Abstract
Significance: The free radical theory of aging has provided a theoretical framework for an enormous amount of work leading to significant advances in our understanding of aging. Up to the turn of the century, the theory received abundant support from observations coming from fields as far apart as comparative physiology or molecular biology. Recent Advances: Work from many laboratories supports the theory, for instance showing that overexpression of antioxidant enzymes results in increases in life-span. But other labs have shown that in some cases, there is an increased oxidative stress and increased longevity. The discovery that free radicals can not only cause molecular damage to cells, but also serve as signals; led to the proposal that they act as modulators of physiological processes. For instance, reactive oxygen species (ROS) stimulate physiological adaptations to physical exercise. Critical Issues: A critical blow to the free radical theory of aging came from epidemiological studies showing that antioxidant supplementation did not lower the incidence of many age-associated diseases but, in some cases, increased the risk of death. Moreover, recent molecular evidence has shown that increasing generation of ROS, in some cases, increases longevity. Future Directions: Gerontologists interested in free radical biology are at a crossroads and clearly new insights are required to clarify the role of ROS in the process of aging. The hurdles are, no doubt, very high, but the intellectual and practical promise of these studies is of such magnitude that we feel that all efforts will be generously rewarding. Antioxid. Redox Signal. 19, 779–787.
Introduction
Much of the research on aging that has taken place in recent decades has been guided by the free radical theory of aging (19). Albert Einstein, in a review paper on the theory of relativity, asked himself “What impels us to devise theories?” Einstein's answer to this question was twofold. He first pointed out that we devise theories because we enjoy comprehending. The second reason was that we strive towards simplification. This literal quotation has been taken from one of his review papers written in 1950 (11). He went on to say that “there exists a passion for comprehension, just as there is a passion for music that is common in children but is lost in most people later on” (10). This is in keeping with the famous dictum by Jacques Monod near his death when he said “Je cherche à comprendre,” I try to understand. According to Karl R. Popper (38) the criterion of demarcation of a scientific theory is not its verifiability but its falsifiability. Popper further added that it must be possible for an empirical scientific system to be refuted by experience. Thus, the fact that, as will become apparent in this review, many experiments have been performed in trying to prove that the free radical theory of aging does not hold true only indicates the importance that this theory has had, and even now has, in gerontological research.
We have recently reviewed the theories of aging (52). There are more than three hundred that could, in general terms, be classified into three big groups: the genetic mutation theories, the wear and tear theories, and the cellular waste accumulation theories (52). Some theories may be included in two groups, for instance the free radical theory of aging shares characteristics of the genetic mutation, as well as the cellular waste accumulation theories. This can also be applied to the telomeres and Hayflick limit theories of aging. Thus, it is even difficult to classify the number of theories that have been postulated to understand the phenomenon of aging. Moreover, the fact that so many theories exist indicates that there is not one clear-cut theory to explain the multifactorial phenomenon of aging. A comprehensive theory of aging must explain the characteristics of aging first clearly formulated by Strehler (47) and summarized in Figure 1. The American gerontologist defined aging by means of the following postulates:
FIG. 1.
Characteristics of aging.
Aging is universal: a phenomenon associated with the process of aging must occur in different degrees in all individuals of a species. Aging must be intrinsic: the causes that are the origin of aging must be endogenous; they must not depend on extrinsic factors. Aging must be progressive: changes that lead to aging must occur progressively throughout the life span (they must also occur in young individuals, albeit in a small proportion). Aging must be deleterious: that is, a phenomenon associated with aging will only be considered as part of the aging process if it is “bad” for the individual.
We have reviewed here literature on aging of multicellular organisms (and not, for instance in the yeast Saccharomyces cerevisiae) and more emphasis has been placed in aging of vertebrates than in invertebrates.
The Free Radical Theory of Aging
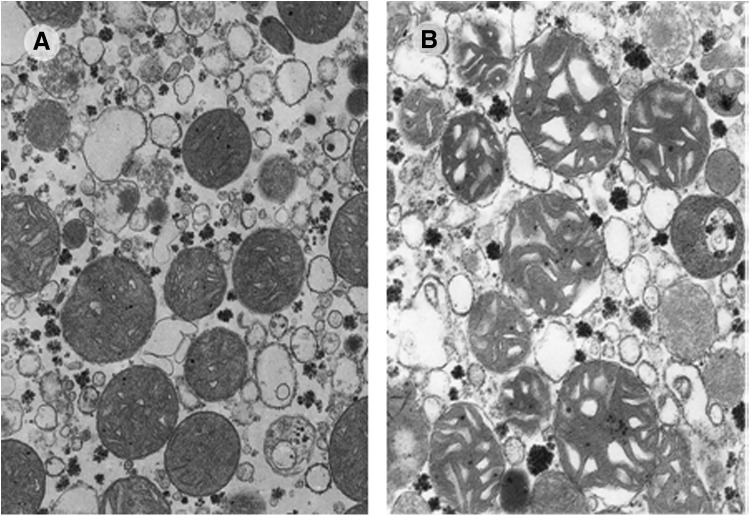
The fact that oxygen poisoning has mechanisms in common with X-irradiation and that free radicals are involved was first postulated by Rebeca Gerschman, an Argentinean researcher working in Rochester in the laboratory of Wallace O. Fenn (13). This critical paper, published in 1954, paved the way for the first postulation by Denham Harman in 1956 of the free radical theory of aging. In his seminal paper, Harman stated that “aging and the degenerative diseases associated with it are attributed basically to the deleterious side attacks of free radicals on cell constituents and on the connected tissues” (18). Harman first suggested that mitochondria are key organelles involved in aging. However, the mitochondrial free radical theory of aging was clearly postulated in the 70's by Miquel et al. (29). Miquel's contribution to the free radical theory of aging was important in pointing out mitochondria as sources of free radicals and mitochondrial deoxyribonucleic acid (DNA) as a critical target to explain the age-associated damage in cells. We (in cooperation with Miquel) provided the first evidence that mitochondria are damaged inside cells and that the effects of aging on mitochondria are not due to the fact that they are more fragile and damaged upon isolation but to intrinsic damage which occurs in cells as they age (see Fig. 2) (45). Our findings were independently confirmed almost simultaneously by the group of Ames showing the characteristics of the mitochondrial decay with aging (17).
FIG. 2.
Mitochondria from old animals are damaged inside cells. (A) Shows liver mitochondria from young rats and (B) shows liver mitochondria from old rats (original magnification 30,000–1). Mitochondria from old animals show more heterogeneity, bigger size, and disrupted cristae; all indications of histological damage. Taken from Sastre et al. (45). Reprinted with permission.
Thus, by the turn of the century, the free radical theory of aging appeared to be established, but new experimental approaches showed that this was not the case (see below). Moreover, an important characteristic of this theory was that it opened up room for intervention because administration of antioxidants could be favorable to delay aging and perhaps even more, to prevent age-associated diseases. The assumption that antioxidant supplements were in general good for your health was to be proved wrong as will be explained later in some detail.
The Controversy (2000–2011)
A major aim of this review is to clarify and provide a balanced position of the literature with regards to the validity of the free radical theory of aging.
We have found, over the last 5 years, a very clear controversy with some authors providing evidence in favor and some against the free radical theory of aging. Of the many papers proposing evidence in favor and against, we have selected only a few to underline this controversy. For instance, Moosmann and Behl published in 2008 that “these results provide distinct support for the free radical theory of aging” (31). In very clear contrast, Arlan Richardson and his group entitled a review paper published in 2009 “Is the oxidative stress theory of aging dead?” and stated that “data call into serious question the hypothesis that alterations in oxidative damage/stress play a role in the longevity of mice” (36). The conclusions of a paper published in 2004, by Nemoto and Finkel stated, “In our view, the most likely sign to hang in any hypothetical aging headquarters at the moment would read: It's the free radicals, stupid! Indeed, the cellular effects of free radicals represent the most likely contender to explain the aging process across a wide range of species” (32). In 2007, in a joint paper by the teams of Prolla, Barja, and Leeuwenburgh it was stated “These results support the mitochondrial free radical theory of aging” (44). Three years later and in the same journal, Ristow and Zarse entitled their review paper “How increased oxidative stress promotes longevity” (40). Finally, Yang and Hekimi (53) stated that “These findings are not consistent with the mitochondrial oxidative stress theory of aging” and this contrasts with a review paper published in Nature in 2011 (24) entitled “Aging theories unified” where increased reactive oxygen species (ROS) occupy a central role in what the author claims to be a unified theory of aging.
Thus, there is a very serious controversy as to the validity of the free radical theory of aging, and in the end, it is important for gerontologists to know the present position and the scientific evidence in favor and against this theory.
Evidence in Favor of the Free Radical Theory of Aging
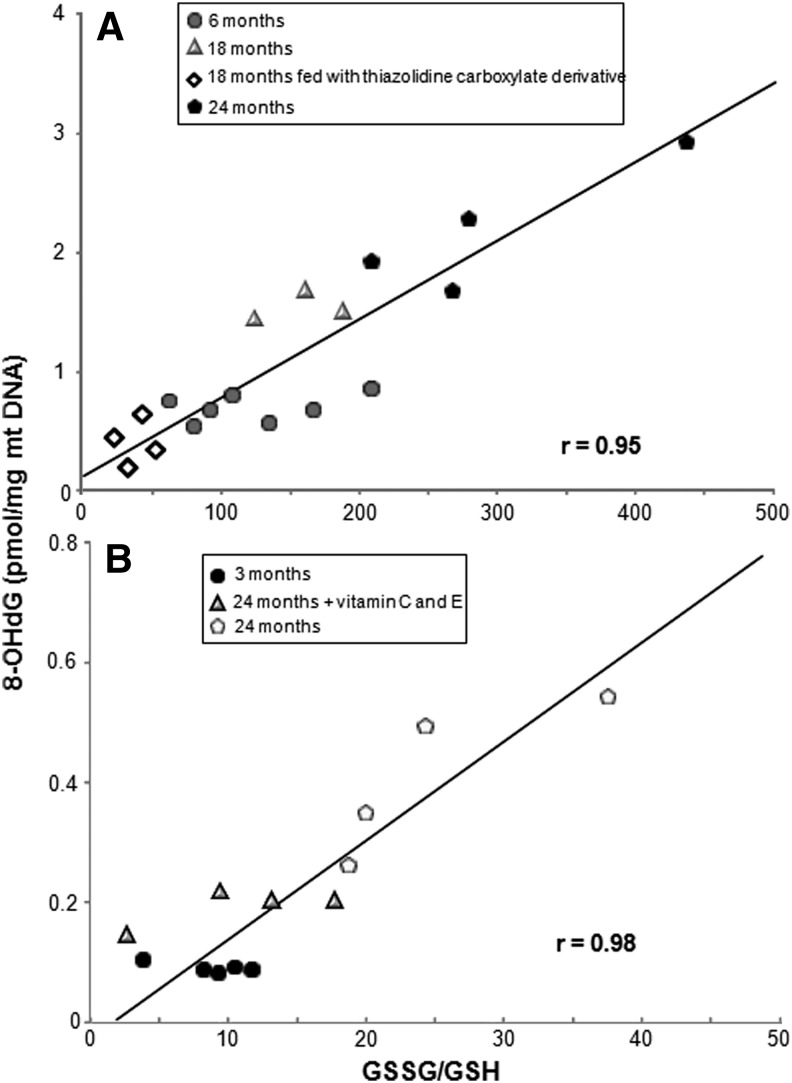
Much of the evidence gathered in the 80's, which was in favor of the free radical theory of aging was based on “Correlation between species-specific levels of antioxidant defenses and lifetime energy expenditure” (8). Cutler, in 1991, reviewed some of this and said “Results suggest a role of oxyradicals in causing aging and that the antioxidant status of an individual could be important in determining the frequency of age-dependent diseases and the duration of general health maintenance” (9). This author found a clear linear correlation between superoxide dismutase (SOD) activity and the maximal life span potential of various species ranging from mice to monkeys, chimpanzees, and humans. Barja and his coworkers analyzed the very different life span of rats (3–4 years), and pigeons (35 years), which was surprising in view of the similar size and similar oxygen consumption in both species. These authors pointed out that the rate of oxidant production by mitochondria from pigeons was less than 30% that of rats. The authors could correlate longevity to the rate of oxidant production and not oxygen utilization. There was indeed support for the free radical theory of aging (22). By then, measurements of oxidative damage to mitochondrial DNA had been established. The levels of oxo-8-deoxyguanosine in mitochondrial DNA of various animals could be performed and we showed that oxidative damage to DNA correlated linearly with oxidation of glutathione (see Fig. 3), the first being a mutagenic lesion and the second a mere index of oxidative stress (12).
FIG. 3.
Relationship between mitochondrial GSSG/GSH and levels of 8-OHdG in mitochondrial DNA from livers of mice (A) and rats (B). Lines of regression and correlation coefficients (r) are shown. Redrawn from García de la Asunción et al. (12). 8-OHdG, oxo-8-deoxyguanosine; DNA, deoxyribonucleic acid; GSH, reduced glutathione; GSSG, oxidized glutathione.
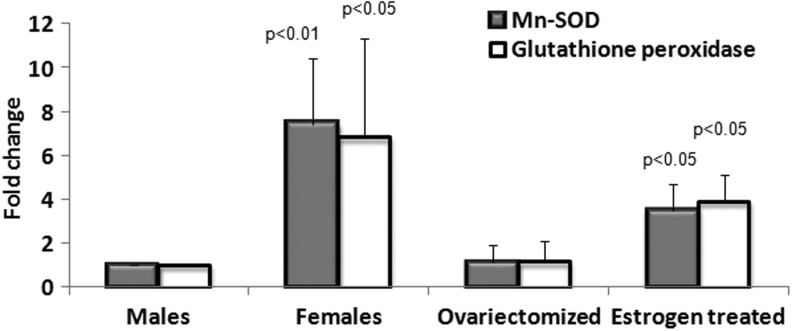
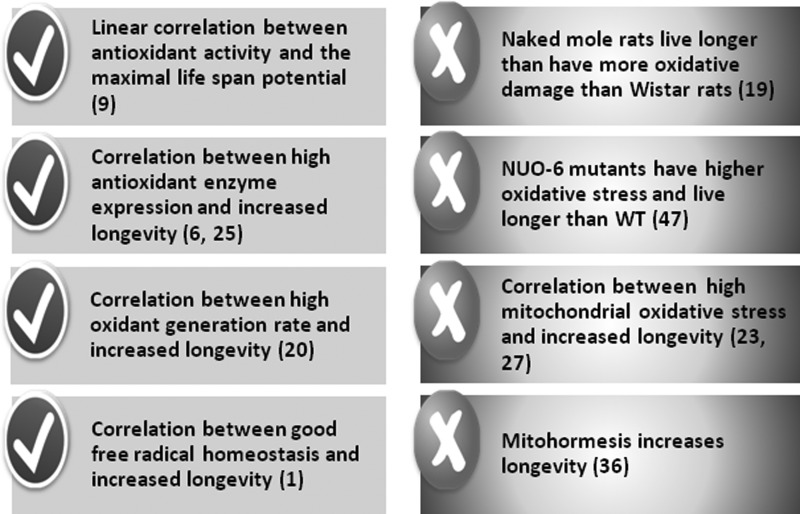
The tools of molecular biology allowed researchers to construct animals with genetically altered levels of antioxidant defence enzymes and test their life span. An important impetus to this line of work came from work by Orr and Sohal (33) in which they showed that over-expression of SOD and catalase could increase life span of Drosophila melanogaster. We observed that females in many species, like rats or humans, live longer than males because estrogens stimulate the over-expression of glutathione peroxidase (GPx) and manganese superoxide dismutase (MnSOD), see Figure 4 (6,51). This again was in support of the free radical theory of aging and further support came from Ali et al. in La Jolla, California. They showed that gender differences in free radical homeostasis during aging were determinant for longevity (1). In species in which females live less than males, then females produce more radicals. This was confirmatory of our previous observation that when the females live long they produce fewer radicals (1). Many papers have manipulated levels of antioxidant enzymes and tested the longevity in several species. We found that over-expressing Arf and p53 led to a significant increase in longevity in animals, which was independent of tumor protection conferred by p53 (28). We could attribute the increase in longevity of these animals to the fact that p53 behaves as an antioxidant because it stimulates the expression of new noncanonical antioxidant enzymes called sestrins (7, 42). Figure 5 summarizes the evidences in favor and against of the free radical theory of aging.
FIG. 4.
Antioxidant gene expression in OF1 mice. Animals that live longer (females) have considerably higher expression of both MnSOD and GPx. Ovariectomy turns values to the equivalent in males and estrogens partially reverse the effect of ovariectomy. These results (previously unpublished from our own laboratory) are in keeping with the free radical theory of aging. Fold change is referred to values for males that arbitrarily are taken to be 1. Values shown are means±SD for five experiments. Significance (according to Tukey test) is indicated by the p-value. We consider that a value is significantly different from control when p-value is lower than 0.05. GPx, glutathione peroxidase; MnSOD, manganese superoxide dismutase; OF1, Oncins France 1; SD, standard deviation.
FIG. 5.
Evidences in favor and against the free radical theory of aging.
Evidence Against the Free Radical Theory of Aging
In pure logical terms, a single result that does not fit with a theory is sufficient to disprove its general validity (38). This, of course, does not indicate that all experiments that support the theory are invalid, or even that the theory is not a “useful” one in that it has fostered research and it has offered a general framework for many experiments, some of which have been discussed above.
When studying the evidence that does not support the free radical theory of aging, it is important to highlight the biology of naked mole rats. These animals live much longer than ordinary rats. The maximal life span of these animals may go as far as 25 years compared with 3–4 years in ordinary rats. According to the free radical theory of aging, oxidative damage should be much less in the naked mole rats than in ordinary rats. This is not the case and, in fact, the naked mole rats show higher oxidative damage than the control, ordinary rats (21). However, one must keep in mind that the naked mole is adapted to limited oxygen tension in the tunnels where it lives and has a low metabolic rate. Moreover, work by Perez et al. has shown that these animals exhibit increased efficiency of cysteine reduction mechanisms leading to improved sensitivity to protein repair and degradation (37). Thus, even if increased longevity coincides with a more oxidized state, the naked mole rat does not constitute an irrefutable proof of failure of the free radical theory of aging.
On the other hand, there are mutants of Caenorhabditis elegans, for instance, the NUO-6 that show higher oxidative stress than the controls and that live longer than the controls (53). However, when NUO-6 mutants are treated with a powerful antioxidant, such as N-acetyl cysteine, then they are under less oxidative stress, but they also live less. Similar evidence has been recently reported regarding the Mclk1 homozygous mutant mice that show higher mitochondrial oxidative stress than controls but live longer than these (26). Evidence has also come from the world of antioxidants, for instance Ristow and Zarse have reported in 2010 that oxidative stress may provoke longevity and metabolic health and these authors have put forward the concept of mitochondrial hormesis or mitohormesis (40). They underscore that by promoting an increase in the ROS and an increase in mild oxidative damage, one can foster stress defenses in mitochondria and increase metabolic health and life span. Some evidence of the lack of correlation between mitochondrial ROS and life span has been recorded in Drosophila, particularly in work from Partridge coworkers (30). These authors show two examples in which mitochondrial free radical production and life span are not correlated. Again lack of correlation (as in the previous paragraph) is not strong proof or disproof of a theory, but in any case the results certainly do not support the free radical theory of aging.
Some work not supporting the free radical theory of aging has recently come from our laboratory. We have, on many occasions, observed that exercise unleashes a powerful antioxidant defense by increasing MnSOD, and inducible nitric oxide synthase, and all this mediated by the activation of the nuclear factor-κB(NF-κB) (14, 23). Since it is well known that exercise promotes health and prevents age-associated diseases of all kinds (related and unrelated to oxidative stress) we thought that up-regulating antioxidant enzymes was in keeping with the fact that exercise was a protection against the ravages of aging. But in all pureness, the free radical theory of aging postulates that it is the aging itself that is mediated by ROS. If this were the case, when antioxidant enzymes are up-regulated, longevity should be promoted, either average life span or maximal life span. It has been reported that mice deficient in both MnSOD and GPx have increased oxidative damage and a greater incidence of pathology but no reduction in longevity (54). Thus, these data do not support a significant role for increased oxidative stress in modulating life span in mice and do not support the free radical theory of aging. Figure 5 summarizes the evidence in favor and against the free radical theory of aging.
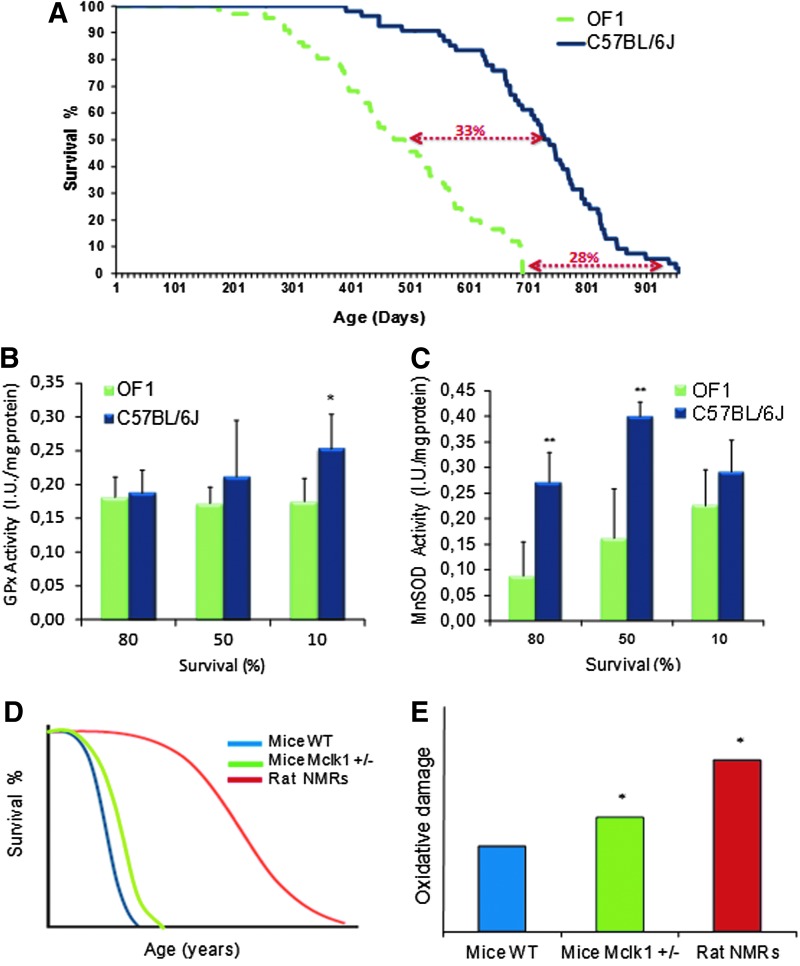
To illustrate the controversy that we have just described, Figure 6 shows evidence in favor of the theory: C57BL6 (C57 Black 6) mice live longer and show higher antioxidant enzyme activities than Oncins France 1 mice (Fig. 6A–C); and evidence against: the naked mole rat lives longer and shows higher oxidative damage than Wild Type or Mclk1+/− mice (Fig. 6D, E).
FIG. 6.
Experimental evidence in favor and against. (A) Shows the average and maximal longevity of two different strains of mice, C57BL6 (shown in blue) and OF1 (shown in green dotted lines). C57BL6 mice live longer than OF1. (B, C) Show that liver antioxidant enzyme expression is higher in C57BL6 mice, which live longer than OF1 (unpublished data from our laboratory). This is in favor of the free radical theory. We measured GPx and MnSOD activity in liver at different survival time points (80%, 50% and 10% of survival). Values are shown as mean±SD for five animals. *Indicates p<0.05, **Indicates p<0.01. (D) Shows longevity curves of Wild Type mice, Mclk1+/− mice and of NMR. Mclk1+/− mice live slightly longer than wild type mice and NMR live much longer than the mice. (E) Shows oxidative damage in mice and NMR. Animals that live longer have higher oxidative damage than wild type. This is squarely against the free radical theory of aging. (D, E) Redrawn from Hekimi et al. (21). C57BL6, C57 Black 6; NMR, naked mole rats. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
An interesting, and indeed, “parallel” set theories of aging are based on Hayflick's limit of cell division. In strict terms, it was proposed that the free radical theory of aging explaines damage associated with aging of post-mitotic cells. The reason for this thinking is that only post-mitotic cells could age because they were not bound to be renewed by mitosis (25). However, experiments performed by Leonard Hayflick in the 1960's led to the formulation of the Hayflick limit of cell divisions (20). Essentially, cells have limited number of divisions before they become completely post-mitotic. An animal can age not only because post-mitotic cells become damaged, but also because Hayflick's limit makes it impossible for cells to renew themselves.
The discovery of telomerase by Blackburn et al. provided a molecular mechanism by which the “end replication problem” of DNA in duplication could be explained (4). Cells lacking telomerase were unable to increase their telomere's length after divisions and therefore, they have a replicative limit. Cells in which telomerase was active maintained their telomere length and therefore, aging would not occur or be delayed. We found that telomerase itself could be related to free radicals because glutathione, a major intracellular reductant in cells, regulates telomerase activity (5). In triple transgenic animals in which p53 and p16 were over-expressed together with telomerase, a very significant (35%) increase in average life span was observed. A significant increase in maximal life span also took place. Thus, telomerase prolonged life span in cancer protected animals (because they were over-expressing p53 and p16). This over-expression could not be traced to an increased level of antioxidant defenses or an increased protection of animals against oxidative stress. So we observed that one could increase life span without affecting oxidative stress (49).
The Corollary of the Free Radical Theory of Aging: Antioxidant Supplements
Linus Pauling, in the 70's, postulated that taking high doses of the antioxidant vitamin C would promote health and would prevent, to some extent, the common cold and even the flu (34). Pauling recommended doses of around 2–3 g/day of vitamin C (and sometimes even higher) (35).
The extraordinary personality and the towering scientific importance of Linus Pauling made many scientists, in both experimental biology and medicine, test the effect of vitamin C supplementation of various age-associated diseases. We have to state at this point that in no case (to our knowledge) has a correct study been performed in which the levels of vitamins were recorded for each patient, or healthy person, to whom antioxidant vitamins were given. Studies that involved a large number of people simply dealt with the administration of the vitamin and the subsequent endpoint. In any case, systematic reviews and meta-analysis of the antioxidant supplements have shown that they do not promote longevity and do not prevent major cardiovascular events (2, 3). Therefore, in our view, the prevalent scientific evidence at this moment is that antioxidant supplementation is not a good practice, at least as advice to the general population. We would like to reiterate that one should be careful to perform correct studies in which level of antioxidants and the antioxidant effect in each of the patient should be tested before one can definitely rule out the validity of antioxidant supplementation in humans. For instance, there was evidence that vitamin E could be treatment for Alzheimer's disease (43). We re-evaluated this by administering vitamin E to a number of Alzheimer's patients and observed a paradoxical effect. We found two populations, one of which was respondent to the antioxidant effect of vitamin E (because these patients had a more reduced glutathione redox ratio in plasma than controls) and another that was nonrespondent to vitamin E and in these patients the glutathione redox ratio was not different from controls. Only those patients, in whom administration of vitamin E had resulted in an antioxidant effect, responded to the treatment of vitamin E and loss of cognition was diminished (27). This indicates that antioxidant administration cannot be completely ruled out in all cases, but the prevalent evidence is that the main corollary for the free radical theory of aging is no longer tenable.
The general view is that by giving powerful antioxidants, one may hamper the useful adaptations to oxidative stress. A clear example of this is our work, in which oral administration of vitamin C was found to decrease muscle mitochondrial biogenesis and to hamper training-induced adaptation to exercise. In our hands, administration of vitamin C was not only useless but, in terms of performance, worse than useless because it prevented adaptation, including mitochondrial biogenesis induced by training (15) (Fig. 7). Peroxisome proliferator-activated receptor gamma, coactivator 1 alpha (PGC-1α) is a master regulator of the cellular response to oxidative stress. This coactivator is also required for mitochondrial biogenesis; thus, we measured not only classical antioxidant responses but also the increase in mitochondriogenesis associated with exercise training and found that antioxidants lower PGC-1α expression and mitochondriogenesis. Thus, the administration of exogenous antioxidants, such as vitamin C, hampers the antioxidant response associated with training in terms of increase in intercellular antioxidant enzymes and also the increase in mitochondriogenesis that occurs associated with the cellular response to mild oxidative stress Work from the groups of Ristow in Germany (41) and Wadley in Australia (48) extended our findings to the beneficial health effects of exercise and these authors observed that supplementation with vitamin E and C prevents an increase in the insulin sensitivity that is normally afforded by exercise.
FIG. 7.
Antioxidants hamper the beneficial effects of training on mitochondriogenesis. These are mediated by ROS and vitamin C blocks partially PGC-1α, NRF1, TFAM, and mitochondrial DNA levels in skeletal muscle. These levels normally increase with exercise training and that effect is blocked by treatment with antioxidants. These results are against the main corollary of the free radical theory of aging, that is, that antioxidant supplementation is good for mitochondriogenesis and in general, good for your health. NRF1, nuclear respiratory factor 1; PGC-1α, peroxisome proliferator-activated receptor gamma, coactivator 1 alpha; ROS, reactive oxygen species; TFAM, mitochondrial transcription factor A.
Conclusions: The Radical Signaling Disruption Theory of Aging
In the introduction to this review we have reproduced the formulation of the free radical theory of aging by Denham Harman in the 50's (19). Harman proposed that aging and age-associated diseases could be attributed to the deleterious effects of free radicals. Present-day evidence does not support this statement. An interesting modification of the free radical theory of aging has been put forward by Sohal and Orr (46). They propose “The redox stress theory of aging.” If free radicals cause a stress that cells can cope with, then damage will not occur because antioxidant defenses will overwhelm such stress. Only if the stress is of such magnitude that it deranges cellular signaling mechanisms, age-associated damage will take place. Keeping this in mind, we would like to propose “The cell signaling disruption theory of aging.” This theory postulates that ROS cause aging inasmuch as they alter—sometimes irreversibly—the signaling network of the cell. If the cell can cope with the stress caused by relatively mild action of ROS, then adaptation takes place and damage does not occur. If, however, the cell is overwhelmed by the action of radicals, subcellular damage and aging will take place. Indeed, radicals serve as signals and interaction between them is tightly balanced. For instance, nitric oxide (NO, a radical) is a powerful vasodilator (16). But it reacts swiftly with superoxide (another radical) yielding peroxynitrite (39). The latter does not have vasodilating properties and thus, interaction with superoxide prevents the vasodilating effects of NO. Steady state levels of superoxide are elevated in aging as shown by our group (45), as well as by that of Ames and coworkers (17). This results in more NO being removed and is important to explain the low vascular reactivity of the old. Free radicals are involved in the age-associated damage to macromolecules and this may cause a derangement in ROS-mediated cell signaling causing stress and diseases. This is exemplified, for instance, by the altered reactivity of NF-κB or activator protein 1 in aging (50) by the lack of reactivity of p38 and PGC-1α (10).
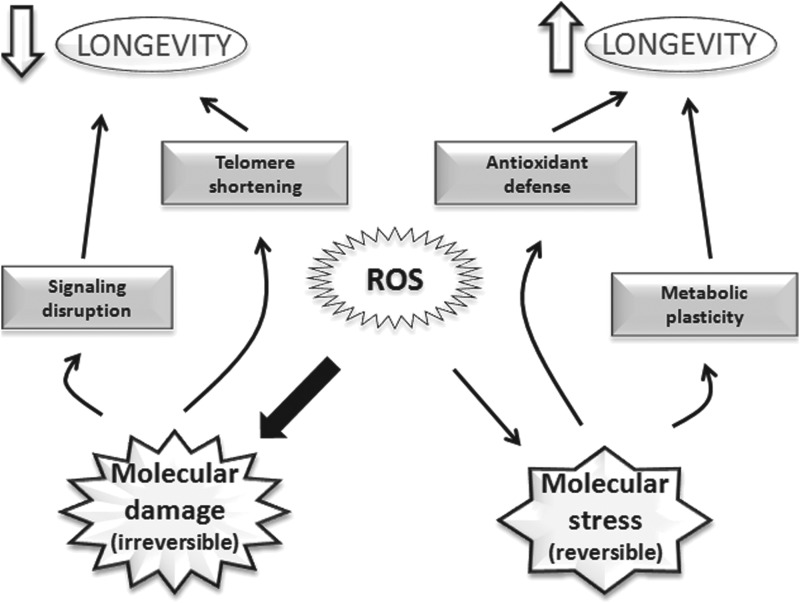
A “modified” free radical theory of aging, which is in keeping with the majority of experiments reported in this review, is that aging is caused by a disruption of the whole signaling network involving ROS. This “modified” theory does not imply that radicals always cause damage (as in the original theory) or that the more oxidative stress the less longevity. There may be interventions, completely independent of ROS (like up-regulation of telomerase) that promote longevity without affecting ROS or oxidative stress (see Fig. 8).
FIG. 8.
The double edge sword of free radicals. They have hormetic effects. When radicals cause severe effects on biomolecules it causes damage (i.e., irreversible alterations), whereas when the aggression is mild, a stress is caused and this may have signalling effects, as well as the already-mentioned hormetic effects.
Finally, our view is that, in general terms, we cannot support the idea of proposing antioxidant supplementation for the general population. There are many cases (like for instance exercise training) in which antioxidant supplementation is bad for you. And in general terms, it is much better to increase endogenous defences by nutritional or physiological manipulation than administering antioxidant compounds, such as vitamin C or E.
These mainly negative considerations do not detract from the free radical theory of aging, which has been extremely useful and has fostered research by providing a general theoretical framework on which many of us have based decades-long experimental research.
In view of the crossroads, at which the authors working in Gerontology now find themselves, we believe that future experiments should try to provide conclusive evidence against the free radical theory of aging (using the Popperian approach, one should not be trying to find more evidence in favor, but rather evidence against that has to be incontrovertible) and if such evidence is provided by molecular mechanisms, one should try and postulate new, more comprehensive theories to understand aging and these theories must be testable. The cell signaling disruption theory of aging is one of such approaches, which we hope may provide a theoretical framework for new experiments that will lead to a more exhaustive understanding of aging.
Abbreviations Used
- 8-OHdG
oxo-8-deoxyguanosine
- C57BL6
C57 Black 6
- DNA
deoxyribonucleic acid
- GPx
glutathione peroxidase
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- MnSOD
manganese superoxide dismutase
- NF-κB
nuclear factor kappa B
- NMR
naked mole rats
- NO
nitric oxide
- NRF1
nuclear respiratory factor 1
- OF1
Oncins France 1
- PGC-1α
peroxisome proliferator-activated receptor gamma, coactivator 1 alpha
- ROS
reactive oxygen species
- SD
standard deviation
- SOD
superoxide dismutase
- TFAM
mitochondrial transcription factor A
Acknowledgments
This work was supported by grants SAF2010-19498, from the Spanish Ministry of Education and Science (MEC); ISCIII2006-RED13-027 from the “Red Temática de investigación cooperativa en envejecimiento y fragilidad (RETICEF), PROMETEO2010/074 from “Conselleria de Sanitat de la Generalitat Valenciana”, 35NEURO GentxGent from “Fundació Gent Per Gent de la Comunitat Valenciana” and EU Funded COSTB35 and CM1001. This study has been cofinanced by FEDER funds from the European Union.
References
- 1.Ali SS. Xiong C. Lucero J. Behrens MM. Dugan LL. Quick KL. Gender differences in free radical homeostasis during aging: shorter-lived female C57BL6 mice have increased oxidative stress. Aging Cell. 2006;5:565–574. doi: 10.1111/j.1474-9726.2006.00252.x. [DOI] [PubMed] [Google Scholar]
- 2.Bjelakovic G. Nikolova D. Gluud LL. Simonetti RG. Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 3.Bjelakovic G. Nikolova D. Simonetti RG. Gluud C. Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis. Lancet. 2004;364:1219–1228. doi: 10.1016/S0140-6736(04)17138-9. [DOI] [PubMed] [Google Scholar]
- 4.Blackburn EH. Greider CW. Henderson E. Lee MS. Shampay J. Shippen-Lentz D. Recognition and elongation of telomeres by telomerase. Genome. 1989;31:553–560. doi: 10.1139/g89-104. [DOI] [PubMed] [Google Scholar]
- 5.Borras C. Esteve JM. Vina JR. Sastre J. Vina J. Pallardo FV. Glutathione regulates telomerase activity in 3T3 fibroblasts. J Biol Chem. 2004;279:34332–34335. doi: 10.1074/jbc.M402425200. [DOI] [PubMed] [Google Scholar]
- 6.Borras C. Sastre J. Garcia-Sala D. Lloret A. Pallardo FV. Vina J. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic Biol Med. 2003;34:546–552. doi: 10.1016/s0891-5849(02)01356-4. [DOI] [PubMed] [Google Scholar]
- 7.Budanov AV. Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134:451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cutler RG. Antioxidants, aging and longevity. In: Pryor WA, editor. Free Radicals in Biology. Orlando, FL: Academic Press; 1984. pp. 371–428. [Google Scholar]
- 9.Cutler RG. Antioxidants and aging. Am J Clin Nutr. 1991;53:373S–379S. doi: 10.1093/ajcn/53.1.373S. [DOI] [PubMed] [Google Scholar]
- 10.Derbre F. Gomez-Cabrera MC. Nascimento AL. Sanchis-Gomar F. Martinez-Bello VE. Tresguerres JA. Fuentes T. Gratas-Delamarche A. Monsalve M. Vina J. Age associated low mitochondrial biogenesis may be explained by lack of response of PGC-1alpha to exercise training. Age (Dordr) 2011;34:669–679. doi: 10.1007/s11357-011-9264-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Einstein A. Physics, philosophy and scientific progress. J Int Coll Surg. 1950;14:755–758. [PubMed] [Google Scholar]
- 12.García de la Asunción J. Millan A. Pla R. Bruseghini L. Esteras A. Pallardo FV. Sastre J. Viña J. Mitochondiral glutathione oxidation correlates with age-associated oxidative damage to mitochondrial DNA. FASEB J. 1996;10:333–338. doi: 10.1096/fasebj.10.2.8641567. [DOI] [PubMed] [Google Scholar]
- 13.Gerschman R. Gilbert DL. Nye SW. Dwyer P. Fenn WO. Oxygen poisoning and x-irradiation: a mechanism in common. Science. 1954;119:623–626. doi: 10.1126/science.119.3097.623. [DOI] [PubMed] [Google Scholar]
- 14.Gomez-Cabrera MC. Borras C. Pallardo FV. Sastre J. Ji LL. Vina J. Decreasing xanthine oxidase mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J Physiol. 2005;567:113–120. doi: 10.1113/jphysiol.2004.080564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez-Cabrera MC. Domenech E. Romagnoli M. Arduini A. Borras C. Pallardo FV. Sastre J. Vina J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr. 2008;87:142–149. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- 16.Gruetter CA. Barry BK. McNamara DB. Kadowitz PJ. Ignarro LJ. Coronary arterial relaxation and guanylate cyclase activation by cigarette smoke, N′-nitrosonornicotine and nitric oxide. J Pharmacol Exp Ther. 1980;214:9–15. [PubMed] [Google Scholar]
- 17.Hagen TM. Yowe DL. Bartholomew JC. Wehr CM. Do KL. Park JY. Ames BN. Mitochondrial decay in hepatocytes from old rats: membrane potential declines, heterogeneity and oxidants increase. Proc Natl Acad Sci U S A. 1997;94:3064–3069. doi: 10.1073/pnas.94.7.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;2:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 19.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 20.Hayflick L. Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 21.Hekimi S. Lapointe J. Wen Y. Taking a “good” look at free radicals in the aging process. Trends Cell Biol. 2011;21:569–576. doi: 10.1016/j.tcb.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrero A. Barja G. Sites and mechanisms responsible for the low rate of free radical production of heart mitochondria in the long-lived pigeon. Mech Ageing Dev. 1997;98:95–111. doi: 10.1016/s0047-6374(97)00076-6. [DOI] [PubMed] [Google Scholar]
- 23.Ji LL. Gomez-Cabrera MC. Steinhafel N. Vina J. Acute exercise activates nuclear factor (NF)-kappaB signaling pathway in rat skeletal muscle. FASEB J. 2004;18:1499–1506. doi: 10.1096/fj.04-1846com. [DOI] [PubMed] [Google Scholar]
- 24.Kelly DP. Cell biology: ageing theories unified. Nature. 2011;470:342–343. doi: 10.1038/nature09896. [DOI] [PubMed] [Google Scholar]
- 25.Lambert AJ. Brand MD. Research on mitochondria and aging, 2006–2007. Aging Cell. 2007;6:417–420. doi: 10.1111/j.1474-9726.2007.00316.x. [DOI] [PubMed] [Google Scholar]
- 26.Lapointe J. Hekimi S. Early mitochondrial dysfunction in long-lived Mclk1+/− mice. J Biol Chem. 2008;283:26217–26227. doi: 10.1074/jbc.M803287200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lloret A. Badia MC. Mora NJ. Pallardo FV. Alonso MD. Vina J. Vitamin E paradox in Alzheimer's disease: it does not prevent loss of cognition and may even be detrimental. J Alzheimers Dis. 2009;17:143–149. doi: 10.3233/JAD-2009-1033. [DOI] [PubMed] [Google Scholar]
- 28.Matheu A. Maraver A. Klatt P. Flores I. Garcia-Cao I. Borras C. Flores JM. Vina J. Blasco MA. Serrano M. Delayed ageing through damage protection by the Arf/p53 pathway. Nature. 2007;448:375–379. doi: 10.1038/nature05949. [DOI] [PubMed] [Google Scholar]
- 29.Miquel J. Economos AC. Fleming J. Johnson JE., Jr. Mitochondrial role in cell aging. Exp Gerontol. 1980;15:575–591. doi: 10.1016/0531-5565(80)90010-8. [DOI] [PubMed] [Google Scholar]
- 30.Miwa S. Riyahi K. Partridge L. Brand MD. Lack of correlation between mitochondrial reactive oxygen species production and life span in Drosophila. Ann N Y Acad Sci. 2004;1019:388–391. doi: 10.1196/annals.1297.069. [DOI] [PubMed] [Google Scholar]
- 31.Moosmann B. Behl C. Mitochondrially encoded cysteine predicts animal lifespan. Aging Cell. 2008;7:32–46. doi: 10.1111/j.1474-9726.2007.00349.x. [DOI] [PubMed] [Google Scholar]
- 32.Nemoto S. Finkel T. Ageing and the mystery at Arles. Nature. 2004;429:149–152. doi: 10.1038/429149a. [DOI] [PubMed] [Google Scholar]
- 33.Orr WC. Sohal RS. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1994;263:1128–1130. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- 34.Pauling L. Freeman S. Francisco. San Franciscom CA: 1973. Vitamin C, the Common Cold and the Flu. [Google Scholar]
- 35.Pauling L. Are recommended daily allowances for vitamin C adequate? Proc Natl Acad Sci U S A. 1974;71:4442–4446. doi: 10.1073/pnas.71.11.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perez VI. Bokov A. Van Remmen H. Mele J. Ran Q. Ikeno Y. Richardson A. Is the oxidative stress theory of aging dead? Biochim Biophys Acta. 2009;1790:1005–1014. doi: 10.1016/j.bbagen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perez VI. Buffenstein R. Masamsetti V. Leonard S. Salmon AB. Mele J. Andziak B. Yang T. Edrey Y. Friguet B. Ward W. Richardson A. Chaudhuri A. Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat. Proc Natl Acad Sci U S A. 2009;106:3059–3064. doi: 10.1073/pnas.0809620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Popper KR. The Logic of Scientific Discovery. London: Hutchinson; 1972. [Google Scholar]
- 39.Radi R. Beckman JS. Bush KM. Freeman BA. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991;266:4244–4250. [PubMed] [Google Scholar]
- 40.Ristow M. Zarse K. How increased oxidative stress promotes longevity and metabolic health: the concept of mitochondrial hormesis (mitohormesis) Exp Gerontol. 2010;45:410–418. doi: 10.1016/j.exger.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 41.Ristow M. Zarse K. Oberbach A. Kloting N. Birringer M. Kiehntopf M. Stumvoll M. Kahn CR. Bluher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sablina AA. Budanov AV. Ilyinskaya GV. Agapova LS. Kravchenko JE. Chumakov PM. The antioxidant function of the p53 tumor suppressor. Nat Med. 2005;11:1306–1313. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sano M. Ernesto C. Thomas RG. Klauber MR. Schafer K. Grundman M. Woodbury P. Growdon J. Cotman CW. Pfeiffer E. Schneider LS. Thal LJ. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer's disease. The Alzheimer''s Disease Cooperative Study. N Engl J Med. 1997;336:1216–1222. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- 44.Sanz A. Hiona A. Kujoth GC. Seo AY. Hofer T. Kouwenhoven E. Kalani R. Prolla TA. Barja G. Leeuwenburgh C. Evaluation of sex differences on mitochondrial bioenergetics and apoptosis in mice. Exp Gerontol. 2007;42:173–182. doi: 10.1016/j.exger.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sastre J. Pallardo FV. Pla R. Pellin A. Juan G. O'Connor JE. Estrela JM. Miquel J. Vina J. Aging of the liver: age-associated mitochondrial damage in intact hepatocytes. Hepatology. 1996;24:1199–1205. doi: 10.1002/hep.510240536. [DOI] [PubMed] [Google Scholar]
- 46.Sohal RS. Orr WC. The redox stress hypothesis of aging. Free Radic Biol Med. 2012;52:539–555. doi: 10.1016/j.freeradbiomed.2011.10.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strehler BL. Time, Cells and Aging. New York; London: Academic Press; 1977. [Google Scholar]
- 48.Strobel NA. Peake JM. Matsumoto A. Marsh SA. Coombes JS. Wadley GD. Antioxidant supplementation reduces skeletal muscle mitochondrial biogenesis. Med Sci Sports Exerc. 2010;43:1017–1024. doi: 10.1249/MSS.0b013e318203afa3. [DOI] [PubMed] [Google Scholar]
- 49.Tomas-Loba A. Flores I. Fernandez-Marcos PJ. Cayuela ML. Maraver A. Tejera A. Borras C. Matheu A. Klatt P. Flores JM. Vina J. Serrano M. Blasco MA. Telomerase reverse transcriptase delays aging in cancer-resistant mice. Cell. 2008;135:609–622. doi: 10.1016/j.cell.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 50.Vasilaki A. McArdle F. Iwanejko LM. McArdle A. Adaptive responses of mouse skeletal muscle to contractile activity: the effect of age. Mech Ageing Dev. 2006;127:830–839. doi: 10.1016/j.mad.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 51.Vina J. Borras C. Gambini J. Sastre J. Pallardo FV. Why females live longer than males? Importance of the upregulation of longevity-associated genes by oestrogenic compounds. FEBS Lett. 2005;579:2541–2545. doi: 10.1016/j.febslet.2005.03.090. [DOI] [PubMed] [Google Scholar]
- 52.Vina J. Borras C. Miquel J. Theories of ageing. IUBMB Life. 2007;59:249–254. doi: 10.1080/15216540601178067. [DOI] [PubMed] [Google Scholar]
- 53.Yang W. Hekimi S. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol. 2010;8:e1000556. doi: 10.1371/journal.pbio.1000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y. Ikeno Y. Qi W. Chaudhuri A. Li Y. Bokov A. Thorpe SR. Baynes JW. Epstein C. Richardson A. Van Remmen H. Mice deficient in both Mn superoxide dismutase and glutathione peroxidase-1 have increased oxidative damage and a greater incidence of pathology but no reduction in longevity. J Gerontol A Biol Sci Med Sci. 2009;64:1212–1220. doi: 10.1093/gerona/glp132. [DOI] [PMC free article] [PubMed] [Google Scholar]