Abstract
Rapid divergence in postmating-prezygotic characters suggests that selection may be responsible for generating reproductive barriers between closely related species. Theoretical models indicate that this rapid divergence could be generated by a series of male adaptations and female counteradaptations by means of sexual selection or conflict, but empirical tests of particular mechanisms are generally lacking. Moreover, although a male–female genotypic interaction in mediating sperm competition attests to an active role of females, molecular or morphological evidence of the female's participation in the coevolutionary process is critically needed. Here we show that postmating-prezygotic variation among populations of cactophilic desert Drosophila reflects divergent coevolutionary trajectories between the sexes. We explicitly test the female's role in intersexual interactions by quantifying differences in a specific postmating-prezygotic reproductive character, the insemination reaction mass, in two species, Drosophila mojavensis and Drosophila arizonae. A series of interpopulation crosses confirmed that population divergence was propelled by male–female interactions, a prerequisite if the selective forces derive from sexual conflicts. An association between the reaction mass and remating and oviposition behavior argues that divergence has been propelled by sexually antagonistic coevolution, and potentially has important implications for speciation.
Recent interest in the role of postmating-prezygotic characters in speciation has focused attention on the processes that generate rapid divergence in these traits among species (1, 2). High rates of sequence divergence and levels of polymorphism in postmating-prezygotic characters, such as fertilization and seminal proteins, show that these differences are selectively driven (3–9) and reflect the potential importance of these reproductive proteins in mate recognition and speciation (10, 11). In Drosophila melanogaster, males produce ≈100 accessory gland proteins (ACPs) that are passed to females in the ejaculate (12). The effects of male genotypic differences on interejaculate competition (13–16) and the influence of the ACPs and sperm themselves on female oviposition and remating behavior (17–19) suggest that these genes are targets of strong selection. Although an interaction between male and female genotypes argues for an active role of the female (20–22), molecular or morphological evidence of the female's participation in the coevolutionary process is critically needed (2, 11–23).
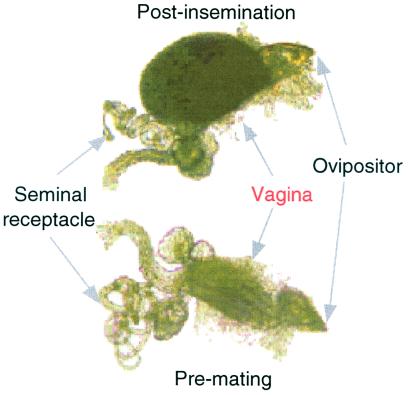
The insemination reaction mass (Fig. 1), a large opaque vaginal mass that forms after mating in a number of Drosophila species from the Repleta group (24), is ideal for testing theories about the evolution of postmating-prezygotic reproductive characters, especially from the female's perspective. Females typically do not oviposit or remate until the mass has subsided (25). Pronounced increases in the size and duration of the mass in heterospecific matings, lasting several days in certain species crosses (26, 27), suggest there is a reproductive, biochemical mismatch between males and females. However, it is unclear whether this coevolutionary breakdown is a consequence of the gradual accumulation of species differences over time, or whether the changes themselves were driven by interactions between the sexes. By quantifying differences in the insemination reaction mass among populations, we can test three predictions that derive from a theory of sexually antagonistic coevolution (10, 28): (i) genetically differentiated populations show divergence in postmating-prezygotic characters; (ii) divergence was generated by male–female interactions, for example, by a process of adaptation and counteradaptation; and (iii) the postmating-prezygotic character is associated with costs in fitness. We characterized the temporal trajectory of the insemination reaction mass (i.e., size and duration of the mass) in six populations of two cactophilic desert Drosophila species, Drosophila mojavensis and Drosophila arizonae. A series of interpopulation crosses was used to determine whether divergence was propelled by male–female interactions. One potential cost associated with the mass, namely effects on offspring production, was evaluated by testing for an association between the duration of the mass and the onset of oviposition.
Figure 1.
Schematic of D. mojavensis female reproductive tract pre- and postmating.
Methods
All flies were collected after eclosion and stored in sex-specific yeast-culture vials. Only mature females and males (i.e., flies of 5 and 9 days of age, respectively) were used. Matings were performed in the morning, which is the typical mating time in natural populations. One female was aspirated into each culture vial with a male, and the time of copulation was recorded after which males were removed. Eggs were counted, and logistic regression was used to test for an association between size of the reaction mass and oviposition. The temporal trajectory of the reaction mass was characterized within each population or cross by dissecting an average of five mated females each hour until the mass was no longer visible (i.e., up to 16 h postmating). The size of the mass (perimeter) was measured from digital images of the female reproductive tracts (Fig. 1) by using National Institutes of Health image software (http://rsb.info.nih.gov/nih-image/). Images were captured by using an Olympus (New Hyde Park, NY) stereomicroscope with an attached digital camera. The mass was characterized for four D. mojavensis populations (AB, Anza Borrego, southern CA; CI, Santa Catalina Island; GU, Guaymas, Sonora, Mexico; EN, Ensenada de los Muertos, Cape region of Baja, CA), two D. arizonae populations (EN; PE, Peralta Canyon, AZ), and reciprocal crosses between the AB and CI, and GU and EN D. mojavensis populations, and between the two D. arizonae populations, PE and EN.
An analysis of covariance was used to compare the temporal trajectories of the reaction mass among populations and between reciprocal crosses and intrapopulation matings, with time as the covariate. Significance of the three effects (i.e., the main, covariate, and interaction effects) was assessed by using randomization tests because the data were not normally distributed and transformations were ineffective. The F ratios for each of the three effects were calculated and compared with the distributions of F ratios derived from 1,000 permutated data sets in which the y values (i.e., the size of the reaction mass) were randomized [i.e., a “full” randomization, sensu Manly (29), which preserves the structure of the x variables]. The data were divided into three discrete groups along the covariate (i.e., early, mid, and late hours postmating), and the adjusted population means of each group were analyzed with ANOVAs (adjusted for multiple comparisons with a Bonferroni correction) to determine specifically how mass duration differed among populations.
Results and Discussions
Divergence Among Populations.
To test this first prediction, we compared the temporal trajectory of the reaction mass among four populations of the Sonoran Desert endemic D. mojavensis, and two populations of its largely sympatric sister species, D. arizonae. When there are reproductive conflicts of interest between the sexes, a perpetual antagonistic coevolution between males and females may cause rapid evolution of postmating-prezygotic characters within a population (28, 30). Among populations, this coevolutionary process is expected to differ because of underlying genetic differences and new mutations. Consequently, postmating-prezygotic characters are expected to diverge rapidly among populations, as well as species (2, 11). As predicted, there were pronounced differences in the reaction mass among populations of D. mojavensis (Table 1 D. mojavensis and Fig. 2). The divergence among populations is not attributable to differences in the initial size of the vagina among females. The size of the vagina of unmated females did not differ significantly among populations (F5, 59 = 1.12, P = 0.35).
Table 1.
Comparisons of the temporal trajectory of the reaction mass among populations (on the left), and between intra- and interpopulation matings (on the right), where the masses from the reciprocal crosses (e.g., AB × CI and CI × AB) were compared to those from intrapopulation matings (e.g., AB × AB) separately (see Fig. 3)
| Comparing among populations
|
Comparing intra- vs. interpopulation matings
|
||||
|---|---|---|---|---|---|
| Source | SS | F | Source | SS | F |
| D. mojavensis | D. mojavensis AB | ||||
| Group × h | 435167.31 | 4.50* | Group × h | 334392.00 | 5.37* |
| Group | 4908479.00 | 48.49** | Group | 980800.98 | 15.11** |
| Hour | 7667450.86 | 227.23** | Hour | 2711797.17 | 83.58** |
| Comparisons at early, mid, and late h | |||||
| 1–5 h postmating | D. mojavensis CI | ||||
| Group × h | 94293.52 | 1.16 | Group × h | 319916.31 | 5.74* |
| Group | 485933.53 | 5.96** | Group | 1723156.54 | 29.45** |
| Hour | 4293.58 | 0.16 | Hour | 4196388.27 | 143.41** |
| 6–10 h postmating | D. mojavensis GU | ||||
| Group × h | 256938.38 | 2.84 | Group × h | 321986.38 | 4.94* |
| Group | 3753902.18 | 39.26** | Group | 608082.83 | 8.69** |
| Hour | 521126.76 | 16.35** | Hour | 1320983.53 | 37.74** |
| 11–16 h postmating | D. mojavensis EN | ||||
| Group × h | 54570.21 | 0.95 | Group × h | 392564.026 | 5.44* |
| Group | 2091674.66 | 36.60** | Group | 614666.99 | 7.85** |
| Hour | 110837.93 | 5.82 | Hour | 1809888.50 | 46.21** |
| D. arizonae | D. arizonae PE | ||||
| Group × h | 9135.94 | 0.88 | Group × h | 62800.03 | 2.38 |
| Group | 19790.28 | 1.91 | Group | 456951.60 | 16.77** |
| Hour | 1004992.64 | 97.085** | Hour | 178363.09 | 13.09** |
| D. arizonae EN | |||||
| Group × h | 26524.26 | 0.82 | |||
| Group | 374658.51 | 11.61** | |||
| Hour | 347875.23 | 21.56** | |||
Significance of F values from ANCOVAs was assessed by using a randomization procedure (see Methods); significant effects are marked with asterisks (*, P < 0.05 and
, P < 0.01 after adjusting for multiple comparisons with a Bonferroni correction). A significant group by hour effect indicates that the rate of decrease of the reaction mass differed among populations, whereas significant main effects of group and hour reflect population differences in the adjusted mean size and duration of the mass, respectively.
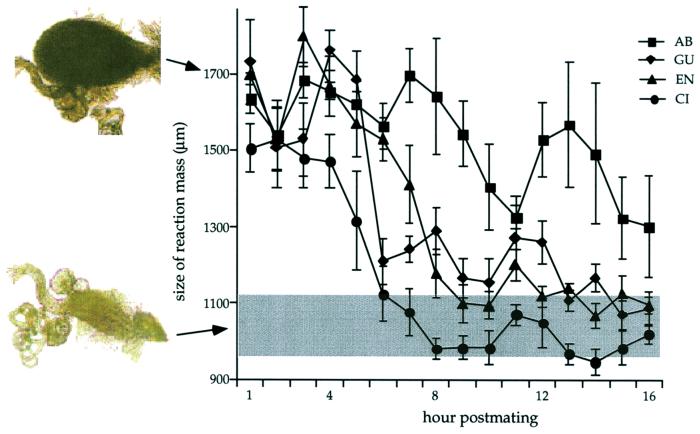
Figure 2.
Divergence among D. mojavensis populations in the temporal trajectory of the reaction mass (means and standard errors are shown). Size of vagina in unmated females represented by shaded area.
The rate of decrease in the size of the reaction mass differed among populations of D. mojavensis (i.e., significant group by hour interaction; Table 1 D. mojavensis and Fig. 2). ANOVAs on the adjusted population means after dividing the data into three groups along the covariate (i.e., early, mid, and late hours after mating) showed that this difference primarily reflected variation in mass duration (Table 1 D. mojavensis). During the 6–10-h postmating period, both mass duration and size differed significantly among populations (Table 1 D. mojavensis). For example, the reaction mass had essentially disappeared in populations such as CI, but it was still quite pronounced in the AB population (Fig. 2). Only in the AB population was a distinct mass present after 10 h postmating, accounting for the significant difference among populations in mass size during this period (Table 1 D. mojavensis). In D. arizonae, there was also a significant decrease in mass size over time, but not in the temporal trajectory of the mass between the two populations (i.e., a nonsignificant group effect and group by hour interaction; Table 1 D. arizonae).
Intra- Vs. Interpopulational Matings.
To test the second prediction that the observed divergence among populations reflects coevolutionary interactions between the sexes, we compared the mass from intra- and interpopulation matings. The results clearly show that the temporal trajectory of the mass differed significantly between the intra- and interpopulation matings and between the reciprocal crosses (Table 1 D. mojavensis and D. arizonae, and Fig. 3). The size of the mass was consistently larger and/or of longer duration in most reciprocal crosses, even when the populations themselves did not differ significantly from each other, such as with the two D. arizonae populations (Table 1 D. arizonae).
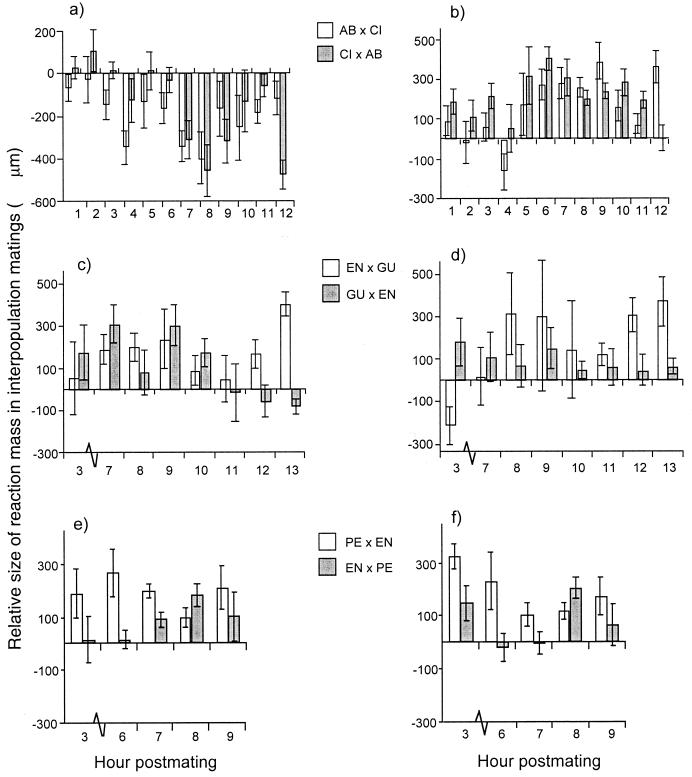
Figure 3.
Size of the reaction mass in reciprocal crosses relative to that from intrapopulation matings, which were scaled to 0. Means and standard errors of mass size relative to intrapopulation matings of D. mojavensis populations [AB (a), CI (b), GU (c), EN (d)] and D. arizonae populations [EN (e) and PE (f)] are shown. The females' population is listed first in the interpopulation crosses.
It is unlikely that these differences reflect variation in the amount of ejaculate transferred by males from the different populations. Otherwise, the size and duration of the mass would be similar regardless of with which female the male mates. For example, if mass duration and size were simply functions of ejaculate quantity, matings involving AB males would be predicted to produce a large mass of long duration and vice versa for CI males (Fig. 2). The mass in CI females mated with AB males did last significantly longer relative to CI females mated with CI males (Fig. 3), although the duration was not as long as that between AB males and AB females. However, the mass in AB females mated with CI males was also significantly larger and of longer duration than that from CI intrapopulation matings (Fig. 3), demonstrating that variation in the mass is not a simple reflection of ejaculate quantity.
Exaggeration of the reaction mass in females from reciprocal interpopulation crosses relative to intrapopulation matings (Table 1 and Fig. 3) demonstrates that the female reproductive tract is not a static, passive environment. Instead, contrasts between the intra- and interpopulation matings indicate that population differences reflect divergent trajectories in the reproductive biochemical coevolution of males and females among populations. Coevolution between the sexes in these and other closely related species is also apparent in correlated morphological changes between the sexes, such as between sperm length and length of the ventral receptacle in females (31).
Potential Costs Associated with Reaction Mass.
Lastly, if the divergence indeed was generated by male–female interactions by means of a process of adaptation and counteradaptation because of differences in the evolutionary interests of the sexes, the reaction mass is predicted to be associated with costs in fitness. In accordance with previous studies on the association between the reaction mass and female oviposition and remating behavior (25), our results also indicated that oviposition did not occur until the mass had subsided. The presence of the reaction mass was significantly negatively associated with the onset of oviposition (χ2 = 85.42, P < 0.0001). To the extent that such behavior is suboptimal (e.g., ref. 19), the results are consistent with the third prediction that sexual conflict has generated the divergence observed among populations. The ability of proteins in the seminal fluid to manipulate female behavior and physiology, despite a cost to females (12, 18, 28), as well as evidence for female resistance (30, 32) in Drosophila, is consistent with sexually antagonistic coevolution.
Conclusions
Our results demonstrate not only that postmating-prezygotic characters can diverge rapidly among populations, but also that this divergence reflects differences in the coevolutionary trajectory between males and females among populations. This coevolutionary divergence among populations indicates that the biochemical reproductive mismatch between males and females from different species, as manifested by differences in the reaction mass from inter- vs. intraspecific matings (refs. 26 and 27; L.L.K. and T.A.M., unpublished data), is most likely selectively driven. Divergence of postmating-prezygotic characters has important implications for the evolution of reproductive isolation and consequently is particularly relevant to the study of speciation. Because postmating interactions can have important fitness consequences for both males and females (2, 23), postmating-prezygotic characters are subject to strong selective pressures. Moreover, divergence in such traits creates the potential for biochemical and morphological incompatibilities between the sexes from different populations that can result in assortative fertilization or species specificity (1, 10, 11, 25, 33). Whether there is an association between the coevolutionary breakdown of males and females from different species, as inferred from the reaction mass, and patterns of hybrid inviability, remains to be determined.
The association between the reaction mass and female oviposition and remating (25) behavior suggests that this character may be a good candidate for divergence by means of antagonistic coevolution. The potential costs associated with the reaction mass, especially effects on offspring production because of sperm limitation, now need to be quantified to confirm that the differences reflect a mode of divergence involving sexual conflict (10, 28) and not some other type of sexual selection (34). Although males also incur a cost if offspring production is reduced in females because of the reaction mass, unlike females, this cost would be offset in males if the reduced remating frequency associated with the mass increases his reproductive success by reducing sperm competition with other males. Our findings underscore the importance of understanding the evolution of the female reproductive tract because it sets the stage for both male–male and male–female postmating-prezygotic interactions, such as sperm competition or female cryptic preferences (23).
Acknowledgments
We thank Dean Adams for statistical advice and for graciously providing the program for the randomization tests, and Douglas J. Futuyma, John Alcock, James F. Crow, and two anonymous reviewers for thoughtful comments that greatly improved the manuscript. This research has been supported by a postdoctoral fellowship from the National Science Foundation Research Training Grant in the Analysis of Biological Diversification at the University of Arizona (to L.L.K.) and by National Science Foundation Grants DEB 95-10645 and DEB-0075312 (to T.A.M.).
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Howard D J. Annu Rev Ecol Syst. 1999;30:109–132. [Google Scholar]
- 2.Rice W R. Proc Natl Acad Sci USA. 2000;97:12953–12955. doi: 10.1073/pnas.97.24.12953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coulthart M B, Singh R S. Biochem Genet. 1988;26:153–164. doi: 10.1007/BF00555496. [DOI] [PubMed] [Google Scholar]
- 4.Thomas S, Singh R. Mol Biol Evol. 1992;9:507–525. doi: 10.1093/oxfordjournals.molbev.a040738. [DOI] [PubMed] [Google Scholar]
- 5.Swanson W J, Vacquier V D. Proc Natl Acad Sci USA. 1995;92:4957–4961. doi: 10.1073/pnas.92.11.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Metz E C, Robles-Sikisaka R, Vacquier V D. Proc Natl Acad Sci USA. 1998;95:10676–10681. doi: 10.1073/pnas.95.18.10676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hellberg M E, Moy G W, Vacquier V D. Mol Biol Evol. 2000;17:458–466. doi: 10.1093/oxfordjournals.molbev.a026325. [DOI] [PubMed] [Google Scholar]
- 8.Tsaur S-C, Ting C-T, Wu C-I. Mol Biol Evol. 2001;18:22–26. doi: 10.1093/oxfordjournals.molbev.a003716. [DOI] [PubMed] [Google Scholar]
- 9.Swanson W J, Yang Z, Wolfner M F, Aquadro C F. Proc Natl Acad Sci USA. 2001;98:2509–2514. doi: 10.1073/pnas.051605998. . (First Published February 20, 2001; 10.1073/pnas.051605998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rice W R. In: Endless Forms: Species and Speciation. Howard D J, Berlocher S H, editors. New York: Oxford Univ. Press; 1998. pp. 261–270. [Google Scholar]
- 11.Markow T A. Proc Natl Acad Sci USA. 1997;94:7756–7760. doi: 10.1073/pnas.94.15.7756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolfner M F. Insect Biochem Mol Biol. 1997;27:179–192. doi: 10.1016/s0965-1748(96)00084-7. [DOI] [PubMed] [Google Scholar]
- 13.Harshman L G, Prout T. Evolution (Lawrence, Kans) 1994;48:758–766. doi: 10.1111/j.1558-5646.1994.tb01359.x. [DOI] [PubMed] [Google Scholar]
- 14.Clark A G, Aguadé M, Prout T, Harshman L G, Langley C H. Genetics. 1995;139:189–201. doi: 10.1093/genetics/139.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark A G, Dermitzakis E T, Civetta A. Evolution (Lawrence, Kans) 2000;54:1030–1035. doi: 10.1111/j.0014-3820.2000.tb00102.x. [DOI] [PubMed] [Google Scholar]
- 16.Chapman T, Neubaum D M, Wolfner M F, Partridge L. Proc R Soc London Ser B. 2000;267:1097–1105. doi: 10.1098/rspb.2000.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tram U, Wolfner M F. Genetics. 1999;153:837–844. doi: 10.1093/genetics/153.2.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xue L, Noll M. Proc Natl Acad Sci USA. 2000;97:3272–3275. doi: 10.1073/pnas.060018897. . (First Published March 21, 2000; 10.1073/pnas.060018897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrés J A, Arnqvist G. Proc R Soc London Ser B. 2001;268:399–405. doi: 10.1098/rspb.2000.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark A G, Begun D J. Genetics. 1998;149:1487–1493. doi: 10.1093/genetics/149.3.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark A G, Begun D J, Prout T. Science. 1999;283:217–220. doi: 10.1126/science.283.5399.217. [DOI] [PubMed] [Google Scholar]
- 22.Civetta A, Clark A G. Proc Natl Acad Sci USA. 2000;97:13162–13165. doi: 10.1073/pnas.230305397. . (First Published November 14, 2000; 10.1073/pnas.230305397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eberhard W G. Female Control: Sexual Selection by Cryptic Female Choice. Princeton: Princeton Univ. Press; 1996. [Google Scholar]
- 24.Patterson J T, Stone W. Evolution in the Genus Drosophila. New York: Macmillan; 1952. [Google Scholar]
- 25.Markow T A, Hocutt G D. In: Endless Forms: Species and Speciation. Howard D J, Berlocher S H, editors. New York: Oxford Univ. Press; 1998. pp. 234–244. [Google Scholar]
- 26.Baker W K. Univ Tex Publ. 1947;4720:126–136. [Google Scholar]
- 27.Patterson J T. Univ Tex Publ. 1947;4720:41–77. [Google Scholar]
- 28.Rice W R. Nature (London) 1996;381:232–234. doi: 10.1038/381232a0. [DOI] [PubMed] [Google Scholar]
- 29.Manly B F J. Randomization, Bootstrap and Monte Carlo Methods in Biology. New York: Chapman & Hall; 1997. [Google Scholar]
- 30.Holland B, Rice W R. Proc Natl Acad Sci USA. 1999;96:5083–5088. doi: 10.1073/pnas.96.9.5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pitnick S, Markow T, Spicer G S. Evolution (Lawrence, Kans) 1999;53:1804–1822. doi: 10.1111/j.1558-5646.1999.tb04564.x. [DOI] [PubMed] [Google Scholar]
- 32.Gavrilets S, Arnqvist G, Friberg U. Proc R Soc London Ser B. 2001;268:531–539. doi: 10.1098/rspb.2000.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swanson W J, Aquadro C F, Vacquier V D. Mol Biol Evol. 2001;18:376–383. doi: 10.1093/oxfordjournals.molbev.a003813. [DOI] [PubMed] [Google Scholar]
- 34.Lande R. Proc Natl Acad Sci USA. 1981;78:3721–3725. doi: 10.1073/pnas.78.6.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]