Abstract
Objective
The purpose of this study was to assess lisdexamfetamine dimesylate (LDX) treatment effects based on baseline emotional control dysfunction in children with attention-deficit/hyperactivity disorder (ADHD) categorized with or without impairments of executive function (EF) emotional control.
Methods
Post-hoc analyses of a 7 week, open-label LDX study in children with ADHD (American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision [DSM-IV-TR] defined) and impairments in EF control of emotional response. At baseline, participants were dichotomized by Behavior Rating Inventory of EF (BRIEF) emotional control domain T-scores of ≥65 (with impairment) or <65 (without impairment). ADHD Rating Scale-IV (ADHD-RS-IV), BRIEF Global Executive Composite and emotional control domain, Expression and Emotion Scale for Children (EESC) scores, Pearson correlations for BRIEF versus ADHD-RS-IV and EESC, and Clinical Global Impressions scores were assessed at baseline and end of study (week 7)/early termination (EOS/ET) by baseline category of BRIEF emotional control impairment. Safety assessments included treatment-emergent adverse events (TEAEs).
Results
At baseline and EOS/ET, respectively, 53.0% and 20.7% met criteria for emotional control impairment. Participants with and without emotional control impairments had similar ADHD-RS-IV change scores. Mean (SD) change from baseline for those with and without emotional control impairments were −20.8 (12.89) and −14.6 (11.25) for BRIEF global scores and −16.0 (13.19) and −5.0 (9.48) for BRIEF emotional control domain scores. Participants with emotional control impairments had greater mean EESC total score changes. BRIEF emotional control domain and all ADHD-RS-IV scores indicated moderate correlations between change scores (all p<0.0001). Overall, 84.9% of participants had TEAEs (mostly mild-to-moderate in severity); 3.8% discontinued because of TEAEs.
Conclusions
The proportion of children with behavioral impairments in EF control of emotional response decreased during LDX treatment. ADHD symptoms improved in both groups. The moderate correlations between EF behaviors and ADHD symptoms suggest there may be utility in evaluating behavioral domains beyond core ADHD symptoms.
Introduction
Although attention-deficit/hyperactivity disorder (ADHD) is commonly conceptualized as a behavioral disorder, it is also associated with significant difficulties in emotional control (Strine et al. 2006). Behavioral aspects of emotional expression can be positive (e.g., happiness, creativity, confidence) and negative (e.g., temper outbursts, mood lability, dysphoria) (Perwien et al. 2008; Findling et al. 2009). Using a parent-reported strengths and difficulties questionnaire, the 2003 National Health Information Survey found that emotional expression in children and adolescents (ages 4–17 years) with ADHD is often more labile than that of those without ADHD (23.0% vs 6.3%, respectively) (Strine et al. 2006).
Sobanski and colleagues reported that the core symptoms of ADHD, especially hyperactivity/impulsivity symptoms, were more pronounced with increasing severity of emotional lability (Sobanski et al. 2010). However, the cause of emotional difficulties in patients with ADHD is not well understood. Emotional symptoms and deficits in emotional control may be integral to ADHD (Barkley 2010; Barkley and Murphy 2010). Conversely, they may also be related to comorbid disorders (Biederman et al. 1996) and/or treatment (psychostimulants) effects (American Academy of Pediatrics Subcommittee on Attention-Deficit/Hyperactivity Disorder and Committee on Quality Improvement 2001; Kratochvil et al. 2007).
Recently, validated clinical questionnaires have been used to assess aspects of emotional expression in ADHD. The Expression and Emotion Scale for Children (EESC) (Perwien et al. 2008) is a validated measure of emotional expression in children. The Behavior Rating Inventory of Executive Function (BRIEF) (Gioia et al. 2000) is a validated measure of executive function (EF) in children and includes an emotional control subscale. This subscale assesses EF behavioral control over emotional expression. A study using the parent-rated BRIEF found that preschoolers with ADHD were rated as having significantly higher T-scores versus controls on BRIEF subscales, including the emotional control subscale (Mahone and Hoffman 2007). Moreover, difficulties in self-regulation of affect or emotion may be another key feature of children with ADHD. They are commonly described by their parents, teachers, and clinicians as having more pronounced highs and lows in emotional expression and regulation, in addition to core ADHD symptoms (Anastopoulos et al. 2010).
Psychostimulants have been reported to improve or worsen emotional symptoms (Ahmann et al. 1993; Gillberg et al. 1997; Short et al. 2004; Katic et al. 2012). Lisdexamfetamine dimesylate (LDX), a long-acting prodrug psychostimulant, is indicated for ADHD treatment in children (6–12 years of age), adolescents (13–17 years of age), and adults (Vyvanse package insert 2012). The EESC, which assessed emotional symptoms, and the BRIEF, which assessed emotional control, were secondary outcome measures in a 7 week, open-label, dose-optimization study of LDX (Findling et al. 2009) in children (6–12 years of age) with ADHD. LDX demonstrated small but statistically significant improvements in emotional lability on the clinician-administered, parent/caregiver-reported EESC total and subscale (positive emotions, emotional flatness, and emotional lability) scores (Findling et al. 2009).
In a further post-hoc analysis of EESC findings, LDX treatment was not associated with overall clinical worsening of emotional expression in most children (Katic et al. 2012). The majority of participants showed no meaningful change in EESC scores. However, more participants were categorized as improved, with an end of study/early termination (EOS/ET) score ≤1 standard error of measurement (SEM) of baseline score, than were categorized as worsened, with an EOS/ET score ≥1 SEM of baseline score.
The aim of the current post-hoc analyses of this 7 week, open-label study (Findling et al. 2009) was to assess treatment outcomes based on participants' status as having, or not having, impaired EF behavioral control of emotional expression. ADHD symptoms, BRIEF Global Executive Composite (GEC) scores, EESC scores, and Clinical Global Impressions (CGI) scores were assessed in children with or without emotional control impairments based on the baseline and EOS/ET BRIEF emotional control classifications.
Methods
Study design
Study methodology was previously reported in detail (Findling et al. 2009). Post-hoc analyses of a 7 week, open-label, multicenter dose-optimization study of LDX (20–70 mg/d) in children with a primary ADHD diagnosis were conducted. The EESC can be used in a clinical setting to determine changes in emotional expression in children receiving pharmacotherapy for ADHD (Kratochvil et al. 2007). However, it is difficult to use the EESC to categorize patients by poor or normal emotional expression, as the EESC lacks established criteria to dichotomize patients by level of emotional expression (poor vs. normal). The variance for EESC scores is large also. However, the BRIEF scale possesses criteria to characterize these differences. For these reasons, the current analysis used the BRIEF scale to dichotomize children as being with or without impairments in EF behavioral control of emotional response prior to treatment. LDX treatment effects were also assessed based on these groups dichotomized by baseline dysfunction in emotional control.
This study was conducted in accordance with current applicable regulations, with International Conference on Harmonization of Good Clinical Practice and local ethical and legal requirements. The study also complied with the principles of the 18th World Medical Assembly (Helsinki 1964) and amendments of the 29th (Tokyo 1975), 35th (Venice 1983), 41st (Hong Kong 1989), and 48th (South Africa 1996) World Medical Assemblies, Declaration of Helsinki. The study protocol and amendments, as well as the parent/legally authorized representative's informed consent and the participant's assent forms, were submitted in writing to the Institutional Review Board/Independent Ethics Committee and approved.
Key inclusion/exclusion criteria
Study participants were children (6–12 years of age) with a primary ADHD diagnosis by Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision (DSM-IV-TR) (American Psychiatric Association 2000) criteria and a baseline ADHD Rating Scale IV (ADHD-RS-IV) total score of ≥28 (DuPaul et al. 1998). Participants exhibited age-appropriate intellectual functioning. They had blood pressure measurements within the 95th percentile for age, sex, and height at screening and/or baseline.
Exclusion criteria included: comorbid conduct disorder; most Axis I or II psychiatric diagnoses; a history of seizures or tic disorders; abnormal thyroid function; any condition that affects cardiac performance; a concurrent chronic or acute illness or unstable medical condition; and a documented allergy, hypersensitivity, or intolerance to amphetamines. Participants taking other medications that have central nervous system effects or affect performance, such as sedating antihistamines, were excluded. Also excluded were those whose ADHD symptoms were well controlled on their current ADHD medication with acceptable tolerability.
Efficacy measures
The primary efficacy measure (ADHD-RS-IV) was evaluated at baseline (week 0) and at each postbaseline weekly visit through the EOS/ET. Of the secondary efficacy assessments in the present analysis, the BRIEF and EESC were evaluated at baseline and EOS/ET. The CGI-Severity (CGI-S) scale was evaluated at baseline and the CGI-Improvement (CGI-I) scale weekly thereafter to EOS/ET. Description of these assessments follows.
Primary efficacy measure: Clinician-reported ADHD-RS-IV
Primary efficacy data, using the clinician-reported ADHD-RS-IV (primary efficacy measure), has been previously reported in detail (Findling et al. 2009). The ADHD-RS-IV is an 18 item scale designed to reflect current ADHD symptomatology. Based on DSM-IV-TR ADHD criteria, the ADHD-RS-IV has 18 symptom items that include 9 inattention and 9 hyperactivity/impulsivity items. Each item is scored from 0 (never or rarely) to 3 (very often), with a total possible score ranging from 0 to 54. Higher scores indicate greater ADHD symptom severity (DuPaul et al. 1998; Faries et al. 2001).
Parent-reported BRIEF
The BRIEF is a parent-reported assessment of EF behavioral impairments, using discrete, observable behaviors. It is categorized into eight domains: inhibition, shifting, emotional control, initiation, working memory, planning/organizing, organizing materials, and monitoring (Gioia et al. 2000). The emotional control domain is a 10 item subscale measuring EF control over behaviors reflecting emotional response. Items include such behaviors as “Has explosive, angry outbursts,” or “Becomes tearful easily.”
The BRIEF is scored using T-scores. The normative population T-score mean is 50 and the standard deviation (SD) is 10. A T-score ≥1.5 SD of the normative mean (≥65) can be used to characterize someone as impaired. At baseline and EOS/ET, participants were classified as either having (T-score ≥65) or not having (T-score <65) impairments in the BRIEF emotional control domain.
Clinician-administered, parent-reported EESC
The clinician-administered EESC is a 29 item measure assessing parents' report of positive and negative aspects of emotion and expression (Perwien et al. 2008). At baseline and EOS/ET, the EESC (original version) was completed. For the EESC, 29 items assess positive (e.g., confidence and friendliness) and negative (e.g., mood swings and flat mood) aspects of emotional expression over the preceding 2 weeks of medication use. These items are scored from 1 (not at all true) to 5 (very much true), with an EESC total score range of 29–145 (Perwien et al. 2008). The EESC has three subscales: Positive emotions (range of scores, 13–65), emotional flatness (range of scores, 10–50), and emotional lability (range of scores, 5–25). Responses to the positive emotions subscale are reversed during scoring so that, on all scales, higher scores indicate poorer emotional expression (Perwien et al. 2008).
CGI
The clinician-rated CGI-S (Guy 1976), completed at baseline, allows for a global evaluation of the patient's illness severity level, using a seven point scale from 1 (normal, not at all ill) to 7 (among the most extremely ill). The clinician-reported CGI-I scale, which was completed postbaseline, rated a participant's global improvement over time. The CGI-I is based on a seven point scale, ranging from 1 (very much improved) to 7 (very much worse). Post-hoc analyses were conducted to assess mean baseline CGI-S for participants with and without BRIEF emotional control impairments at baseline and EOS/ET. The CGI-I evaluated those with and without BRIEF emotional control impairments at EOS/ET.
Statistical analysis
ADHD-RS-IV, BRIEF GEC, and EESC scores at baseline and EOS/ET were examined by BRIEF emotional control baseline classifications. Because of the post-hoc nature of these analyses and the relatively small subgroups, no comparative statistical analyses of dichotomized emotional control categories were performed between groups. However, summary statistics were generated.
Post-hoc Pearson product-moment correlation was used to assess the linear relationship between BRIEF emotional control domain scores and ADHD-RS-IV scores, and between BRIEF emotional control scores and EESC scores at baseline, at EOS/ET, and for change from baseline to EOS/ET. Participants with both baseline and EOS/ET assessments were included in these analyses. Pearson positive correlations range from 0.1 to 0.3 for small correlations, 0.3 to 0.5 for moderate correlations, and 0.5 to 1.0 for strong correlations. Efficacy data in the present analysis are reported as mean (SD) values.
Safety
Safety measures have been previously reported in detail and included treatment-emergent adverse events (TEAEs) (Findling et al. 2009). As the present analysis is focused on emotional lability, additional reporting of TEAEs relating to emotional lability are presented.
Results
Disposition, baseline demographics
Detailed disposition and baseline demographics were previously reported for the overall population (Findling et al. 2009). In summary, 317 of 318 (99.7%) participants enrolled in the overall population were included in the safety population, 316 (99.4%) were included in the intention-to-treat (ITT) population, and 278 (87.4%) completed the study. Forty participants were discontinued. Most participants in the safety population were male (70.7%), non-Hispanic/non-Latino (82.6%), white (70.7%), and of the combined ADHD subtype (81.7%).
There were 315 participants dichotomized by BRIEF emotional control at baseline for ADHD-RS-IV total scores, ADHD-RS-IV inattention and hyperactivity/impulsivity subscale scores, and the BRIEF GEC and emotional control subscale T-score assessments. For the EESC total score assessment, 312 participants were dichotomized by baseline BRIEF emotional control category.
Clinician-reported ADHD-RS-IV
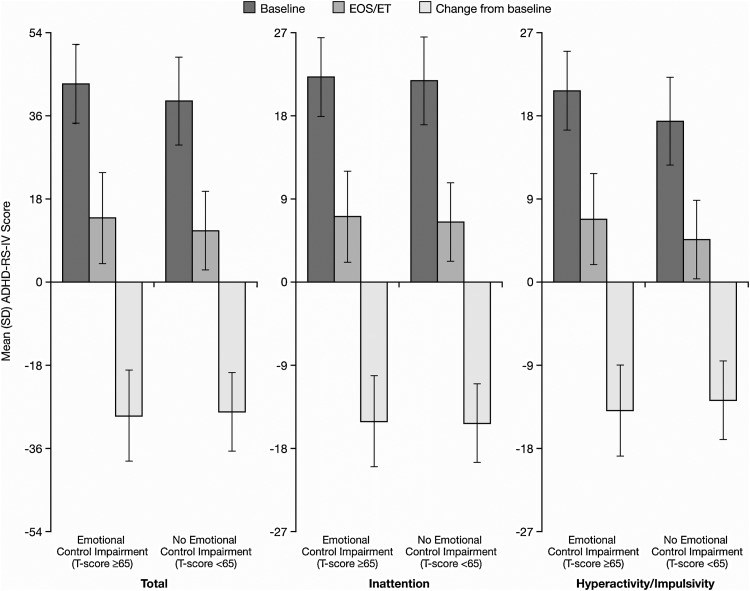
Among participants with baseline and EOS/ET assessments for ADHD-RS-IV total and subscale scores, 167 of 315 children (53.0%) had impairments, and 148 (47.0%) did not have impairments in BRIEF emotional control at baseline. The mean (SD) change scores from baseline to EOS/ET in ADHD-RS-IV total and subscale scores were similar for participants with and without impairments in BRIEF emotional control (Fig. 1).
Fig. 1.
ADHD-RS-IV total and subscale scores by baseline BRIEF emotional control T-score category. ADHD-RS-IV, Attention-Deficit/Hyperactivity Disorder Rating Scale IV; EOS/ET, end of study/early termination.
Parent-reported BRIEF
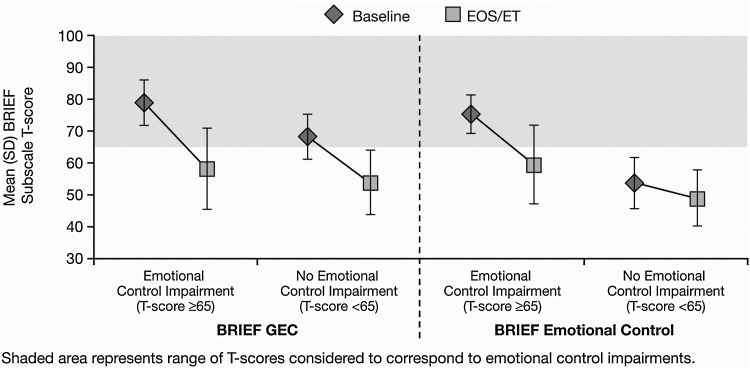
Participants categorized as having emotional control impairments had a baseline mean (SD) BRIEF GEC T-score of 78.9 (7.20), which was numerically greater than that of children without emotional control impairments (68.4 [7.24]). Improvement was observed in both groups at EOS/ET, with a mean (SD) GEC T-score of 58.1 (12.95) and 53.7 (10.28) for those with and without emotional control impairments, respectively. The impaired emotional control group demonstrated a greater mean (SD) numerical change at EOS/ET from baseline relative to the unimpaired emotional control group. Change scores were −20.8 (12.89) and −14.6 (11.25), respectively (Fig. 2).
Fig. 2.
Mean (SD) BRIEF GEC and BRIEF emotional control T-scores by baseline BRIEF emotional control T-score category at baseline and EOS/ET. BRIEF, Behavior Rating Inventory of Executive Function; EC, emotional control; EOS/ET, end of study/early termination; GEC, Global Executive Composite.
At baseline, the mean (SD) BRIEF emotional control subscale T-score for participants categorized with emotional control impairments (75.3 [6.16]) was numerically greater than for those not impaired (53.7 [8.17]). The mean (SD) T-score change from baseline at EOS/ET on the BRIEF emotional control domain was numerically greater for those with impaired emotional control (−16.0 [13.19]) than for those without emotional control impairments (−5.0 [9.48]).
Clinician-administered, parent-reported EESC
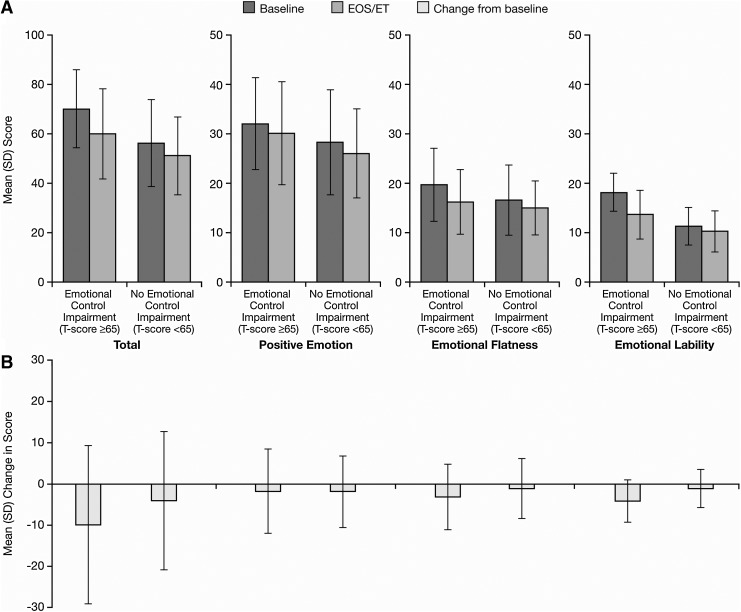
The EESC was recorded for 312 participants at baseline. When stratified by baseline BRIEF emotional control T-scores, 164 (52.6%) of these participants had emotional control impairments and 148 (47.4%) had no emotional control impairments. Mean baseline, EOS/ET, and change from baseline EESC total and subscale scores were generally numerically larger in the group of participants with (vs. without) baseline impairments by BRIEF emotional control scores (Fig. 3). No difference in mean change from baseline for EESC positive emotions subscale scores was noted between those with and those without baseline BRIEF emotional control impairments. The EESC was recorded for 305 children at EOS/ET. When stratified by EOS/ET BRIEF emotional control impairments, 63 (20.7%) children had emotional control impairments and 242 (79.3%) had no emotional control impairments.
Fig. 3.
Mean (SD) EESC total and subscale scores by baseline BRIEF emotional control T-score category at baseline and EOS/ET. BRIEF, Behavior Rating Inventory of Executive Function; EESC, Expression and Emotion Scale for Children; EOS/ET, end-of-study/early termination.
Pearson correlation coefficients
At baseline, Pearson correlation coefficients were moderate between the BRIEF emotional control domain and the ADHD-RS-IV total and hyperactivity/impulsivity subscale scores (Table 1). However, there was no significant correlation observed with BRIEF emotional control domain T-scores and ADHD-RS-IV inattention subscale scores (Table 1).
Table 1.
Pearson Correlation Between Baseline BRIEF Emotional Control Domain T-Scores and ADHD-RS-IV Scores (n=316)
| |
ADHD-RS-IV Score: Pearson correlation coefficient (p value) |
||
|---|---|---|---|
| BRIEF Emotional Control Subscale | Total | Inattention Subscale | Hyperactivity/Impulsivity Subscale |
| Baseline | 0.3159 | 0.0729 | 0.3314 |
| (<0.0001) | (0.1969) | (<0.0001) | |
| EOS/ET | 0.4717 | 0.4133 | 0.4553 |
| (<0.0001) | (<0.0001) | (<0.0001) | |
| Change from baseline | 0.4170 | 0.3425 | 0.3823 |
| (<0.0001) | (<0.0001) | (<0.0001) | |
ADHD-RS-IV, Attention-Deficit/Hyperactivity Disorder Rating Scale IV; BRIEF, Behavior Rating Inventory of Executive Function; EOS/ET, end of study/early termination.
Changes from baseline to EOS/ET showed moderate correlations for ADHD-RS-IV total score and inattention and hyperactivity/impulsivity subscale scores (all p<0.0001) versus the BRIEF emotional control domain T-scores. At baseline, weak-to-moderate correlations were seen between the BRIEF emotional control domain T-scores and EESC total, positive emotions, and emotional flatness scores (p≤0.0006). Emotional lability scores, however, were strongly correlated (∼0.7) (Table 2). Moderate correlation coefficients in change from baseline to EOS/ET were noted between the BRIEF emotional control domain T-scores and the EESC total, positive emotions, and emotional flatness scores (p<0.0001) (Table 2). Also, correlations were relatively strong (∼0.7) between the BRIEF emotional control domain T-scores and the EESC emotional lability scores in change from baseline to EOS/ET.
Table 2.
Pearson Correlation Between Baseline BRIEF Emotional Control Domain T-Scores and EESC Scores (n=316)
| |
EESC Score: Pearson correlation coefficient (p value) |
|||
|---|---|---|---|---|
| BRIEF Emotional Control Domain | Total | Positive emotions | Emotional flatness | Emotional lability |
| Baseline | 0.4167 | 0.1939 | 0.2262 | 0.7388 |
| (<0.0001) | (0.0006) | (<0.0001) | (<0.0001) | |
| EOS/ET | 0.5621 | 0.4040 | 0.3481 | 0.7726 |
| (<0.0001) | (<0.0001) | (<0.0001) | (<0.0001) | |
| Change from baseline | 0.4735 | 0.3008 | 0.2879 | 0.6691 |
| (<0.0001) | (<0.0001) | (<0.0001) | (<0.0001) | |
BRIEF, Behavior Rating Inventory of Executive Function; EESC, Expression and Emotion Scale for Children; EOS/ET, end of study/early termination.
CGI
As categorized by baseline BRIEF emotional control T-scores, the mean (SD) baseline CGI-S ratings, were 4.8 (0.69) for those participants with emotional control impairments (n=167) and 4.6 (0.68) for those without emotional control impairments (n=148). As categorized by EOS/ET BRIEF emotional control T-scores, the mean (SD) EOS/ET CGI-I ratings were 2.0 (1.02) for those with emotional control impairments (n=65) and 1.4 (0.65) for those without emotional control impairments (n=243).
Safety
The safety findings of this study have been previously reported in detail (Findling et al. 2009). The majority of study participants in the overall safety population (269/317; 84.9%) experienced TEAEs with LDX treatment (all doses). Most reported TEAEs were mild to moderate in severity, and TEAEs resulted in discontinuation for 12 of 317 (3.8%) participants. TEAEs reported by ≥10% of study participants with LDX treatment (all doses) included decreased appetite (43.2%), decreased weight (17.0%), insomnia (16.1%), irritability (16.1%), headache (13.9%), upper abdominal pain (13.2%), and initial insomnia (11.4%).
TEAEs reported by study participants that may have been related to emotional control impairment included irritability (16.1%), affect lability (7.3%), anxiety (1.3%), tearfulness (1.3%), aggression (0.9%), agitation (0.9%), flat affect (0.9%), mood swings (0.6%), restlessness (0.6%), crying (0.3%), dysphoria (0.3%), and nervousness (0.3%). Moreover, there were six participants who were discontinued because of these types of emotional control-related TEAEs. All six participants had baseline BRIEF GEC T-scores ≥65 (66, 70, 74, 77, 78, and 84), indicating impairment. Two participants, both treated with 20 mg/day LDX, were discontinued because of treatment-related, mild affect/emotional lability. For each event, safety concerns were resolved, as assessed by the investigator, within 1 week of treatment cessation (1 day and 4 days after participants were discontinued). Other TEAEs that were associated with emotional control and led to discontinuation included severe irritability (20 mg/day LDX), moderate irritability (30 mg/day LDX), moderate aggression and irritability (40 mg/day LDX), and mild aggression/temper outburst (20 mg/day LDX). All TEAEs but the last were considered resolved at EOS/ET as assessed by the investigator.
Discussion
A greater proportion of children with ADHD were categorized with BRIEF emotional control impairments prior to treatment with LDX (53.0%), than after 7 weeks of LDX treatment (20.7%). Improvements observed in ADHD symptoms were similar irrespective of BRIEF emotional control impairments at baseline. As might be expected, children with impairments in the BRIEF emotional control domain had higher initial BRIEF GEC T-scores than those without impairments. They also showed greater improvement, so that at EOS/ET, the two groups had similar GEC T-scores. Moreover, the large proportion of children with emotional control impairments at baseline suggests that an assessment of emotional status should be considered in addition to assessments of core ADHD symptoms when evaluating clinical outcomes.
Moderate correlations were noted between the BRIEF emotional control domain and all ADHD-RS-IV scores, EESC total, and most EESC subscale scores at baseline and EOS/ET. Correlations between BRIEF emotional control subscale T-scores and EESC emotional lability subscale scores were relatively higher compared with other domains and scores. These findings suggest a closer relationship for these aspects of emotional behavior. Global illness severity and improvement appeared similar for children categorized with and without BRIEF emotional control impairments.
Results from the present analysis were consistent with data from recent post-hoc analyses (Childress et al. 2012) of a double-blind, placebo-controlled 4 week LDX trial. This trial stratified children with ADHD into categories of “with” and “without” prominent emotional lability impairments at baseline (e.g., based on scores of ≤3 or >3 on Conners' Parent Rating Scale items of anger, loss of temper, and irritability). Childress and colleagues found that both groups had improved ADHD symptoms with LDX treatment versus placebo. However, emotional lability symptoms were significantly improved only in children with prominent emotional lability at baseline. In the present study, despite the greater improvement in BRIEF GEC T-scores in the subgroup of children with baseline emotional control impairments, both subgroups improved over the course of the study. Although participants without baseline emotional control impairments improved, their change scores were less than those of participants with baseline emotional control impairments. This difference was possibly the result of a floor effect (less room for improvement) for those without baseline emotional control impairments. Additionally, mean EOS/ET BRIEF T-scores for those with baseline emotional control impairments were within the normal range (i.e., without emotional control impairments). These scores, however, were still higher than the mean baseline scores of those without baseline BRIEF emotional control impairments.
The potential of floor effects to limit observed changes was also noted in a previous analysis of ADHD treatment effects on emotional expression using the EESC scale (Kratochvil et al. 2007). In a post-hoc analysis of EESC data sets based on the Jacobson and Truax method (Jacobson and Truax 1991), ≥99% of participants did not show emotional control impairments and method-defined, reliable change in emotional expression during LDX treatment (Katic et al. 2012).
In this study, LDX also demonstrated a safety profile generally consistent with that of long-acting psychostimulant use. No new, clinically relevant emotional control-related TEAEs were observed with LDX treatment in children with ADHD.
Limitations
Several limitations should be considered when evaluating data from this study. This was an exploratory study that used post-hoc assessments; therefore, it is important to consider that the results were not powered to show quantitative statistical effects. Other study constraints are the open-label design and the short study duration that limits the ability to extrapolate LDX effects on emotional lability over the long term. Also, the limited number of participants with comorbid disorders in this study may not be reflective of the general clinical population of patients with ADHD.
Conclusions
Children with ADHD showed improvement in emotional expression (by EESC scores) and ADHD symptoms (by ADHD-RS-IV scores) during LDX treatment regardless of BRIEF emotional control domain impairments at baseline. The proportion of children with impairments in EF control of emotional response decreased during LDX treatment. Moderate correlations were evident between global and emotional control domains of EF behaviors and ADHD symptoms, suggesting value in measuring additional outcomes beyond assessment of core ADHD symptoms.
Study results also demonstrated the overall utility of the BRIEF to measure emotional control impairments based on strong correlation with the EESC. Impaired emotional expression at baseline, as measured by EESC total and subscale scores, was more prominent in children with baseline BRIEF emotional control impairments than in those without. These data provide support for the BRIEF emotional control threshold T-score of ≥65 as an indication of emotional control impairment used in the current study.
Clinical Significance
The large proportion of children with emotional control impairments at baseline suggests that, when evaluating clinical outcomes, an assessment of emotional status by a clinician should be considered in addition to assessments for core ADHD symptoms. There may be only moderate overlap between ADHD treatment measures. Therefore, the use of a single outcome measure may not be sufficient to capture all relevant ADHD symptom domains and to measure clinically relevant treatment response. Additional assessments will allow clinicians to evaluate the overall treatment response that may not be fully observed with a single outcome measure.
Acknowledgments
Departments and institutions where work was done: Scottsdale, AZ, El Centro, CA, Rolling Hills Estates, CA, San Francisco, CA, Spring Valley, CA, Wildomar, CA, Jacksonville, FL, Gainesville, FL, Hialeah, FL, Maitland, FL, Miami, FL, Orlando, FL, West Palm Beach, FL, Libertyville, IL, Indianapolis, IN, Terre Haute, IN, Newton, KS, Overland Park, KS, Bardstown, KY, Lexington, KY, Owensboro, KY, Paducah, KY, Durham, NC, Rochester, NY, Cleveland, OH, Moore, OK, Oklahoma City, OK, Portland, OR, Salem, OR, Pittsburgh, PA, Jackson, TN, Memphis, TN, Austin, TX, Bellaire, TX, Houston, TX, Lake Jackson, TX, San Antonio, TX, Herndon, VA, Midlothian, VA, Richmond, VA, and Kirkland, WA.
Disclosures
Dr. Katic receives or has received research grant support from Abbott Laboratories, Alexza, AstraZeneca, Bristol-Myers Squibb, Cephalon, Corcept, Cyberonics, Dainippon Sumitomo, Eli Lilly, Forest, GlaxoSmithKline, Hisamitsu, Lundbeck, Merck, Novartis, Noven, Organon, Otsuka, Pfizer, Sanofi-Aventis, Sepracor, Shire, Takeda, Targacept, and Wyeth, and has served on a speaker's bureau for Novartis, Pfizer, and Shire. Dr. Dirks is an employee of Shire and holds stock and/or stock options in Johnson & Johnson and Shire. Dr. Babcock is an employee of Shire and holds stock and/or stock options in Shire. Dr. Scheckner is an employee of Shire and holds stock and/or stock options in Shire. Mr. Adeyi is an employee of Shire and holds stock and/or stock options in Shire. Dr. Richards is an employee of Shire and owns stock and/or stock options in Shire. Dr. Findling receives or has received research support, acted as a consultant, received royalties from, and/or served on a speaker's bureau for Abbott, Addrenex, Alexza, American Psychiatric Press, AstraZeneca, Biovail, Bristol-Myers Squibb, Dainippon Sumitomo Pharma, Forest, GlaxoSmithKline, Guilford Press, Johns Hopkins University Press, Johnson & Johnson, KemPharm Lilly, Lundbeck, Merck, National Institutes of Health, Neuropharm, Novartis, Noven, Organon, Otsuka, Pfizer, Physicians' Post-Graduate Press, Rhodes Pharmaceuticals, Roche, Sage, Sanofi-Aventis, Schering-Plough, Seaside Therapeutics, Sepracor, Shionogi, Shire, Solvay, Stanley Medical Research Institute, Sunovion, Supernus Pharmaceuticals, Transcept Pharmaceuticals, Validus, WebMD, and Wyeth.
Under the direction of the authors, Michael Pucci, PhD, an employee of SCI Scientific Communications & Information (SCI), and Asha Phillip, PharmD, and Huda Abdullah, PhD, former employees of SCI, provided writing assistance for this manuscript. Editorial assistance in formatting, proofreading, copy editing, and fact checking was also provided by SCI. Shire Development LLC provided funding to SCI for support in writing and editing this manuscript. Although the sponsor was involved in the design, collection, analysis, interpretation, and fact checking of information, the content of this manuscript, the ultimate interpretation, and the decision to submit it for publication in the Journal of Child and Adolescent Psychopharmacology were made by the authors independently.
References
- Ahmann PA. Waltonen SJ. Olson KA. Theye FW. Van Erem AJ. LaPlant RJ. Placebo-controlled evaluation of Ritalin side effects. Pediatrics. 1993;91:1101–1106. [PubMed] [Google Scholar]
- American Academy of Pediatrics Subcommittee on Attention-Deficit/Hyperactivity Disorder and Committee on Quality Improvement: Clinical practice guideline: Treatment of the school-aged child with attention-deficit/hyperactivity disorder. Pediatrics. 2001;108:1033–1044. doi: 10.1542/peds.108.4.1033. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association: Attention-deficit and disruptive behavior disorders. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: American Psychiatric Association; 2000. pp. 85–93. Text Revision. [Google Scholar]
- Anastopoulos AD. Smith TF. Garrett ME. Morrissey–Kane E. Schatz NK. Sommer JL. Kollins SH. Ashley–Koch A. Self-regulation of emotion, functional impairment, and comorbidity among children with ADHD. J Atten Disord. 2010;15:583–592. doi: 10.1177/1087054710370567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA. Deficient emotional self-regulation: A core component of attention-deficit/hyperactivity disorder. J ADHD Related Disord. 2010;1:5–37. [Google Scholar]
- Barkley RA. Murphy KR. Deficient emotional self-regulation in adults with attention-deficit/hyperactivity disorder (ADHD): The relative contributions of emotional impulsiveness and ADHD symptoms to adaptive impairments in major life activities. J ADHD Relat Disord. 2010;1:5–28. [Google Scholar]
- Biederman J. Faraone S. Milberger S. Guite J. Mick E. Chen L. Mennin D. Marrs A. Ouellette C. Moore P. Spencer T. Norman D. Wilens T. Kraus I. Perrin J. A prospective 4-year follow-up study of attention-deficit hyperactivity and related disorders. Arch Gen Psychiatry. 1996;53:437–446. doi: 10.1001/archpsyc.1996.01830050073012. [DOI] [PubMed] [Google Scholar]
- Childress AC. Arnold V. Dirks B. Babcock T. Scheckner B. Lasser R. Lopez FA. The effects of lisdexamfetamine dimesylate on emotional lability in children 6 to 12 years of age with ADHD in a double-blind placebo-controlled trial. J Atten Disord. 2012 doi: 10.1177/1087054712448252. [ePub ahead of print]. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ. Power TJ. Anastopoulos AD. Reid R. New York: Guilford Press; 1998. ADHD Rating Scale-IV: Checklists, Norms, Clinical Interpretation. [Google Scholar]
- Faries DE. Yalcin I. Harder D. Heiligenstein JH. Validation of the ADHD Rating Scale as a clinician administered and scored instrument. J Atten Disord. 2001;5:107–115. [Google Scholar]
- Findling RL. Ginsberg LD. Jain R. Gao J. Effectiveness, safety, and tolerability of lisdexamfetamine dimesylate in children with attention-deficit/hyperactivity disorder: An open-label, dose-optimization study. J Child Adolesc Psychopharmacol. 2009;19:649–662. doi: 10.1089/cap.2008.0165. [DOI] [PubMed] [Google Scholar]
- Gillberg C. Melander H. von Knorring A-L. Janols LO. Thernlund G. Hagglof B. Eidevall–Wallin L. Gustafsson P. Kopp S. Long-term stimulant treatment of children with attention-deficit hyperactivity disorder symptoms. A randomized, double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1997;54:857–864. doi: 10.1001/archpsyc.1997.01830210105014. [DOI] [PubMed] [Google Scholar]
- Gioia GA. Isquith PK. Guy SC. Kenworthy L. Lutz, FL: Psychological Assessment Resources, Inc.; 2000. BRIEF: Behavior Rating Inventory of Executive Function: Professional Manual. [Google Scholar]
- Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Health, Education, and Welfare; Public Health Service, Alcohol, Drug Abuse and Mental Health Administration, NIMH Psychopharmacology Research Branch; 1976. Clinical global impressions; pp. 218–222. [Google Scholar]
- Jacobson NS. Truax P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59:12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- Katic A. Ginsberg L. Jain R. Adeyi B. Dirks B. Babcock T. Scheckner B. Richards C. Lasser R. Turgay A. Findling RL. Clinically relevant changes in emotional expression in children with ADHD treated with lisdexamfetamine dimesylate. J Atten Disord. 2012;16:384–397. doi: 10.1177/1087054710389990. [DOI] [PubMed] [Google Scholar]
- Kratochvil CJ. Faries D. Vaughan B. Perwien A. Busner J. Saylor K. Kaplan S. Buermeyer C. Swindle R. Emotional expression during attention-deficit/hyperactivity disorders treatment: initial assessment of treatment effects. J Child Adolesc Psychopharmacol. 2007;17:51–62. doi: 10.1089/cap.2006.0018. [DOI] [PubMed] [Google Scholar]
- Mahone EM. Hoffman J. Behavior ratings of executive function among preschoolers with ADHD. Clin Neuropsychol. 2007;21:569–586. doi: 10.1080/13854040600762724. [DOI] [PubMed] [Google Scholar]
- Perwien AR. Kratochvil CJ. Faries D. Vaughan B. Busner J. Saylor KE. Buermeyer CM. Kaplan S. Swindle R. Emotional expression in children treated with ADHD medication: development of a new measure. J Atten Disord. 2008;11:568–579. doi: 10.1177/1087054707306117. [DOI] [PubMed] [Google Scholar]
- Short EJ. Manos MJ. Findling RL. Schubel EA. A prospective study of stimulant response in preschool children: insights from ROC analyses. J Am Acad Child Adolesc Psychiatry. 2004;43:251–259. doi: 10.1097/00004583-200403000-00005. [DOI] [PubMed] [Google Scholar]
- Sobanski E. Banaschewski T. Asherson P. Buitelaar J. Chen W. Franke B. Holtmann M. Krumm B. Sergeant J. Sonuga–Barke E. Stringaris A. Taylor E. Anney R. Ebstein RP. Gill M. Miranda A. Mulas F. Oades RD. Roeyers H. Rothenberger A. Steinhausen HC. Faraone SV. Emotional lability in children and adolescents with attention deficit/hyperactivity disorder (ADHD): Clinical correlates and familial prevalence. J Child Psychol Psychiatry. 2010;51:915–923. doi: 10.1111/j.1469-7610.2010.02217.x. [DOI] [PubMed] [Google Scholar]
- Strine TW. Lesesne CA. Okoro CA. McGuire IC. Chapman DP. Balluz LS. Mokdad AH. Emotional, behavioral difficulties, impairments in everyday functioning among children with a history of attention-deficit/hyperactivity disorder. Prev Chronic Dis. 2006;3:A52. [PMC free article] [PubMed] [Google Scholar]
- Vyvanse [package insert] Wayne, PA: Shire US Inc.; 2012. [Google Scholar]