gene in Drosophila melanogaster. Arch Biochem Biophys. 1997;339:323–334. doi: 10.1006/abbi.1996.9872. [DOI] [PubMed] [Google Scholar]
- 12.Cao J. Schulte J. Knight A. Leslie NR. Zagozdzon A. Bronson R. Manevich Y. Beeson C. Neumann CA. Prdx1 inhibits tumorigenesis via regulating PTEN/AKT activity. EMBO J. 2009;28:1505–1517. doi: 10.1038/emboj.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chae HZ. Oubrahim H. Park JW. Rhee SG. Chock PB. Protein glutathionylation in the regulation of peroxiredoxins: a family of thiol-specific peroxidases that function as antioxidants, molecular chaperones, and signal modulators. Antioxid Redox Signal. 2012;16:506–523. doi: 10.1089/ars.2011.4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang TS. Jeong W. Woo HA. Lee SM. Park S. Rhee SG. Characterization of mammalian sulfiredoxin and its reactivation of hyperoxidized peroxiredoxin through reduction of cysteine sulfinic acid in the active site to cysteine. J Biol Chem. 2004;279:50994–51001. doi: 10.1074/jbc.M409482200. [DOI] [PubMed] [Google Scholar]
- 15.Cheng Z. Arscott LD. Ballou DP. Williams CH., Jr. The relationship of the redox potentials of thioredoxin and thioredoxin reductase from Drosophila melanogaster to the enzymatic mechanism: reduced thioredoxin is the reductant of glutathione in Drosophila. Biochemistry. 2007;46:7875–7885. doi: 10.1021/bi700442r. [DOI] [PubMed] [Google Scholar]
- 16.Clancy DJ. Gems D. Harshman LG. Oldham S. Stocker H. Hafen E. Leevers SJ. Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- 17.Cunningham JT. Rodgers JT. Arlow DH. Vazquez F. Mootha VK. Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. 2007;450:736–740. doi: 10.1038/nature06322. [DOI] [PubMed] [Google Scholar]
- 18.Curtis C. Landis GN. Folk D. Wehr NB. Hoe N. Waskar M. Abdueva D. Skvortsov D. Ford D. Luu A. Badrinath A. Levine RL. Bradley TJ. Tavare S. Tower J. Transcriptional profiling of MnSOD-mediated lifespan extension in Drosophila reveals a species-general network of aging and metabolic genes. Genome Biol. 2007;8:R262. doi: 10.1186/gb-2007-8-12-r262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dames SA. Mulet JM. Rathgeb-Szabo K. Hall MN. Grzesiek S. The solution structure of the FATC domain of the protein kinase target of rapamycin suggests a role for redox-dependent structural and cellular stability. J Biol Chem. 2005;280:20558–20564. doi: 10.1074/jbc.M501116200. [DOI] [PubMed] [Google Scholar]
- 20.Dansen TB. Smits LM. van Triest MH. de Keizer PL. van Leenen D. Koerkamp MG. Szypowska A. Meppelink A. Brenkman AB. Yodoi J. Holstege FC. Burgering BM. Redox-sensitive cysteines bridge p300/CBP-mediated acetylation and FoxO4 activity. Nat Chem Biol. 2009;5:664–672. doi: 10.1038/nchembio.194. [DOI] [PubMed] [Google Scholar]
- 21.Davies SM. Poljak A. Duncan MW. Smythe GA. Murphy MP. Measurements of protein carbonyls, ortho- and meta-tyrosine and oxidative phosphorylation complex activity in mitochondria from young and old rats. Free Radic Biol Med. 2001;31:181–190. doi: 10.1016/s0891-5849(01)00576-7. [DOI] [PubMed] [Google Scholar]
- 22.de Candia P. Blekhman R. Chabot AE. Oshlack A. Gilad Y. A combination of genomic approaches reveals the role of FOXO1a in regulating an oxidative stress response pathway. PLoS One. 2008;3:e1670. doi: 10.1371/journal.pone.0001670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Keizer PL. Burgering BM. Dansen TB. Forkhead box o as a sensor, mediator, and regulator of redox signaling. Antioxid Redox Signal. 2011;14:1093–1106. doi: 10.1089/ars.2010.3403. [DOI] [PubMed] [Google Scholar]
- 24.DeGennaro M. Hurd TR. Siekhaus DE. Biteau B. Jasper H. Lehmann R. Peroxiredoxin stabilization of DE-cadherin promotes primordial germ cell adhesion. Dev Cell. 2011;20:233–243. doi: 10.1016/j.devcel.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doyle K. Fitzpatrick FA. Redox signaling, alkylation (carbonylation) of conserved cysteines inactivates class I histone deacetylases 1, 2, and 3 and antagonizes their transcriptional repressor function. J Biol Chem. 2010;285:17417–17424. doi: 10.1074/jbc.M109.089250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eisenberg T. Knauer H. Schauer A. Buttner S. Ruckenstuhl C. Carmona-Gutierrez D. Ring J. Schroeder S. Magnes C. Antonacci L. Fussi H. Deszcz L. Hartl R. Schraml E. Criollo A. Megalou E. Weiskopf D. Laun P. Heeren G. Breitenbach M. Grubeck-Loebenstein B. Herker E. Fahrenkrog B. Frohlich KU. Sinner F. Tavernarakis N. Minois N. Kroemer G. Madeo F. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11:1305–1314. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- 27.Ferguson M. Mockett RJ. Shen Y. Orr WC. Sohal RS. Age-associated decline in mitochondrial respiration and electron transport in Drosophila melanogaster. Biochem J. 2005;390:501–511. doi: 10.1042/BJ20042130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fontana L. Partridge L. Longo VD. Extending healthy life span—from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forquet S GR. Biard D. Toledano MB. Activation of NRF2 by nitrosative agents and H2O2 involves KEAP1 disulfide formation. J Biol Chem. 2010;285:8463–8471. doi: 10.1074/jbc.M109.051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallogly MM. Shelton MD. Qanungo S. Pai HV. Starke DW. Hoppel CL. Lesnefsky EJ. Mieyal JJ. Glutaredoxin regulates apoptosis in cardiomyocytes via NFkappaB targets Bcl-2 and Bcl-xL: implications for cardiac aging. Antioxid Redox Signal. 2010;12:1339–1353. doi: 10.1089/ars.2009.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilmer LK. Ansari MA. Roberts KN. Scheff SW. Age-related changes in mitochondrial respiration and oxidative damage in the cerebral cortex of the Fischer 344 rat. Mech Ageing Dev. 2010;131:133–143. doi: 10.1016/j.mad.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giordano E. Peluso I. Rendina R. Digilio A. Furia M. The clot gene of Drosophila melanogaster encodes a conserved member of the thioredoxin-like protein superfamily. Mol Genet Genomics. 2003;268:692–697. doi: 10.1007/s00438-002-0792-0. [DOI] [PubMed] [Google Scholar]
- 33.Hamilton ML. Guo Z. Fuller CD. Van Remmen H. Ward WF. Austad SN. Troyer DA. Thompson I. Richardson A. A reliable assessment of 8-oxo-2-deoxyguanosine levels in nuclear and mitochondrial DNA using the sodium iodide method to isolate DNA. Nucleic Acids Res. 2001;29:2117–2126. doi: 10.1093/nar/29.10.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 35.Haugaard N. Cellular mechanism of oxygen toxicity. Physiol Rev. 1968;48:311–373. doi: 10.1152/physrev.1968.48.2.311. [DOI] [PubMed] [Google Scholar]
- 36.Hourihan JM. Kenna JG. Hayes JD. The gasotransmitter hydrogen sulfide induces Nrf2-target genes by inactivating the Keap1 ubiquitin ligase substrate adaptor through formation of a disulfide bond between Cys-226 and Cys-613. Antioxid Redox Signal. 2013;19:465–481. doi: 10.1089/ars.2012.4944. [DOI] [PubMed] [Google Scholar]
- 37.Hochmuth CE. Biteau B. Bohmann D. Jasper H. Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila. Cell Stem Cell. 2011;8:188–199. doi: 10.1016/j.stem.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holland R. Hawkins AE. Eggler AL. Mesecar AD. Fabris D. Fishbein JC. Prospective type 1 and type 2 disulfides of Keap1 protein. Chem Res Toxicol. 2008;21:2051–2060. doi: 10.1021/tx800226m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeong W. Bae SH. Toledano MB. Rhee SG. Role of sulfiredoxin as a regulator of peroxiredoxin function and regulation of its expression. Free Radic Biol Med. 2012;53:447–456. doi: 10.1016/j.freeradbiomed.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 40.Jin LH. Shim J. Yoon JS. Kim B. Kim J. Kim-Ha J. Kim YJ. Identification and functional analysis of antifungal immune response genes in Drosophila. PLoS Pathog. 2008;4:e1000168. doi: 10.1371/journal.ppat.1000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol. 2008;295:C849–C868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joza N. Galindo K. Pospisilik JA. Benit P. Rangachari M. Kanitz EE. Nakashima Y. Neely GG. Rustin P. Abrams JM. Kroemer G. Penninger JM. The molecular archaeology of a mitochondrial death effector: AIF in Drosophila. Cell Death Differ. 2008;15:1009–1018. doi: 10.1038/cdd.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Junger MA. Rintelen F. Stocker H. Wasserman JD. Vegh M. Radimerski T. Greenberg ME. Hafen E. The Drosophila forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signaling. J Biol. 2003;2:20. doi: 10.1186/1475-4924-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kabe Y. Ando K. Hirao S. Yoshida M. Handa H. Redox regulation of NF-kappaB activation: distinct redox regulation between the cytoplasm and the nucleus. Antioxid Redox Signal. 2005;7:395–403. doi: 10.1089/ars.2005.7.395. [DOI] [PubMed] [Google Scholar]
- 45.Kanzok SM. Fechner A. Bauer H. Ulschmid JK. Muller HM. Botella-Munoz J. Schneuwly S. Schirmer R. Becker K. Substitution of the thioredoxin system for glutathione reductase in Drosophila melanogaster. Science. 2001;291:643–646. doi: 10.1126/science.291.5504.643. [DOI] [PubMed] [Google Scholar]
- 46.Katewa SD. Kapahi P. Role of TOR signaling in aging and related biological processes in Drosophila melanogaster. Exp Gerontol. 2011;46:382–390. doi: 10.1016/j.exger.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kenyon C. Chang J. Gensch E. Rudner A. Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 48.Knoops B. Goemaere J. Van der Eecken V. Declercq JP. Peroxiredoxin 5: structure, mechanism, and function of the mammalian atypical 2-Cys peroxiredoxin. Antioxid Redox Signal. 2011;15:817–829. doi: 10.1089/ars.2010.3584. [DOI] [PubMed] [Google Scholar]
- 49.Landis GN. Abdueva D. Skvortsov D. Yang J. Rabin BE. Carrick J. Tavare S. Tower J. Similar gene expression patterns characterize aging and oxidative stress in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2004;101:7663–7668. doi: 10.1073/pnas.0307605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Larson K. Yan SJ. Tsurumi A. Liu J. Zhou J. Gaur K. Guo D. Eickbush TH. Li WX. Heterochromatin formation promotes longevity and represses ribosomal RNA synthesis. PLoS Genet. 2012;8:e1002473. doi: 10.1371/journal.pgen.1002473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee KS. Iijima-Ando K. Iijima K. Lee WJ. Lee JH. Yu K. Lee DS. JNK/FOXO-mediated neuronal expression of fly homologue of peroxiredoxin II reduces oxidative stress and extends life span. J Biol Chem. 2009;284:29454–29461. doi: 10.1074/jbc.M109.028027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee RY. Hench J. Ruvkun G. Regulation of C. elegans DAF-16 and its human ortholog FKHRL1 by the daf-2 insulin-like signaling pathway. Curr Biol. 2001;11:1950–1957. doi: 10.1016/s0960-9822(01)00595-4. [DOI] [PubMed] [Google Scholar]
- 53.Lee W. Choi KS. Riddell J. Ip C. Ghosh D. Park JH. Park YM. Human peroxiredoxin 1 and 2 are not duplicate proteins: the unique presence of CYS83 in Prx1 underscores the structural and functional differences between Prx1 and Prx2. J Biol Chem. 2007;282:22011–22022. doi: 10.1074/jbc.M610330200. [DOI] [PubMed] [Google Scholar]
- 54.Legan SK. Rebrin I. Mockett RJ. Radyuk SN. Klichko VI. Sohal RS. Orr WC. Overexpression of glucose-6-phosphate dehydrogenase extends the life span of Drosophila melanogaster. J Biol Chem. 2008;283:32492–32499. doi: 10.1074/jbc.M805832200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lei K. Townsend DM. Tew KD. Protein cysteine sulfinic acid reductase (sulfiredoxin) as a regulator of cell proliferation and drug response. Oncogene. 2008;27:4877–4887. doi: 10.1038/onc.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li XD. Rebrin I. Forster MJ. Sohal RS. Effects of age and caloric restriction on mitochondrial protein oxidative damage in mice. Mech Ageing Dev. 2012;133:30–36. doi: 10.1016/j.mad.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Libert S. Chao Y. Chu X. Pletcher SD. Trade-offs between longevity and pathogen resistance in Drosophila melanogaster are mediated by NFkappaB signaling. Aging Cell. 2006;5:533–543. doi: 10.1111/j.1474-9726.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- 58.Lim MJ. Chae HZ. Rhee SG. Yu DY. Lee KK. Yeom YI. The type II peroxiredoxin gene family of the mouse: molecular structure, expression and evolution. Gene. 1998;216:197–205. doi: 10.1016/s0378-1119(98)00290-x. [DOI] [PubMed] [Google Scholar]
- 59.Lim YS. Cha MK. Kim HK. Uhm TB. Park JW. Kim K. Kim IH. Removals of hydrogen peroxide and hydroxyl radical by thiol-specific antioxidant protein as a possible role in vivo. Biochem Biophys Res Commun. 1993;192:273–280. doi: 10.1006/bbrc.1993.1410. [DOI] [PubMed] [Google Scholar]
- 60.Lin K. Hsin H. Libina N. Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Genet. 2001;28:139–145. doi: 10.1038/88850. [DOI] [PubMed] [Google Scholar]
- 61.Loeb J. Northrop JH. On the influence of food and temperature on the duration of life. J Biol Chem. 1917;32:103–121. [Google Scholar]
- 62.Luchak JM. Prabhudesai L. Sohal RS. Radyuk SN. Orr WC. Modulating longevity in Drosophila by over- and underexpression of glutamate-cysteine ligase. Ann N Y Acad Sci. 2007;1119:260–273. doi: 10.1196/annals.1404.000. [DOI] [PubMed] [Google Scholar]
- 63.Luo X. Puig O. Hyun J. Bohmann D. Jasper H. Foxo and Fos regulate the decision between cell death and survival in response to UV irradiation. EMBO J. 2007;26:380–390. doi: 10.1038/sj.emboj.7601484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Magwere T. Pamplona R. Miwa S. Martinez-Diaz P. Portero-Otin M. Brand MD. Partridge L. Flight activity, mortality rates, and lipoxidative damage in Drosophila. J Gerontol A Biol Sci Med Sci. 2006;61:136–145. doi: 10.1093/gerona/61.2.136. [DOI] [PubMed] [Google Scholar]
- 65.Mavis CK. Morey Kinney SR. Foster BA. Karpf AR. Expression level and DNA methylation status of glutathione-S-transferase genes in normal murine prostate and TRAMP tumors. Prostate. 2009;69:1312–1324. doi: 10.1002/pros.20976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McArthur MC. Sohal R.S. Relationship between metabolic rate, aging, lipid peroxidation and fluorescent age pigment in the milkweed bug, Oncopeltus fasciatus (hemiptera) J Gerontol. 1982;37:268–274. doi: 10.1093/geronj/37.3.268. [DOI] [PubMed] [Google Scholar]
- 67.Miquel J. Lundgren PR. Bensch KG. Atlan H. Effects of temperature on the life span, vitality and fine structure of Drosophila melanogaster. Mech Ageing Dev. 1976;5:347–370. doi: 10.1016/0047-6374(76)90034-8. [DOI] [PubMed] [Google Scholar]
- 68.Missirlis F. Rahlfs S. Dimopoulos N. Bauer H. Becker K. Hilliker A. Phillips JP. Jackle H. A putative glutathione peroxidase of Drosophila encodes a thioredoxin peroxidase that provides resistance against oxidative stress but fails to complement a lack of catalase activity. Biol Chem. 2003;384:463–472. doi: 10.1515/BC.2003.052. [DOI] [PubMed] [Google Scholar]
- 69.Missirlis F. Ulschmid JK. Hirosawa-Takamori M. Gronke S. Schafer U. Becker K. Phillips JP. Jackle H. Mitochondrial and cytoplasmic thioredoxin reductase variants encoded by a single Drosophila gene are both essential for viability. J Biol Chem. 2002;277:11521–11526. doi: 10.1074/jbc.M111692200. [DOI] [PubMed] [Google Scholar]
- 70.Mitsui A. Hamuro J. Nakamura H. Kondo N. Hirabayashi Y. Ishizaki-Koizumi S. Hirakawa T. Inoue T. Yodoi J. Overexpression of human thioredoxin in transgenic mice controls oxidative stress and life span. Antioxid Redox Signal. 2002;4:693–696. doi: 10.1089/15230860260220201. [DOI] [PubMed] [Google Scholar]
- 71.Mockett RJ. Sohal RS. Orr WC. Overexpression of glutathione reductase extends survival in transgenic Drosophila melanogaster under hyperoxia but not normoxia. FASEB J. 1999;13:1733–1742. doi: 10.1096/fasebj.13.13.1733. [DOI] [PubMed] [Google Scholar]
- 72.Mockett RJ. Sohal RS. Orr WC. Roles of Oxidative Stress in the Aging Process of Drosophila melanogaster. In: Miwa S, editor; Beckman KB, editor; Muller FL, editor. Totowa, NJ: Humana Press; 2008. [Google Scholar]
- 73.Oka S. Ago T. Kitazono T. Zablocki D. Sadoshima J. The role of redox modulation of class II histone deacetylases in mediating pathological cardiac hypertrophy. J Mol Med (Berl) 2009;87:785–791. doi: 10.1007/s00109-009-0471-2. [DOI] [PubMed] [Google Scholar]
- 74.Orr WC. Radyuk SN. Prabhudesai L. Toroser D. Benes JJ. Luchak JM. Mockett RJ. Rebrin I. Hubbard JG. Sohal RS. Overexpression of glutamate-cysteine ligase extends life span in Drosophila melanogaster. J Biol Chem. 2005;280:37331–37338. doi: 10.1074/jbc.M508272200. [DOI] [PubMed] [Google Scholar]
- 75.Orr WC. Sohal RS. Does overexpression of Cu,Zn-SOD extend life span in Drosophila melanogaster? Exp Gerontol. 2003;38:227–230. doi: 10.1016/s0531-5565(02)00263-2. [DOI] [PubMed] [Google Scholar]
- 76.Park JW. Mieyal JJ. Rhee SG. Chock PB. Deglutathionylation of 2-Cys peroxiredoxin is specifically catalyzed by sulfiredoxin. J Biol Chem. 2009;284:23364–23374. doi: 10.1074/jbc.M109.021394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pearl R. The Rate of Living. Alfred A. Knopf; New York: 1928. [Google Scholar]
- 78.Perez VI. Cortez LA. Lew CM. Rodriguez M. Webb CR. Van Remmen H. Chaudhuri A. Qi W. Lee S. Bokov A. Fok W. Jones D. Richardson A. Yodoi J. Zhang Y. Tominaga K. Hubbard GB. Ikeno Y. Thioredoxin 1 overexpression extends mainly the earlier part of life span in mice. J Gerontol A Biol Sci Med Sci. 2011;66:1286–1299. doi: 10.1093/gerona/glr125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Radyuk SN. Gambini J. Borras C. Serna E. Klichko VI. Vina J. Orr WC. Age-dependent changes in the transcription profile of long-lived Drosophila over-expressing glutamate cysteine ligase. Mech Ageing Dev. 2012;133:401–413. doi: 10.1016/j.mad.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 80.Radyuk SN. Michalak K. Klichko VI. Benes J. Orr WC. Peroxiredoxin 5 modulates immune response in Drosophila. Biochim Biophys Acta. 2010;1800:1153–1163. doi: 10.1016/j.bbagen.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Radyuk SN. Michalak K. Klichko VI. Benes J. Rebrin I. Sohal RS. Orr WC. Peroxiredoxin 5 confers protection against oxidative stress and apoptosis and also promotes longevity in Drosophila. Biochem J. 2009;419:437–445. doi: 10.1042/BJ20082003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Radyuk SN. Rebrin I. Klichko VI. Sohal BH. Michalak K. Benes J. Sohal RS. Orr WC. Mitochondrial peroxiredoxins are critical for the maintenance of redox state and the survival of adult Drosophila. Free Radic Biol Med. 2010;49:1892–1902. doi: 10.1016/j.freeradbiomed.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ragland SS. Sohal RS. Mating behavior, physical activity and aging in the housefly, Musca domestica. Exp Gerontol. 1973;8:135–145. doi: 10.1016/0531-5565(73)90003-x. [DOI] [PubMed] [Google Scholar]
- 84.Ragland SS. Sohal RS. Ambient temperature, physical activity and aging in the housefly, Musca domestica. Exp Gerontol. 1975;10:279–289. doi: 10.1016/0531-5565(75)90005-4. [DOI] [PubMed] [Google Scholar]
- 85.Ralat LA. Manevich Y. Fisher AB. Colman RF. Direct evidence for the formation of a complex between 1-cysteine peroxiredoxin and glutathione S-transferase pi with activity changes in both enzymes. Biochemistry. 2006;45:360–372. doi: 10.1021/bi0520737. [DOI] [PubMed] [Google Scholar]
- 86.Rebrin I. Bayne AC. Mockett RJ. Orr WC. Sohal RS. Free aminothiols, glutathione redox state and protein mixed disulphides in aging Drosophila melanogaster. Biochem J. 2004;382:131–136. doi: 10.1042/BJ20040506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rebrin I. Kamzalov S. Sohal RS. Effects of age and caloric restriction on glutathione redox state in mice. Free Radic Biol Med. 2003;35:626–635. doi: 10.1016/s0891-5849(03)00388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rebrin I. Sohal RS. Comparison of thiol redox state of mitochondria and homogenates of various tissues between two strains of mice with different longevities. Exp Gerontol. 2004;39:1513–1519. doi: 10.1016/j.exger.2004.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rebrin I. Sohal RS. Comparison between the effects of aging and hyperoxia on glutathione redox state and protein mixed disulfides in Drosophila melanogaster. Mech Ageing Dev. 2006;127:869–874. doi: 10.1016/j.mad.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rebrin I. Sohal RS. Pro-oxidant shift in glutathione redox state during aging. Adv Drug Deliv Rev. 2008;60:1545–1552. doi: 10.1016/j.addr.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ren JC. Rebrin I. Klichko V. Orr WC. Sohal RS. Cytochrome c oxidase loses catalytic activity and structural integrity during the aging process in Drosophila melanogaster. Biochem Biophys Res Commun. 2010;401:64–68. doi: 10.1016/j.bbrc.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rhee SG. Chae HZ. Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005;38:1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 93.Rhee SG. Woo HA. Multiple functions of peroxiredoxins: peroxidases, sensors and regulators of the intracellular messenger H(2)O(2), and protein chaperones. Antioxid Redox Signal. 2011;15:781–794. doi: 10.1089/ars.2010.3393. [DOI] [PubMed] [Google Scholar]
- 94.Rhee SG. Woo HA. Kil IS. Bae SH. Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides. J Biol Chem. 2012;287:4403–4410. doi: 10.1074/jbc.R111.283432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rodriguez J. Agudo M. Van Damme J. Vandekerckhove J. Santaren JF. Polypeptides differentially expressed in imaginal discs define the peroxiredoxin family of genes in Drosophila. Eur J Biochem. 2000;267:487–497. doi: 10.1046/j.1432-1327.2000.01022.x. [DOI] [PubMed] [Google Scholar]
- 96.Rogina B. Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rogina B. Helfand SL. Frankel S. Longevity regulation by Drosophila Rpd3 deacetylase and caloric restriction. Science. 2002;298:1745. doi: 10.1126/science.1078986. [DOI] [PubMed] [Google Scholar]
- 98.Rubner M. Das Problem der Lebensdauer und seine Beziehunger zum Wachstum und Ernaibrung. Oldenburg; Munchen: 1908. [Google Scholar]
- 99.Schwarze SR. Weindruch R. Aiken JM. Oxidative stress and aging reduce COX I RNA and cytochrome oxidase activity in Drosophila. Free Radic Biol Med. 1998;25:740–747. doi: 10.1016/s0891-5849(98)00153-1. [DOI] [PubMed] [Google Scholar]
- 100.Seong KH. Ogashiwa T. Matsuo T. Fuyama Y. Aigaki T. Application of the gene search system to screen for longevity genes in Drosophila. Biogerontology. 2001;2:209–217. doi: 10.1023/a:1011517325711. [DOI] [PubMed] [Google Scholar]
- 101.Siebold AP. Banerjee R. Tie F. Kiss DL. Moskowitz J. Harte PJ. Polycomb Repressive Complex 2 and Trithorax modulate Drosophila longevity and stress resistance. Proc Natl Acad Sci U S A. 2010;107:169–174. doi: 10.1073/pnas.0907739107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sohal RS. Arnold L. Orr WC. Effect of age on superoxide dismutase, catalase, glutathione reductase, inorganic peroxides, TBA-reactive material, GSH/GSSG, NADPH/NADP+ and NADH/NAD+ in Drosophila melanogaster. Mech Ageing Dev. 1990;56:223–235. doi: 10.1016/0047-6374(90)90084-s. [DOI] [PubMed] [Google Scholar]
- 103.Sohal RS. Buchan P.B. Relationship between physical activity and life span in the adult housefly, Musca domestica. Exp Gerontol. 1981;16:157–162. doi: 10.1016/0531-5565(81)90040-1. [DOI] [PubMed] [Google Scholar]
- 104.Sohal RS. Orr WC. The redox stress hypothesis of aging. Free Radic Biol Med. 2012;52:539–555. doi: 10.1016/j.freeradbiomed.2011.10.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sohal RS. Sohal BH. Orr WC. Mitochondrial superoxide and hydrogen peroxide generation, protein oxidative damage, and longevity in different species of flies. Free Radic Biol Med. 1995;19:499–504. doi: 10.1016/0891-5849(95)00037-x. [DOI] [PubMed] [Google Scholar]
- 106.Sohal RS. Toroser D. Bregere C. Mockett RJ. Orr WC. Age-related decrease in expression of mitochondrial DNA encoded subunits of cytochrome c oxidase in Drosophila melanogaster. Mech Ageing Dev. 2008;129:558–561. doi: 10.1016/j.mad.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Soriano FX. Leveille F. Papadia S. Higgins LG. Varley J. Baxter P. Hayes JD. Hardingham GE. Induction of sulfiredoxin expression and reduction of peroxiredoxin hyperoxidation by the neuroprotective Nrf2 activator 3H-1,2-dithiole-3-thione. J Neurochem. 2008;107:533–543. doi: 10.1111/j.1471-4159.2008.05648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stacey MM. Vissers MC. Winterbourn CC. Oxidation of 2-cys peroxiredoxins in human endothelial cells by hydrogen peroxide, hypochlorous acid, and chloramines. Antioxid Redox Signal. 2012;17:411–421. doi: 10.1089/ars.2011.4348. [DOI] [PubMed] [Google Scholar]
- 109.Stuart JA. Bourque BM. de Souza-Pinto NC. Bohr VA. No evidence of mitochondrial respiratory dysfunction in OGG1-null mice deficient in removal of 8-oxodeoxyguanine from mitochondrial DNA. Free Radic Biol Med. 2005;38:737–745. doi: 10.1016/j.freeradbiomed.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 110.Susin SA. Lorenzo HK. Zamzami N. Marzo I. Snow BE. Brothers GM. Mangion J. Jacotot E. Costantini P. Loeffler M. Larochette N. Goodlett DR. Aebersold R. Siderovski DP. Penninger JM. Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 111.Svensson MJ. Chen JD. Pirrotta V. Larsson J. The ThioredoxinT and deadhead gene pair encode testis- and ovary-specific thioredoxins in Drosophila melanogaster. Chromosoma. 2003;112:133–143. doi: 10.1007/s00412-003-0253-5. [DOI] [PubMed] [Google Scholar]
- 112.Svensson MJ. Larsson J. Thioredoxin-2 affects lifespan and oxidative stress in Drosophila. Hereditas. 2007;144:25–32. doi: 10.1111/j.2007.0018-0661.01990.x. [DOI] [PubMed] [Google Scholar]
- 113.Sykiotis GP. Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev Cell. 2008;14:76–85. doi: 10.1016/j.devcel.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tatar M. Kopelman A. Epstein D. Tu MP. Yin CM. Garofalo RS. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- 115.Tettweiler G. Miron M. Jenkins M. Sonenberg N. Lasko PF. Starvation and oxidative stress resistance in Drosophila are mediated through the eIF4E-binding protein, d4E-BP. Genes Dev. 2005;19:1840–1843. doi: 10.1101/gad.1311805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Toroser D. Orr WC. Sohal RS. Carbonylation of mitochondrial proteins in Drosophila melanogaster during aging. Biochem Biophys Res Commun. 2007;363:418–424. doi: 10.1016/j.bbrc.2007.08.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tower J. Aging mechanisms in fruit files. Bioessays. 1996;18:799–807. doi: 10.1002/bies.950181006. [DOI] [PubMed] [Google Scholar]
- 118.Tsuda M. Ootaka R. Ohkura C. Kishita Y. Seong KH. Matsuo T. Aigaki T. Loss of Trx-2 enhances oxidative stress-dependent phenotypes in Drosophila. FEBS Lett. 2010;584:3398–3401. doi: 10.1016/j.febslet.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 119.Umeda-Kameyama Y. Tsuda M. Ohkura C. Matsuo T. Namba Y. Ohuchi Y. Aigaki T. Thioredoxin suppresses Parkin-associated endothelin receptor-like receptor-induced neurotoxicity and extends longevity in Drosophila. J Biol Chem. 2007;282:11180–11187. doi: 10.1074/jbc.M700937200. [DOI] [PubMed] [Google Scholar]
- 120.Van Remmen H. Ikeno Y. Hamilton M. Pahlavani M. Wolf N. Thorpe SR. Alderson NL. Baynes JW. Epstein CJ. Huang TT. Nelson J. Strong R. Richardson A. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics. 2003;16:29–37. doi: 10.1152/physiolgenomics.00122.2003. [DOI] [PubMed] [Google Scholar]
- 121.Wang MC. Bohmann D. Jasper H. JNK signaling confers tolerance to oxidative stress and extends lifespan in Drosophila. Dev Cell. 2003;5:811–816. doi: 10.1016/s1534-5807(03)00323-x. [DOI] [PubMed] [Google Scholar]
- 122.Wang MC. Bohmann D. Jasper H. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell. 2005;121:115–125. doi: 10.1016/j.cell.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 123.Wood JG. Hillenmeyer S. Lawrence C. Chang C. Hosier S. Lightfoot W. Mukherjee E. Jiang N. Schorl C. Brodsky AS. Neretti N. Helfand SL. Chromatin remodeling in the aging genome of Drosophila. Aging Cell. 2010;9:971–978. doi: 10.1111/j.1474-9726.2010.00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xiong Y. Uys JD. Tew KD. Townsend DM. S-glutathionylation: from molecular mechanisms to health outcomes. Antioxid Redox Signal. 2011;15:233–270. doi: 10.1089/ars.2010.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yoshida S. Hong S. Suzuki T. Nada S. Mannan AM. Wang J. Okada M. Guan KL. Inoki K. Redox regulates mammalian target of rapamycin complex 1 (mTORC1) activity by modulating the TSC1/TSC2-Rheb GTPase pathway. J Biol Chem. 2011;286:32651–32660. doi: 10.1074/jbc.M111.238014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yu S. Khor TO. Cheung KL. Li W. Wu TY. Huang Y. Foster BA. Kan YW. Kong AN. Nrf2 expression is regulated by epigenetic mechanisms in prostate cancer of TRAMP mice. PLoS One. 2010;5:e8579. doi: 10.1371/journal.pone.0008579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhao Y. Sun H. Lu J. Li X. Chen X. Tao D. Huang W. Huang B. Lifespan extension and elevated hsp gene expression in Drosophila caused by histone deacetylase inhibitors. J Exp Biol. 2005;208:697–705. doi: 10.1242/jeb.01439. [DOI] [PubMed] [Google Scholar]
- 128.Zheng J. Edelman SW. Tharmarajah G. Walker DW. Pletcher SD. Seroude L. Differential patterns of apoptosis in response to aging in Drosophila. Proc Natl Acad Sci U S A. 2005;102:12083–12088. doi: 10.1073/pnas.0503374102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhou J. Bi C. Cheong LL. Mahara S. Liu SC. Tay KG. Koh TL. Yu Q. Chng WJ. The histone methyltransferase inhibitor, DZNep, up-regulates TXNIP, increases ROS production, and targets leukemia cells in AML. Blood. 2011;118:2830–2839. doi: 10.1182/blood-2010-07-294827. [DOI] [PubMed] [Google Scholar]
- 130.Zou S. Meadows S. Sharp L. Jan LY. Jan YN. Genome-wide study of aging and oxidative stress response in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2000;97:13726–13731. doi: 10.1073/pnas.260496697. [DOI] [PMC free article] [PubMed] [Google Scholar]