Abstract
There are a limited number of therapies available to prevent heart failure following myocardial infarction. One novel therapy that is currently being pursued is the implantation of cardiac progenitor cells (CPCs); however, their responses to oxidative stress during differentiation have yet to be elucidated. The objective of this study was to determine the effect of hydrogen peroxide (H2O2) treatment on CPC differentiation in vitro, as well as the effect of H2O2 preconditioning before implantation following ischemia-reperfusion (I/R) injury. CPCs were isolated and cloned from adult rat hearts, and then cultured in the absence or presence of H2O2 for 2 or 5 days. CPC survival was assessed with Annexin V, and cellular differentiation was evaluated through mRNA expression for cardiogenic genes. We found that 100 μM H2O2 decreased serum withdrawal-induced apoptosis by at least 45% following both 2 and 5 days of treatment. Moreover, 100 μM H2O2 treatment for 2 days significantly increased endothelial and smooth muscle markers compared to time-matched untreated CPCs. However, continued H2O2 treatment significantly decreased these markers. Left ventricular cardiac function was assessed 28 days after I/R and I/R with the implantation of Luciferase/GFP+ CPCs, which were preconditioned with 100 μM H2O2 for 2 days. Hearts implanted with Luciferase/GFP+ CPCs had significant improvement in both positive and negative dP/dT over I/R. Furthermore, cardiac fibrosis was significantly decreased in the preconditioned cells versus both I/R alone and I/R with control cells. We also observed a significant increase in endothelial cell density in the preconditioned CPC hearts compared to untreated CPC hearts, which also coincided with a higher density of Luciferase+ vessels. These findings suggest that preconditioning of CPCs with H2O2 for 2 days stimulates neoangiogenesis in the peri-infarct area following I/R injury and could be a viable therapeutic option to prevent heart failure.
Highlights
Cardiac progenitor cells are resistant to acute oxidative insults in culture, while differentiating. Hydrogen peroxide (H2O2) treatment acutely increases proangiogenic markers in vitro. H2O2 preconditioned cardiac progenitor cells implanted into the left ventricle following ischemia-reperfusion injury led to decreased cardiac fibrosis and a higher number of endothelial cells and vascular density.
Introduction
Recent statistics demonstrate that over 8 million Americans have been diagnosed with cardiovascular disease and there are an estimated 785,000 new myocardial infarction (MI) and another 450,000 recurrent MI patients annually [1]. The loss of tissue is highly localized and the human body cannot replace the damaged tissue, leading to cardiac hypertrophy and eventual heart failure, which accounts for an estimated one in nine deaths. The increasing levels of obesity and diabetes, as well as diet, exercise, and lifestyle choices in the United States, suggest that the incidence of MI and heart failure will increase in the future. Presently, the only definitive treatment to reverse heart failure is through transplantation, but the donor waiting list is longer than the predicted survival time for most disease sufferers. In recent years, many clinical studies have been initiated to deliver localized therapy in the form of various cell types for reconstitution of the myocardium [2,3]. However, there is much debate on the optimal cell type, whether or not stem cells can differentiate into a functional myocardium, and the long-term effects of these nonmyocytes.
Recent evidence in multiple species indicates that the heart contains self-renewing capabilities, since it possesses a population of stem cells called cardiac progenitor cells (CPCs) [4–7]. These cells possess qualities of stemness (clonogenic), but can only be differentiated into cardiac cell types. Studies demonstrate successful regeneration of the myocardium by delivery of CPCs that differentiate into endothelial cells (enhancing angiogenesis) and myocytes (enhancing contractility) [6,8]. Furthermore, preliminary findings from a Phase I clinical trial support the animal studies and demonstrate the efficacy of infusing CPCs intravenously into patients suffering from ischemic cardiomyopathy [NCT00474461] [9,10]. These findings suggest that CPCs could be the optimal cell type to improve cardiac function following MI in both animal models and humans, but it is still unclear if the beneficial effects are due to the differentiation of the cells into new cardiomyocytes, vascular and smooth muscle (SM) cells, or the release of paracrine factors to support the injured myocardium. Additionally, in many clinical trials where improvements in acute function are seen, the benefits are not carried to long-term recovery of function, suggesting that improvements still need to be made.
While excitement over CPC therapy builds, one caveat with CPC implantation is the reduced number of cells that are retained in the heart [11] demonstrating the need for adjuvant therapy. A potential treatment used in multiple stem/progenitor cell types has been to precondition cells with a burst of reactive oxygen species (ROS). Incubation with ROS has been shown to stimulate various cell survival pathways that include antiapoptotic proteins and antioxidative enzymes [12–15]. Multiple studies evaluating the effect of hydrogen peroxide (H2O2) preconditioning on cell function have been inconclusive, in which differentiation into cardiomyocytes occurred in embryonic stem cells, while mesenchymal stem cells showed a decrease in adhesion and survival following transplantation [16,17]. Additionally, H2O2 has different effects on cardiogenic differentiation of stem/progenitor cells depending on the cell type [18,19]. However, the effect of H2O2 preconditioning on CPC differentiation has yet to be elucidated. In the present study, we sought to determine whether preconditioning CPCs with H2O2 during an early differentiation period would induce cell-signaling mechanisms that enhance CPC survival, differentiation, and engraftment and the amount of viable myocardium following ischemia/reperfusion (I/R) injury.
Materials and Methods
CPC isolation and culture and H2O2 treatment
Endogenous c-Kit+ CPCs were isolated from the myocardium of adult Sprague-Dawley rats, and cells were generated from single-cell clones and cultured as previously described [20] and detailed in the Supplementary Methods; Supplementary Data are available online at www.liebertpub.com/scd.
Before H2O2 (30% stabilized with sodium stannate, Fisher Scientific) treatment, CPCs were serum starved overnight in Ham's F-12 media supplemented with l-glutamine, penicillin/streptomycin, insulin/transferrin/selenium (ITS), and the leukemia inhibitory factor (LIF). CPCs were allowed to differentiate in Ham's F-12 media without the LIF, supplemented with l-Glutamine, penicillin/streptomycin, ITS, and in the absence or presence of various H2O2 concentrations (1, 10, or 100 μM) for basal (0 day), acute (2 days), or chronic (5 days) treatment. Media H2O2 doses during treatment were replenished once daily. Each experimental time point had its own time-matched control group.
Annexin V staining
CPC apoptosis at the indicated time points was assessed by flow cytometry with the Annexin V Alexa-647 conjugate antibody kit (Invitrogen) as described in the Supplementary Methods.
mRNA isolation and real-time reverse transcription-polymerase chain reaction
Gene expression was measured in CPCs following the treatment protocol by real-time reverse transcription polymerase chain reaction (PCR) as detailed in the Supplementary Methods.
CPC transduction
cKit+ CPCs were transduced with a firefly luciferase/green fluorescent protein (GFP) lentivirus (Gentarget) according to the manufacturer's protocol. Cloned CPCs were plated in a 24-well plate and grown to confluency of 50% in growth media. At the time of transduction, 50 μL of viral stock was added to each well in growth media in the absence of penicillin and streptomycin and incubated for 3 days at 37°C with occasional mixing. After 3 days of viral treatment, CPCs were cultured in growth media with antibiotics and the addition of Blasticidin (10 μg/mL) for 2 days. Transduction was verified by the presence of GFP in cells with an Olympus IX71 fluorescence microscope. GFP+ cells were expanded in growth media.
Self-assembling peptide hydrogels for cell delivery
RAD16-II self-assembling peptides were utilized during intramyocardial placement of non-pretreated and pretreated CPCs as previously described with slight modifications [5,21]. Luciferase+ (Luc+) CPCs were plated and allowed to differentiate for 2 days in the presence or absence of 100 μM H2O2 after the removal of LIF. Cells were dissociated from the plate following 2 days of CPC differentiation. CPCs (5×105) were resuspended in 50 μL of 1% w/v self-assembling peptides in 295 mM sterile sucrose for injection.
MI and CPC implantation
Adult, male Sprague-Dawley rats were obtained from Charles River Laboratories. The animals were anesthetized with isoflurane and, after tracheal intubation, MI was induced by occlusion of the left anterior descending artery for 30 min followed by reperfusion [22]. Immediately following reperfusion, three injections of Luc+ GFP+ CPCs in self-assembling peptides were made in the border zone of the left ventricular free wall, while the heart was beating. After injections, the chests were closed and animals recovered on a heating pad. Sham animals had the surgery to open the chest and the left anterior descending artery was visualized before the chest was closed. Briefly, all animals received surgery and were assigned a number. Following 28 days of recovery, an individual without knowledge of the numbering system would perform the echocardiography and immunohistochemical studies in a blinded manner. All studies were performed in a randomized and double-blind manner and were approved by the Emory University Institutional Animal Care and Use Committee.
Invasive left ventricular measurements
Twenty eight days after CPC implantation in vivo ventricular dimensions and function were assessed as previously described [23] and detailed in the Supplementary Methods.
Immunohistochemistry and confocal microscopy
After 28 days, animals were euthanized, hearts were excised and fixed with 4% paraformaldehyde, embedded in paraffin, and 5-μm sections were made as previously described [24]. Collagen deposition was measured with picrosirius red staining as previously described [24], and antibody labeling was measured with a fluorescent confocal microscope. Both procedures are detailed in the Supplementary Methods.
Statistics
All statistical analyses were performed using Graphpad Prism 5.0 software. The following statistical tests were utilized according to the study layout: Unpaired two-tailed Students' t-test or One-Way Analysis of Variance followed by the appropriate post-test. All data are expressed as mean±SEM. P-values of<0.05 were considered significant.
Results
H2O2 provides antiapoptotic protection to CPCs
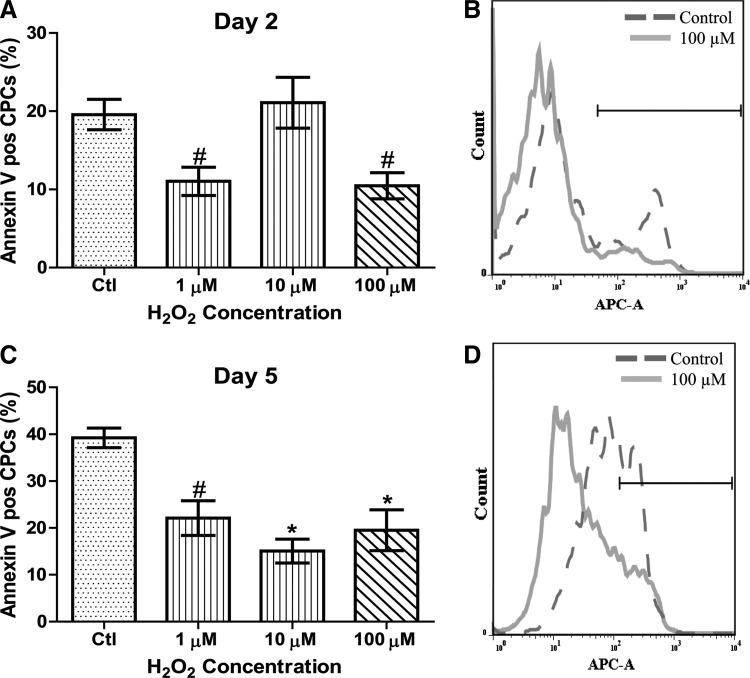
To determine CPC viability after H2O2 treatment, CPCs in culture were allowed to differentiate for 2 and 5 days in the presence or absence of daily administrations of 1, 10, and 100 μM H2O2. The extent of apoptosis was measured with Annexin V and the percentage of apoptotic cells was quantified by flow cytometry. Representative flow cytometric histograms shown in Fig. 1B and D depict Annexin V experiments that show untreated and 100 μM H2O2-treated CPCs following 2 and 5 days of serum withdrawal, respectively. As shown in Fig. 1A, treatment with either 1 or 100 μM H2O2 for 2 days resulted in a significant decrease in basal apoptosis by 50% (P<0.01) and 45% (P<0.01) compared to time-matched untreated CPCs. As shown in Fig. 1C, CPCs treated for 5 days with 100 μM H2O2 exhibited a significantly lower apoptosis (52%; P<0.01) compared to time-matched untreated CPCs. We also observed a significant decrease in apoptosis with both 1 and 10 μM H2O2 after 5 days of treatment. These data demonstrate that H2O2 treatment improves basal CPC survival at 2 and 5 days of treatment.
FIG. 1.
H2O2 enhances CPC survival following serum withdrawal. CPCs were cultured in media with 1, 10, or 100 μM H2O2 or without H2O2 replaced daily for 2 (A) or 5 (C) days and apoptosis was measured by Annexin V staining with flow cytometric analysis. Figures B and D are representative histograms of Annexin V-positive stained cells. The area under the black bar is indicative of the percentage of apoptosis in CPCs. At 2 days, both 1 and 100 μM significantly decreased apoptosis, while at 5 days, all H2O2 concentrations significantly improved survival. Data are mean±SEM. Statistical significance was determined by One-Way ANOVA followed by the Tukey–Kramer Post hoc test. *P<0.05, #P<0.01, n=3–7. Ctl=control cells; H2O2, hydrogen peroxide; CPC=cardiac progenitor cell.
To determine if H2O2 scavenging genes were regulated by H2O2 treatment, we measured catalase and glutathione peroxidase 1 (Gpx1), thioredoxin 1 (Txn1), and peroxiredoxin 6 (Prdx6) gene expression levels over time. While the results are summarized in Supplementary Figs. 1A–D and 2A and B, we found no effect of the H2O2 treatment on H2O2 scavenging enzyme gene expression at 2 or 5 days.
Effect of H2O2 treatment on cardiac gene expression
To determine the effect of H2O2 treatment on cardiogenic gene expression, CPCs were treated for 2 or 5 days in the presence or absence of 1 or 100 μM H2O2 and gene expression was determined by PCR. We examined levels of Troponin C and T to determine cardiac differentiation/maturation. After 2 days of treatment, untreated cells significantly increased the levels of Troponin C as compared to day 0 baseline controls (Supplementary Fig. 2A; P<0.05). There was no further increase with H2O2 treatment. There was no increase in Troponin T in control cells after 2 days as compared to day 0 baseline controls (Supplementary Fig. 2B). While there was a significant increase in Troponin T with 1 μM H2O2 after 2 days (P<0.01 vs. day 0 baseline control), and all other groups (P<0.05), however, there was no effect of 100 μM H2O2. Moreover, there was no significant change in Troponin C in H2O2-treated groups as compared to their time-matched control. With 5 days of treatment, there was a significant increase in Troponin C gene expression in both untreated controls and 1 μM H2O2 as compared to day 0 baseline controls (Supplementary Fig. 2C; P<0.01 and P<0.05, respectively). Treatment with 100 μM H2O2 significantly decreased Troponin C as compared to time-matched untreated controls. After 5 days of H2O2 treatment, there was no difference in Troponin T for any treatment (Supplementary Fig. 2D).
Effect of H2O2 treatment on vascular gene expression
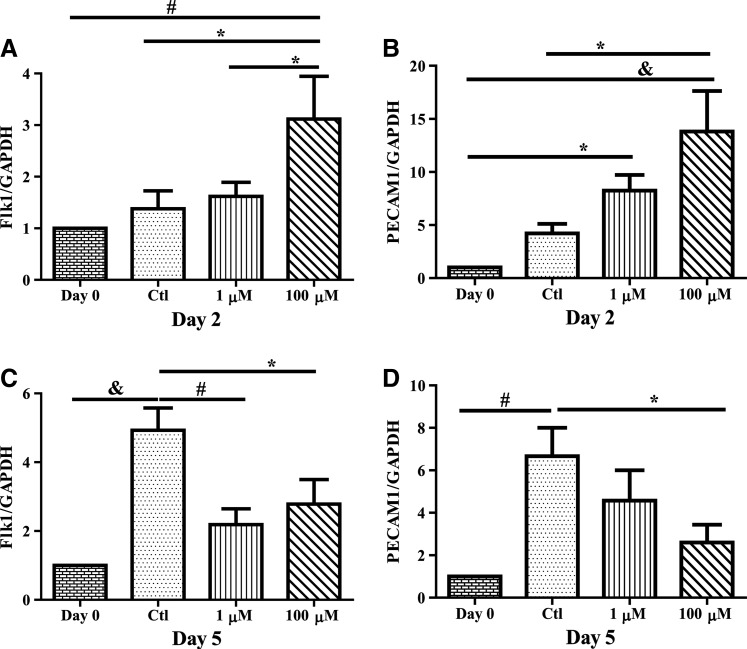
To determine differentiation/maturation toward endothelial cells, mRNA expression of the vascular endothelial growth factor (VEGF) receptor 2 (Flk1) and platelet endothelial cell adhesion molecule (PECAM1), was assessed following H2O2 treatment. For Flk1, there was no increase in expression in untreated cells after 2 days of treatment as compared to day 0 baseline controls (Fig. 2A). While there was no change with 1 μM H2O2, 100 μM after 2 days significantly elevated Flk1 as compared to both day 0 baseline levels and time-matched controls (P<0.01). Similarly, with PECAM1, there was no change in untreated controls, but significant increases in gene expression with 1 and 100 μM treatments after 2 days (Fig. 2B, P<0.05 and P<0.001, respectively). In contrast, after 5 days of treatment, there was a significant increase (P<0.01) in both Flk1 and PECAM1 gene expression in untreated controls as compared with day 0 baseline levels (Fig. 2C, D). For Flk1, both 1 and 100 μM H2O2 significantly decreased expression compared with time-matched control (P<0.05), while only 100 μM significantly decreased PECAM1 levels compared with time-matched control (P<0.05).
FIG. 2.
H2O2 increases endothelial gene expression acutely. CPCs were cultured in media with 1 or 100 μM H2O2 or without H2O2 replaced daily for 2 (A, B) or 5 (C, D) days and mRNA was harvested and gene expression levels were measured through RT-PCR. At 2 days, 100 μM H2O2 increased both Flk1 and PECAM1 mRNA transcripts compared to untreated cells. However, chronic (5 days) 100 μM H2O2 treatment decreased gene expression below that of untreated cells. Data are mean±SEM. Statistical significance was determined by One-Way ANOVA followed by the Tukey–Kramer Post hoc test. *P<0.05, #P<0.01, and &P<0.001, n=4–10. PCR, polymerase chain reaction; GAPDH=glyceraldehyde 3-phosphate dehydrogenase.
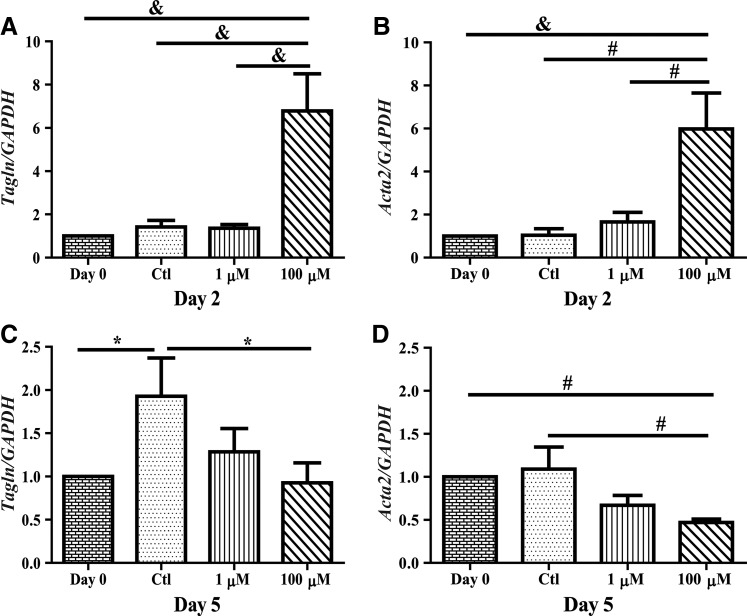
For SM markers, SM 22 alpha (SM22α) and alpha SM actin (α SM actin) gene expressions were assessed in the CPCs following H2O2 treatment during differentiation. For both genes, there was no significant change in expression following 2 days in untreated controls as compared with day 0 baseline levels (Fig. 3A, B). There was no change with 1 μM H2O2 treatment, but 100 μM H2O2 treatment significantly increased expression of both markers as compared to time-matched controls (P<0.001). After 5 days of treatment, there was a significant increase in SM22α gene expression in untreated controls as compared with day 0 baseline levels (Fig. 3C; P<0.05). While there was no effect with 1 μM H2O2 treatment, 100 μM significantly decreased this response (P<0.05). Similar to SM22α, 100 μM H2O2 treatment significantly decreased α SM actin levels compared with time-matched untreated controls (Fig. 3D; P<0.01).
FIG. 3.
H2O2 increases SM gene expression acutely. CPCs were cultured in media with 1 or 100 μM H2O2 or without H2O2 replaced daily for 2 (A, B) or 5 (C, D) days and mRNA was harvested and gene expression levels were measured through RT-PCR. At 2 days, 100 μM H2O2 increased both Tagln and Acta2 mRNA transcripts compared to untreated cells. However, chronic (5 days) 100 μM H2O2 treatment decreased gene expression below that of untreated cells. Data are mean±SEM. Statistical significance was determined by One-Way ANOVA followed by the Tukey–Kramer Post hoc test. *P<0.05, #P<0.01, and &P<0.001, n=4–9. SM=smooth muscle; GAPDH=glyceraldehyde 3-phosphate dehydrogenase.
Implanted CPCs pretreated with H2O2 enhance function following I/R
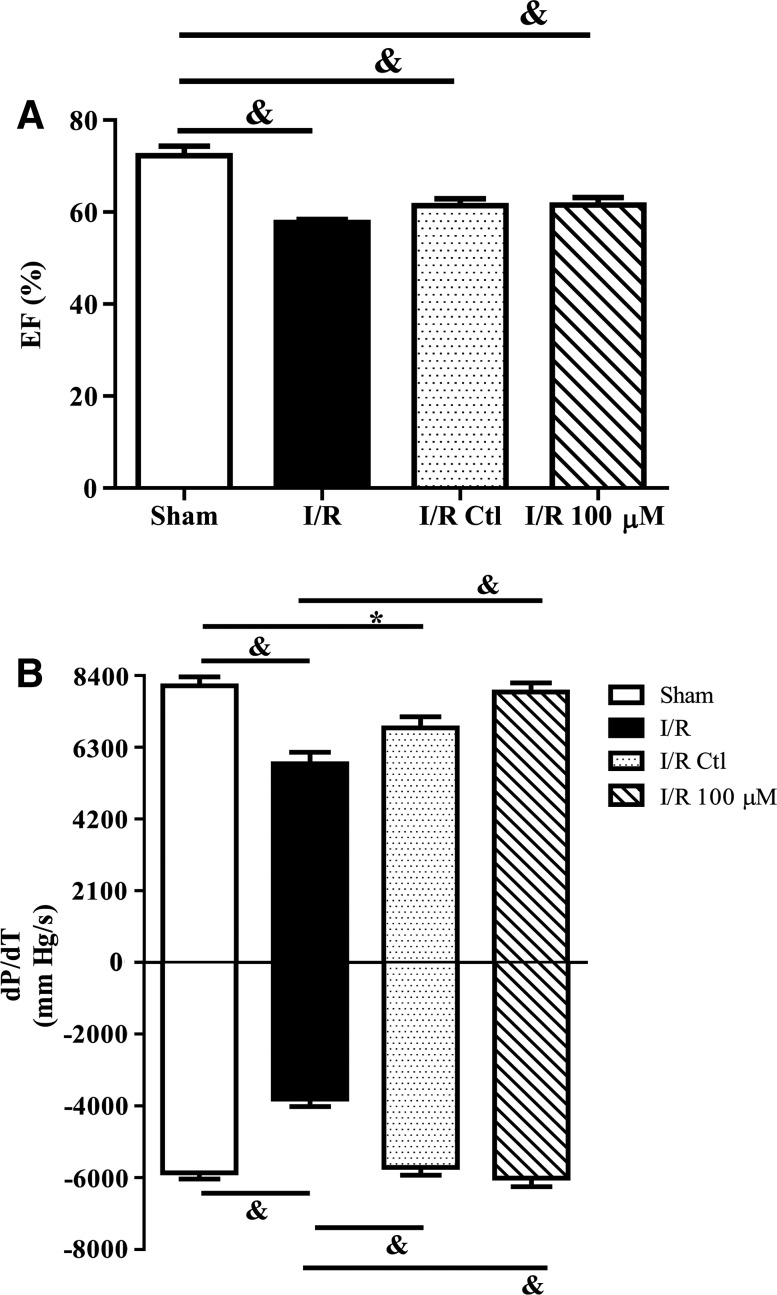
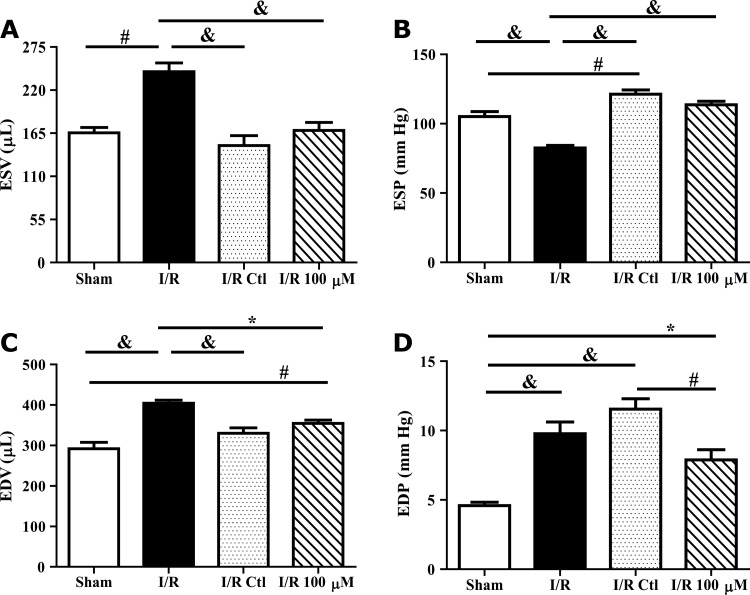
To determine whether the increases in survival and vascular gene expression enhanced efficacy of cell therapy, CPCs were pretreated with media alone or media containing 100 μM H2O2 before implantation in rat hearts subjected to I/R injury. Five hundred thousand CPCs differentiated in the absence or presence of daily doses of 100 μM H2O2 for 2 days were implanted within self-assembling peptide scaffolds immediately following reperfusion. Echocardiography performed 7 days following treatment demonstrated no significant improvement in either group of rats treated with cells (data not shown). Invasive hemodynamic measurements were taken 28 days following injury. The ejection fraction was significantly decreased in I/R rats as compared to sham operated animals (Fig. 4A; P<0.001). Moreover, treatment with control cells and H2O2 pretreated cells did not significantly improve the ejection fraction compared with I/R alone. Similar to the ejection fraction, there was a significant decrease in dP/dT following I/R as compared with sham animals (Fig. 4B; P<0.001). While treatment with control cells significantly improved the−dP/dT (P<0.001), but not the +dP/dT compared to I/R alone, and treatment with H2O2 preconditioned cells significantly improved both measures (P<0.001). End-systolic and -diastolic volumes were both significantly increased in I/R rats as compared with sham operated animals (Fig. 5A, C; P<0.01). Treatment with control and H2O2 preconditioned cells both significantly improved ventricular volumes compared to I/R hearts (P<0.05). End-systolic pressure was decreased in I/R rats as compared with sham operated animals (Fig. 5B; P<0.001). Treatment with control and H2O2 preconditioned cells both significantly restored end-systolic pressure. In contrast, end-diastolic pressure was significantly increased in I/R rats as compared to sham operated animals (Fig. 5D; P<0.001). While there was no effect of control cells on this parameter as compared to I/R, rats treated with H2O2 preconditioned cells significantly improved end-diastolic pressure compared to IR with untreated cells (P<0.01).
FIG. 4.
H2O2 preconditioned CPCs improve cardiac contractility and relaxation following I/R. Left ventricular function was evaluated by invasive pressure–volume hemodynamic measurements 28 days after I/R and CPC implantation. Ejection fraction (A) was depressed in all I/R groups, but+and–dP/dT (B) were significantly improved in 100 μM H2O2 preconditioned CPC hearts compared to I/R alone. Data are mean±SEM. Statistical significance was determined by One-Way ANOVA followed by the Tukey–Kramer Post hoc test. *P<0.05 and &P<0.001, n=6–13. EF=ejection fraction; I/R=ischemia/reperfusion.
FIG. 5.
H2O2 preconditioned CPCs improve end-diastolic pressure after I/R injury. Left ventricular function was evaluated by invasive pressure–volume hemodynamic measurements 28 days after I/R and CPC implantation. CPC-treated hearts had similar improvements in cardiac volumes (A, C) compared to I/R alone. End-systolic pressure (B) was significantly elevated in both CPC-treated groups compared to I/R hearts. About 100 μM H2O2 preconditioned CPC hearts had a lower end-diastolic pressure (D) compared to untreated CPC I/R hearts. Data are mean±SEM. Statistical significance was determined by One-Way ANOVA followed by the Tukey–Kramer Post hoc test. *P<0.05, #P<0.01, and &P<0.001, n=5–12. ESV and ESP=end-systolic volume and pressure; EDV and EDP=end-diastolic volume and pressure.
Implanted CPCs pretreated with H2O2 improve fibrosis and angiogenesis
To determine whether H2O2 preconditioning of CPCs improved fibrosis, collagen deposition was measured using picrosirius red staining. Rats subjected to I/R had a fibrotic area of 38.9%±2.9% of the left ventricle (Fig. 6C). While there was a 20% reduction in control CPC-treated rats, animals treated with H2O2 preconditioned cells had a significant 60% decrease in fibrosis (P<0.05 compared to both groups).
FIG. 6.
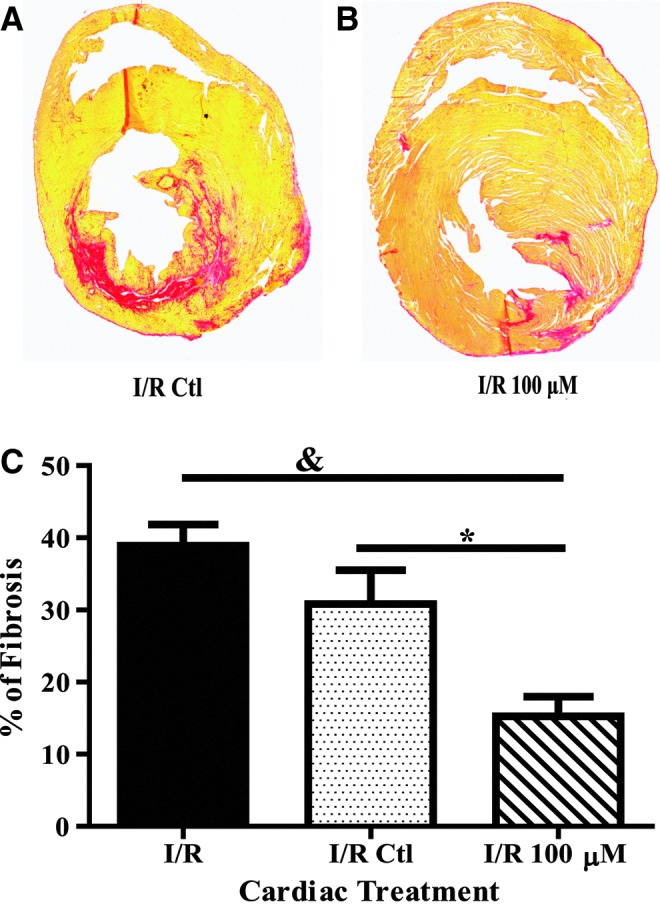
H2O2 preconditioned CPCs decrease cardiac fibrosis following I/R. Representative images of control CPC (A) and 100 μM H2O2 (B) preconditioned CPC hearts were taken with a 2.5×objective and stitched together using Adobe Photoshop, with collagen staining in red. Hearts treated with 100 μM H2O2 preconditioned CPCs exhibited decreased fibrosis compared to both I/R alone and untreated CPC I/R hearts shown in the bar graph (C). Data are mean±SEM. Statistical significance was determined by One-Way ANOVA followed by the Tukey–Kramer Post hoc test. *P<0.05 and &P<0.001, n=6–9.
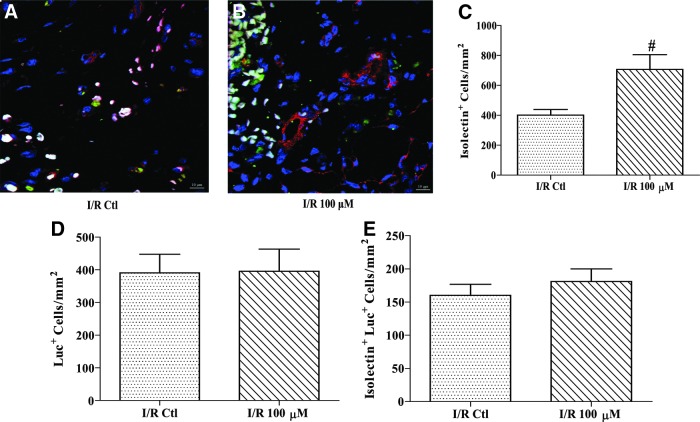
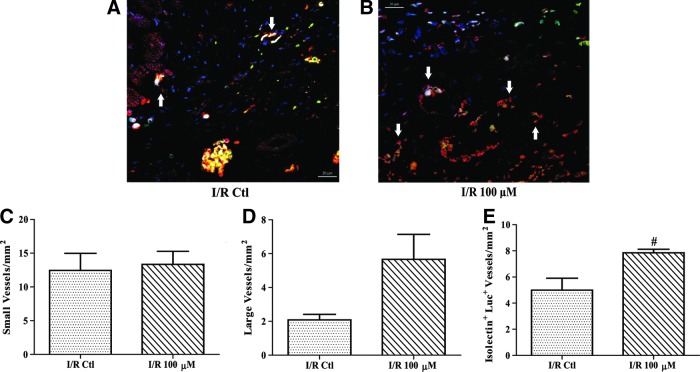
To determine the fate of the implanted cells and whether an angiogenic response was induced, tissue sections were probed with isolectin to stain endothelial cells and an antibody against firefly luciferase to identify the implanted cells. Images were taken using confocal microscopy and quantified as cell number/mm2, or vessel number/mm2. There was a significant increase of 76% in total isolectin-positive cells in the H2O2 preconditioned group as compared to rats implanted with control cells (Fig. 7A–C; P<0.01). Luciferase-positive cells were counted and no significant difference was found (Fig. 7D). Additionally, there was no significant increase in cell staining for both luciferase and isolectin, indicating the difference in isolectin-positive cells was likely attributed to endogenous cells (Fig. 7E). In addition to counting isolectin-positive cells, both small and large vessels were also counted in a similar manner. As the representative images in Fig. 8A and B show, there was no significant difference in the number of small vessels (Fig. 8C). In contrast, the H2O2 preconditioned group exhibited a 171% higher density of large vessels compared to control CPCs, but this comparison did not reach significance (Fig. 8D; P=0.08). Furthermore, vessels that contained luciferase-positive cells were 60% higher in the H2O2 preconditioned group as compared to rats implanted with control cells (Fig. 8E; P=0.01).
FIG. 7.
H2O2 preconditioned CPCs increase endothelial cell density following I/R. After 28 days, hearts were incubated with isolectin and an antiluciferase antibody to determine the density of endothelial and implanted cells, respectively. Isolectin-positive cells are shown in red, firefly luciferase-positive cells are shown in green, and 4′,6-diamidino-2-phenylindole (DAPI) stained nuclei are shown in blue. Representative images of control CPC (A) and H2O2 (B) preconditioned CPC hearts were taken using confocal microscopy (40×objective). Sections from 100 μM H2O2 preconditioned CPC hearts exhibited a greater number of total endothelial cells (C) compared to untreated CPC I/R hearts. There were no significant differences in total Luciferase+ cells (D) or endothelial cells that stained for luciferase (E). Data are mean±SEM. Statistical significance was determined by One-Way ANOVA followed by the Tukey–Kramer Post hoc test. #P<0.01, n=4–8. Scale bar=10 μM.
FIG. 8.
H2O2 preconditioned CPCs increase neoangiogenesis following I/R. After 28 days, hearts were harvested, fixed in paraformaldehyde, sectioned, and incubated with isolectin and an antiluciferase antibody to determine the number of vessels and Luciferase+ (Luc+) vessels, respectively. Isolectin-positive vessels are shown in red, firefly luciferase-positive vessels are shown in green, and DAPI stained nuclei are shown in blue. Representative images of control CPC (A) and H2O2 (B) preconditioned CPC hearts were taken using confocal microscopy (63×objective). White arrows indicate double-positive vessels. There was no significant change in small vessels (C) and large vessels (D) were increased by over 170% although this was not significant (P=0.08). Sections from 100 μM H2O2 preconditioned CPC hearts exhibited a significantly higher number of Luc+ vessels (E) compared to untreated CPC I/R hearts. Data are mean±SEM. Statistical significance was determined by One-Way ANOVA followed by the Tukey–Kramer Post hoc test. #P<0.01, n=4–6. Scale bar=20 μM.
Discussion
Cell therapy following MI has emerged as a promising therapeutic treatment, as evidenced by the number of ongoing clinical trials. Despite the early clinical successes, a major impediment to progenitor cell implantation is the survival and differentiation/maturation of implanted cells in the damaged tissue. Both animal and clinical studies have shown that a significant majority of the implanted cells were not viable following the therapy [9,11]. The current paradigm is that implanted cells such as CPCs have paracrine actions that aid in the preservation or regeneration of the damaged myocardium. Preconditioning of cells has emerged as a viable adjuvant to improving the efficacy of cell therapy. We have previously shown that H2O2 is significantly elevated in the myocardium following I/R injury [24]. Despite this, the role of oxidative stress on CPC survival and differentiation has not yet been studied.
Various methods to precondition cells have been used, such as growth factor exposure as well as modulating the redox levels of the cells through hypoxia and/or oxidative stress. Yan et al. [25] exposed CPCs to hypoxic conditions in vitro, and found that apoptosis was not increased at early time points and Akt phosphorylation was increased due to hypoxia in cKit+ CPCs. In contrast, endothelial progenitor cells exposed to H2O2 demonstrated decreased survival and function, indicating that the response to ROS is cell specific [26]. In our study, acute H2O2 treatment protected CPCs from serum withdrawal-induced apoptosis, while the cells were allowed to differentiate in culture. CPCs acutely treated with H2O2 were protected from apoptosis comparable to studies examining other progenitor and stem cells [27]. The 100 μM H2O2 treatment, which is thought to be more pathophysiological, was protective at both 2 and 5 days compared to untreated CPCs. Other cells increase oxygen scavenging enzymes, like catalase and Gpx1, following hypoxic conditioning as an antiapoptotic mechanism [28]. However, our gene expression data indicated that catalase, Gpx1, Txn1, and Prdx6 were not increased in CPCs in response to H2O2. Our studies determined that 100 μM H2O2, the highest oxidant concentration tested, provided cytoprotection over the time course evaluated in the current study and did not cause an increase in antioxidative scavenging enzyme gene expression. Our preliminary studies showed that this response was not evident at lower doses of H2O2 (<1 μM) and that CPCs basally produce about 0.5–1 μM H2O2 in culture (data not shown). Previous results from our laboratory demonstrated that CPCs acutely exposed to superoxide increased gene and protein expression of superoxide dismutase enzyme isoforms as a compensatory mechanism, and this was in an Akt-dependent manner [20]. While a specific mechanism of enhanced survival was not investigated in this study, our prior results with Akt and studies showing that H2O2 can induce Akt activation may be an area for future studies. Despite this, our data imply that the enhanced survival is not a result of increased scavenging mechanisms as has been shown for other progenitor cells [20,27].
CPCs can differentiate into endothelial, SM cells, and cardiomyocytes, however, the effect of oxidative stress on CPC differentiation is unknown. It is known that bone marrow-derived mesenchymal stem cells migrate to sites of injury and form vascular structures following MI and hindlimb ischemia [29,30]. Previous reports in bone marrow-derived mesenchymal stem cells demonstrated that hypoxic preconditioning stimulated a proangiogenic phenotype to a greater extent than nontreated cells in in vitro studies [31]. When these hypoxia-preconditioned bone marrow-derived mesenchymal stem cells were injected into a model of hindlimb ischemia, the cells induced angiogenesis, leading to an improvement in regional blood flow. In CPCs, H2O2 treatment for 2 days caused a significant increase in gene expression of early endothelial markers, such as Kdr and Pecam1, compared to untreated CPCs. However, chronic exposure to 100 μM H2O2, but not 1 μM led to a marked decrease in both genes. Su et al. [32] found that H2O2 directed the differentiation of immature proliferating human vascular SM cells toward a mature contracting vascular SM cell phenotype with an increase in SM genes and proteins. Interestingly, in CPCs both Tagln and Acta2 were significantly increased following 2 days of 100 μM H2O2; however, chronic H2O2 treatment lead to an inhibition of the genes below that of untreated differentiating CPCs. Future studies will determine if this increase is mediated by VEGF release into the media similar to [30], although our media was replaced daily with fresh media containing more H2O2 and this may be a confounding factor.
The effect of H2O2 treatment on embryonic stem cell differentiation into cardiomyocytes has been explored previously [18,19]. These studies indicated that an acute incubation period of H2O2 (6 h; <1 μM) stimulated the PI3 kinase-dependent pathway and nicotinamide adenine dinucleotide phosphate (NOX) enzymes, leading to increased expression of cardiomyocyte proteins and contracting cells. We found that low concentrations of H2O2 acutely during CPC differentiation produced significantly higher mRNA levels for only Troponin T compared to nondifferentiated CPCs with no increase in Troponin C (Supplementary Fig. 3). Moreover, no increase in gene expression for the early markers Gata4 and Nkx2-5 was observed following 2 days of treatment (Supplementary Fig. 4). Prolonged exposure of CPCs to 100 μM H2O2 treatments caused a significant decrease in Troponin C gene expression, indicating potential negative effects of prolonged H2O2 exposure on cardiogenic differentiation, although this was the only gene to show this response. These data are in contrast to both endothelial and SM cell gene expressions that show sensitivity to H2O2 treatment both acutely and chronically.
As our in vitro data suggested that acute H2O2 (100 μM) treatment directed CPCs to an antiapoptotic, provascular differentiation, we sought to determine whether H2O2 preconditioning of CPCs would improve cardiac function following MI. Luciferase-transduced CPCs suspended in self-assembling nanofiber hydrogels were injected into the peri-infarct zone following a 30-min I/R model of myocardial injury. Peptide hydrogels were used to enhance retention in the myocardium and eliminate the retention of cells as a potential confounding variable as we did not measure adhesion previously. Prior studies using the self-assembling peptide nanofibers formed from RAD-II showed that hydrogel injection without cells into the myocardium did not improve cardiac function following I/R [5,21]. Additionally, while it has been shown that implantation of nondifferentiated CPCs following I/R with the same self-assembling hydrogels did improve left ventricular function, this response was enhanced by codelivery of the insulin-like growth factor-1 indicating the potential for improved cell therapy with additional stimuli [5]. In the present studies, 28 days after CPC implantation, we evaluated whether cardiac function was improved through the use of invasive catheterization in the left ventricle. The ejection fraction was decreased after I/R, and both groups that received CPC therapy showed a modest, but not significant improvement. We also determined additional indices of cardiac function, such as positive and negative dP/dT, as well as left ventricular volume and pressure. Only hearts treated with 100 μM H2O2 preconditioned cells significantly improved the maximal contraction velocity as compared to I/R alone, with values similar to the sham group, while hearts treated with control CPCs had a significantly lower velocity compared to sham hearts with no improvement over I/R alone. Negative dP/dT velocities exhibited a significant improvement in both of the CPC-treated heart groups compared to I/R hearts. Furthermore, we found that the increase of ventricular volumes in I/R hearts was abolished following implantation of CPCs with no effect of preconditioning. Also, end-systolic pressure was significantly higher in both CPC-treated hearts compared to I/R hearts, and the 100 μM H2O2 preconditioned CPC-treated group was similar to sham animals. However, rats treated with 100 μM H2O2 preconditioned CPCs demonstrated a significant improvement in end-diastolic pressure over control CPC-treated I/R hearts. These data suggest that there may be improvements in certain measures of heart function with H2O2 preconditioned CPCs, specifically those related to diastolic contractility/relaxation, but these did not translate to global changes in the ejection fraction. While this may initially seem confusing, some prior studies have demonstrated that changes in function may be more apparent at longer time points [33–35]. Moreover, the fact that H2O2 preconditioned CPCs are not generating large amounts of new myocytes may also contribute to this phenomenon. As the present studies were terminated at 28 days to ensure that changes in implanted CPCs could be measured, future studies will examine whether functional improvements are enhanced at longer time points.
We used immunohistochemistry to determine whether there were underlying structural changes in the myocardial architecture. Prior studies demonstrate that implanted cells are capable of reducing collagen deposition and fibrosis following I/R [34,36]. We assessed cardiac fibrosis using picrosirius red staining, and found that while there was no effect of control CPC-treated hearts on cardiac fibrosis, CPCs preconditioned with 100 μM H2O2 had significantly less myocardial collagen deposition compared to I/R alone or I/R treated with control CPCs. As fibrosis and angiogenesis are often linked, and our in vitro results showed changes in endothelial gene expression, we measured endothelial cells in the infarcted tissue. We found significantly higher numbers of isolectin-positive cells and a trend for a higher density of large vessels in hearts treated with H2O2 preconditioned CPCs compared to hearts treated with control cells. Additionally, there was a significant increase in double-positive vessels localized in the infarct area of hearts treated with H2O2 preconditioned cells compared to hearts that received control CPCs. Interestingly, we found no difference in double-positive endothelial cells, indicating that the H2O2 preconditioned cells attracted more endogenous endothelial cells. Another explanation is that the double-positive cells were much more quickly incorporated into vessels (or formed new vessels) than control CPCs. These results indicate that the preconditioning of CPCs with H2O2 induce an in vivo angiogenic effect that may be mediated through the paracrine actions, although we did not measure this. A recent study demonstrated the ability of bone marrow-derived c-Kit+ cells, but not mesenchymal stem cells, to induce endogenous neovascularization following I/R, although the mechanism was not fully elucidated [37,38]. In our study, whether the improvement in fibrosis led to improvements in angiogenesis, or vice versa, was not determined, but remains an interesting potential area for investigation.
In summary, we found that acute H2O2 preconditioning of c-kit+ CPCs stimulates endothelial and SM gene expression, while chronic H2O2 exposure reverses these trends. Additionally, preconditioning of CPCs with H2O2 enhances the angiogenic response following I/R injury and reduces fibrosis, leading to improvements in certain measures of cardiac function. Given the current status of CPC therapy in early clinical trials, this may lead to future studies of H2O2 preconditioning as a potential therapeutic avenue in patients.
Supplementary Material
Acknowledgments
This work was supported by grant HL094527 to M.E.D. from the National Heart, Lung, and Blood Institute, a Merck/United Negro College Fund postdoctoral fellowship UNCF/Merck Science Initiative as well as a National Institutes of Health LRP to K.D.P. 1 L60 MD006426-01, and an American Heart Association predoctoral fellowship 11PRE7840078 to A.V.B. Additionally, support was received from a T32 Research in Academic Cardiology training grant HL007745. The authors wish to acknowledge the assistance of the Emory University Microscopy in Medicine Core facility (MiMCORE) under the direction of Dr. Lula Hilenski (National Institutes of Health, National Heart, Lung, and Blood Institute PO1 HL095070).
Author Disclosure Statement
The authors have no conflicts to report.
References
- 1.Roger VL. Go AS. Lloyd-Jones DM. Benjamin EJ. Berry JD. Borden WB. Bravata DM. Dai S. Ford ES, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kang HJ. Kim HS. Zhang SY. Park KW. Cho HJ. Koo BK. Kim YJ. Soo Lee D. Sohn DW, et al. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: the MAGIC cell randomised clinical trial. Lancet. 2004;363:751–756. doi: 10.1016/S0140-6736(04)15689-4. [DOI] [PubMed] [Google Scholar]
- 3.Assmus B. Schachinger V. Teupe C. Britten M. Lehmann R. Dobert N. Grunwald F. Aicher A. Urbich C, et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) Circulation. 2002;106:3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 4.Bearzi C. Rota M. Hosoda T. Tillmanns J. Nascimbene A. De Angelis A. Yasuzawa-Amano S. Trofimova I. Siggins RW, et al. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Padin-Iruegas ME. Misao Y. Davis ME. Segers VF. Esposito G. Tokunou T. Urbanek K. Hosoda T. Rota M, et al. Cardiac progenitor cells and biotinylated insulin-like growth factor-1 nanofibers improve endogenous and exogenous myocardial regeneration after infarction. Circulation. 2009;120:876–887. doi: 10.1161/CIRCULATIONAHA.109.852285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smits AM. van Laake LW. den Ouden K. Schreurs C. Szuhai K. van Echteld CJ. Mummery CL. Doevendans PA. Goumans MJ. Human cardiomyocyte progenitor cell transplantation preserves long-term function of the infarcted mouse myocardium. Cardiovasc Res. 2009;83:527–535. doi: 10.1093/cvr/cvp146. [DOI] [PubMed] [Google Scholar]
- 7.Steele A. Jones OY. Gok F. Marikar Y. Steele P. Chamizo W. Scott M. Boucek RJ., Jr. Stem-like cells traffic from heart ex vivo, expand in vitro, and can be transplanted in vivo. J Heart Lung Transplant. 2005;24:1930–1939. doi: 10.1016/j.healun.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Tillmanns J. Rota M. Hosoda T. Misao Y. Esposito G. Gonzalez A. Vitale S. Parolin C. Yasuzawa-Amano S, et al. Formation of large coronary arteries by cardiac progenitor cells. Proc Natl Acad Sci U S A. 2008;105:1668–1673. doi: 10.1073/pnas.0706315105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolli R. Chugh AR. D'Amario D. Loughran JH. Stoddard MF. Ikram S. Beache GM. Wagner SG. Leri A, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Chugh AR. Beache GM. Loughran JH. Mewton N. Elmore JB. Kajstura J. Pappas P. Tatooles A. Stoddard MF, et al. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126:S54–S64. doi: 10.1161/CIRCULATIONAHA.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Z. Lee A. Huang M. Chun H. Chung J. Chu P. Hoyt G. Yang P. Rosenberg J. Robbins RC. Wu JC. Imaging survival and function of transplanted cardiac resident stem cells. J Am Coll Cardiol. 2009;53:1229–1240. doi: 10.1016/j.jacc.2008.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma RK. Zhou Q. Netland PA. Effect of oxidative preconditioning on neural progenitor cells. Brain Res. 2008;1243:19–26. doi: 10.1016/j.brainres.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 13.Bartosh TJ. Wang Z. Rosales AA. Dimitrijevich SD. Roque RS. 3D-model of adult cardiac stem cells promotes cardiac differentiation and resistance to oxidative stress. J Cell Biochem. 2008;105:612–623. doi: 10.1002/jcb.21862. [DOI] [PubMed] [Google Scholar]
- 14.Li S. Deng Y. Feng J. Ye W. Oxidative preconditioning promotes bone marrow mesenchymal stem cells migration and prevents apoptosis. Cell Biol Int. 2009;33:411–418. doi: 10.1016/j.cellbi.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Guo YL. Chakraborty S. Rajan SS. Wang R. Huang F. Effects of oxidative stress on mouse embryonic stem cell proliferation, apoptosis, senescence, and self-renewal. Stem Cells Dev. 2010;19:1321–1331. doi: 10.1089/scd.2009.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao Q. Luo Z. Pepe AE. Margariti A. Zeng L. Xu Q. Embryonic stem cell differentiation into smooth muscle cells is mediated by Nox4-produced H2O2. Am J Physiol Cell Physiol. 2009;296:C711–C723. doi: 10.1152/ajpcell.00442.2008. [DOI] [PubMed] [Google Scholar]
- 17.Song H. Cha MJ. Song BW. Kim IK. Chang W. Lim S. Choi EJ. Ham O. Lee SY, et al. Reactive oxygen species inhibit adhesion of mesenchymal stem cells implanted into ischemic myocardium via interference of focal adhesion complex. Stem Cells. 2010;28:555–563. doi: 10.1002/stem.302. [DOI] [PubMed] [Google Scholar]
- 18.Buggisch M. Ateghang B. Ruhe C. Strobel C. Lange S. Wartenberg M. Sauer H. Stimulation of ES-cell-derived cardiomyogenesis and neonatal cardiac cell proliferation by reactive oxygen species and NADPH oxidase. J Cell Sci. 2007;120:885–894. doi: 10.1242/jcs.03386. [DOI] [PubMed] [Google Scholar]
- 19.Sauer H. Rahimi G. Hescheler J. Wartenberg M. Role of reactive oxygen species and phosphatidylinositol 3-kinase in cardiomyocyte differentiation of embryonic stem cells. FEBS Lett. 2000;476:218–223. doi: 10.1016/s0014-5793(00)01747-6. [DOI] [PubMed] [Google Scholar]
- 20.Seshadri G. Che PL. Boopathy AV. Davis ME. Characterization of superoxide dismutases in cardiac progenitor cells demonstrates a critical role for manganese superoxide dismutase. Stem Cells Dev. 2012;21:3136–3146. doi: 10.1089/scd.2012.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis ME. Motion JP. Narmoneva DA. Takahashi T. Hakuno D. Kamm RD. Zhang S. Lee RT. Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells. Circulation. 2005;111:442–450. doi: 10.1161/01.CIR.0000153847.47301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seshadri G. Sy JC. Brown M. Dikalov S. Yang SC. Murthy N. Davis ME. The delivery of superoxide dismutase encapsulated in polyketal microparticles to rat myocardium and protection from myocardial ischemia-reperfusion injury. Biomaterials. 2010;31:1372–1379. doi: 10.1016/j.biomaterials.2009.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bradley JM. Nguyen JB. Fournett AC. Gardner JD. Cigarette smoke exacerbates ventricular remodeling and dysfunction in the volume overloaded heart. Microsc Microanal. 2012;18:91–98. doi: 10.1017/S1431927611012207. [DOI] [PubMed] [Google Scholar]
- 24.Pendergrass KD. Varghese ST. Maiellaro-Rafferty K. Brown ME. Taylor WR. Davis ME. Temporal effects of catalase overexpression on healing after myocardial infarction. Circ Heart Fail. 2011;4:98–106. doi: 10.1161/CIRCHEARTFAILURE.110.957712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan F. Yao Y. Chen L. Li Y. Sheng Z. Ma G. Hypoxic preconditioning improves survival of cardiac progenitor cells: role of stromal cell derived factor-1alpha-CXCR4 axis. PLoS One. 2012;7:e37948. doi: 10.1371/journal.pone.0037948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ingram DA. Krier TR. Mead LE. McGuire C. Prater DN. Bhavsar J. Saadatzadeh MR. Bijangi-Vishehsaraei K. Li F. Yoder MC. Haneline LS. Clonogenic endothelial progenitor cells are sensitive to oxidative stress. Stem Cells. 2007;25:297–304. doi: 10.1634/stemcells.2006-0340. [DOI] [PubMed] [Google Scholar]
- 27.Dernbach E. Urbich C. Brandes RP. Hofmann WK. Zeiher AM. Dimmeler S. Antioxidative stress-associated genes in circulating progenitor cells: evidence for enhanced resistance against oxidative stress. Blood. 2004;104:3591–3597. doi: 10.1182/blood-2003-12-4103. [DOI] [PubMed] [Google Scholar]
- 28.Aly A. Peterson K. Lerman A. Lerman L. Rodriguez-Porcel M. Role of oxidative stress in hypoxia preconditioning of cells transplanted to the myocardium: a molecular imaging study. J Cardiovasc Surg (Torino) 2011;52:579–585. [PMC free article] [PubMed] [Google Scholar]
- 29.Musialek P. Tekieli L. Kostkiewicz M. Majka M. Szot W. Walter Z. Zebzda A. Pieniazek P. Kadzielski A, et al. Randomized transcoronary delivery of CD34(+) cells with perfusion versus stop-flow method in patients with recent myocardial infarction: early cardiac retention of (9)(9)(m)Tc-labeled cells activity. J Nucl Cardiol. 2011;18:104–116. doi: 10.1007/s12350-010-9326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kubo M. Li TS. Kurazumi H. Takemoto Y. Ohshima M. Murata T. Katsura S. Morikage N. Furutani A. Hamano K. Hypoxic preconditioning enhances angiogenic potential of bone marrow cells with aging-related functional impairment. Circ J. 2012;76:986–994. doi: 10.1253/circj.cj-11-0605. [DOI] [PubMed] [Google Scholar]
- 31.Chacko SM. Ahmed S. Selvendiran K. Kuppusamy ML. Khan M. Kuppusamy P. Hypoxic preconditioning induces the expression of prosurvival and proangiogenic markers in mesenchymal stem cells. Am J Physiol Cell Physiol. 2010;299:C1562–C1570. doi: 10.1152/ajpcell.00221.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su B. Mitra S. Gregg H. Flavahan S. Chotani MA. Clark KR. Goldschmidt-Clermont PJ. Flavahan NA. Redox regulation of vascular smooth muscle cell differentiation. Circ Res. 2001;89:39–46. doi: 10.1161/hh1301.093615. [DOI] [PubMed] [Google Scholar]
- 33.Mohsin S. Khan M. Toko H. Bailey B. Cottage CT. Wallach K. Nag D. Lee A. Siddiqi S, et al. Human cardiac progenitor cells engineered with pim-I kinase enhance myocardial repair. J Am Coll Cardiol. 2012;60:1278–1287. doi: 10.1016/j.jacc.2012.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carr CA. Stuckey DJ. Tan JJ. Tan SC. Gomes RS. Camelliti P. Messina E. Giacomello A. Ellison GM. Clarke K. Cardiosphere-derived cells improve function in the infarcted rat heart for at least 16 weeks—an MRI study. PLoS One. 2011;6:e25669. doi: 10.1371/journal.pone.0025669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee ST. White AJ. Matsushita S. Malliaras K. Steenbergen C. Zhang Y. Li TS. Terrovitis J. Yee K, et al. Intramyocardial injection of autologous cardiospheres or cardiosphere-derived cells preserves function and minimizes adverse ventricular remodeling in pigs with heart failure post-myocardial infarction. J Am Coll Cardiol. 2011;57:455–465. doi: 10.1016/j.jacc.2010.07.049. [DOI] [PubMed] [Google Scholar]
- 36.Dai B. Huang W. Xu M. Millard RW. Gao MH. Hammond HK. Menick DR. Ashraf M. Wang Y. Reduced collagen deposition in infarcted myocardium facilitates induced pluripotent stem cell engraftment and angiomyogenesis for improvement of left ventricular function. J Am Coll Cardiol. 2011;58:2118–2127. doi: 10.1016/j.jacc.2011.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li TS. Cheng K. Malliaras K. Smith RR. Zhang Y. Sun B. Matsushita N. Blusztajn A. Terrovitis J, et al. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol. 2012;59:942–953. doi: 10.1016/j.jacc.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loffredo FS. Steinhauser ML. Gannon J. Lee RT. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell. 2011;8:389–398. doi: 10.1016/j.stem.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.