Abstract
Emerging hypotheses suggest that efficient cognitive functioning requires the integration of separate, but interconnected cortical networks in the brain. Although task-related measures of brain activity suggest that a frontoparietal network is associated with the control of attention, little is known regarding how components within this distributed network act together or with other networks to achieve various attentional functions. This review considers both functional and structural studies of brain connectivity, as complemented by behavioral and task-related neuroimaging data. These studies show converging results: The frontal and parietal cortical regions are active together, over time, and identifiable frontoparietal networks are active in relation to specific task demands. However, the spontaneous, low-frequency fluctuations of brain activity that occur in the resting state, without specific task demands, also exhibit patterns of connectivity that closely resemble the task-related, frontoparietal attention networks. Both task-related and resting-state networks exhibit consistent relations to behavioral measures of attention. Further, anatomical structure, particularly white matter pathways as defined by diffusion tensor imaging, places constraints on intrinsic functional connectivity. Lastly, connectivity analyses applied to investigate cognitive differences across individuals in both healthy and diseased states suggest that disconnection of attentional networks is linked to deficits in cognitive functioning, and in extreme cases, to disorders of attention. Thus, comprehensive theories of visual attention and their clinical translation depend on the continued integration of behavioral, task-related neuroimaging, and brain connectivity measures.
Key words: attention, cognitive control, diffusion tensor imaging (DTI), functional magnetic resonance imaging (fMRI), human brain connectivity, visual perception
Introduction
Since the advent of neuroimaging, this family of techniques has been used to investigate the structural and functional neuroanatomy of cognitive abilities such as perception, memory, and attention (Buckner, 2003; Cabeza and Nyberg, 2000; D'Esposito et al., 1999; Pessoa et al., 2003; Prichard and Rosen, 1994; Raichle and Mintun, 2006). Compared with sensory/motor functions, complex cognitive abilities such as attention have a widely distributed representation in the brain (Buckner, 2010; Gitelman et al., 1999; LaBerge, 2000; McIntosh, 2000; Nobre et al., 1997; Posner and Rothbart, 2007). An initial and continuing trend in neuroimaging research is the estimation of the mean level of activation for brain regions that are associated with experimental conditions, which is valuable for identifying the nodes of task-relevant networks. Research from the last decade, in particular, has revealed a broadly distributed network of frontal and parietal regions that is active during attention-demanding tasks (Corbetta and Shulman, 2002; Fan et al., 2005; Kastner and Ungerleider, 2000; Li et al., 2010; Pessoa and Ungerleider, 2004). However, since task-related data are typically averaged across trials, the relations among network regions are not always clear. Different components of the network could be active on the same or different trials, and the activations of such components could be independent of each other or have a pattern of causal influence within the network. Thus, the development of valid and informative theories of networks of cognition and, specifically, attention will require exploration of the concept of connectivity.
In this review, we are concerned with the neural architecture of attention, particularly within the visual modality. We consider the complementary roles of behavioral studies, which define the components of visual attention, and task-related neuroimaging studies (using magnetic resonance imaging [MRI]; and positron emission tomography [PET]), which define the relevant brain regions. The critical issue that we consider here is brain connectivity, and the ways in which recent studies of brain connectivity extend and advance task-related studies of mean activation, in the context of defining functional and structural networks of attention in the brain. These studies include both functional connectivity, in terms of different regions of activation that covary across time (or trials), and structural connectivity, in terms of the anatomic constraints on functionally connected regions. Of critical importance here is how patterns of connectivity relate to behavioral measures of attention, and how such patterns vary in relation to individual differences and disease. We propose that data from both task-related and resting-state measures of functional connectivity will contribute to improved theories of the brain networks that are associated with visual attention. We consider the potential contribution of diffusion tensor imaging (DTI) of cerebral white matter, in particular, to define the anatomical properties of the white matter pathways that are relevant for cortical networks of attention. In addition, the concept of disconnection among these network components has long been applied to the interpretation of neurological disorders (Catani and ffytche, 2005; Geschwind, 1965a, 1965b), and, more recently, to the interpretation of developmental differences (Bartzokis, 2004; Bartzokis et al., 2004; Bennett and Rypma, 2013; Buckner et al., 2008; Carmichael and Lockhart, 2012; Madden et al., 2010, 2012; Salat, 2011; Stevens et al., 2009b). Thus, we propose that the connectivity of attentional networks may vary systematically, across individuals, in a manner that will be informative for understanding human development and the effects of brain injury and disease. Across this review, an emerging theme is that connectivity reflects the functional parcellation of separate, but interconnected cortical networks that interact to mediate cognitive function.
Components of Visual Attention
Feature selection and attentional allocation
The human cognitive system is limited and can support the conscious awareness of only a small portion of visual information. A fundamental assumption of many laboratory-based, behavioral investigations of visual attention, over the last several decades, is that object identification, while seemingly instantaneous, extends over time (though often at a millisecond scale) and relies on the extraction of basic visual features such as size, color, and orientation (Schweickert, 1993; Shiffrin, 1988; Theeuwes, 1993; Wolfe and Horowitz, 2004). This extraction of featural information leads to the binding or conjunction of individual features and the priming or partial activation of associated perceptual and behavioral responses (Eriksen and Schultz, 1979; Quinlan, 2003; Treisman, 2006). Since information gradually leads to perception of one or more specific objects, response priming becomes increasingly selective. Competition between perceptual responses continues until priming for a particular response reaches a threshold level (which varies with context), ultimately leading to visual object identification and the selection of the associated response.
The cognitive resources available for this sequence of visual information processing stages are limited (e.g., Broadbent, 1958; Kahneman, 1973). When the visual environment comprises multiple objects, these objects compete for selective access to the finite pool of processing resources. Thus, processing should be biased in some manner in order for some objects and not others to be selected for access to conscious recognition (Desimone and Duncan, 1995; Duncan, 1984; Luck et al., 1996). This selection process is the core of the concept of attention. Computationally, attentional biasing may be viewed as increased weighting for some dimensions (e.g., color, orientation) or feature values (e.g., red, vertical), rather than others, during the process of object identification (Bundesen, 1990; Gramann et al., 2010; Müller et al., 1995; Müller and Krummenacher, 2006a; Wolfe and Horowitz, 2004). This variation in the computational weighting may involve either the enhancement of a target signal (Carrasco et al., 2000; Luck et al., 1996) or the suppression of distractor noise (Lavie et al., 2004; Leber and Egeth, 2006), or both, with the end result being a higher signal-to-noise ratio for attended events than for non-attended ones. The sources of attentional biasing may be categorized, broadly, as either bottom-up influences related to the physical salience of local contrasts within the display (Theeuwes, 2010), or top-down influences related to the observer's goals and expectations (Wolfe, 1994).
Beyond the specific task of visual object identification, the allocation of limited-capacity resources also provides a boundary condition for the efficiency of information processing at a more global level of task control. That is, attentional selection is required for the coordination of task components and response selection as well as for object identification. For example, the requirement to switch between two tasks (e.g., semantic categorization and size discrimination), across trials, typically leads to worse performance in either task, relative to the performance of that task in isolation (Meiran et al., 2000; Monsell, 2003). These task-switching costs are evident both at a global level (i.e., performance decrements within a dual-task context relative to a single-task context) and at a local level (i.e., decrements on switch trials relative to non-switch trials). Task-switching costs reflect the attentional demands related to updating task-set information for the appropriate task and establishing the appropriate rules that map between an object and a response (Logan and Bundesen, 2003; Monsell and Mizon, 2006; Rogers and Monsell, 1995).
Spatial orienting and visual search
A complete survey of behavioral measures of attention and associated theoretical models is beyond the scope of this review (Knudsen, 2007; Kramer et al., 1996; Luck and Vecera, 2002; Pashler, 1998). Virtually all behavioral investigations of attention rely on some form of cognitive subtraction, in which the attentional demands of a task are inferred from the differences in reaction time (RT), or error rate, across task conditions that vary in the stimuli presented or responses required (Gottsdanker and Shragg, 1985; Nickerson, 1972; Pachella, 1974; Sternberg, 1969). Particularly relevant for attention are RT cost/benefit analyses, in which a cue or task-relevant signal is presented before the display requiring a response. By subtracting the RT associated with a neutral cue condition, the improvement in performance (benefit) associated with an informative cue and the performance decrement (cost) associated with an incorrect or misleading cue can be measured (Posner, 1980; Posner et al., 1980). For example, when an arrow cue indicates the probable spatial location of an upcoming visual target, participants respond more quickly and accurately relative to trials in which a cue occurs but does not provide any spatial information. These changes in behavior reflect the benefit of orienting attention to a relevant location in space. Once a specific spatial location has been attended, however, subsequent responses to items at that location are typically slowed as compared with other locations in the visual field (an effect termed “inhibition of return”) (Klein, 2000; Posner and Cohen, 1984). Focusing on accuracy rather than RT as a measure of performance, investigators have also used computational models to develop quantitative indices of the information processing components of attention, such as speed, working memory capacity, the spatial distribution of attention, and top-down control (Bundesen, 1990; Bundesen et al., 2005; Finke et al., 2005).
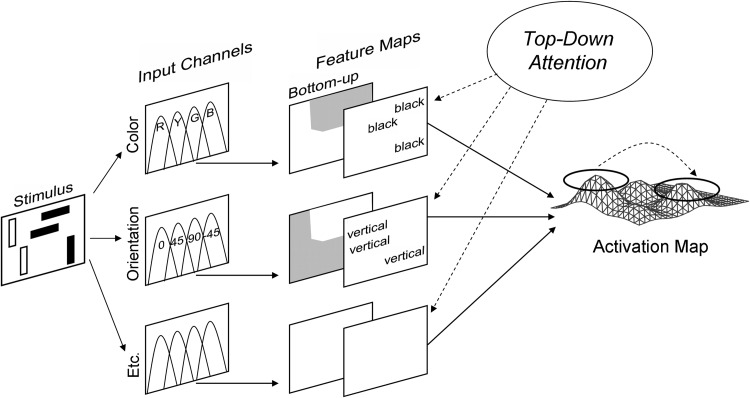
These methods of behavioral research have been applied not only to the detection and identification of individual items, but also to more complex attentional issues that are associated with locating and identifying objects in multi-item displays. A widely investigated laboratory task is visual search, which measures an observer's ability to detect or discriminate a target item among one or more nontarget (distractor) items (Duncan and Humphreys, 1989; Eckstein, 2011; Müller and Krummenacher, 2006b; Schneider and Shiffrin, 1977; Shiffrin and Schneider, 1977; Wolfe, 1998). A useful index of the overall attentional demands or difficulty of target detection is the degree to which RT or error rate increases with the number of items in either the display presented or the set of search targets. In a feature search task, for example, the target differs from all of the distractors by a single feature (e.g., a black T among white, rotated Ts and Ls), the bottom-up salience of the target is high, and search is efficient, as reflected in RT that is independent of the number of display items. In a conjunction search task, in contrast, the target is a conjunction of features in the distractor items (e.g., a black T among black, rotated Ts and Ls), and RT increases as a function of increasing display size, implying a less efficient search that depends on top-down attention. Currently, competing models of visual search rely on the general framework illustrated in Figure 1, in which target identification occurs as the result of a match between a target template held in memory and the features selected from the visual input. Theories of attention are concerned with characterizing aspects of the selection process, in terms of the emphasis (weighting) given to different features of the display, the relation between target and distractor features, and the relations between the top-down and bottom-up sources of biasing.
FIG. 1.
The architecture of the Guided Search Model of human visual search performance. Attention is guided to the most highly activated combination of features. Attentional biasing may reflect bottom-up sources related to the salience of local contrasts within the display, and/or top-down sources related to the observer's goals and expectations. Modified from Wolfe (1994) and reproduced with permission from Kramer and Madden (2008).
A central theme of current research on visual search is that attentional selection involves the interaction of bottom-up and top-down effects. Top-down attention is necessary during conjunction search, because the target search object is defined by a particular combination of features, and featural salience is comparable across individual items. The observer's internal representation or template of target features is the basis of attentional guidance to the target (Wolfe, 1994, 2007). Increasing the salience of a conjunction search target, however, can improve search efficiency (Proulx, 2007). Conversely, top-down attentional guidance can facilitate target identification when search is highly efficient (e.g., during a feature search) (Wolfe et al., 2003). The stage at which the interaction between top-down and bottom-up processing occurs is under debate. Evidence suggests that top-down selection biases visual processing only after selection of the most salient item (i.e., after an initial sweep of stimulus-driven processing) (Hickey et al., 2006; Theeuwes, 2010; Theeuwes et al., 2006), whereas other findings indicate that the initial sweep of visual processing is modulated by top-down effects (Folk et al., 1992; Müller et al., 1995; Tollner et al., 2012). Lavie (1995, 2004) proposed that these disparate views reflect differences in the perceptual load imposed by relevant information. Specifically, Lavie suggested that perceptual processing is capacity limited, but proceeds automatically until no processing resources are available. When processing resources are exhausted (i.e., when perceptual load is high), non-selected objects are not fully processed. Conversely, when relevant objects do not require all available attentional capacity (i.e., when perceptual load is low), the remaining attentional resources are allocated to the irrelevant objects, resulting in their full perceptual processing. Active inhibition of distractors may, thus, only be necessary in this latter case.
Priming, working memory, and executive function
As previously noted, behavioral investigations of visual attention typically involve a series of measurements across different types of displays, or trials, that vary in their physical structure and response assignment. The identification of a visual target, within any individual trial, will be enhanced by the selective weighting of target-relevant features, whereas target identification will also be enhanced by repetition priming. That is, if the current target is repeated from a recent presentation, this repetition may prime individual features independently of any attentional allocation (Geyer et al., 2010; Kristjánsson et al., 2002; Maljkovic and Nakayama, 1994). Research on priming suggests that top-down attention contributes to target identification independently of repetition priming (Wolfe et al., 2003), but the sequence of trials leading to a response to the current target and the associated reward history (Awh et al., 2012; Hutchinson and Turk-Browne, 2012) are also important determinants of target identification performance.
Similarly, the role of attention in visual object identification cannot be easily separated from memory. In a feature search task, the detection of any local contrast is sufficient for a target identification response, whereas a conjunction search task relies on some internal template, maintained over time, which defines the constellation of target features (e.g., a bar that is both red and vertical). Working memory, the maintenance and organization of items in current awareness, is, thus, intimately related to attentional processes, leading Baddeley (1993) to introduce the concept of working attention. Visual search performance is significantly impaired, for example, when working memory is engaged, illustrating the close dependence between memory and attention (Woodman and Luck, 2004). The processes of rehearsal, task preparation, scheduling of task components, inhibition of irrelevant information, and response selection are referred to as executive functioning, which interacts closely with working memory (Baddeley, 2002; Gopher, 1996; Hartley and Speer, 2000; Miyake et al., 2000). Further, the memory status of a display item (e.g., previously studied or new) as compared to that of temporally surrounding items influences the duration of attentional dwell time on that item during search (Parks and Hopfinger, 2008), and the repetition of priming effects in visual search interact with memory load (Kristjánsson et al., 2013). Within the domain of memory retrieval, Cabeza and coworkers (2008) proposed that the distinction between top-down and bottom-up processing that was developed in attentional theories is also relevant for understanding memory retrieval. That is, memory retrieval may be guided by either the salience of the individual memory, which is analogous to bottom-up attentional selection, or by goals and expectation, which are analogous to top-down attentional selection. Thus, although here we focus primarily on attention in the context of visual object identification, attentional processes contribute to a wide range of cognitive abilities, including memory and decision making.
Task-Related Activation in Neuroimaging Studies of Attention
Neuroimaging studies of attention complement the behavioral research by identifying the brain networks that are relevant for attention-related processes, such as top-down and bottom-up forms of biasing, enhancement of relevant sensory information, the inhibition of irrelevant information, the binding of stimulus features, and priming. Early neuroimaging studies of attention relied on PET measures of mean changes in task-related activation, in conjunction with cuing paradigms, to determine brain regions that are responsive during attention-demanding tasks. These studies defined activation as the difference between task conditions that were designed to isolate component processes of attention, using the cognitive subtraction methodology from behavioral research (D'Esposito et al., 1999; Friston et al., 1991; Price et al., 1997). Since the measurement of activation from PET depends on the half-life of the radionuclide used to measure cerebral blood flow, the minimum time scale for PET is in the order of minutes, and subtractions between task conditions are conducted between blocks of trials containing many individual events. By subtracting PET activity recorded during a central detection task, Corbetta and colleagues (1993) localized the brain regions associated with spatial shifts of attention. Activity in the bilateral dorsal frontal and parietal cortex, in particular, was greater when attention was shifted than when maintained at fixation. The dorsal frontal and parietal cortical regions were later associated with other attention-demanding tasks, such as visual search (Corbetta and Shulman, 1998; Corbetta et al., 1995). The parietal component of these brain responses, in particular, was activated during search, requiring the conjunction of two target features, but not during efficient search driven by the bottom-up salience of a target (Corbetta et al., 1995).
It is difficult to define the time course of specific attentional mechanisms, from PET, with the relatively coarse time scale. Event-related functional MRI (fMRI), in contrast, which is sensitive to changes in the blood oxygen level dependent (BOLD) hemodynamic response, has a time scale of seconds rather than minutes. As a result, the development of the event-related fMRI methodology provided the opportunity to measure regional brain activity at the level of individual trials and to apply subtraction techniques to attention, memory, and other cognitive abilities (D'Esposito et al., 1999). In particular, it has been possible, from event-related fMRI, to more clearly distinguish the sources of attention (i.e., the networks of attention-related activation) from the sites of attention (i.e., the sensory processing that is modulated by attention) (Hopfinger et al., 2000; Kastner et al., 1999; Kastner and Ungerleider, 2000).
Converging evidence from single cell recordings, fMRI, and transcranial magnetic stimulation (TMS) points to the dorsal frontoparietal attention network, including the intraparietal sulcus (IPS) and the frontal eye fields (FEFs), as specific sources of top-down attentional effects (Corbetta and Shulman, 2002; Giesbrecht et al., 2003; Hopfinger et al., 2000; Kastner et al., 1999; Moore and Armstrong, 2003; Ruff et al., 2006, 2009; Shulman et al., 2003). Results from a meta-analysis of 31 fMRI and PET studies suggest that a common set of frontoparietal brain regions is active in diverse executive control operations, including attentional shifting and working memory processes (Wager et al., 2004). Shulman and associates (1999, 2003) demonstrated that the dorsal frontoparietal activation related to the onset of attention-directing cues could be distinguished from target-related activation, which allowed the discrimination of attentional orienting from the subsequent effect of attention on sensory processes. Later fMRI work replicated these findings and further demonstrated that the target-related activation of occipital cortex was largely independent of that activated by directional cues (Corbetta and Shulman, 2002; Hopfinger et al., 2000). Additional studies revealed that the frontoparietal regions are activated during both covert shifts of attention (without eye movements) and overt eye movements, suggesting that attentional and oculomotor processes are tightly linked at the neural level (Corbetta et al., 1998). Kastner and colleagues (1999) demonstrated that a covert shift of attention led not only to activation of frontoparietal cortical regions but also to extrastriate cortex, even in the absence of visual stimulation. This result supports the role of the frontoparietal network as a source of top-down attention that enhanced activation at the site of target-relevant processing, in extrastriate cortex, independently of actual target identification.
In addition to the dorsal frontoparietal attention network for top-down attention, a more ventral component, including the temporoparietal junction (TPJ) and the ventral frontal cortex, operates as a source of bottom-up attentional effects. When, for example, a salient or unexpected item occurs in the visual environment, the ventral network may act to interrupt current top-down attention to reorient attention to the novel item or event (Corbetta et al., 2000, 2008; Corbetta and Shulman, 2002). Efficient attentional processing, thus, relies on the interaction of the dorsal and ventral attention networks. For example, Shulman and associates (2003) used a go/no-go version of search tasks, in which a target display could appear at a particular time point within a series of sequentially presented displays. Changes in fMRI regional activation, across the time course of search and target detection, illustrated the interaction of the dorsal and ventral attentional networks. Both the FEF and IPS were found to be activated during search through nontargets, which is consistent with a role of these regions in maintaining attention-related signals during search. However, unlike the FEF, the IPS also showed stimulus-related activations, and, therefore, may act to combine signals related to sensory and task-dependent components of salience. The IPS was active from the onset of the search display, whereas the ventral component of the network, in particular the TPJ, was recruited only when the target was detected, indicating a bottom-up interruption of search, from a detection process. Shulman and colleagues suggested that the TPJ acts as a circuit breaker that interrupts ongoing processes when a target is detected, and that a filter, possibly set by the dorsal frontoparietal attention network, determines the range of stimuli which can activate the TPJ. Thus, the frontoparietal network, in conjunction with the TPJ, acts to enhance task-relevant activation, while inhibiting task-irrelevant activation.
Further defining the role of the parietal cortex in attentional selection, Wei and colleagues (2011) compared activation across different forms of target detection, including feature search and three types of conjunction search. These authors found that parietal activation was enhanced in response to conjunction relative to feature search, suggesting that the parietal cortex represents a “master map” for binding individual features coded in distinct feature maps by a common location. Findings from TMS also support a critical role of the parietal cortex in the feature binding process required by conjunction search (Beck et al., 2006; Muggleton et al., 2008; Rosenthal et al., 2006). However, other evidence indicates that the parietal cortex, and in particular the IPS, is also related to the attentional set for external information even within a feature search (Imaruoka et al., 2003). The role of parietal cortical regions, particularly the IPS, in the integration of spatial and feature-based information (Egner et al., 2008), in the suppression of irrelevant distractors (Humphreys et al., 2004; Melloni et al., 2012), and in the grouping of visual elements (Xu and Chun, 2007) has also been highlighted. Further, several findings suggest functional distinctions within other regions of the parietal cortex. Pollmann and associates (2003), for example, proposed that during visual search, one region within the parietal cortex, the superior parietal lobule (SPL) mediates the selection biases against old stimuli to benefit the selection of new objects. Activation of the TPJ, in contrast, was associated with target detection, which is in line with the results of Shulman and colleagues (2003).
Within visual search and other experimental tasks, activity in the frontal and parietal cortex has been associated with the allocation of attention to goal-relevant objects. Less is known, however, with regard to how the processing of relevant objects is enhanced under conditions of distraction, when the processing of irrelevant objects should be inhibited. To begin addressing this issue, de Fockert and colleagues (2004) correlated behavioral measures of interference induced by distractors in a visual search task with the neural activity related to those distractors (i.e., between RT and fMRI signal specific to distractors). Search for a shape singleton target was disrupted by the presence of an irrelevant, color singleton distractor, and this disruption was paralleled by an increase in frontoparietal activation (the bilateral SPL and the left lateral precentral gyrus). Critically, there was a significant negative correlation between activity in the frontal region and the magnitude of the distractor interference in RT, indicating that greater frontal activity was associated with attenuated distractor interference. Lawrence and associates (2003) revealed similar findings using a rapid serial visual presentation (RSVP) task designed to isolate sustained attention (vigilance) effects. Weissman and associates (2005) argued that, in the context of a cued global/local selective attention task, fMRI activation in the dorsal anterior cingulate cortex (ACC) is critical for enhancing attention to relevant objects when behavioral goals are threatened by distracting events. Thus, although the different behavioral tasks elicited different patterns of activation overall, increased activation within frontal cortical regions appears to correlate with selective attention when distraction is present.
More generally, the frontal and parietal cortical regions may also be involved in “resetting” executive control systems in preparation for a switch in task demands. Using a cued categorization task for single word targets, Braver and associates (2003) compared switch and non-switch trials and found that switch trials produced activation in the dorsolateral prefrontal cortex (dlPFC) and ventrolateral PFC and the superior, primarily left, parietal cortex. During the instructive cue interval before target onset, increased activation in these prefrontal regions was associated with faster semantic categorization responses to target words for both switch and non-switch trials. In contrast, left superior parietal activations during this interval were selectively associated with faster responses in switch trials, revealing a possible role for this region in switch costs. Braver and colleagues also examined global switch costs (comparing sustained activations for dual-task switch blocks to single-task blocks), and found that anterior regions within the frontoparietal network demonstrated greater sustained activation during dual-task blocks, relative to single-task blocks. Similarly, across trials within a feature search task, Kristjánsson and associates (2007) found that repeating either the location or the color of a singleton target led to decreased activation (i.e., repetition suppression) in regions of the frontoparietal network (the FEF and IPS). Thus, data from both switching across tasks and stimulus repetition within tasks suggest that frontoparietal activation corresponds to the degree to which perceptual systems require resetting in preparation for upcoming visual demands.
In sum, neuroimaging studies measuring task-related activation have revealed widely distributed networks of brain regions in the frontal and parietal cortex that are associated with attentional functioning, including the enhancement of sensory processing, the inhibition of irrelevant information, the binding of stimulus features, and aspects of task control. These regions act to modulate visual feature processing regions in the occipital cortex, and several lines of evidence suggest that the parietal cortex serves as the primary source of this top-down biasing, as well as feature binding. Still in question is how the spatially distinct regions within the frontoparietal network act together to orchestrate attentional selection, and how these processes differentially represent top-down versus bottom-up mechanisms that interact with other cognitive functions such as memory, executive function, and cognitive control. Of further interest is how “sources” of attention (e.g., the frontoparietal network) modulate the neural “sites” at which attention acts (e.g., visual cortex), and, thus, modulate visual perception.
Task-Related Functional Connectivity of Attentional Networks
In contrast to studies of task-related differences in mean activation, discussed in the previous section, investigations of functional connectivity take the additional step of attempting to define which regions are active together, over the time course of the fMRI hemodynamic response (Friston, 2009; Friston et al., 1993, 2003). When spatially remote brain regions demonstrate strongly correlated patterns of BOLD signal, these regions are considered functionally connected. Such coordinated fluctuations are hypothesized to reflect a history of co-activation that leads to the strengthening or reorganizing of frequently utilized connections (i.e., Hebbian connectivity) and the removal of unused connections (i.e., synaptic pruning) (Hebb, 1949).
Several methods for measuring functional connectivity have emerged to quantify the degree of co-activation among brain regions, each varying in the types of assumptions required and the types of conclusions that may be drawn. One technique, introduced by Rissman and colleagues (2004), measures inter-regional correlations during distinct stages of a cognitive task. To estimate stage-specific activity, this approach adapts a standard general linear model such that separate parameter estimates (beta values) are determined for each individual trial and then used as the dependent measure in a correlation analysis. The correlated fluctuations in the time series beta values imply functional connectivity.
Other techniques focus more explicitly on effective connectivity, that is, the directional influence between the spatiotemporal covariation in regional brain activity. Granger causal modeling (GCM), for example, defines connectivity in terms of the temporal dependence between regional activations over time (Deshpande and Hu, 2012; Goebel et al., 2003). This dependence, for example, the statistical conclusion that activation of Region A reliably precedes the activation of Region B, refers only to data sets comprising the fMRI hemodynamic responses. Further, this form of Granger causality does not directly incorporate the experimental task as an input and, thus, does not reveal whether functional connectivity is caused by the task demands (Friston, 2009). Instead, GCM identifies voxels that are sources or targets of directed influence for any selected regions of interest (ROI), without testing or contrasting hypotheses about neuronal interactions. As an exploratory method, GCM explores directed influences between neuronal populations without a priori specification of an anatomically based model that contains preselected regions and connections between them (Roebroeck et al., 2005).
Using a different technique to explore effective connectivity, McIntosh and associates (1994) applied structural equation modeling and principal component analysis (PCA) to PET data, obtaining path coefficients representing the magnitude of the influence of each directional path. The resulting networks of interregional correlations, particularly in the right hemisphere, indicated that in an object vision (face matching) task, dominant path influences occurred along ventral pathways extending from occipital to temporal cortex; whereas in a spatial vision (dot-location matching), dorsal interactions from occipital to parietal cortex were stronger. McIntosh and colleagues later extended this approach to a more general analytic framework termed partial least squares, which operates on the covariance between brain voxels and experimental design (behavioral task conditions) to identify latent variables that optimally relate the brain data and behavioral data (McIntosh et al., 1996, 2004; McIntosh and Lobaugh, 2004). Two related approaches, psychophysiological interaction (PPI) (Friston et al., 1997; Gitelman et al., 2003) and dynamic causal modeling (DCM) (Friston et al., 2003), address the causal role of neuronal and behavioral events in the functional connectivity within the fMRI data sets. In PPI, the goal is to estimate the modulation of effective connectivity by the experimental or psychological context (i.e., an interaction). The DCM approach relies on a specific model of how this influence is mediated (based on biophysical constraints), which yields parameter estimations between regions based on Bayesian inference.
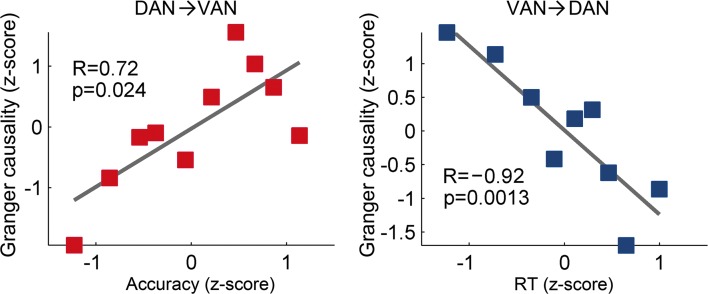
Using these methods, several fMRI studies have applied functional connectivity analyses to measures of visual attention, examining how attention-related brain regions interact to orchestrate top-down and bottom-up selection processes. For example, Wen and colleagues (2012) recorded BOLD responses during a cued visual spatial attention task and correlated measures of functional connectivity, based on GCM, with task performance (Fig. 2). Stronger Granger causal influences from the dorsal attention network to the ventral attention network were correlated with improved task performance, with the main source of these enhancements originating from the bilateral IPS and the right FEF. In addition, stronger Granger causal influences from the ventral attention network to the dorsal attention network were generally associated with worse performance, primarily driven by the right TPJ. Wen and associates concluded that signals from the dorsal network to the ventral network suppress and filter out unimportant distracter information, whereas signals in the reverse direction (from ventral to dorsal) break the attentional set maintained by the dorsal attention network to enable attentional reorienting.
FIG. 2.
Behavioral performance varies with the direction of Granger causal influences between the dorsal attention network (DAN) and ventral attention network (VAN). Linear fits are shown, where R is the Spearman correlation coefficient and p is the significance level. Stronger Granger causal influences from the DAN to the VAN were positively correlated with improved task performance (red), while stronger Granger causal influences from the VAN to the DAN were negatively correlated with task performance (blue). Reproduced with permission from Wen et al. (2012).
Vossel and associates (2012) also investigated cued spatial attention, but used a DCM approach, with Bayesian model selection. This study, however, focused on the connections between the visual cortex and the dorsal and ventral attention networks, respectively. Their results suggest that top-down (feedback) connections from dorsal parietal to visual cortex were modulated by the spatial deployment of attention, whereas invalid cueing led to changes in the bottom-up (feedforward) connections from visual areas to right TPJ. This latter effect, presumably reflecting attentional reorienting, is consistent with the ventral connectivity pattern observed by Wen and associates (2012). Prado and colleagues (2011) used PPI to examine whether trial-by-trial RT variations in a selective attention task also mirror fluctuations in functional connectivity between attention-related brain regions. Increasing RT was associated with reduced functional connectivity between frontal and parietal regions and within frontal regions, despite increases in the task-related activation of these regions. Using DCM, Wang and associates (2010) further characterized the functional relationship among frontoparietal regions during executive control processing: a flanker task either with or without a pre-target cue. Based on Bayesian selection procedures, the optimal model was that in which the processing of unexpected targets (those without anticipatory cues) mediated the influence of the IPS over prefrontal cortical regions (ACC; and dlPFC), and the conflict processing of incongruent flankers mediated the influence of these prefrontal cortical regions over the IPS. Thus, the IPS may play a key role in the processing of unexpected targets, whereas the PFC may act to resolve conflict through attentional modulation of the IPS. In conjunction with the findings of Wen and associates (2012), Vossel and associates (2012), and Prado and associates (2011), these results suggest that frontal and parietal regions are active together, over time, during executive control processing, and that the directional influence of these regions depends on whether attentional demands are driven by bottom-up or top-down influences.
Chica and associates (2013) further proposed that, within the dorsal attention network, functional connectivity among frontoparietal regions modulates the interaction between spatial attention and conscious perception. These authors focused on the fMRI activity during the attentional orienting period of a visual discrimination task in which near-threshold targets were preceded by peripheral cues. The authors determined whether different attentional processes (orienting versus reorienting) were associated with the subsequent conscious perception of the near-threshold targets. The results indicated that frontoparietal regions demonstrated higher connectivity for consciously perceived targets than for unperceived targets at attended locations (i.e., when the cues validly signaled the target location). However, connectivity was lower for consciously perceived targets than for unperceived targets when reorienting was required (i.e., when cues were spatially invalid). This pattern is consistent with an effective orienting of spatial attention toward the cued location. In other words, when a valid cue correctly guided attention to the upcoming target location, higher frontoparietal connectivity was associated with subjective reports of targets as being seen. In contrast, when an invalid cue required a reorientation of attention away from the incorrectly cued location, higher connectivity was associated with subjective reports of targets as being unseen. Thus, before the occurrence of a target object, functional connectivity among frontoparietal regions facilitates the access of the object to consciousness.
Attentional processing depends not only on efficient connectivity within frontoparietal regions, but also on connectivity between these source regions and the sites of attentional control in visual sensory cortex. For example, Imamoglu and associates (2012) found that the conscious recognition of objects is dependent on functional connectivity between the dlPFC and the extrastriate visual cortex, as measured by GCM. Connections between the frontal and visual cortical regions have also been established during a visual search of naturalistic scenes using DCM and independent component analysis (ICA) in conjunction with PPI (Pantazatos et al., 2012). In that study, task-related activations were isolated in the ventro-medial PFC (vmPFC) and the lateral occipital cortex (LOC). Functional connectivity between these regions, based on temporal correlations between independent components, was enhanced during visual search, with results from DCM suggesting bidirectional connections between the vmPFC and the LOC that were positively modulated by the task.
The studies described here investigate how patterns of functional connectivity are related to behavioral measures of attention. They provide initial evidence that frontal and parietal regions are active together, over time, during attention-demanding tasks, and that the directional influence of these regions depends on whether attentional demands are driven by bottom-up or top-down influences. Analyses of functional and effective connectivity suggest that signals from the ventral to the dorsal network may act to interrupt the attentional set maintained by the dorsal attention network to enable attentional reorienting. Variations in functional connectivity between frontal and parietal regions may contribute to trial-by-trial fluctuations in behavioral performance; and, further, attentional modulation of the visual cortex by these regions may depend on changes in functional connectivity. Overall, these results indirectly support the view that efficient information processing depends on the coordination of integrated, yet distinct functional brain networks (e.g., dorsal frontoparietal, ventral frontoparietal, visual processing networks, etc.).
Resting-State Functional Connectivity of Attentional Networks
Examination of task-related changes in functional connectivity has led to significant insights regarding how brain regions activate together, over time, in response to attentional demands. Recent findings also suggest, however, that brain regions are functionally connected in the resting brain, in the absence of any task, stimuli, or defined attentional demands (Fox et al., 2006b; Raichle and Mintun, 2006; Yeo et al., 2011). Activity in the resting brain is referred to as intrinsic or resting-state activity and comprises spontaneous, low-frequency oscillations (<0.1 Hz) in the BOLD signal that are highly correlated with concurrent fluctuations in neuronal spiking (Shmuel and Leopold, 2008). Regional correlations can be obtained either from pre-defined ROIs (Biswal et al., 1995; Greicius et al., 2003) or from whole-brain methods such as ICA (Greicius et al., 2004; McKeown et al., 1998). Identification of brain regions demonstrating strong correlations within the resting-state time-series helps define functionally coherent brain networks (Biswal et al., 1995). Resting-state data consistently suggest a default mode network (DMN), which includes a set of brain regions (in particular, medial PFC [mPFC]; posterior cingulate cortex [PCC]; lateral parietal cortex; and parahippocampal cortex). These regions exhibit not only highly correlated brain activity during resting state but also a reduced level of task-induced activation, with BOLD signal level often below baseline, across a wide range of tasks (Biswal et al., 2010; Greicius et al., 2003; Raichle and Mintun, 2006; van den Heuvel and Pol, 2010). Activity in the DMN appears to represent self-referential memory and spontaneous cognition that should be suppressed to respond efficiently to external events (Anticevic et al., 2012; Buckner et al., 2008; Buckner and Vincent, 2007; Fransson, 2005).
Resting-state measures have revealed connectivity not only within the DMN but also within the frontoparietal network associated with task-related measures of visual attention. Fox and colleagues (2005) demonstrated that resting-state fluctuations within regions of the dorsal frontoparietal attention network (including the IPS, FEF, and a middle temporal region [MT+]) were positively correlated. Thus, the attentional network that is activated in a functionally connected manner, during task performance, also exhibits connectivity at rest, or at least in the absence of specific behavioral task demands. The functional organization of the brain into dorsal and ventral attentional systems consistently demonstrated in task-related imaging studies (Corbetta and Shulman, 2002) is also present in intrinsic measures of connectivity “at rest” (Fox et al., 2006a). Specifically, Fox and associates (2006a) identified a bilateral dorsal attention system and a distinct right-lateralized ventral attention system solely on the basis of correlations between spontaneous fluctuations in the BOLD signal. Resting-state activity of a prefrontal region correlated with both attentional systems, potentially reflecting a mechanism by which the two systems interact.
Further, Fox and colleagues (2005) reported that time course data within the dorsal frontoparietal attention network correlate negatively with the DMN time course (Fig. 3), providing additional support for the functional separation of attentional and DMN resting-state activity. Some degree of overlap between the DMN and attentional networks, however, has also been observed using both ROI-based (He et al., 2009) and whole-brain (Tomasi and Volkow, 2011) analyses. The magnitude of the negative correlation between the DMN and attentional networks exhibits a continuous gradient across network subcomponents (Anderson et al., 2011), and some evidence suggests that switching between the DMN and attentional networks may be controlled by specific brain regions such as the right frontoinsula (Sridharan et al., 2008).
FIG. 3.
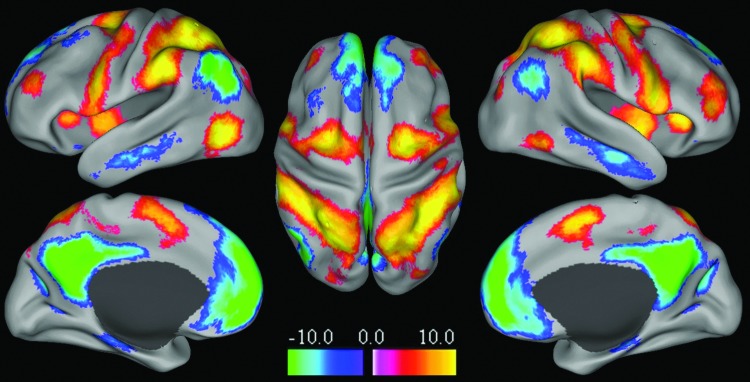
Resting-state functional connectivity data reveals two widely distributed, anticorrelated brain networks. Positive nodes are significantly correlated with seed regions within the frontoparietal attention network (task-positive seeds) and significantly anticorrelated with seed regions in the default mode network (DMN) that routinely deactivate during attention-demanding cognitive tasks (task-negative seeds). Negative nodes are significantly correlated with task-negative seed regions and significantly anticorrelated with task-positive seed regions. (Left) Lateral and medial views of left hemisphere. (Center) Dorsal view. (Right) Lateral and medial views of right hemisphere. Reproduced with permission from Fox et al. (2005).
Resting-state connectivity and behavior
Although spontaneous fluctuations in the brain have been identified in cortical regions associated with attention, the behavioral significance of the intrinsic functional connectivity is less clear. Some authors have argued that functional connectivity at rest may help functional systems to maintain an active state that is primed to respond to unpredictable but behaviorally relevant events (van den Heuvel et al., 2009). Fox and associates (2006b), for example, demonstrated that resting-state connectivity accounts for variability in event-related BOLD signals: Spontaneous activity measured in the right somatomotor cortex accounted for fluctuations in BOLD responses in the left somatomotor cortex following a right-hand button press. Further, fluctuations in the regional resting-state connectivity correlated with trial-to-trial variability in a behavioral measure, button press force (Fox et al., 2007). When resting-state activity in the somatomotor cortex was low, participants pressed a button with more force than when the intrinsic activity was high.
Resting-state connectivity also accounts for variability in performance during cognitive tasks. For example, Baldassarre and colleagues (2012) demonstrated that resting-state functional connectivity in task-relevant networks, measured before training, is predictive of subsequent performance in a visual discrimination task (a visual conjunction search for an inverted T among randomly oriented Ts). These authors defined task fitness (search efficiency) by the first component of a PCA combining the rate of performance improvement and the amount of practice required to reach criterion. Baldassarre and associates noted two patterns regarding the relation between functional connectivity and subsequent visual task fitness. First, stronger connectivity within visual cortical regions (e.g., linking dorsal and ventral subregions) was associated with higher fitness. A high degree of coherence between regions of the visual cortex, before any training, may facilitate the dynamic reweighting of the functional connections that occur with training, as a skill is acquired. The second pattern was an inverse correlation between spontaneous activity in the visual cortex and regions of the DMN and the task-positive network, for those individuals with better task performance; that is, enhanced anticorrelation of functional connectivity between visual cortex and DMN, and between visual cortex and a task-positive region previously associated with executive control processes (e.g., anterior insula). Baldassarre and associates concluded that this latter pattern, while complex, may represent a contribution of the DMN to distractor filtering, which becomes less important as target selection becomes more automatic.
Recently, Meier and associates (2012b) developed a novel analytic method to further investigate the association between fluctuations in resting-state networks and behavior. This method, termed parallel ICA, identifies complex relationships between resting-state fMRI networks and behavioral data by simultaneously performing ICA on each data set and finding the mutual information between the data sets. In a sample of 24 healthy younger adults, these authors identified the relation between several resting-state networks and neuropsychological test performance. In particular, the precuneus (a region previously implicated in visuospatial attention) exhibited increased connectivity with a ventral attention network when visual discrimination was relatively efficient (Stroop congruent trials) and decreased connectivity with this network when discrimination was inefficient (Stroop incongruent trials).
In addition to variability in behavior, resting-state connectivity also accounts for variability in task-dependent functional connectivity. Mennes and associates (2010), for example, explored the relationship between resting-state and task-dependent functional connectivity, in the context of an attention-demanding Eriksen flanker task (responding to the direction of an arrow flanked by same- or differently-oriented arrows). These authors used independently defined seeds for DMN and task-positive networks (from Fox et al., 2005) and found that resting-state connectivity with these seeds exhibited a network-dependent pattern of correlation to overall task-related activity. Specifically, resting-state connectivity with the task-positive network seed regions (left IPS, left MT+, right FEF) was related positively to overall task-induced activity, whereas DMN connectivity (left lateral parietal, mPFC, PCC) was related negatively to overall task-induced activity. That is, the more strongly a region was either connected to the task-positive network or segregated from the DMN network, the greater the magnitude of task-related activity. Mennes and colleagues found that the task-related activity associated with the congruency effect (i.e., activation for differently-oriented arrows minus same-oriented arrows) was positively correlated with resting-state connectivity with the task-positive network seed regions. This relationship was primarily driven by the incongruent trials, suggesting that resting-state connectivity may be particularly relevant when task demands are high.
Across these studies, a common theme is that although connectivity exists within both the DMN and task-positive networks, these networks are also segregated from each other and differentially relate to behavior. Functional dissociations have also been identified within the task-positive network. For example, one task-positive network, termed the cognitive control network, has been associated with more broadly defined attentional functions involved in executive control processes (Cole et al., 2010). Analysis of high-resolution resting-state data revealed that this network represents a set of regions that are contiguous, yet anatomically distinct from those of the dorsal frontoparietal attention network (Vincent et al., 2008). Seeley and associates (2007) demonstrated that interhemispheric intrinsic connectivity within a similar, ICA-derived control network is positively correlated with enhanced executive functioning, as measured by the Trail Making test (a visuomotor search task). This connectivity pattern may reflect attentional control operations that act to incorporate context and changing task demands.
In addition to the overlapping control networks mentioned here, a network known as the cinguloopercular network has been linked to another form of top-down control. Specifically, graph theory analysis of resting-state data revealed that the cinguloopercular network contains signals that provide stable “set-maintenance” throughout an attentionally demanding task; whereas the frontoparietal attention network acts to initiate and adjust control on a trial-to-trial basis (Dosenbach et al., 2007). Further, graph theory analyses suggest that the cinguloopercular and frontoparietal networks exhibit a small-world architecture of dense short-range connections within networks and weaker long-range connections between networks (Dosenbach et al., 2008), which is consistent with other resting-state analyses (Achard and Bullmore, 2007; Shen et al., 2012). The development of a comprehensive theory of attention should consider how these networks both co-engage and interact to mediate the various cognitive functions highlighted across differing experimental paradigms.
In conclusion, although resting-state connectivity exists within both task-positive and task-negative brain networks (leading to functional integration within a network), these sets of networks are also functionally distinct from each other (leading to functional segregation between networks). Intrinsic connectivity within distinct networks has been differentially related to task-dependent functional connectivity and to behavioral task demands. However, research is needed to determine the degree to which specific cognitive tasks are related to resting-state functional connectivity strength within and between brain networks.
Anatomical Constraints on Attentional Networks
The studies discussed in the preceding sections have established that functional connectivity occurs between widely dispersed brain regions, both during attention-demanding tasks and at rest. The cortical regions within the DMN and task-positive networks are anatomically separated, whereas their fMRI time series are highly correlated. It is likely that the integrity of white matter pathways is critical for the support of functional connectivity, both task-related and resting-state. Evidence suggests that the specialization of cortical neurons is predominantly determined by their connective inputs (Sharma et al., 2000), and that increased functional connectivity between regions may reflect increased myelination (Giedd et al., 1999). DTI and related techniques for assessing the microstructural properties of white matter tracts have contributed to an improved characterization of the anatomical constraints on functional connectivity in humans (Beaulieu, 2002; Jones, 2011; Mori and Zhang, 2006). It is important to note, however, when interpreting the relation between fMRI and DTI data, that DTI-related measures reflect the displacement of water molecules, which, in turn, reflect the combined influences of many variables (e.g., number and orientation of axons, myelination). Thus, DTI is informative regarding relevant anatomical constraints but does not directly yield a measure of structural connectivity (Jones et al., 2013; Wheeler-Kingshott and Cercignani, 2009).
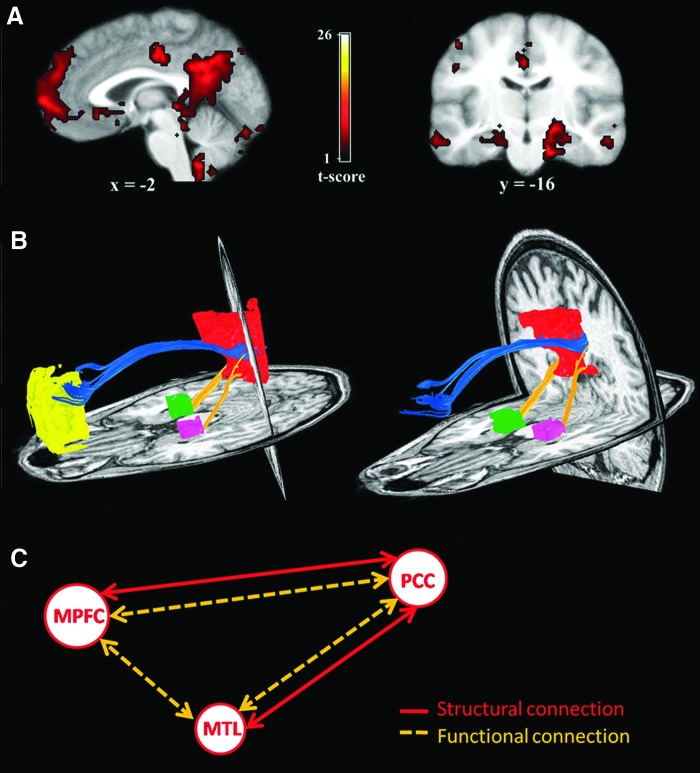
The first study to directly compare anatomical and functional connectivity demonstrated that when structural connectivity was high, as defined by the probability that DTI would identify a tract between two voxels, resting-state functional connectivity between those voxels also tended to be high (Koch et al., 2002). Only along the central sulcus, however, was there a significant positive correlation between functional and anatomical connectivity in this study. Later work focused on the relationship between structural and functional connectivity within a specific network, namely the DMN, rather than across all voxels within a hemisphere (Greicius et al., 2009) (Fig. 4). Using DMN seed regions obtained from intrinsic functional connectivity maps identified with an ICA, DTI tractography analysis revealed that the cingulum bundle connected the PCC and the mPFC seed regions, and that the descending cingulum bundles connected the PCC and the medial temporal lobe. Van den Heuvel and associates (2009) employed a similar method, but identified a bilateral resting-state network, including the superior parietal and frontal cortical regions, that closely overlapped the task-related frontoparietal attention network. By combining the resting-state functional connectivity map for this network with DTI-based fiber tracking, these authors demonstrated that the superior longitudinal fasciculus (SLF) serves as a structural bridge between the parietal and frontal regions identified in the resting-state data. The tight relation between functional and structural connectivity observed in these studies provided initial evidence for the critical role of white matter pathways in the support of efficient, coordinated processing between distant brain regions.
FIG. 4.
Diffusion tensor imaging (DTI)-based structural connectivity overlaps functional connectivity in the default mode network (DMN). (A) Intrinsic functional connectivity in the DMN in a group of six participants. The sagittal view depicts the posterior cingulate cortex/retrosplenial cortex (PCC/RSC) and medial prefrontal cortex (mPFC) clusters. Prominent bilateral medial temporal lobe (MTL) clusters are visible in the coronal image. (B) DTI fiber tractography in a single subject demonstrates the cingulum bundle (blue tracts) connecting the PCC/RSC to the mPFC. (C) Schematic representation of the structural and functional connections between these three nodes of the DMN. Modified from Greicius et al. (2009) and reproduced with permission from Damoiseaux and Greicius (2009).
While results from the multi-modal imaging approaches described earlier suggest that DTI-measured structural networks were consistent with resting-state functional networks, no direct comparisons across modalities were conducted. Van den Heuvel and colleagues (2008) directly compared a DTI measure of the directionality of water diffusion (fractional anisotropy [FA]) within the cingulum bundle to the functional correlation between these regions at rest. In a sample of healthy young adults, a positive correlation between the structural and functional measures was evident, such that increasing FA was associated with enhanced functional connectivity at rest.
Honey and colleagues (2009) later combined fMRI, diffusion spectrum imaging tractography of white matter, and computational modeling, to investigate the way in which the properties of anatomical networks can account for the systems-level properties of functional brain networks. These authors found that although resting-state functional connectivity is variable and frequently present between regions without direct structural linkage, the large-scale anatomical structure of the brain constrained the strength, persistence, and spatial statistics of functional connectivity. Similarly, Damoiseaux and Greicius (2009) reviewed eight studies that directly compared intrinsic functional and structural connectivity and concluded that the results exhibited largely convergent findings, which is consistent with a positive correlation between white matter tract integrity and functional connectivity. Parallel findings were also observed in the primate brain using a more direct measure of anatomical connectivity: axonal tract tracing (Shen et al., 2012). Shen and associates also noted, as did Honey and associates (2009) and Damoiseaux and Greicius (2009), that structural mediation of the functional connectivity between two regions may be indirect (i.e., dependent on a third region).
In addition to this general relation between anatomy and functional connectivity, a more specific relation also exists, which is relevant for visual attention: Networks for the sources of attention (i.e., frontal and parietal cortex) and the sites of attention (i.e., visual sensory cortex) are dependent on the integrity of relevant white matter tracts. Greenberg and associates (2012), for example, correlated fMRI-based measures of attentional modulation with the strength of structural connectivity between the posterior IPS and subregions of the visual sensory cortex, during a visual discrimination task. Structural connectivity was based on a method combining high-direction reconstruction of the orientation distribution function (from high angular resolution, diffusion spectrum imaging) and deterministic fiber tracking. Participants viewed RSVPs presented across the visual field, but attended to only one RSVP location at a time. Using this approach, Greenberg and associates found that attentional modulations in the visual cortex (i.e., increasing effects of attention from V1 to V3) were positively correlated with structural connectedness to the posterior IPS, indicating that white matter tracts between the parietal and visual cortex may mediate the attention signals that resolve competition among stimuli for representation in visual cortex. Bennett et al. (2012) further demonstrated that DTI-based measures of white matter tract integrity within the frontoparietal network (e.g., FA along the genu, SLF, and inferior longitudinal fasciculus; ILF) predict visual search performance during both feature and conjunction search tasks. This brain-behavior relationship did not significantly differ across younger and older adults despite age-related declines in conjunction search performance and in FA along the ILF and genu. Thus, the integrity of white matter within the frontoparietal attention network provides an anatomical foundation for attentional performance in younger and older adults.
In sum, intrinsic functional connectivity between widely dispersed brain regions, comprising both the DMN and the frontoparietal attention network, is constrained by the integrity of white matter structures as characterized by DTI and other related techniques. Although resting-state functional connectivity is variable and may occur between regions without direct structural linkage, the large-scale anatomical structure of the brain constrains the characteristics of functional connectivity. Recent evidence suggests that the degree of structural connectivity within attentional networks (e.g., occipital to parietal, and frontal to parietal) facilitates the task-related allocation of attention. Whether the integrity of white matter structures also constrains task-dependent functional connectivity is unknown.
Individual Differences in Attentional Network Connectivity
As we have emphasized in the preceding sections, a central theme of several recent studies of functional connectivity, using both resting-state and task-dependent measures, is that connectivity reflects separate but interrelated networks with distinct functional goals, ranging from the coordination of search and detection processes in individual trials to the maintenance of a stable task set across trials (Dosenbach et al., 2007, 2008; Fox et al., 2006a). The successful clinical translation of functional connectivity measures requires that these measures vary in a reliable manner with developmental and neuropathological conditions, and several recent investigations suggest that connectivity measures are in fact sensitive to these types of individual differences.
Child developmental differences in brain connectivity
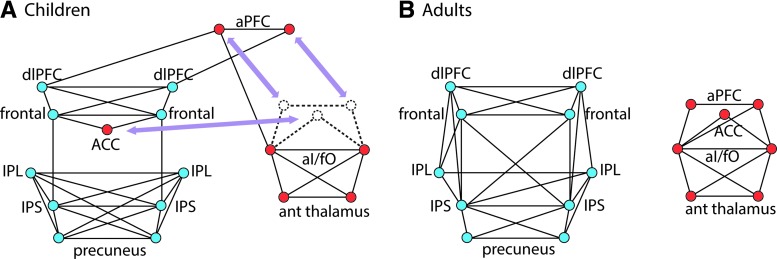
Fair and colleagues (2007) investigated developmental changes in the frontoparietal and cinguloopercular networks (Dosenbach et al., 2007) between adolescence and adulthood (Fig. 5). These authors analyzed the regional correlations of the fMRI resting-state, time-series data, across three age groups: 7–9, 10–15, and 20–31 years of age. The frontoparietal and cinguloopercular networks were clearly distinguishable in the adults' data, but the children's data exhibited three noteworthy differences from the adult layout. First, although regions within the frontoparietal network were connected, additional connections were evident in the children's data between the frontoparietal and cinguloopercular networks. Second, a close relation was present between a part of the cinguloopercular network and the frontoparietal network in children. Finally, additional links between frontal and parietal regions developed with increased age. Overall, the data demonstrated that with age, there was segregation of the two networks and integration of regions within each network. Fair and associates suggested that this developmental pattern may represent a learning mechanism by which precursors to adult task sets are originally derived from more available signals generated by regions of the more rapidly adaptive control network (frontoparietal).
FIG. 5.
The development of two proposed adult control networks involves both the segregation and integration of the brain regions that comprise them. Graphs formed from putative task-control regions in children and adults. Regions of interest (ROI) locations are drawn to correspond to topographic brain locations. Right-sided ROIs are displayed on the right and anterior ROIs at the top of each graph. Resting-state functional connectivity revealed a significant deviation between children and adults in two previously described control networks (Dosenbach et al., 2007). (A) The top 75 connections in children revealed that the two control networks were connected by a bridge connection: anterior prefrontal cortex–dorsolateral prefrontal cortex (aPFC-dlPFC). The dorsal ACC/msFC region was incorporated into the frontoparietal network. Children lacked connections from the dlPFC to intraparietal sulcus (IPS) and inferior parietal lobule (IPL). (B) In adults, resting-state functional connectivity revealed two separate control networks. Modified with permission from Fair et al. (2007).
This general theme of the developmental segregation of networks, with concomitant integration of connectivity within networks, derived from resting-state data, is consistent with other studies that have focused more directly on the relation between functional connectivity and behavioral measures of attentional control. Stevens and associates (2007) used DCM, with a go/no-go task, to define three response inhibition-related functional networks related to response inhibition, forming a hierarchical, inter-dependent system. A frontoparietal circuit exerted a top-down influence over a mediofrontal-striatal-parietal network in adult participants, but this relationship was absent in adolescents. Further, the ability of the frontoparietal network to influence the other circuit was inversely correlated with the percentage of false alarm errors on the go/no-go task errors in adolescents, but not adults, suggesting that maturation of top-down attentional control is associated with behavioral improvement on executive tasks.
Focusing more specifically on network engagement during error processing, Stevens and colleagues (2009a) found evidence for multiple error-processing networks in the brain in an ICA of task-dependent fMRI data. One of these networks, associated with greater activity during error responses, comprised anterior temporal lobe, limbic, and pregenual cingulate cortex, possibly reflecting an affective response to errors. This latter network, which was more active in adults than in adolescents, may reflect the increased ability to guide ongoing behavior on the basis of a learned emotional response. More generally, these investigations suggest that the development of executive ability depends on the ability of anatomically late-maturing PFC regions to exert control over other systems (Luna and Sweeney, 2004; Stevens, 2009).
Adult developmental differences in brain connectivity: resting-state
Investigations of both resting-state and task-based connectivity have also exhibited age-related differences over the course of adult development, in relation to attention and cognitive functioning. To date, the most consistent finding from resting-state studies is a decline, during increasing adult age, in the connectivity within individual networks. In a seminal study of 93 healthy adults who were 18–93 years of age, Andrews-Hanna and colleagues (2007) examined resting-state connectivity within both the DMN and dorsal frontoparietal networks, using an ROI approach, with primary visual cortex as a control region. The low-frequency fluctuations were obtained by filtering task-related fMRI data associated with a semantic classification task. These authors also investigated the correlation between the strength of resting-state connectivity and both behavioral performance and the integrity of white matter (from DTI).
Across both the DMN and dorsal frontoparietal attention networks, Andrews-Hanna and colleagues (2007) found that significant age-related decline was evident in the strength of connectivity, whereas the connectivity of the visual cortical regions did not vary substantially with age. For a group of 40 of the older adult participants who had completed neuropsychological testing, decreasing DMN connectivity was associated with decreasing performance in tests representing the domains of memory, perceptual speed, and executive functioning. Further, within the older adult group, decreasing DMN connectivity was associated with decreasing white matter integrity, averaged throughout a wide region, including corona radiata, SLF, and cingulum bundle. Chen and colleagues (2009) also found that, within a group of healthy older adults, decreasing white matter integrity within the genu of the corpus callosum (connecting left and right PFC) was correlated with decreasing resting-state connectivity of a prefrontal network (centered on the inferior frontal gyrus). Overall, Andrews-Hanna and associates (2007) concluded that aging is associated with widespread disruption of the DMN and dorsal frontoparietal attention systems, with minimal disruption within the visual system. This pattern complements the sequence noted in the studies of child development, in which the higher-order systems associated with attentional control are the latest to exhibit functional and structural connectivity (Fair et al., 2007; Luna and Sweeney, 2004; Stevens, 2009). Thus, aging may lead to some degree of disconnection of the structural and functional networks that emerge during the course of healthy development (Bartzokis et al., 2004; Davis et al., 2009; Madden et al., 2012; O'Sullivan et al., 2001; Sullivan and Pfefferbaum, 2011).
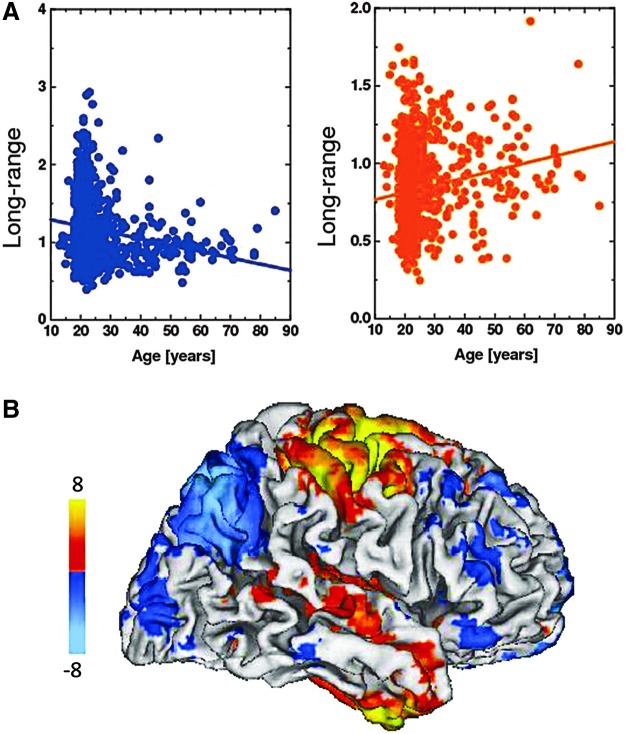
The trend of age-related decline in resting-state connectivity has appeared in several other investigations (Biswal et al., 2010; Damoiseaux et al., 2008; Esposito et al., 2008; Grady et al., 2010; Tomasi and Volkow, 2012). Most notably, Biswal and associates (2010) combined the resting-state data sets from 35 international centers and a total of 1414 research participants (the 1000 Functional Connectomes Project), thus allowing a fine-grained analysis of individual difference effects. Analysis of these data also revealed an age-related decline in the strength of DMN connectivity, but a new trend was the presence of an age-related increase in connectivity, in some regions outside of the DMN (e.g., premotor cortex). Tomasi and Volkow (2012) extended these findings, in an analysis of 913 participants from the 1000 Functional Connectomes Project, using functional connectivity density mapping, and graph theory measures, which provided separate estimation of the hubs (regions with high functional connectivity density) for short-range and long-range functional connections. With this approach, Tomasi and Volkow found that aging was associated with a decline in connectivity for the DMN and dorsal frontoparietal attention network (Fig. 6), as previously observed (Andrews-Hanna et al., 2007; Biswal et al., 2010; Damoiseaux et al., 2008; Esposito et al., 2008), but that this decline was more pronounced for long-range connectivity than for short-range connectivity. In addition, Tomasi and Volkow found that age-related increases in connectivity were evident for somatosensory and subcortical networks. Thus, given sufficient statistical power, both age-related increases and decreases in resting-state functional connectivity can be observed.
FIG. 6.
Analysis of 913 healthy participants reveals both age-related increases and decreases in resting-state functional connectivity. Normal aging is associated with pronounced decreases (A, left panel) in long-range functional connectivity density that map into the default mode network (DMN) and dorsal frontoparietal attention networks (B, light blue pattern) and with increases (A, right panel) that map into somatosensory and cerebellar networks (B, orange pattern). Reproduced with permission from Tomasi and Volkow (2012).
The Tomasi and Volkow data suggest that the later maturing networks of attentional control (Fair et al., 2007; Luna and Sweeney, 2004; Stevens, 2009) are among the most vulnerable to decline with aging. Although the neurophysiological basis for this adult developmental pattern is not clear, age-related decline in both glucose metabolism, as measured by PET (Madden and Hoffman, 1997; Volkow et al., 1998), and in striatal dopamine D2 receptors (Bäckman et al., 2000; Volkow et al., 2000), is most prominent in prefrontal and anterior cingulate cortical regions, which are associated with the dorsal frontoparietal attention network. The observed increased connectivity was located in somatosensory regions, which typically exhibit less age-related functional decline and, thus, may be candidate regions for compensatory recruitment for supporting cognitive function.
Meier and associates (2012a) demonstrated that an analytic method involving the selection of independent variables to predict the class membership of individual examples (i.e., a technique termed support vector machine) could successfully discriminate younger and older adults on the basis of resting-state network data. The classification was 84% accurate in classifying individual younger and older adults, and the majority of the connections used by the classifier came from seed regions associated with the sensorimotor and cinguloopercular networks. Positive correlations within both the DMN and cinguloopercular networks decreased with increasing adult age, which is consistent with the previously reported age-related trends (Andrews-Hanna et al., 2007; Biswal et al., 2010; Damoiseaux et al., 2008; Esposito et al., 2008). Further, the analyses revealed both an age-related weakening of long-range connections (Tomasi and Volkow, 2012) and an age-related strengthening of short-range connections, suggesting that networks become less differentiated during adult aging (Park et al., 2004).
As noted previously, age-related differences in the correlation between intrinsic functional connectivity and cognitive performance have been investigated (e.g., Andrews-Hanna et al., 2007; Chen et al., 2009), but these investigations have focused primarily on individual cognitive measures. More complex, attentional tasks, however, typically comprise several different task conditions, and a critical issue is the potential change in the relation between functional connectivity, across task conditions, and the difference in this correlational pattern with age. Few investigations addressing this issue have been conducted, but initial findings indicate that the relation between functional connectivity varies reliably across both adult age and the attentional demands of different task conditions. Campbell and associates (2012) revealed reduced intrinsic connectivity within a task-relevant frontoparietal network for older relative to younger adults during a 1-back working memory task that required the suppression of distracting information. This decreased connectivity pattern for older adults was associated with increased behavioral distraction. Grady and colleagues (2010), however, found that greater intrinsic connectivity with a frontal seed in the task positive network (the right dlPFC) predicted improved performance in older adults across four visual tasks representing: detection, perceptual matching, attentional cuing, and working memory. Chou and colleagues (2013) recently identified sets of regions, spanning somatomotor, orbitofrontal, and subcortical networks, for which increasing intrinsic functional connectivity was associated with faster responding (lower RT) in a visual search task. Relative to younger adults, older adults exhibited a lower strength of this RT-connectivity relation and greater disruption of this relation by a salient but irrelevant display item (color singleton distractor).
Adult developmental differences in brain connectivity: task-dependent
Several studies of adult age-related differences in task-dependent functional connectivity have also been conducted, although the results are variable because different tasks engage different networks of cognition. The majority of the findings to date are more directly relevant to the cognitive domain of memory than to attention. In a study of younger adults, Gazzaley and colleagues (2007) reported that a visual association cortex seed region was functionally connected to a prefrontal region (left middle frontal gyrus) during a working memory task for sequentially presented scenes. In a related approach, Clapp and colleagues (2011) found that when a delayed recognition task for scenes (the primary task) was interrupted by a secondary task (face categorization), both younger and older adults exhibited an attenuation of a scene-relevant component of an occipital-prefrontal network and an increase in a face-relevant component of the network. Unlike younger adults, however, the functional connections associated with the primary task remained weak in older adults, after the interrupting stimulus onset, suggesting that task re-engagement is decreased in aging. Other investigations, focusing more specifically on adult age-related differences in episodic memory, indicate that age-related declines in functional connectivity, particularly in medial temporal regions during encoding, are accompanied by increased connectivity of prefrontal networks providing additional top-down resources (Daselaar et al., 2006; Dennis et al., 2008).
Madden and colleagues (2010) addressed age-related differences in executive control, by assessing functional connectivity in a cued, task-switching paradigm that required different forms of semantic categorization for words. From interspersed cue-only trials, the functional connectivity of the preparatory processing associated with the cue could be distinguished from that associated with the target word. Functional connectivity, within a distributed frontoparietal network, differed for cue- and target-related processing. Critically, the functional connectivity of switch-related regions, during cue processing, was higher for younger adults than for older adults, whereas functional connectivity during target processing was comparable across the age groups. Further, individual differences in cue-related functional connectivity shared a substantial portion of the age-related variability in the efficiency of target categorization response (drift rate). This age-related difference in functional connectivity, however, was independent of white matter integrity within task-relevant regions. Although individual differences in white matter integrity accounted for a substantial portion of age-related variance in the categorization response in this task (Madden et al., 2009), the mediating effects of functional connectivity and white matter integrity, in this instance, were independent of each other.
In a study of top-down visual attention that included both fMRI and DTI measures, Madden et al. (2007) found that older adults, but not younger adults, exhibited a significant correlation between FA and parietal activation, such that individuals with lower FA of frontal pericallosal white matter exhibited greater activation of the SPL. Older adults also exhibited a significant association between increasing parietal activation and more efficient search performance (lower RT), but the relation between FA and parietal activation, and between RT and parietal activation, were independent statistically. In contrast, Bennett et al. (2012) found that increasing FA within frontoparietal white matter tracts was associated with lower visual search RT for both younger and older adults, but that no age-related difference in the FA-performance relation was evident.
Resolving these discrepant patterns in the initial investigations of age-related differences in task-dependent connectivity will require multi-modal investigations that combine structural imaging, functional imaging, and behavioral measures of visual attention. In a review of recent studies that have taken a multi-modal approach, Bennett and Rypma (2013) have proposed that the relation between structural brain integrity and functional activation may differ qualitatively with age, reflecting neural efficiency in the case of younger adults but neural compensation in the case of older adults (cf. Davis et al., 2012). In light of previous studies reporting a correlation between white matter integrity and resting-state functional connectivity (Andrew-Hanna et al., 2007; Chen et al., 2009), it is also possible that task-dependent functional connectivity is less related to anatomical integrity than is intrinsic functional connectivity.
From the studies of child and adult development, it is clear that changes in the strength and composition of functional and structural connectivity occur over time. The initial findings have indicated that the development of efficient attentional control is associated with the segregation of functional networks and the integration of connections within networks (Fair et al., 2007; Luna and Sweeney, 2004; Stevens, 2009). Some degree of decreased connectivity occurs during the course of normal aging, although the pattern associated with aging may not directly mirror the child developmental pattern, and increased connectivity within and between network components, with age, also occurs (Andrews-Hanna et al., 2007; Biswal et al., 2010; Tomasi and Volkow, 2012). Understanding the basis for age-related differences in connectivity of attentional networks will require multi-modal studies that combine behavioral measures of attention with structural and functional indices of brain connectivity (Bennett and Rypma, 2013; Davis et al., 2012; Madden et al., 2012; Salat, 2011).
Brain connectivity in disorders of visual attention
A promising application of connectivity analyses is the comparison between groups of healthy participants and those with neurological or psychiatric disease. Resting-state data, in particular, may provide a neuroimaging biomarker for diagnosis and treatment of neurodegenerative diseases (Anticevic et al., 2012; Buckner et al., 2005; Corbetta, 2012; Fox and Greicius, 2010; Palop et al., 2006). Greicius and colleagues (2004), for example, demonstrated that functional connectivity of the DMN, as defined by ICA, is decreased in patients with mild Alzheimer's disease (AD) as compared with healthy age-matched controls during a simple sensory-motor processing task. At the individual subject level, goodness-of-fit analysis of the DMN between the groups discriminated patients at a sensitivity of 85% and a specificity of 77%, suggesting that DMN connectivity may reflect a biomarker of incipient AD.
Investigation of other neurodegenerative diseases indicates disease-specific targeting of functional brain networks. Seeley and associates (2009) examined functional connectivity profiles across distinct neurodegenerative syndromes: behavioral variant of frontotemporal dementia (bvFTD), semantic dementia, progressive fluent aphasia, and corticobasal syndrome. The syndrome-associated regional degeneration patterns mirrored the intrinsic functional network architecture in the healthy brain identified with seed-based ICA (with seeds placed in the cortical region most atrophied in patients with that syndrome). Zhou and colleagues (2012) used graph theoretical analyses to investigate how intrinsic connectivity in health predicts region-by-region vulnerability to disease, in this same set of syndromes. For each illness, these authors identified regional “epicenters,” regions whose healthy connectivity profiles most resembled the diseased-specific atrophy pattern. Regions that were highly interconnected (i.e., with higher “total connection flow”) and that were closer to the epicenters (i.e., had shorter functional paths to the epicenters) exhibited greater disease-related vulnerability, suggesting that network damage may result from some toxic agent propagating along network connections.
One of the disorders investigated in both Seeley and colleagues (2009) and Zhou and colleagues (2012), bvFTD, has been associated with functional connectivity disruptions in a recently identified network known as the “salience network.” Intrinsic coactivations within this network, including the ACC, orbito-frontal-insular cortex, and subcortical structures, are dissociable from an executive control network (within the dorsolateral frontoparietal cortex) and are related to individual differences in a measure of anxiety (stressor-associated anticipatory anxiety) (Seeley et al., 2007). Using ICA, Filippi and colleagues (2012) reported that resting-state connectivity in bvFTD is decreased in the salience network, primarily within the anterior cingulum, as compared to patients with probable AD and healthy controls. Within a right-lateralized network previously associated with attention and working memory processes, a divergent connectivity pattern was also observed in bvFTD versus controls, with decreased connectivity in the dlPFC and enhanced connectivity in the precuneus. Finally, as compared with controls, bvFTD was characterized by decreased connectivity between a dorsal attention network and the DMN, and also an executive control network. Intrinsic functional abnormalities present in diseases such as bvFTD may, thus, represent altered interactions among many resting-state networks.
Relating these functional connectivity differences to alterations in a relevant behavioral variable is critical for the clinical application of resting-state data. He and colleagues (2007), for instance, investigated longitudinal functional connectivity changes in stroke patients who exhibited right-hemisphere lesions leading to spatial neglect, relating these changes to behavioral disease symptoms (e.g., RT in a spatial attention task) and severity. Resting-state functional connectivity was estimated by regressing out the deterministic task-related responses associated with event-related fMRI activity in a cued visual target detection task. The authors found that lesioned areas within the ventral (bottom-up) frontoparietal attention network displayed an expected pattern of disrupted connectivity in patients at both acute and chronic stages of neglect. The structurally intact dorsal (top-down) attention network also exhibited reduced connectivity as compared with age-matched controls in the acute stage, although this effect disappeared with recovery (i.e., in the chronic stage). Further, the degree of disruption positively correlated with the ability to reorient attention toward the neglected visual field in the acute, but not the chronic stage of neglect. Thus, in line with behavioral evidence (Snow and Mattingley, 2006), neglect may represent a transient imbalance between top-down and bottom-up attention mechanisms that can be observed through measures of functional connectivity.
Structural disconnection of attention-related networks may also parallel aberrant patterns of brain activation and performance. Bonnelle and associates (2012), for example, examined impairments in inhibitory control during a stop-signal task in patients with traumatic brain injury. Unlike healthy controls, patients failed to deactivate the DMN during the task, and the extent of this effect was predicted by the amount of white matter damage in a tract within the cognitive control network (i.e., in a tract connecting the right anterior insula to the pre-supplementary motor area and the dorsal ACC). Attenuation of white matter integrity within several tracts (the SLF, the ILF, and the inferior fronto-occipital fasciculus) has also been demonstrated in patients with another attentional disorder, simultanagnosia (Chechlacz et al., 2012).
Measures of connectivity applied to investigate cognitive differences across individuals suggest that disconnection of attentional networks contributes to attenuated cognitive functioning, and, in extreme cases, to disorders of attention. A network-based perspective should, therefore, be helpful in the development of biomarkers which are relevant for the diagnosis of neurodegenerative disease and the identification of healthy networks that may support recovery and rehabilitation.
Conclusions
Investigations of brain connectivity have confirmed and extended task-related studies of mean activation by defining functional and structural networks of attention. The task-related studies have revealed a distributed network of frontal and parietal cortical regions, with separable but interacting patterns of functional connectivity between dorsal and ventral frontoparietal regions. The directional influence among regions of the dorsal and ventral components of the attentional network depends on whether the task-relevant attentional demands are relatively top-down (i.e., dorsal) or bottom-up (i.e., ventral). These attention-dependent patterns of brain activity, however, are also distinguishable during resting-state measures of the spontaneous, low-frequency fluctuations in the fMRI signal, obtained in the absence of a specific behavioral task. That is, intrinsic functional connectivity among attention networks is both reliable and distinct from the intrinsic connectivity among other (default-mode) regions whose activity is suppressed during attentional processing. A constraint on functional network connectivity is anatomical connectivity, and individual differences in the integrity of white matter pathways appear to converge with the predicted anatomical connectivity for intrinsic functional networks. A critical challenge for future research is defining the relative roles for structural and functional (task-related and intrinsic) connectivity in different forms of attention.
Research on individual differences in connectivity has demonstrated that the development of efficient attentional control throughout childhood and adulthood is associated with the segregation of intrinsic functional networks and the integration of connections within networks. With increasing age in adulthood, some degree of decreased connectivity occurs, although this pattern may not directly mirror the child developmental pattern, and increased connectivity within and between network components is also evident with aging. Individual differences in the integrity of white matter pathways may contribute to these age-related effects. Similarly, in the context of neurodegenerative disease, intrinsic functional connectivity networks appear to interact in a reliable manner with different attentional demands, and neuropathology appears to lead to disconnection among networks identified in normative studies. Thus, understanding brain connectivity of visual attention may contribute to improved biomarkers for diagnosis and treatment. The success of this clinical translation, however, will require continued advances in understanding the relation between structural and functional indices of brain connectivity, in relation to visual attention performance.
Acknowledgments
The preparation of this article was supported by research grant R01 AG039684 from the National Institute on Aging. We are grateful to David Hoagey and Payal Chakraborty for assistance with figure preparation.
Author Disclosure Statement
The authors state that no competing financial interests or commercial associations exist which might create a conflict of interest in connection with the submission of this article.
References
- Achard S. Bullmore E. Efficiency and cost of economical brain functional networks. PLoS Comput Biol. 2007;3:e17. doi: 10.1371/journal.pcbi.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JS. Ferguson MA. Lopez-Larson M. Yurgelun-Todd D. Connectivity gradients between the default mode and attention control networks. Brain Connect. 2011;1:147–157. doi: 10.1089/brain.2011.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR. Snyder AZ. Vincent JL. Lustig C. Head D. Raichle ME. Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A. Cole MW. Murray JD. Corlett PR. Wang X-J. Krystal JH. The role of default network deactivation in cognition and disease. Trends Cogn Sci. 2012;16:584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh E. Belopolsky AV. Theeuwes J. Top-down versus bottom-up attentional control: a failed theoretical dichotomy. Trends Cogn Sci. 2012;16:437–443. doi: 10.1016/j.tics.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckman L. Ginovart N. Dixon RA. Wahlin TB. Wahlin A. Halldin C. Farde L. Age-related cognitive deficits mediated by changes in the striatal dopamine system. Am J Psychiatry. 2000;157:635–637. doi: 10.1176/ajp.157.4.635. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory or working attention? In: Baddeley A, editor; Weiskrantz L, editor. Attention: Selection, Awareness, and Control. Oxford: Claredon Press; 1993. pp. 152–170. [Google Scholar]
- Baddeley A. Fractionating the central executive. In: Stuss DT, editor; Knight RT, editor. Principles of Frontal Lobe Function. New York: Oxford University Press; 2002. pp. 246–260. [Google Scholar]
- Baldassarre A. Lewis CM. Committeri G. Snyder AZ. Romani GL. Corbetta M. Individual variability in functional connectivity predicts performance of a perceptual task. Proc Natl Acad Sci U S A. 2012;109:3516–3521. doi: 10.1073/pnas.1113148109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer's disease. Neurobiol Aging. 2004;25:5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Sultzer D. Lu PH. Nuechterlein KH. Mintz J. Cummings JL. Heterogeneous age-related breakdown of white matter structural integrity: Implications for cortical “disconnection” in aging and Alzheimer's disease. Neurobiol Aging. 2004;25:843–851. doi: 10.1016/j.neurobiolaging.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Beck DM. Muggleton N. Walsh V. Lavie N. Right parietal cortex plays a critical role in change blindness. Cereb Cortex. 2006;16:712–717. doi: 10.1093/cercor/bhj017. [DOI] [PubMed] [Google Scholar]
- Bennett IJ. Motes MA. Rao NK. Rypma B. White matter tract integrity predicts visual search performance in young and older adults. Neurobiol Aging. 2012;33:433e421–431. doi: 10.1016/j.neurobiolaging.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett IJ. Rypma B. Advances in functional neuroanatomy: a review of combined DTI and fMRI studies in healthy younger and older adults. Neurosci Biobehav Rev. 2013 doi: 10.1016/j.neubiorev.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B. Yetkin FZ. Haughton VM. Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Biswal BB. Mennes M. Zuo XN. Gohel S. Kelly C. Smith SM. Beckmann CF. Adelstein JS. Buckner RL. Colcombe S. Dogonowski AM. Ernst M. Fair D. Hampson M. Hoptman MJ. Hyde JS. Kiviniemi VJ. Kotter R. Li SJ. Lin CP. Lowe MJ. Mackay C. Madden DJ. Madsen KH. Margulies DS. Mayberg HS. McMahon K. Monk CS. Mostofsky SH. Nagel BJ. Pekar JJ. Peltier SJ. Petersen SE. Riedl V. Rombouts SA. Rypma B. Schlaggar BL. Schmidt S. Seidler RD. Siegle GJ. Sorg C. Teng GJ. Veijola J. Villringer A. Walter M. Wang L. Weng XC. Whitfield-Gabrieli S. Williamson P. Windischberger C. Zang YF. Zhang HY. Castellanos FX. Milham MP. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnelle V. Ham TE. Leech R. Kinnunen KM. Mehta MA. Greenwood RJ. Sharp DJ. Salience network integrity predicts default mode network function after traumatic brain injury. Proc Natl Acad Sci U S A. 2012;109:4690–4695. doi: 10.1073/pnas.1113455109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS. Reynolds JR. Donaldson DI. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39:713–726. doi: 10.1016/s0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- Broadbent DE. Perception and Communication. London: Pergamon; 1958. [Google Scholar]
- Buckner RL. Functional-anatomic correlates of control processes in memory. J Neurosci. 2003;23:3999–4004. doi: 10.1523/JNEUROSCI.23-10-03999.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL. Human functional connectivity: new tools, unresolved questions. Proc Natl Acad Sci U S A. 2010;107:10769–10770. doi: 10.1073/pnas.1005987107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL. Andrews-Hanna JR. Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Snyder AZ. Shannon BJ. LaRossa G. Sachs R. Fotenos AF. Sheline YI. Klunk WE. Mathis CA. Morris JC. Mintun MA. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL. Vincent JL. Unrest at rest: default activity and spontaneous network correlations. Neuroimage. 2007;37:1091–1096. doi: 10.1016/j.neuroimage.2007.01.010. discussion 1097–1099. [DOI] [PubMed] [Google Scholar]
- Bundesen C. A theory of visual attention. Psychol Rev. 1990;97:523–547. doi: 10.1037/0033-295x.97.4.523. [DOI] [PubMed] [Google Scholar]
- Bundesen C. Habekost T. Kyllingsbaek S. A neural theory of visual attention: bridging cognition and neurophysiology. Psychol Rev. 2005;112:291–328. doi: 10.1037/0033-295X.112.2.291. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Ciaramelli E. Olson IR. Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nat Rev Neurosci. 2008;9:613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R. Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Campbell KL. Grady CL. Ng C. Hasher L. Age differences in the frontoparietal cognitive control network: implications for distractibility. Neuropsychologia. 2012;50:2212–2223. doi: 10.1016/j.neuropsychologia.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael O. Lockhart S. The role of diffusion tensor imaging in the study of cognitive aging. Curr Top Behav Neurosci. 2012;11:289–320. doi: 10.1007/7854_2011_176. [DOI] [PubMed] [Google Scholar]
- Carrasco M. Penpeci-Talgar C. Eckstein M. Spatial covert attention increases contrast sensitivity across the CSF: support for signal enhancement. Vis Res. 2000;40:1203–1215. doi: 10.1016/s0042-6989(00)00024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M. Ffytche DH. The rises and falls of disconnection syndromes. Brain. 2005;128:2224–2239. doi: 10.1093/brain/awh622. [DOI] [PubMed] [Google Scholar]
- Chechlacz M. Rotshtein P. Hansen PC. Riddoch JM. Deb S. Humphreys GW. The neural underpinings of simultanagnosia: disconnecting the visuospatial attention network. J Cogn Neurosci. 2012;24:718–735. doi: 10.1162/jocn_a_00159. [DOI] [PubMed] [Google Scholar]
- Chen NK. Chou YH. Song AW. Madden DJ. Measurement of spontaneous signal fluctuations in fMRI: adult age differences in intrinsic functional connectivity. Brain Struct Funct. 2009;213:571–585. doi: 10.1007/s00429-009-0218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chica AB. Paz-Alonso PM. Valero-Cabre A. Bartolomeo P. Neural bases of the interactions between spatial attention and conscious perception. Cereb Cortex. 2013;23:1269–1279. doi: 10.1093/cercor/bhs087. [DOI] [PubMed] [Google Scholar]
- Chou YH. Chen NK. Madden DJ. Functional brain connectivity and cognition: effects of adult age and task demands. Neurobiol Aging. 2013 doi: 10.1016/j.neurobiolaging.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp WC. Rubens MT. Sabharwal J. Gazzaley A. Deficit in switching between functional brain networks underlies the impact of multitasking on working memory in older adults. Proc Natl Acad Sci U S A. 2011;108:7212–7217. doi: 10.1073/pnas.1015297108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW. Pathak S. Schneider W. Identifying the brain's most globally connected regions. Neuroimage. 2010;49:3132–3148. doi: 10.1016/j.neuroimage.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Corbetta M. Functional connectivity and neurological recovery. Dev Psychobiol. 2012;54:239–253. doi: 10.1002/dev.20507. [DOI] [PubMed] [Google Scholar]
- Corbetta M. Akbudak E. Conturo TE. Snyder AZ. Ollinger JM. Drury HA. Linenweber MR. Petersen SE. Raichle ME. Van Essen DC. Shulman GL. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Corbetta M. Kincade JM. Ollinger JM. McAvoy MP. Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Corbetta M. Miezin FM. Shulman GL. Petersen SE. A PET study of visuospatial attention. J Neurosci. 1993;13:1202–1226. doi: 10.1523/JNEUROSCI.13-03-01202.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M. Patel G. Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M. Shulman GL. Human cortical mechanisms of visual attention during orienting and search. Philos Trans R Soc Lond B Biol Sci. 1998;353:1353–1362. doi: 10.1098/rstb.1998.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M. Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corbetta M. Shulman GL. Miezin FM. Petersen SE. Superior parietal cortex activation during spatial attention shifts and visual feature conjunction. Science. 1995;270:802–805. doi: 10.1126/science.270.5237.802. [DOI] [PubMed] [Google Scholar]
- D'Esposito M. Zarahn E. Aguirre GK. Event-related functional MRI: Implications for cognitive psychology. Psychol Rev. 1999;125:155–164. doi: 10.1037/0033-2909.125.1.155. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS. Beckmann CF. Arigita EJ. Barkhof F. Scheltens P. Stam CJ. Smith SM. Rombouts SA. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 2008;18:1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS. Greicius MD. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain Struct Funct. 2009;213:525–533. doi: 10.1007/s00429-009-0208-6. [DOI] [PubMed] [Google Scholar]
- Daselaar SM. Fleck MS. Dobbins IG. Madden DJ. Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: an event-related fMRI study. Cereb Cortex. 2006;16:1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW. Dennis NA. Buchler NG. White LE. Madden DJ. Cabeza R. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage. 2009;46:530–541. doi: 10.1016/j.neuroimage.2009.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW. Kragel JE. Madden DJ. Cabeza R. The architecture of cross-hemispheric communication in the aging brain: linking behavior to functional and structural connectivity. Cereb Cortex. 2012;22:232–242. doi: 10.1093/cercor/bhr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Fockert J. Rees G. Frith C. Lavie N. Neural correlates of attentional capture in visual search. J Cogn Neurosci. 2004;16:751–759. doi: 10.1162/089892904970762. [DOI] [PubMed] [Google Scholar]
- Dennis NA. Hayes SM. Prince SE. Madden DJ. Huettel SA. Cabeza R. Effects of aging on the neural correlates of successful item and source memory encoding. J Exp Psychol Learn Mem Cogn. 2008;34:791–808. doi: 10.1037/0278-7393.34.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G. Hu X. Investigating effective brain connectivity from FMRI data: past findings and current issues with reference to granger causality analysis. Brain Connect. 2012;2:235–245. doi: 10.1089/brain.2012.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R. Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU. Fair DA. Cohen AL. Schlaggar BL. Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU. Fair DA. Miezin FM. Cohen AL. Wenger KK. Dosenbach RA. Fox MD. Snyder AZ. Vincent JL. Raichle ME. Schlaggar BL. Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. Selective attention and the organization of visual information. J Exp Psychol Gen. 1984;113:501–517. doi: 10.1037//0096-3445.113.4.501. [DOI] [PubMed] [Google Scholar]
- Duncan J. Humphreys GW. Visual search and stimulus similarity. Psychol Rev. 1989;96:433–458. doi: 10.1037/0033-295x.96.3.433. [DOI] [PubMed] [Google Scholar]
- Eckstein MP. Visual search: a retrospective. J Vis. 2011;11 doi: 10.1167/11.5.14. [DOI] [PubMed] [Google Scholar]
- Egner T. Monti JM. Trittschuh EH. Wieneke CA. Hirsch J. Mesulam MM. Neural integration of top-down spatial and feature-based information in visual search. J Neurosci. 2008;28:6141–6151. doi: 10.1523/JNEUROSCI.1262-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen CW. Schultz DW. Information processing in visual search: a continuous flow conception and experimental results. Percept Psychophys. 1979;25:249–263. doi: 10.3758/bf03198804. [DOI] [PubMed] [Google Scholar]
- Esposito F. Aragri A. Pesaresi I. Cirillo S. Tedeschi G. Marciano E. Goebel R. Di Salle F. Independent component model of the default-mode brain function: combining individual-level and population-level analyses in resting-state fMRI. Magn Reson Imaging. 2008;26:905–913. doi: 10.1016/j.mri.2008.01.045. [DOI] [PubMed] [Google Scholar]
- Fair DA. Dosenbach NU. Church JA. Cohen AL. Brahmbhatt S. Miezin FM. Barch DM. Raichle ME. Petersen SE. Schlaggar BL. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J. McCandliss BD. Fossella J. Flombaum JI. Posner MI. The activation of attentional networks. Neuroimage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Filippi M. Agosta F. Scola E. Canu E. Magnani G. Marcone A. Valsasina P. Caso F. Copetti M. Comi G. Cappa SF. Falini A. Functional network connectivity in the behavioral variant of frontotemporal dementia. Cortex. 2012 doi: 10.1016/j.cortex.2012.09.017. [DOI] [PubMed] [Google Scholar]
- Finke K. Bublak P. Krummenacher J. Kyllingsbaek S. Müller HJ. Schneider WX. Usability of a theory of visual attention (TVA) for parameter-based measurement of attention I: evidence from normal subjects. J Int Neuropsychol Soc. 2005;11:832–842. doi: 10.1017/s1355617705050976. [DOI] [PubMed] [Google Scholar]
- Folk CL. Remington RW. Johnston JC. Involuntary covert orienting is contingent on attentional control settings. J Exp Psychol Hum Percept Perform. 1992;18:1030–1044. [PubMed] [Google Scholar]
- Fox MD. Corbetta M. Snyder AZ. Vincent JL. Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006a;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD. Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4:1–13. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD. Snyder AZ. Vincent JL. Corbetta M. Van Essen DC. Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD. Snyder AZ. Vincent JL. Raichle ME. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56:171–184. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Fox MD. Snyder AZ. Zacks JM. Raichle ME. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nat Neurosci. 2006b;9:23–25. doi: 10.1038/nn1616. [DOI] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K. Causal modelling and brain connectivity in functional magnetic resonance imaging. PLoS Biol. 2009;7:e33. doi: 10.1371/journal.pbio.1000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ. Buechel C. Fink GR. Morris J. Rolls E. Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Frith CD. Liddle PF. Frackowiak RS. Functional connectivity: the principal-component analysis of large (PET) data sets. J Cereb Blood Flow Metab. 1993;13:5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Frith CD. Liddle PF. Frackowiak RSJ. Comparing functional (PET) images: the assessment of significant change. J Cereb Blood Flow Metab. 1991;11:690–699. doi: 10.1038/jcbfm.1991.122. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Harrison L. Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Gazzaley A. Rissman J. Cooney J. Rutman A. Seibert T. Clapp W. D'Esposito M. Functional interactions between prefrontal and visual association cortex contribute to top-down modulation of visual processing. Cereb Cortex. 2007;17(Suppl 1):i125–135. doi: 10.1093/cercor/bhm113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. I. Brain. 1965a;88:237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. II. Brain. 1965b;88:585–644. doi: 10.1093/brain/88.3.585. [DOI] [PubMed] [Google Scholar]
- Geyer T. Zehetleitner M. Muller HJ. Contextual cueing of pop-out visual search: when context guides the deployment of attention. J Vis. 2010;10:20. doi: 10.1167/10.5.20. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Blumenthal J. Jeffries NO. Castellanos FX. Liu H. Zijdenbos A. Paus T. Evans AC. Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giesbrecht B. Woldorff MG. Song AW. Mangun GR. Neural mechanisms of top-down control during spatial and feature attention. Neuroimage. 2003;19:496–512. doi: 10.1016/s1053-8119(03)00162-9. [DOI] [PubMed] [Google Scholar]
- Gitelman DR. Nobre AC. Parrish TB. LaBar KS. Kim YH. Meyer JR. Mesulam M. A large-scale distributed network for covert spatial attention: further anatomical delineation based on stringent behavioural and cognitive controls. Brain. 1999;122(Pt 6):1093–1106. doi: 10.1093/brain/122.6.1093. [DOI] [PubMed] [Google Scholar]
- Gitelman DR. Penny WD. Ashburner J. Friston KJ. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage. 2003;19:200–207. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- Goebel R. Roebroeck A. Kim DS. Formisano E. Investigating directed cortical interactions in time-resolved fMRI data using vector autoregressive modeling and Granger causality mapping. Magn Reson Imaging. 2003;21:1251–1261. doi: 10.1016/j.mri.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Gopher D. Attention control: explorations of the work of an executive controller. Cogn Brain Res. 1996;5:23–38. doi: 10.1016/s0926-6410(96)00038-9. [DOI] [PubMed] [Google Scholar]
- Gottsdanker R. Shragg GP. Verification of Donders' subtraction method. Journal of Experimental Psychology: Human Perception and Performance. 1985;11:765–776. doi: 10.1037//0096-1523.11.6.765. [DOI] [PubMed] [Google Scholar]
- Grady CL. Protzner AB. Kovacevic N. Strother SC. Afshin-Pour B. Wojtowicz M. Anderson JA. Churchill N. McIntosh AR. A multivariate analysis of age-related differences in default mode and task-positive networks across multiple cognitive domains. Cereb Cortex. 2010;20:1432–1447. doi: 10.1093/cercor/bhp207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramann K. Tollner T. Müller HJ. Dimension-based attention modulates early visual processing. Psychophysiology. 2010;47:968–978. doi: 10.1111/j.1469-8986.2010.00998.x. [DOI] [PubMed] [Google Scholar]
- Greenberg AS. Verstynen T. Chiu YC. Yantis S. Schneider W. Behrmann M. Visuotopic cortical connectivity underlying attention revealed with white-matter tractography. J Neurosci. 2012;32:2773–2782. doi: 10.1523/JNEUROSCI.5419-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD. Krasnow B. Reiss AL. Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD. Srivastava G. Reiss AL. Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD. Supekar K. Menon V. Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley AA. Speer NK. Locating and fractionating working memory using functional neuroimaging: storage, maintenance, and executive functions. Microsc Res Tech. 2000;51:45–53. doi: 10.1002/1097-0029(20001001)51:1<45::AID-JEMT5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- He BJ. Snyder AZ. Vincent JL. Epstein A. Shulman GL. Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007;53:905–918. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- He Y. Wang J. Wang L. Chen ZJ. Yan C. Yang H. Tang H. Zhu C. Gong Q. Zang Y. Evans AC. Uncovering intrinsic modular organization of spontaneous brain activity in humans. PLoS One. 2009;4:e5226. doi: 10.1371/journal.pone.0005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. The Organization of Behavior: A Neuropsychological Theory. New York: Wiley; 1949. [Google Scholar]
- Hickey C. McDonald JJ. Theeuwes J. Electrophysiological evidence of the capture of visual attention. J Cogn Neurosci. 2006;18:604–613. doi: 10.1162/jocn.2006.18.4.604. [DOI] [PubMed] [Google Scholar]
- Honey CJ. Sporns O. Cammoun L. Gigandet X. Thiran JP. Meuli R. Hagmann P. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfinger JB. Buonocore MH. Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Humphreys GW. Kyllingsbaek S. Watson DG. Olivers CN. Law I. Paulson OB. Parieto-occipital areas involved in efficient filtering in search: a time course analysis of visual marking using behavioural and functional imaging procedures. Q J Exp Psychol A. 2004;57:610–635. doi: 10.1080/02724980343000620. [DOI] [PubMed] [Google Scholar]
- Hutchinson JB. Turk-Browne NB. Memory-guided attention: control from multiple memory systems. Trends Cogn Sci. 2012;16:576–579. doi: 10.1016/j.tics.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamoglu F. Kahnt T. Koch C. Haynes J-D. Changes in functional connectivity support conscious object recognition. Neuroimage. 2012;63:1909–1917. doi: 10.1016/j.neuroimage.2012.07.056. [DOI] [PubMed] [Google Scholar]
- Imaruoka T. Yanagida T. Miyauchi S. Attentional set for external information activates the right intraparietal area. Brain Res Cogn Brain Res. 2003;16:199–209. doi: 10.1016/s0926-6410(02)00274-4. [DOI] [PubMed] [Google Scholar]
- Jones DK. Diffusion MRI: Theory, Methods, and Applications. New York: Oxford University Press; 2011. [Google Scholar]
- Jones DK. Knosche TR. Turner R. White matter integrity, fiber count, and other fallacies: The do's and don'ts of diffusion MRI. Neuroimage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Kahneman D. Attention and Effort. Englewood Cliffs, NJ: Prentice Hall; 1973. [Google Scholar]
- Kastner S. Pinsk MA. De Weerd P. Desimone R. Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Kastner S. Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Klein RM. Inhibition of return. Trends Cogn Sci. 2000;4:138–147. doi: 10.1016/s1364-6613(00)01452-2. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Fundamental components of attention. Annu Rev Neurosci. 2007;30:57–78. doi: 10.1146/annurev.neuro.30.051606.094256. [DOI] [PubMed] [Google Scholar]
- Koch MA. Norris DG. Hund-Georgiadis M. An investigation of functional and anatomical connectivity using magnetic resonance imaging. Neuroimage. 2002;16:241–250. doi: 10.1006/nimg.2001.1052. [DOI] [PubMed] [Google Scholar]
- Kramer AF. Coles MGH. Logan GD. Converging Operations in the Study of Visual Selective Attention. Washington, DC: American Psychological Association; 1996. [Google Scholar]
- Kramer AF. Madden DJ. Attention. In: Craik FIM, editor; Salthouse TA, editor. The Handbook of Aging and Cognition. New York: Psychology Press; 2008. pp. 189–249. [Google Scholar]
- Kristjánsson Á. Saevarsson S. Driver J. The boundary conditions of priming of visual search: from passive viewing through task-relevant working memory load. Psychon Bull Rev. 2013 doi: 10.3758/s13423-013-0375-6. [DOI] [PubMed] [Google Scholar]
- Kristjánsson Á. Vuilleumier P. Schwartz S. Macaluso E. Driver J. Neural basis for priming of pop-out during visual search revealed with fMRI. Cereb Cortex. 2007;17:1612–1624. doi: 10.1093/cercor/bhl072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristjánsson Á. Wang D. Nakayama K. The role of priming in conjunctive visual search. Cognition. 2002;85:37–52. doi: 10.1016/s0010-0277(02)00074-4. [DOI] [PubMed] [Google Scholar]
- LaBerge D. Networks of attention. In: Gazzaniga MS, editor. The New Cognitive Neurosciences. 2nd. Cambridge, MA: MIT Press; 2000. pp. 711–723. [Google Scholar]
- Lavie N. Perceptual load as a necessary condition for selective attention. J Exp Psychol Hum Percept Perform. 1995;21:451–468. doi: 10.1037//0096-1523.21.3.451. [DOI] [PubMed] [Google Scholar]
- Lavie N. Hirst A. de Fockert JW. Viding E. Load theory of selective attention and cognitive control. J Exp Psychol Gen. 2004;133:339–354. doi: 10.1037/0096-3445.133.3.339. [DOI] [PubMed] [Google Scholar]
- Lawrence NS. Ross TJ. Hoffmann R. Garavan H. Stein EA. Multiple neuronal networks mediate sustained attention. J Cogn Neurosci. 2003;15:1028–1038. doi: 10.1162/089892903770007416. [DOI] [PubMed] [Google Scholar]
- Leber AB. Egeth HE. It's under control: top-down search strategies can override attentional capture. Psychon Bull Rev. 2006;13:132–138. doi: 10.3758/bf03193824. [DOI] [PubMed] [Google Scholar]
- Li L. Gratton C. Yao D. Knight RT. Role of frontal and parietal cortices in the control of bottom-up and top-down attention in humans. Brain Res. 2010;1344:173–184. doi: 10.1016/j.brainres.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD. Bundesen C. Clever homunculus: is there an endogenous act of control in the explicit task-cuing procedure? J Exp Psychol Hum Percept Perform. 2003;29:575–599. doi: 10.1037/0096-1523.29.3.575. [DOI] [PubMed] [Google Scholar]
- Luck SJ. Hillyard SA. Mouloua M. Hawkins HL. Mechanisms of visual-spatial attention: resource allocation or uncertainty reduction? J Exp Psychol Hum Percept Perform. 1996;22:725–737. doi: 10.1037//0096-1523.22.3.725. [DOI] [PubMed] [Google Scholar]
- Luck SJ. Vecera SP. Attention. In: Pashler H, editor; Yantis S, editor. Stevens' Handbook of Experimental Psychology. Sensation and Perception. 3rd. Vol. 1. New York: Wiley; 2002. pp. 235–286. [Google Scholar]
- Luna B. Sweeney JA. The emergence of collaborative brain function: FMRI studies of the development of response inhibition. Ann N Y Acad Sci. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- Madden DJ. Bennett IJ. Burzynska A. Potter GG. Chen NK. Song AW. Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochim Biophys Acta. 2012;1822:386–400. doi: 10.1016/j.bbadis.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ. Bennett IJ. Song AW. Cerebral white matter integrity and cognitive aging: contributions from diffusion tensor imaging. Neuropsychol Rev. 2009;19:415–435. doi: 10.1007/s11065-009-9113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ. Costello MC. Dennis NA. Davis SW. Shepler AM. Spaniol J. Bucur B. Cabeza R. Adult age differences in functional connectivity during executive control. Neuroimage. 2010;52:643–657. doi: 10.1016/j.neuroimage.2010.04.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ. Hoffman JM. Application of positron emission tomography to age-related cognitive changes. In: Krishnan KRR, editor; Doraiswamy PM, editor. Brain Imaging and Clinical Psychiatry. New York: Marcel Dekker; 1997. pp. 575–613. [Google Scholar]
- Madden DJ. Spaniol J. Whiting WL. Bucur B. Provenzale JM. Cabeza R. White LE. Huettel SA. Adult age differences in the functional neuroanatomy of visual attention: a combined fMRI and DTI study. Neurobiol Aging. 2007;28:459–476. doi: 10.1016/j.neurobiolaging.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maljkovic V. Nakayama K. Priming of pop-out: I. Role of features. Mem Cognit. 1994;22:657–672. doi: 10.3758/bf03209251. [DOI] [PubMed] [Google Scholar]
- McIntosh AR. Towards a network theory of cognition. Neural Netw. 2000;13:861–870. doi: 10.1016/s0893-6080(00)00059-9. [DOI] [PubMed] [Google Scholar]
- McIntosh AR. Bookstein FL. Haxby JV. Grady CL. Spatial pattern analysis of functional brain images using partial least squares. Neuroimage. 1996;3:143–157. doi: 10.1006/nimg.1996.0016. [DOI] [PubMed] [Google Scholar]
- McIntosh AR. Chau WK. Protzner AB. Spatiotemporal analysis of event-related fMRI data using partial least squares. Neuroimage. 2004;23:764–775. doi: 10.1016/j.neuroimage.2004.05.018. [DOI] [PubMed] [Google Scholar]
- McIntosh AR. Grady CL. Ungerleider LG. Haxby JV. Rapoport SI. Horwitz B. Network analysis of cortical visual pathways mapped with PET. J Neurosci. 1994;14:655–666. doi: 10.1523/JNEUROSCI.14-02-00655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR. Lobaugh NJ. Partial least squares analysis of neuroimaging data: applications and advances. Neuroimage. 2004;23(Suppl 1):S250–S263. doi: 10.1016/j.neuroimage.2004.07.020. [DOI] [PubMed] [Google Scholar]
- McKeown MJ. Makeig S. Brown GG. Jung TP. Kindermann SS. Bell AJ. Sejnowski TJ. Analysis of fMRI data by blind separation into independent spatial components. Hum Brain Mapp. 1998;6:160–188. doi: 10.1002/(SICI)1097-0193(1998)6:3<160::AID-HBM5>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier TB. Desphande AS. Vergun S. Nair VA. Song J. Biswal BB. Meyerand ME. Birn RM. Prabhakaran V. Support vector machine classification and characterization of age-related reorganization of functional brain networks. Neuroimage. 2012a;60:601–613. doi: 10.1016/j.neuroimage.2011.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier TB. Wildenberg JC. Liu J. Chen J. Calhoun VD. Biswal BB. Meyerand ME. Birn RM. Prabhakaran V. Parallel ICA identifies sub-components of resting state networks that covary with behavioral indices. Front Hum Neurosci. 2012b;6:281. doi: 10.3389/fnhum.2012.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiran N. Chorev Z. Sapir A. Component processes in task switching. Cognit Psychol. 2000;41:211–253. doi: 10.1006/cogp.2000.0736. [DOI] [PubMed] [Google Scholar]
- Melloni L. van Leeuwen S. Alink A. Muller NG. Interaction between bottom-up saliency and top-down control: how saliency maps are created in the human brain. Cereb Cortex. 2012;22:2943–2952. doi: 10.1093/cercor/bhr384. [DOI] [PubMed] [Google Scholar]
- Mennes M. Kelly C. Zuo X-N. Di Martino A. Biswal BB. Castellanos FX. Milham MP. Inter-individual differences in resting-state functional connectivity predict task-induced BOLD activity. Neuroimage. 2010;50:1690–1701. doi: 10.1016/j.neuroimage.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A. Friedman NP. Emerson MJ. Witzki AH. Howerter A. Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Monsell S. Task switching. Trends Cogn Sci. 2003;7:134–140. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Monsell S. Mizon GA. Can the task-cuing paradigm measure an endogenous task-set reconfiguration process? J Exp Psychol Hum Percept Perform. 2006;32:493–516. doi: 10.1037/0096-1523.32.3.493. [DOI] [PubMed] [Google Scholar]
- Moore T. Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421:370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- Mori S. Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51:527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Muggleton NG. Cowey A. Walsh V. The role of the angular gyrus in visual conjunction search investigated using signal detection analysis and transcranial magnetic stimulation. Neuropsychologia. 2008;46:2198–2202. doi: 10.1016/j.neuropsychologia.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Müller HJ. Heller D. Ziegler J. Visual search for singleton feature targets within and across feature dimensions. Percept Psychophys. 1995;57:1–17. doi: 10.3758/bf03211845. [DOI] [PubMed] [Google Scholar]
- Müller HJ. Krummenacher J. Locus of dimension weighting: preattentive or postselective? Vis Cogn. 2006a;14:490–513. [Google Scholar]
- Müller HJ. Krummenacher J. Visual Search and Attention. Hove, UK: Psychology Press; 2006b. [Google Scholar]
- Nickerson RS. Binary-classification reaction time: a review of some studies of human information-processing capabilities. Psychonomic Monogr Suppl. 1972;4:275–318. [Google Scholar]
- Nobre AC. Sebestyen GN. Gitelman DR. Mesulam MM. Frackowiak RS. Frith CD. Functional localization of the system for visuospatial attention using positron emission tomography. Brain. 1997;120(Pt 3):515–533. doi: 10.1093/brain/120.3.515. [DOI] [PubMed] [Google Scholar]
- O'Sullivan M. Jones DK. Summers PE. Morris RG. Williams SC. Markus HS. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology. 2001;57:632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- Pachella RG. The interpretation of reaction time in information-processing research. In: Kantowitz B, editor. Human Information Processing: Tutorials in Performance and Cognition. Hillsdale, NJ: Erlbaum; 1974. pp. 41–82. [Google Scholar]
- Palop JJ. Chin J. Mucke L. A network dysfunction perspective on neurodegenerative diseases. Nature. 2006;443:768–773. doi: 10.1038/nature05289. [DOI] [PubMed] [Google Scholar]
- Pantazatos SP. Yanagihara TK. Zhang X. Meitzler T. Hirsch J. Frontal-occipital connectivity during visual search. Brain Connect. 2012;2:164–175. doi: 10.1089/brain.2012.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC. Polk TA. Park R. Minear M. Savage A. Smith MR. Aging reduces neural specialization in ventral visual cortex. Proc Natl Acad Sci U S A. 2004;101:13091–13095. doi: 10.1073/pnas.0405148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks EL. Hopfinger JB. Hold it! Memory affects attentional dwell time. Psychon Bull Rev. 2008;15:1128–1134. doi: 10.3758/PBR.15.6.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashler HE. The Psychology of Attention. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- Pessoa L. Kastner S. Ungerleider LG. Neuroimaging studies of attention: from modulation of sensory processing to top-down control. J Neurosci. 2003;23:3990–3998. doi: 10.1523/JNEUROSCI.23-10-03990.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. Ungerleider LG. Top-down mechanisms for working memory and attentional processes. In: Gazzaniga MS, editor. The Cognitive Neurosciences III. Cambridge, MA: The MIT Press; 2004. pp. 919–930. [Google Scholar]
- Pollmann S. Weidner R. Humphreys GW. Olivers CN. Müller K. Lohmann G. Wiggins CJ. Watson DG. Separating distractor rejection and target detection in posterior parietal cortex—an event-related fMRI study of visual marking. Neuroimage. 2003;18:310–323. doi: 10.1016/s1053-8119(02)00036-8. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI. Cohen Y. Components of visual orienting. In: Bouma H, editor; Bouwhis D, editor. Attention and Performance. Hillsdale, NJ: Erlbaum Associates; 1984. pp. 531–556. [Google Scholar]
- Posner MI. Rothbart MK. Research on attention networks as a model for the integration of psychological science. Annu Rev Psychol. 2007;58:1–23. doi: 10.1146/annurev.psych.58.110405.085516. [DOI] [PubMed] [Google Scholar]
- Posner MI. Snyder CRR. Davidson BJ. Attention and the detection of signals. J Exp Psychol Gen. 1980;109:160–174. [PubMed] [Google Scholar]
- Prado J. Carp J. Weissman DH. Variations of response time in a selective attention task are linked to variations of functional connectivity in the attentional network. Neuroimage. 2011;54:541–549. doi: 10.1016/j.neuroimage.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ. Moore CJ. Friston KJ. Subtractions, conjunctions, and interactions in experimental design of activation studies. Hum Brain Mapp. 1997;5:264–272. doi: 10.1002/(SICI)1097-0193(1997)5:4<264::AID-HBM11>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Prichard JW. Rosen BR. Functional study of the brain by NMR. J Cereb Blood Flow Metab. 1994;14:365–372. doi: 10.1038/jcbfm.1994.47. [DOI] [PubMed] [Google Scholar]
- Proulx MJ. Bottom-up guidance in visual search for conjunctions. J Exp Psychol Hum Percept Perform. 2007;33:48–56. doi: 10.1037/0096-1523.33.1.48. [DOI] [PubMed] [Google Scholar]
- Quinlan PT. Visual feature integration theory: past, present, and future. Psychol Bull. 2003;129:643–673. doi: 10.1037/0033-2909.129.5.643. [DOI] [PubMed] [Google Scholar]
- Raichle ME. Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- Rissman J. Gazzaley A. D'Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23:752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Roebroeck A. Formisano E. Goebel R. Mapping directed influence over the brain using Granger causality and fMRI. Neuroimage. 2005;25:230–242. doi: 10.1016/j.neuroimage.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Rogers RD. Monsell S. Costs of a predictable switch between simple cognitive tasks. J Exp Psychol Gen. 1995;124:207–231. [Google Scholar]
- Rosenthal CR. Walsh V. Mannan SK. Anderson EJ. Hawken MB. Kennard C. Temporal dynamics of parietal cortex involvement in visual search. Neuropsychologia. 2006;44:731–743. doi: 10.1016/j.neuropsychologia.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Ruff CC. Blankenburg F. Bjoertomt O. Bestmann S. Freeman E. Haynes JD. Rees G. Josephs O. Deichmann R. Driver J. Concurrent TMS-fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex. Curr Biol. 2006;16:1479–1488. doi: 10.1016/j.cub.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Ruff CC. Blankenburg F. Bjoertomt O. Bestmann S. Weiskopf N. Driver J. Hemispheric differences in frontal and parietal influences on human occipital cortex: direct confirmation with concurrent TMS-fMRI. J Cogn Neurosci. 2009;21:1146–1161. doi: 10.1162/jocn.2009.21097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH. The declining infrastructure of the aging brain. Brain Connect. 2011;1:279–293. doi: 10.1089/brain.2011.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W. Shiffrin RM. Controlled and automatic human information processing: I. Detection, search, and attention. Psychol Rev. 1977;84:1–66. [Google Scholar]
- Schweickert R. Information, time, and the structure of mental events: a twenty-five year review. In: Meyer DE, editor; Kornblum S, editor. Attention and Performance XIV: Synergies in Experimental Psychology, Artificial Intelligence, and Cognitive Neuroscience. Cambridge, MA: MIT Press; 1993. pp. 535–566. [Google Scholar]
- Seeley WW. Crawford RK. Zhou J. Miller BL. Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW. Menon V. Schatzberg AF. Keller J. Glover GH. Kenna H. Reiss AL. Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma J. Angelucci A. Sur M. Induction of visual orientation modules in auditory cortex. Nature. 2000;404:841–847. doi: 10.1038/35009043. [DOI] [PubMed] [Google Scholar]
- Shen K. Bezgin G. Hutchison RM. Gati JS. Menon RS. Everling S. McIntosh AR. Information processing architecture of functionally defined clusters in the macaque cortex. J Neurosci. 2012;32:17465–17476. doi: 10.1523/JNEUROSCI.2709-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffrin RM. Attention. In: Atkinson RC, editor; Herrnstein RJ, editor; Lindzey G, editor; Luce RD, editor. Stevens' Handbook of Experimental Psychology. New York: Wiley; 1988. pp. 739–811. [Google Scholar]
- Shiffrin RM. Schneider W. Controlled and automatic human information processing: II. Perceptual learning, automatic attending, and a general theory. Psychol Rev. 1977;84:127–190. [Google Scholar]
- Shmuel A. Leopold DA. Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: implications for functional connectivity at rest. Hum Brain Mapp. 2008;29:751–761. doi: 10.1002/hbm.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL. McAvoy MP. Cowan MC. Astafiev SV. Tansy AP. d'Avossa G. Corbetta M. Quantitative analysis of attention and detection signals during visual search. J Neurophysiol. 2003;90:3384–3397. doi: 10.1152/jn.00343.2003. [DOI] [PubMed] [Google Scholar]
- Shulman GL. Ollinger JM. Akbudak E. Conturo TE. Snyder AZ. Petersen SE. Corbetta M. Areas involved in encoding and applying directional expectations to moving objects. J Neurosci. 1999;19:9480–9496. doi: 10.1523/JNEUROSCI.19-21-09480.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow JC. Mattingley JB. Stimulus- and goal-driven biases of selective attention following unilateral brain damage: implications for rehabilitation of spatial neglect and extinction. Restor Neurol Neurosci. 2006;24:233–245. [PubMed] [Google Scholar]
- Sridharan D. Levitin DJ. Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg S. The discovery of processing stages: extensions of Donders' method. In: Koster WG, editor. Attention and Performance II. Amsterdam: North Holland; 1969. pp. 276–315. [Google Scholar]
- Stevens MC. The developmental cognitive neuroscience of functional connectivity. Brain Cogn. 2009;70:1–12. doi: 10.1016/j.bandc.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Stevens MC. Kiehl KA. Pearlson GD. Calhoun VD. Functional neural networks underlying response inhibition in adolescents and adults. Behav Brain Res. 2007;181:12–22. doi: 10.1016/j.bbr.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC. Kiehl KA. Pearlson GD. Calhoun VD. Brain network dynamics during error commission. Hum Brain Mapp. 2009a;30:24–37. doi: 10.1002/hbm.20478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC. Skudlarski P. Pearlson GD. Calhoun VD. Age-related cognitive gains are mediated by the effects of white matter development on brain network integration. Neuroimage. 2009b;48:738–746. doi: 10.1016/j.neuroimage.2009.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV. Pfefferbaum A. Diffusion tensor imaging in aging and age-related neurodegenerative disorders. In: Jones DK, editor. Diffusion MRI: Theory, Methods, and Applications. New York: Oxford University Press; 2011. pp. 624–643. [Google Scholar]
- Theeuwes J. Visual selective attention: a theoretical analysis. Acta Psychol. 1993;83:93–154. doi: 10.1016/0001-6918(93)90042-p. [DOI] [PubMed] [Google Scholar]
- Theeuwes J. Top-down and bottom-up control of visual selection. Acta Psychol (Amst) 2010;135:77–99. doi: 10.1016/j.actpsy.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Theeuwes J. Reimann B. Mortier K. Visual search for featural singletons: No top-down modulation, only bottom-up priming. Vis Cogn. 2006;14:466–489. [Google Scholar]
- Tollner T. Muller HJ. Zehetleitner M. Top-down dimensional weight set determines the capture of visual attention: evidence from the PCN component. Cereb Cortex. 2012;22:1554–1563. doi: 10.1093/cercor/bhr231. [DOI] [PubMed] [Google Scholar]
- Tomasi D. Volkow ND. Association between functional connectivity hubs and brain networks. Cereb Cortex. 2011;21:2003–2013. doi: 10.1093/cercor/bhq268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D. Volkow ND. Aging and functional brain networks. Mol Psychiatry. 2012;17:549–558. doi: 10.1038/mp.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman A. How the deployment of attention determines what we see. Vis Cogn. 2006;14:411–443. doi: 10.1080/13506280500195250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M. Mandl R. Luigjes J. Hulshoff Pol H. Microstructural organization of the cingulum tract and the level of default mode functional connectivity. J Neurosci. 2008;28:10844–10851. doi: 10.1523/JNEUROSCI.2964-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP. Mandl RC. Kahn RS. Hulshoff Pol HE. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp. 2009;30:3127–3141. doi: 10.1002/hbm.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP. Pol HEH. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharm. 2010;20:519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Vincent JL. Kahn I. Snyder AZ. Raichle ME. Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND. Gur RC. Wang GJ. Fowler JS. Moberg PJ. Ding YS. Hitzemann R. Smith G. Logan J. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry. 1998;155:344–349. doi: 10.1176/ajp.155.3.344. [DOI] [PubMed] [Google Scholar]
- Volkow ND. Logan J. Fowler JS. Wang GJ. Gur RC. Wong C. Felder C. Gatley SJ. Ding YS. Hitzemann R. Pappas N. Association between age-related decline in brain dopamine activity and impairment in frontal and cingulate metabolism. Am J Psychiatry. 2000;157:75–80. doi: 10.1176/ajp.157.1.75. [DOI] [PubMed] [Google Scholar]
- Vossel S. Weidner R. Driver J. Friston KJ. Fink GR. Deconstructing the architecture of dorsal and ventral attention systems with dynamic causal modeling. J Neurosci. 2012;32:10637–10648. doi: 10.1523/JNEUROSCI.0414-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD. Jonides J. Reading S. Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage. 2004;22:1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Wang L. Liu X. Guise KG. Knight RT. Ghajar J. Fan J. Effective connectivity of the fronto-parietal network during attentional control. J Cogn Neurosci. 2010;22:543–553. doi: 10.1162/jocn.2009.21210. [DOI] [PubMed] [Google Scholar]
- Wei P. Müller HJ. Pollmann S. Zhou X. Neural correlates of binding features within- or cross-dimensions in visual conjunction search: an fMRI study. Neuroimage. 2011;57:235–241. doi: 10.1016/j.neuroimage.2011.04.024. [DOI] [PubMed] [Google Scholar]
- Weissman DH. Gopalakrishnan A. Hazlett CJ. Woldorff MG. Dorsal anterior cingulate cortex resolves conflict from distracting stimuli by boosting attention toward relevant events. Cereb Cortex. 2005;15:229–237. doi: 10.1093/cercor/bhh125. [DOI] [PubMed] [Google Scholar]
- Wen X. Yao L. Liu Y. Ding M. Causal interactions in attention networks predict behavioral performance. J Neurosci. 2012;32:1284–1292. doi: 10.1523/JNEUROSCI.2817-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler-Kingshott CA. Cercignani M. About “axial” and “radial” diffusivities. Magn Reson Med. 2009;61:1255–1260. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- Wolfe JM. Guided search 2.0: A revised model of visual search. Psychon Bull Rev. 1994;1:202–238. doi: 10.3758/BF03200774. [DOI] [PubMed] [Google Scholar]
- Wolfe JM. Visual search. In: Pashler H, editor. Attention. East Sussex, UK: Psychology Press; 1998. pp. 13–73. [Google Scholar]
- Wolfe JM. Guided search 4.0: current progress with a model of visual search. In: Gray W, editor. Integrated Models of Visual Systems. New York: Oxford University Press; 2007. pp. 99–119. [Google Scholar]
- Wolfe JM. Butcher SJ. Lee C. Hyle M. Changing your mind: on the contributions of top-down and bottom-up guidance in visual search for feature singletons. J Exp Psychol Hum Percept Perform. 2003;29:483–502. doi: 10.1037/0096-1523.29.2.483. [DOI] [PubMed] [Google Scholar]
- Wolfe JM. Horowitz TS. What attributes guide the deployment of visual attention and how do they do it? Nat Rev Neurosci. 2004;5:495–501. doi: 10.1038/nrn1411. [DOI] [PubMed] [Google Scholar]
- Woodman GF. Luck SJ. Visual search is slowed when visuospatial working memory is occupied. Psychon Bull Rev. 2004;11:269–274. doi: 10.3758/bf03196569. [DOI] [PubMed] [Google Scholar]
- Xu Y. Chun MM. Visual grouping in human parietal cortex. Proc Natl Acad Sci U S A. 2007;104:18766–18771. doi: 10.1073/pnas.0705618104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT. Krienen FM. Sepulcre J. Sabuncu MR. Lashkari D. Hollinshead M. Roffman JL. Smoller JW. Zollei L. Polimeni JR. Fischl B. Liu H. Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J. Gennatas ED. Kramer JH. Miller BL. Seeley WW. Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron. 2012;73:1216–1227. doi: 10.1016/j.neuron.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]