Abstract
Objectives:
The objective of this article is to update previous evidence-based recommendations for evaluation and management of individuals with solid pulmonary nodules and to generate new recommendations for those with nonsolid nodules.
Methods:
We updated prior literature reviews, synthesized evidence, and formulated recommendations by using the methods described in the “Methodology for Development of Guidelines for Lung Cancer” in the American College of Chest Physicians Lung Cancer Guidelines, 3rd ed.
Results:
We formulated recommendations for evaluating solid pulmonary nodules that measure > 8 mm in diameter, solid nodules that measure ≤ 8 mm in diameter, and subsolid nodules. The recommendations stress the value of assessing the probability of malignancy, the utility of imaging tests, the need to weigh the benefits and harms of different management strategies (nonsurgical biopsy, surgical resection, and surveillance with chest CT imaging), and the importance of eliciting patient preferences.
Conclusions:
Individuals with pulmonary nodules should be evaluated and managed by estimating the probability of malignancy, performing imaging tests to better characterize the lesions, evaluating the risks associated with various management alternatives, and eliciting their preferences for management.
Summary of Recommendations
General Approach
2.3.1. In the individual with an indeterminate nodule that is visible on chest radiography and/or chest CT, we recommend that prior imaging tests should be reviewed (Grade 1C).
2.3.2. In the individual with a solid, indeterminate nodule that has been stable for at least 2 years, we suggest that no additional diagnostic evaluation need be performed (Grade 2C).
Remark: This recommendation applies only to solid nodules. For guidance about follow-up of subsolid nodules, see Recommendations 6.5.1 to 6.5.4.
2.3.3. In the individual with an indeterminate nodule that is identified by chest radiography, we recommend that CT of the chest should be performed (preferably with thin sections through the nodule) to help characterize the nodule (Grade 1C).
Solid Nodules > 8 mm
4.1.1.1. In the individual with a solid, indeterminate nodule that measures > 8 mm in diameter, we suggest that clinicians estimate the pretest probability of malignancy either qualitatively by using their clinical judgment and/or quantitatively by using a validated model (Grade 2C).
4.2.4.1. In the individual with a solid, indeterminate nodule that measures > 8 mm in diameter and low to moderate pretest probability of malignancy (5%-65%), we suggest that functional imaging, preferably with PET, should be performed to characterize the nodule (Grade 2C).
4.2.4.2. In the individual with a solid, indeterminate nodule that measures > 8 mm in diameter and a high pretest probability of malignancy (> 65%), we suggest that functional imaging should not be performed to characterize the nodule (Grade 2C).
Remark: PET may be indicated for pretreatment staging among those patients with nodules in whom malignancy is strongly suspected or confirmed.
4.4.1.1. In the individual with a solid, indeterminate nodule that measures > 8 mm in diameter, we recommend that clinicians discuss the risks and benefits of alternative management strategies and elicit patient preferences for management (Grade 1C).
4.5.1.1. In the individual with a solid, indeterminate nodule that measures > 8 mm in diameter, we suggest surveillance with serial CT scans in the following circumstances (Grade 2C):
When the clinical probability of malignancy is very low (< 5%)
When clinical probability is low (< 30% to 40%) and the results of a functional imaging test are negative (ie, the lesion is not hypermetabolic by PET or does not enhance > 15 Hounsfield units on dynamic contrast CT), resulting in a very-low posttest probability of malignancy
When needle biopsy is nondiagnostic and the lesion is not hypermetabolic by PET
When a fully informed patient prefers this nonaggressive management approach.
Remark: CT surveillance of solid nodules ≥ 8 mm should use low-dose, noncontrast techniques.
4.5.1.2. In the individual with a solid, indeterminate nodule that measures > 8 mm in diameter who undergoes surveillance, we suggest that serial CT scans should be performed at 3 to 6, 9 to 12, and 18 to 24 months, using thin sections and noncontrast, low-dose techniques (Grade 2C).
Remark: Serial CT scans should be compared with all available prior studies, especially the initial (index) CT scan.
Remark: Where available, manual and/or computer-assisted measurements of area, volume, and/or mass may facilitate early detection of growth.
4.5.1.3. In the individual with a solid, indeterminate nodule that shows clear evidence of malignant growth on serial imaging, we recommend nonsurgical biopsy and/or surgical resection unless specifically contraindicated (Grade 1C).
Remark: Solid nodules that decrease in size but do not disappear completely should be followed to resolution or lack of growth over 2 years.
4.6.2.1.1. In the individual with a solid, indeterminate nodule that measures > 8 mm in diameter, we suggest nonsurgical biopsy in the following circumstances (Grade 2C):
When clinical pretest probability and findings on imaging tests are discordant
When the probability of malignancy is low to moderate (~ 10% to 60%)
When a benign diagnosis requiring specific medical treatment is suspected
When a fully informed patient desires proof of a malignant diagnosis prior to surgery, especially when the risk of surgical complications is high.
Remark: The type of biopsy should be selected based on nodule size, location, and relation to a patent airway; the risk of complications in the individual patient; and available expertise.
4.6.3.1.1. In the individual with a solid, indeterminate nodule that measures > 8 mm in diameter, we suggest surgical diagnosis in the following circumstances (Grade 2C):
When the clinical probability of malignancy is high (> 65%)
When the nodule is intensely hypermetabolic by PET or markedly positive by another functional imaging test
When nonsurgical biopsy is suspicious for malignancy
When a fully informed patient prefers undergoing a definitive diagnostic procedure.
4.6.3.1.2. In the individual with a solid, indeterminate nodule measuring > 8 mm in diameter who chooses surgical diagnosis, we recommend thoracoscopy to obtain a diagnostic wedge resection (Grade 1C).
Remark: Use of advanced localization techniques or open thoracotomy may be necessary when resecting small or deep nodules.
Solid Nodules ≤ 8 mm
5.3.1. In the individual with a solid nodule that measures ≤ 8 mm in diameter and no risk factors for lung cancer, we suggest that the frequency and duration of CT surveillance be chosen according to the size of the nodule (Grade 2C):
Nodules measuring ≤ 4 mm in diameter need not be followed, but the patient should be informed about the potential benefits and harms of this approach
Nodules measuring > 4 mm to 6 mm should be reevaluated at 12 months without the need for additional follow-up if unchanged
Nodules measuring > 6 mm to 8 mm should be followed sometime between 6 and 12 months, and then again at between 18 and 24 months if unchanged.
Remark: For the individual with multiple small, solid nodules, the frequency and duration of follow-up should be based on the size of the largest nodule.
Remark: CT surveillance of solid nodules ≤ 8 mm should use low-dose, noncontrast techniques.
5.3.2. In the individual with a solid nodule that measures ≤ 8 mm in diameter who has one or more risk factors for lung cancer, we suggest that the frequency and duration of CT surveillance be chosen according to the size of the nodule (Grade 2C):
Nodules measuring ≤ 4 mm in diameter should be reevaluated at 12 months without the need for additional follow-up if unchanged
Nodules measuring > 4 mm to 6 mm should be followed sometime between 6 and 12 months and then again between 18 and 24 months if unchanged
Nodules measuring > 6 mm to 8 mm should be followed initially sometime between 3 and 6 months, then subsequently between 9 and 12 months, and again at 24 months if unchanged.
Remark: For the individual with multiple small, solid nodules, the frequency and duration of follow-up should be based on the size of the largest nodule.
Remark: CT surveillance of solid nodules ≤ 8 mm should use low-dose, noncontrast techniques.
Nonsolid (Pure Ground Glass) Nodules
6.5.1. In the individual with a nonsolid (pure ground glass) nodule measuring ≤ 5 mm in diameter, we suggest no further evaluation (Grade 2C).
6.5.2. In the individual with a nonsolid (pure ground glass) nodule measuring > 5 mm in diameter, we suggest annual surveillance with chest CT for at least 3 years (Grade 2C).
Remark: CT surveillance of nonsolid nodules should use noncontrast techniques with thin sections through the nodule of interest.
Remark: Nonsolid nodules that grow or develop a solid component are often malignant, prompting further evaluation and/or consideration of resection.
Remark: Early follow-up at 3 months may be indicated for nonsolid nodules measuring > 10 mm (followed by nonsurgical biopsy and/or surgical resection for nodules that persist).
Remark: Limited duration or no follow-up may be preferred by individuals with life-limiting comorbidities in whom a low-grade malignancy would be of little consequence or by others who place a high value on avoiding treatment of possibly indolent lung cancer.
Part-Solid (> 50% Ground Glass) Nodules
6.5.3. In the individual with a part-solid nodule measuring ≤ 8 mm in diameter, we suggest CT surveillance at approximately 3, 12, and 24 months, followed by annual CT surveillance for an additional 1 to 3 years (Grade 2C).
Remark: CT surveillance of part-solid nodules should use noncontrast techniques with thin sections through the nodule of interest.
Remark: Part-solid nodules that grow or develop a solid component are often malignant, prompting further evaluation and/or consideration of resection.
Remark: Limited duration or no follow-up may be preferred by individuals with life-limiting comorbidities in whom a low-grade malignancy would be of little consequence or by others who place a high value on avoiding treatment of possibly indolent lung cancer.
6.5.4. In the individual with a part-solid nodule measuring > 8 mm in diameter, we suggest repeat chest CT at 3 months followed by further evaluation with PET, nonsurgical biopsy, and/or surgical resection for nodules that persist (Grade 2C).
Remark: PET should not be used to characterize part-solid lesions in which the solid component measures ≤ 8 mm.
Remark: Nonsurgical biopsy can be used to establish the diagnosis and/or be combined with wire, radioactive seed, or dye localization to facilitate subsequent resection. A nondiagnostic biopsy result does not exclude the possibility of malignancy.
Remark: Part-solid nodules measuring > 15 mm in diameter should proceed directly to further evaluation with PET, nonsurgical biopsy, and/or surgical resection.
One or More Additional Nodules Detected During Nodule Evaluation
7.1.1. In the individual with a dominant nodule and one or more additional small nodules, we suggest that each nodule be evaluated individually and curative treatment not be denied unless there is histopathological confirmation of metastasis (Grade 2C).
Remark: The classification and appropriate treatment of patients with more than one pulmonary focus of lung cancer is difficult and requires multidisciplinary consideration.
Pulmonary nodules are small, focal, rounded radiographic opacities that may be solitary or multiple. By definition, the solitary pulmonary nodule is a single, well-circumscribed, radiographic opacity that measures up to 3 cm in diameter and is surrounded completely by aerated lung.2,3 There is no associated atelectasis, hilar enlargement, or pleural effusion. Individuals with solitary nodules are typically asymptomatic. Focal pulmonary lesions that are > 3 cm in diameter are called lung masses and are presumed to represent bronchogenic carcinoma until proven otherwise. The management of individuals who present with lung masses and symptomatic nodules are discussed elsewhere.4
We exclude from consideration individuals with diffuse or multiple nodules, arbitrarily defined as those with > 10 nodules. Diffuse nodules are more likely to be accompanied by symptoms and caused by either metastasis from extrathoracic malignancies or active infection or inflammation. Because they rarely represent bronchogenic carcinoma, they will not be discussed further. However, we include abnormalities that appear as a single dominant nodule accompanied by one or more smaller, incidental nodules, which are increasingly common and may represent the new normal given that they may be present in ≥ 50% of patients having thin-section chest CT scans. We use the term “dominant” to refer to a nodule that manifests in this pattern.
We further distinguish small, subcentimeter nodules from larger nodules because nodules that measure ≤ 8 mm in diameter are much less likely to be malignant, typically defy accurate characterization by imaging tests, and often are difficult to approach by nonsurgical biopsy. We also distinguish solid nodules from subsolid nodules. Subsolid nodules are further categorized as pure ground glass or part solid in attenuation.
Throughout this article, we use the term “indeterminate” to describe a nodule that is not calcified in a benign pattern or that does not have other features strongly suggestive of a benign etiology, such as intranodular fat that is pathognomonic of hamartoma or a feeding artery and vein typical for arteriovenous malformation. Our recommendations apply only to indeterminate nodules. We do not distinguish screening-detected nodules from nodules that are detected incidentally, nor do we distinguish nodules that are detected by chest radiography vs chest CT scan. When evaluating individuals with lung nodules, it is more important to consider the size and morphology of the lesions as well as risk factors for malignancy and suitability for curative treatment.
We begin by updating recommendations from the second edition of these guidelines for individuals with solid nodules measuring > 8 mm in diameter (including both solitary and dominant nodules). Next, we update recommendations for evaluating solid nodules measuring ≤ 8 mm. Finally, we present a new set of recommendations for individuals with subsolid nodules.
Most of the evidence described in this article comes from uncontrolled studies of diagnostic accuracy. Although many were methodologically rigorous, all were limited by the use of accuracy as a surrogate outcome. Few randomized controlled trials or studies of higher-level outcomes have been performed. As a result, most of the recommendations are based on evidence that is relatively low in quality.5
1.0 Methods
To update previously published guidelines for evaluation of individuals with pulmonary nodules,6 we repeated prior searches of Medline for studies of chest CT imaging, PET imaging, and transthoracic needle biopsy (TTNB) and performed new searches for studies of subsolid nodules, bronchoscopy, surgical complications, and methods to detect nodule growth (Appendix S1 (629KB, pdf) ). All searches were performed in October 2011 and subsequently updated through May 2012. We identified additional articles by searching our own personal files and by reviewing reference lists of included studies. We included all randomized controlled trials, controlled observational studies, uncontrolled studies of diagnostic accuracy, and cross-sectional studies that examined relationships between nodule morphology and outcomes. A multidisciplinary writing committee comprising four pulmonologists, two thoracic surgeons, and one radiologist formulated questions (Table S1 (895KB, doc) ), synthesized and reviewed available evidence, developed or revised recommendations, rated the quality of evidence, and graded the strength of the recommendations by using a standardized approach, as described by Lewis et al1 in “Methodology for Development of Guidelines for Lung Cancer” in the American College of Chest Physicians (ACCP) Lung Cancer Guidelines. The writing committee reviewed all recommendations and reached consensus by iterative discussion and debate. The manuscript was extensively revised, although some sections of text (including much of the section on solid nodules measuring ≤ 8 mm in diameter) were considered to be current and, therefore, retained from the previous version. The resulting guideline supersedes the previous version. The guideline was reviewed by all members of the ACCP Lung Cancer Guidelines Panel prior to approval by the Thoracic Oncology NetWork, the Guidelines Oversight Committee, and the Board of Regents of the ACCP.
2.0 Anatomic Imaging
Pulmonary nodule diagnosis begins with imaging studies. Recent attention has focused on studies of computer-assisted detection, computer-assisted diagnosis, volumetric measurement of growth, and functional imaging, as described in this section.
2.1 Chest Radiography
Although most nodules are now detected by CT scan, many are still detected incidentally on chest radiographs that were ordered for some other purpose. Our updated literature review identified 18 potentially relevant studies of chest radiography published since 2005, including six studies of dual-energy techniques, nine studies of computer-assisted detection, and four studies of other methods to improve nodule detection (Table S2 (117KB, xls) ). However, none of the studies examined whether specific chest radiographic features were helpful in characterizing nodules as malignant or benign.
Occasionally, a presumptive benign diagnosis can be established when a characteristic pattern of calcification is noted on the chest radiograph. Diffuse, central, laminated, and popcorn patterns of calcification are considered to be benign.7,8 Although often missing, the presence of intranodular fat density and popcorn calcification are specific for hamartoma.9 If one of these patterns of calcification is clearly evident on the chest radiograph, no additional evaluation is necessary. However, other patterns of calcification, including the stippled and eccentric patterns, do not exclude malignancy. Further evaluation of these nodules is considered mandatory.
2.2 Chest CT Scan
As is true for nodules identified by chest radiography, all previous CT scans should be reviewed when a nodule is first identified by CT scan. Chest CT scan also provides specific information about the location, shape, margins, and attenuation characteristics of nodules. In addition, CT scan sometimes identifies unsuspected lymphadenopathy, synchronous parenchymal lesions, or invasion of the chest wall or mediastinum. Selected morphologic characteristics are described next. We discuss nodule size and attenuation characteristics (solid vs subsolid) in greater detail in a subsequent section.
Morphologic characteristics on chest CT scan that suggest malignancy include spiculated margins10‐12; vascular convergence (which suggests vascular and lymphatic invasion)13; and either a dilated bronchus leading into the nodule14 or the presence of pseudocavitation, which has a bubbly appearance believed to represent air bronchiolograms.12 True cavitation, especially when associated with a thick and irregular wall, is a strong predictor of malignancy.15
Our search identified seven recent studies of CT image characteristics (summarized in Appendix S2 (629KB, pdf) ). One such study of 213 patients with nodules (92% solid) from Denmark confirmed many of the distinguishing characteristics first reported by Siegelman et al10 and Zerhouni et al.11 In the Danish study, a malignant (vs benign) diagnosis was more than five times more likely for nodules with spiculated or ragged margins (likelihood ratio [LR], 5.5), almost twice as likely when pleural retraction was present (LR, 1.9), and 70% more likely when a vessel sign was present (LR, 1.7) but only 10% more likely when margins were lobulated (LR, 1.1).16 Malignancy was 30% less likely when a bronchus sign was present (LR, 0.7) and five times less likely for smooth or polygonal margins (LR, 0.2). Noncalcified nodules were equally likely to be malignant or benign (LR, 1.0). Qualitative assessment and subjective weighting of these features yielded a sensitivity of 98% for identifying malignancy but a specificity of only 23%.
Data from the NELSON (Dutch Belgian Randomised Lung Cancer Screening) trial of CT scan screening showed that for solid nodules, malignancy was associated with larger nodule size, spiculated margins, and irregular shape but not with attenuation characteristics.17 In this study, the combination of round shape, smooth margins, and low attenuation (solid nodule with a negative CT scan number) was 100% predictive of benignity. Two other screening studies reported conflicting results about potential predictors of resolving (and therefore benign) nodules.18,19
Other studies have used computer-assisted techniques or novel CT scan parameters to discriminate between malignant and benign nodules, but these have been limited by small size, imprecision, and lack of external validation.20,21 Consequently, although CT scan morphology often helps to estimate the probability of malignancy, it is rarely conclusive.
Risks associated with CT scan include radiation exposure and adverse effects because of administration of iodinated contrast material. The risk of radiation-induced cancer is uncertain in magnitude. Most studies used models that are based on nonmedical sources of ionizing radiation and concluded that risks were small but nontrivial at the population level.22,23 Although controversy exists, less radiation exposure is obviously preferable, and the use of radiation-limiting innovations, including dose modulation and iterative reconstruction techniques, should be used when available to minimize the risks associated with repeated exposures.24 IV contrast is relatively or absolutely contraindicated in patients with renal insufficiency or allergy to iodine, and it is usually not necessary to administer contrast when performing follow-up CT scans to identify growth.
2.3 Recommendations
2.3.1. In the individual with an indeterminate nodule that is visible on chest radiography and/or chest CT, we recommend that prior imaging tests should be reviewed (Grade 1C).
2.3.2. In the individual with a solid, indeterminate nodule that has been stable for at least 2 years, we suggest that no additional diagnostic evaluation need be performed (Grade 2C).
Remark: This recommendation applies only to solid nodules. For guidance about follow-up of subsolid nodules, see recommendations 6.5.1 to 6.5.4.
2.3.3. In the individual with an indeterminate nodule that is identified by chest radiography, we recommend that CT of the chest should be performed (preferably with thin sections through the nodule) to help characterize the nodule (Grade 1C).
3.0 Suitability for Surgery or Other Curative-Intent Treatment
Before embarking on a potentially inconvenient, risky, and expensive evaluation, it is important to establish the individual’s suitability and desire for curative treatment. Although therapeutic lobectomy frequently is contraindicated in individuals with advanced comorbid conditions, relatively few individuals will be excluded from consideration for sublobar resection or other less-invasive treatments (see Brunelli et al25 “Physiologic Evaluation of the Patient With Lung Cancer Being Considered for Resectional Surgery” in the ACCP Lung Cancer Guidelines). However, some individuals may prefer no treatment, particularly those with life-limiting comorbid conditions. In such individuals, it does not make sense to pursue biopsy or aggressive CT scan surveillance, although it is always prudent to monitor for symptoms that may be palliated.
For individuals who desire treatment but either refuse or cannot tolerate surgery (even limited resection), surgical diagnosis is precluded. Other options for evaluation include functional imaging, CT scan surveillance, and nonsurgical biopsy. Prior to beginning nonsurgical treatment, the diagnosis of lung cancer ideally should be confirmed by biopsy specimen. Alternatives to surgical treatment include external beam radiation, stereotactic radiotherapy, and radiofrequency ablation. Except for the section on surgical diagnosis (which applies only to surgical candidates), the remainder of this guideline applies to individuals who are candidates for curative treatment with either surgery or one of these other alternatives.
4.0 Solid Nodules Measuring > 8 mm in Diameter
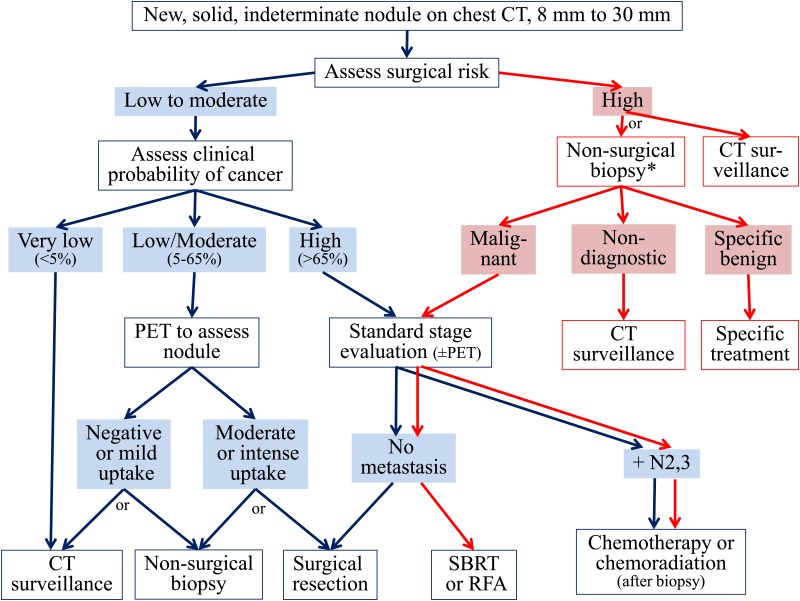
Among individuals with a solid nodule measuring > 8 mm in diameter (either solitary or dominant), steps in the evaluation include estimating the probability of cancer; further characterizing the lesion with CT scan, PET scan, or another functional imaging test; and choosing among nonsurgical biopsy, surgical resection, and active surveillance with serial CT scans (Figs 1, 2).
Figure 1.
[Sections 4.0, 4.3] Management algorithm for individuals with solid nodules measuring 8 to 30 mm in diameter. Branches indicate steps in the algorithm following nonsurgical biopsy. *Among individuals at high risk for surgical complications, we recommend either CT scan surveillance (when the clinical probability of malignancy is low to moderate) or nonsurgical biopsy (when the clinical probability of malignancy is moderate to high). RFA = radiofrequency ablation; SBRT = stereotactic body radiotherapy.
Figure 2.
[Section 4.0] Factors that influence choice between evaluation and management alternatives for indeterminate, solid nodules ≥ 8 to 30 mm in diameter.
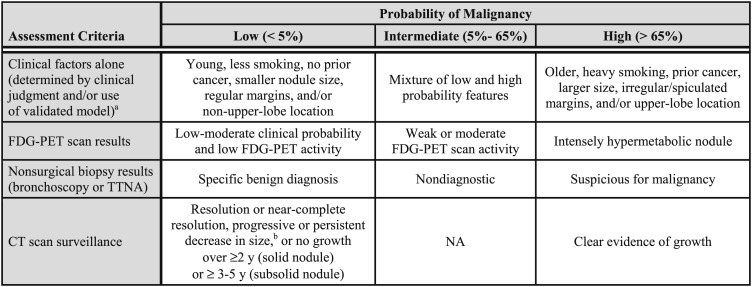
4.1 Clinical Probability of Cancer
Although clinical and radiographic characteristics cannot reliably distinguish between benign and malignant nodules in most individuals, it is nevertheless important to estimate the clinical probability of malignancy before ordering imaging tests or biopsy procedures (Fig 3). Estimating pretest probability facilitates the selection and interpretation of subsequent diagnostic tests. Common sense argues that different management approaches are called for in a 30-year-old nonsmoker with an 8-mm smooth-bordered nodule and a 70-year-old heavy smoker with a 2.5-cm spiculated nodule. Most individuals with nodules have characteristics that fall somewhere between these two extremes.
Figure 3.
[Section 4.1] Assessment of the probability of malignancy.
Although many clinicians estimate pretest probability intuitively, several quantitative models have been developed to assist in this task,26,30,31 including four new models developed since 2005 (Tables S3, S4 (895KB, doc) ).27‐29,32 Three models have undergone external validation since 2005 (Tables S5, S6 (895KB, doc) ).33‐35 Another used data from the PLCO (Prostate, Lung, Colorectal, Ovarian Cancer Screening Trial) of lung cancer screening with chest radiography and found that although a lung mass (not surprisingly) was highly predictive of malignancy (OR, 11.2; 95% CI, 6.3-19.9), the finding of a lung nodule was not (OR, 1.4; 95% CI, 0.8-2.5).36
The most extensively validated model was developed by investigators at the Mayo Clinic who used multiple logistic regression analysis to identify six independent predictors of malignancy in 419 patients with noncalcified nodules that measured between 4 and 30 mm in diameter on chest radiography.26,33 Independent predictors of malignancy included older age (OR, 1.04 for each year), current or past smoking history (OR, 2.2), history of extrathoracic cancer > 5 years before nodule detection (OR, 3.8), nodule diameter (OR, 1.14 for each millimeter), spiculation (OR, 2.8), and upper lobe location (OR, 2.2). The prediction model is described by the following equations:
| (Equation 1) |
| (Equation 2) |
where e is the base of natural logarithms, age is the patient’s age in years, smoke = 1 if the patient is a current or former smoker (otherwise = 0), cancer = 1 if the patient has a history of an extrathoracic cancer that was diagnosed > 5 years ago (otherwise = 0), diameter is the diameter of the nodule in millimeters, spiculation = 1 if the edge of the nodule has spicules (otherwise = 0), and location = 1 if the nodule is located in an upper lobe (otherwise = 0).
Of note, the accuracy of models for predicting malignancy appears to be similar to that of expert clinicians, although the correlation between models and experts is poor, suggesting that the models may provide unique information.37,38 The choice of model might best be guided by the characteristics of the target population, ease of use, and the extent of validation.
4.1.1 Recommendation
4.1.1.1. In the individual with a solid, indeterminate nodule that measures > 8 mm in diameter, we suggest that clinicians estimate the pretest probability of malignancy either qualitatively by using their clinical judgment and/or quantitatively by using a validated model (Grade 2C).
4.2 Functional Imaging
Most functional imaging of lung nodules is done with PET scan, but other modalities include dynamic contrast-enhanced CT scan, dynamic MRI, and single-photon emission CT (SPECT) scan.
4.2.1 Dynamic CT Scan:
CT scan with dynamic contrast enhancement is rarely used in the United States, yet it is highly sensitive (albeit nonspecific) for identifying malignant nodules.39 A multicenter study enrolled 356 participants with normal renal function and noncalcified nodules that measured 0.5 to 4 cm in diameter, 48% of which were malignant.40 With a threshold for enhancement of 15 Hounsfield units (HUs), the sensitivity and specificity of contrast-enhanced CT scan were 98% and 58%, respectively. Absence of lung nodule enhancement was strongly predictive of a benign diagnosis (negative predictive value, 96.5%). Allowing for slight differences in technique, similar results have been reported by others.41‐45 However, later studies highlighted the lack of specificity. Even with the use of novel parameters to measure enhancement, contrast-enhanced CT scan does not reliably discriminate between malignant and active inflammatory or infectious nodules (Appendix S3 (629KB, pdf) ).46,47
4.2.2 Dynamic MRI and SPECT Scan:
A 2008 meta-analysis summarized results of studies of the diagnostic accuracy of PET scan (22 studies), dynamic CT scan (10 studies), dynamic MRI (six studies), and SPECT scan (seven studies).48 Pooled estimates of sensitivity ranged from 93% for dynamic CT scan to 94% for dynamic MRI to 95% for both PET and SPECT scans, whereas pooled specificity ranged from 76% for dynamic CT scan to 79% for dynamic MRI to 82% for both PET and SPECT scans. Comparison of summary receiver operating characteristic curves showed that there were no significant differences in accuracy among all four modalities.
4.2.3 PET Scanning With Fluorodeoxyglucose:
The 2008 meta-analysis48 yielded an estimate for the sensitivity of PET scan that was somewhat higher (95%) than the estimate of 87% that we reported in the second edition of these guidelines.6,39 Among more recent studies identified by our updated literature review, estimates of sensitivity ranged from 72% to 94% (Table S7 (117KB, xls) ).49‐54
A limitation of most studies of diagnostic accuracy is the use of a single threshold for distinguishing malignant from benign nodules. A prospective study of 344 US veterans with lung nodules addressed this limitation by reporting LRs ratios for five different categories of PET results.55 In this study, LRs for definitely benign, probably benign, indeterminate, probably malignant, and definitely malignant PET results were 0.03 (95% CI, 0.01-0.12), 0.15 (95% CI, 0.09-0.25), 1.01 (95% CI, 1.00-1.02), 3.2 (95% CI, 1.9-5.3), and 9.9 (95% CI, 5.4-18.3), respectively, confirming the intuition that greater degrees of fluorodeoxyglucose (FDG) uptake are more strongly associated with malignancy. In this study, FDG uptake that was slightly greater than that of the mediastinal blood pool was considered to be probably malignant, whereas substantially greater uptake was considered to be definitely malignant. Although indeterminate findings on PET scan did not help to distinguish malignant from benign nodules, very few participants (1%) had indeterminate findings.
Some have proposed using dual time point measurements of FDG uptake to improve diagnostic accuracy (Table S8 (117KB, xls) ). However, a systematic review of 816 patients with 890 nodules in 10 studies concluded that dual time point measurement was no better than single time point measurement.56 In this study, the pooled sensitivity and specificity of dual-time FDG-PET scan for identifying malignancy were 85% (95% CI, 82%-89%) and 77% (95% CI, 72%-81%), respectively.
False-negative findings on PET scan can be seen in patients with less metabolically active tumors, including lepidic-predominant adenocarcinomas (minimally invasive or in situ), mucinous adenocarcinomas, and carcinoid tumors. False-positive findings often are the result of infections or inflammatory conditions, including (but not limited to) endemic mycoses, TB, rheumatoid nodules, and sarcoidosis. Paradoxically, false-positive PET scan results can sometimes be helpful because they alert the clinician to the presence of an active infectious or inflammatory condition that might require specific treatment. In some circumstances, FDG-PET scan can be helpful in directing tissue biopsy. As a metabolic biopsy tool, PET scan can identify which lesions or portions of lesions are metabolically active and most likely to yield a definitive tissue result.
Use of FDG-PET scanning may be most cost-effective when clinical pretest probability and CT scan results are discordant, especially when pretest probability is relatively low and CT image characteristics are indeterminate (ie, not clearly benign).57 Among patients with indeterminate nodules (by CT scan) and high pretest probability, negative PET scan results do not reliably exclude malignancy. However, FDG uptake in the primary tumor has been shown to be inversely correlated with survival,58,59 and patients with nonhypermetabolic malignant tumors may have a favorable prognosis, even when definitive surgical treatment is delayed by a period of observation as long as 238 days.60,61 Hence, patients with solid nodules and negative (nonhypermetabolic) PET scan results are believed to require continued surveillance for at least 2 years to confirm benignity. An even more cautious approach would be to perform needle biopsy in high-probability tumors with negative PET scan results.
Integrated PET/CT scanners combine CT and FDG imaging capability in a single patient gantry, facilitating the precise localization of areas of FDG uptake to normal structures or abnormal soft tissue masses. Of three studies that compared dedicated PET scan with integrated PET/CT scan for pulmonary nodule characterization (Table S9 (117KB, xls) ), integrated PET/CT scan was slightly more accurate in two of them, but none of the studies compared integrated PET/CT scan with standard care (side-by-side interpretation of dedicated PET scan and dedicated CT scan).62‐64
Although we view nodule characterization and lung cancer staging as separate indications for PET scanning, we favor PET scan over other functional imaging modalities for nodule characterization in part because PET scan often provides additional information about stage among individuals with malignant nodules. Recommendations about the use of PET scanning for staging are described by Silvestri et al65 in the “Methods for Staging Non-small Cell Lung Cancer” article in the ACCP Lung Cancer Guidelines.
Although exposure to ionizing radiation from dedicated FDG-PET imaging is at least moderate (about 5-7 mSv), the addition of integrated CT scanning for purposes of attenuation correction and anatomic correlation results in doses that are much higher, especially if a full diagnostic CT scan is performed (about 10-25 mSv).66 The widespread belief that PET/CT imaging is without risk is not correct.
4.2.4 Recommendations
4.2.4.1. In the individual with a solid, indeterminate nodule that measures > 8 mm in diameter and low to moderate pretest probability of malignancy (5%-65%), we suggest that functional imaging, preferably with PET, should be performed to characterize the nodule (Grade 2C).
4.2.4.2. In the individual with a solid, indeterminate nodule that measures > 8 mm in diameter and a high pretest probability of malignancy (> 65%), we suggest that functional imaging should not be performed to characterize the nodule (Grade 2C).
Remark: PET may be indicated for pretreatment staging among those patients with nodules in whom malignancy is strongly suspected or confirmed.
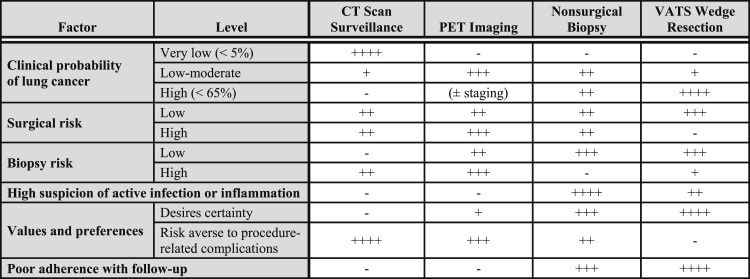
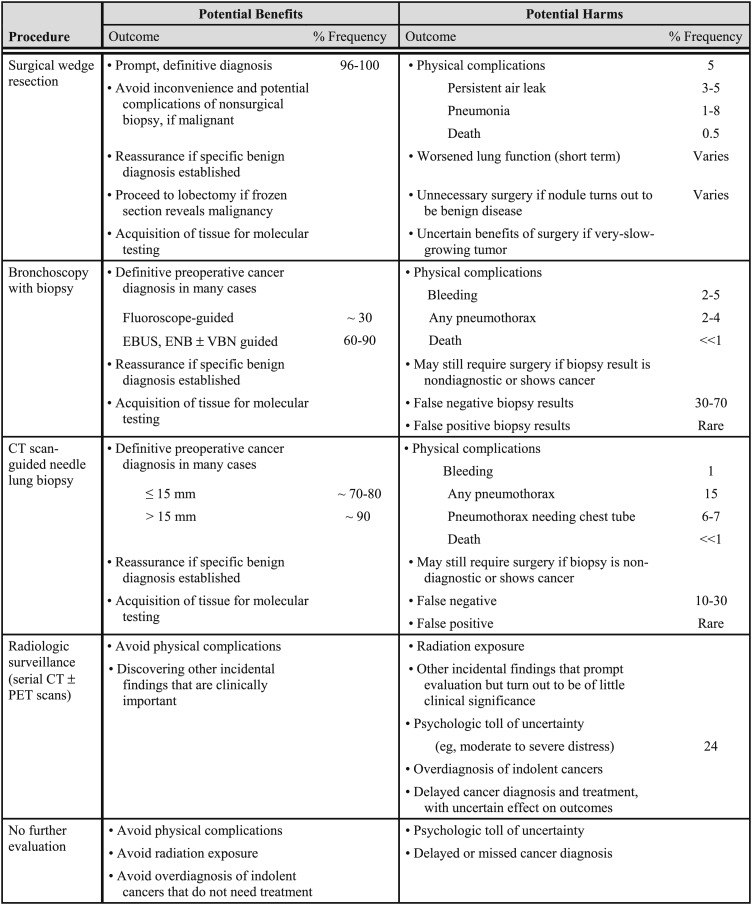
4.3 Management Strategies
Once imaging tests have been performed, management alternatives include surgical diagnosis, nonsurgical biopsy, and surveillance with serial CT scans. Each approach has advantages and disadvantages (Fig 4). Surgery is the diagnostic gold standard and the definitive treatment of malignant nodules, but surgery should be avoided in patients with benign nodules. Nonsurgical biopsy often is used to establish a specific benign or malignant diagnosis, but biopsy is invasive, potentially risky, and frequently nondiagnostic. CT scan surveillance avoids unnecessary surgery in patients with benign nodules, but surveillance delays diagnosis and treatment in cases of malignancy. A decision analysis found that the choice of strategy was a close call across a range of probabilities for malignancy.67 In this analysis, surveillance was favored when the probability of malignancy was < 3%, and surgical diagnosis was preferred when the probability was > 68%. Biopsy was the recommended strategy when the probability of malignancy fell between 3% and 68%. A management algorithm that is based in part on this analysis and a subsequent cost-effectiveness analysis57 is presented in Figure 1. More-specific recommendations are outlined next.
Figure 4.
[Section 4.3] Balance sheet of pros and cons of alternatives for evaluation and management of pulmonary nodule.
4.4 Shared Decision-Making and Patient Preferences
Because different strategies are associated with similar expected outcomes, individual preferences should be elicited and used to guide decisions. Some individuals may be uncomfortable with adopting a strategy of surveillance when told that a potentially cancerous lung nodule is present. Others are similarly risk averse about undergoing surgery unless they are certain that cancer is present.68 All individuals with lung nodules should be provided with an estimate of the probability of cancer and informed about the specific risks and benefits associated with alternative management strategies. Clinicians should elicit preferences for management and be sensitive to the preferred participatory decision-making style of the patient.69
4.4.1 Recommendation
4.4.1.1. In the individual with a solid, indeterminate nodule that measures > 8 mm in diameter, we recommend that clinicians discuss the risks and benefits of alternative management strategies and elicit patient preferences for management (Grade 1C).
4.5 CT Scan Surveillance
In some individuals with lung nodules, surveillance with serial imaging tests may be used as a diagnostic tool. When this strategy is used, detection of growth strongly suggests malignancy, and surgical resection should be performed in patients who are operative candidates. However, very rapidly growing nodules are commonly infectious or inflammatory, necessitating estimation of the growth rate. The growth rate typically is expressed in terms of the doubling time, or the time it takes for the nodule to double in volume. Because the volume of a sphere equals 4πr3/3, one doubling in tumor volume corresponds approximately to an increase in nodule diameter of 26%. The doubling time can be calculated by using the formula, dt = (t × log 2)/[3 × (log (d2/d1)], where dt = doubling time in days, t = time in days between radiographs, d2 = diameter of the nodule at the time of the current radiograph, and d1 = diameter of the nodule at the time of the previous radiograph.70
Two-year radiographic stability is strong presumptive evidence of a benign cause because malignant solid nodules typically double in volume within 400 days.71,72 Longer duration follow-up is advisable for ground glass nodules, which generally have longer volume doubling times (VDTs) when malignant.
Surveillance is virtually always performed with CT scanning, which is more sensitive than chest radiography for detecting growth. Although it may be possible to detect growth on serial chest radiographs when the nodule is large (> 1.5-2 cm) and has sharp, clearly demarcated borders, the surveillance strategy is seldom used for nodules of this size because of the relatively high probability of malignancy. For solid, indeterminate nodules measuring > 8 mm in diameter, the optimal time interval between imaging tests has not been determined, but standard practice is to obtain follow-up CT scans at about 3 to 6, 9 to 12, and 18 to 24 months.73 Less-frequent follow-up is indicated in patients with smaller nodules, as discussed in subsequent sections.
The advantage of the surveillance strategy in avoiding unnecessary invasive procedures among individuals with benign nodules is weighed against the disadvantage of delaying diagnosis and treatment among patients with malignant nodules. Depending on the growth rate and metastatic potential of the nodule and the length of surveillance, some malignant tumors will progress from resectable to unresectable disease during the observation period, and opportunities for surgical cure will be missed. Empirical data relevant to the hazard of delay are scarce, although a Scottish study found that maximum cross-sectional tumor area increased by > 50% in almost 25% of patients who had delays in radiotherapy treatment lasting between 18 and 131 days.74 In contrast, most studies of timeliness of care in lung cancer did not detect an association between timeliness and survival.75,76 Nevertheless, the surveillance strategy should be avoided when the clinical probability of cancer is moderate to high; it is most appropriate in individuals with a very low probability of malignancy and in those who are at high risk for complications of surgical resection and nonsurgical biopsy.
Methods to detect growth on serial CT scans are evolving rapidly (Appendix S4 (629KB, pdf) ). Manual measurements of diameter are limited by poor reliability and accuracy. In one study of 63 patients with lung nodules and 93 pairs of CT scans, manual and electronic measures of diameter and cross-sectional area incorrectly assessed the presence or absence of growth in 27% to 37% of CT scan pairs compared with a reference standard of manual volumetric measurement.77
Measurement of diameter, therefore, is likely to be supplanted by measurement of volume or mass, but available studies are not yet conclusive, being limited by small samples and retrospective, uncontrolled designs. For example, a small retrospective study of 63 participants (including only 11 participants with malignant nodules) used volumetric software to measure nodules on CT scans performed a median of 3.7 months apart and found that a threshold VDT of > 500 days had a sensitivity of 91% and a specificity of 90% for identifying malignancy.78 In another small study of 13 malignant ground glass nodules detected by screening over a mean time of 33 months between the first and last CT scans, diameter increased by 53%, volume by 202%, and mass by 254%, with measurements of mass having the largest signal-to-noise ratio, suggesting that measurements of volume or mass may improve detection of growth.79 Finally, in a study of 69 patients with 87 nodules (92% solid), volumetric measurement of growth changed the management decision from observation to biopsy in seven patients, although only three of these patients were proven to have lung cancer, with VDTs ranging from 347 to 670 days in these three cases.80
However, size measurements are fraught with error. Various measurement methods of solid nodules all have false-positive and false-negative assessments of growth.81 There is poor interobserver and intraobserver consistency for size differences of < 1.5 to 2 mm.82,83 In a study of 100 patients with 233 screening-detected benign nodules all measuring at least 4.8 mm in diameter,84 variability in automated volumetric measurements between nodules seen on CT scans performed at baseline, 3 months, and 12 months was ± 27%, and 70% of measurements had volume differences > 10%. Even in phantom studies, there is an error rate of about 20% in determining the presence or absence of one volume doubling for 5-mm nodules with a slice thickness of 2.5 mm.82 Finally, emerging data suggest that the rate of growth may not be constant and that a decrease in size is observed in about 20% of malignant lesions.85 These issues need to be better understood and standards developed.
Doubling times for malignant nodules are highly variable, but solid nodules usually have doubling times between 20 and 400 days. Because of this, 2-year radiographic stability of a solid nodule strongly implies a benign etiology.
4.5.1 Recommendations
4.5.1.1. In the individual with a solid, indeterminate nodule that measures > 8 mm in diameter, we suggest surveillance with serial CT scans in the following circumstances (Grade 2C):
When the clinical probability of malignancy is very low (< 5%)
When clinical probability is low (< 30% to 40%) and the results of a functional imaging test are negative (ie, the lesion is not hypermetabolic by PET or does not enhance > 15 HUs on dynamic contrast CT), resulting in a very-low posttest probability of malignancy
When needle biopsy is nondiagnostic and the lesion is not hypermetabolic by PET
When a fully informed patient prefers this nonaggressive management approach.
Remark: CT surveillance of solid nodules > 8 mm should use low-dose, noncontrast techniques with thin sections through the nodule of interest.
4.5.1.2. In the individual with a solid, indeterminate nodule that measures > 8 mm in diameter who undergoes surveillance, we suggest that serial CT scans should be performed at 3 to 6, 9 to 12, and 18 to 24 months, using thin sections and noncontrast, low-dose techniques (Grade 2C).
Remark: Serial CT scans should be compared with all available prior studies, especially the initial (index) CT scan.
Remark: Where available, manual and/or computer-assisted measurements of area, volume, and/or mass may facilitate early detection of growth.
4.5.1.3. In the individual with a solid, indeterminate nodule that shows clear evidence of malignant growth on serial imaging, we recommend nonsurgical biopsy and/or surgical resection unless specifically contraindicated (Grade 1C).
Remark: Solid nodules that decrease in size but do not disappear completely should be followed to resolution or lack of growth over 2 years.
4.6 Nonsurgical Biopsy
Options for nonsurgical tissue diagnosis include CT scan-guided TTNB and bronchoscopy guided by fluoroscopy, endobronchial ultrasound (EBUS), electromagnetic navigation bronchoscopy (ENB), and virtual bronchoscopy navigation (VBN).
4.6.1 Transthoracic Needle Biopsy:
TTNB of the pulmonary nodule usually is performed under CT scan guidance. In general, the sensitivity of TTNB depends on the size of the nodule, the size of the needle (especially for identifying lymphoma or benign disease), the number of needle passes, and the presence of onsite cytopathologic examination. In our previous review,39 we identified 11 studies of TTNB performed between 1998 and 2003. In these studies, the prevalence of malignancy was high (median, 75%; range, 63%-85%). Nondiagnostic results were seen in 4% to 41% of cases (median, 20.5%), but specific benign or malignant results were nearly always correct (although not all malignant diagnoses were confirmed surgically).
Our updated literature search identified 11 additional studies of TTNB for pulmonary nodule diagnosis performed between 2005 and 2011 (Table S10-S13 (895KB, doc) ). Once again, the prevalence of malignancy was high (median, 68%; range, 46%-83%), and the frequency of nondiagnostic results was highly variable, ranging from < 1% to 55%, although the median value was lower than what we found previously (6%). In most studies, sensitivity for identifying malignancy was ≥ 90%, but it was somewhat lower (70%-82%) in three studies that analyzed results for patients with nodules measuring ≤ 15 mm in diameter.62,86,87 More importantly, a sensitivity of 90% in a high-prevalence population (about 70%) translates to a risk of nondiagnostic results in about 20% of individuals with malignant nodules.
Our search did not identify any randomized controlled trials comparing TTNB with other approaches, but a 2002 study used case vignettes from 114 patients with solitary nodules (71% malignant) to determine the frequency with which TTNB results changed management.88 In this study, the addition of TTNB results to clinical history and chest CT scan findings reduced the frequency of missed surgical cure from 10% to 7% and reduced the frequency of unnecessary surgery for a benign lesion from 39% to 15%.
Complications of TTNB include pneumothorax and hemorrhage. In a population-based study of all TTNB procedures performed in California, Florida, Michigan, and New York in 2006, the risk of hemorrhage was low (1%), but the risks of any pneumothorax (15%) and pneumothorax requiring chest tube insertion (6.6%) were substantial.89 In this study, risk factors for pneumothorax included age 60 to 69 years, tobacco use, and COPD. In other single-center studies, risk factors for pneumothorax included older age,90 smaller lesion size,91‐94 deeper location,90‐96 the need to traverse fissures,91 the presence of emphysema,95,96 and the number of needle punctures.97
Use of needle biopsy is probably most appropriate when there is discordance among the clinical probability of cancer, imaging test results, patient preferences, and risk of surgical complications, as described in recommendation 4.6.2.1.1. It is important to emphasize that a nondiagnostic needle biopsy result does not rule out the possibility of malignancy.
4.6.2 Bronchoscopy:
Until recently, bronchoscopy played a limited role in pulmonary nodule management outside investigational settings. In older studies, the sensitivity of fluoroscopy-guided bronchoscopy with transbronchial biopsy (TBB) for identifying malignant nodules measuring < 2 cm in diameter ranged from 5% to 76% (median, 31%).98 The likelihood of obtaining a specific benign diagnosis is even lower. However, the presence of an air bronchogram in a pulmonary nodule is associated with a higher diagnostic yield, especially if this provides a specific road map to the bronchial location.99,100
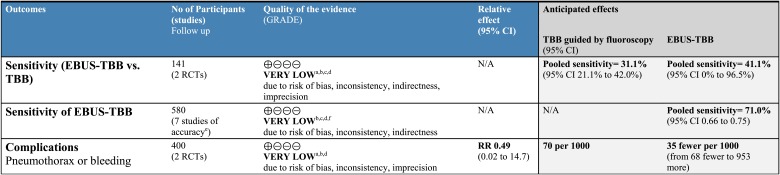
Newer techniques for bronchoscopic guidance include radial EBUS (Appendix S5 (629KB, pdf) ), ENB (Appendix S6 (629KB, pdf) ), and VBN. EBUS facilitates bronchoscopic sampling of smaller peripheral nodules. A recent systematic review identified 13 studies that reported the sensitivity of EBUS-TBB for identifying malignancy in 1,090 patients with peripheral lung lesions.101 For lesions of any size, pooled sensitivity was 0.73 (95% CI, 0.70-0.76); sensitivity was similar when pooled across seven studies that enrolled 580 patients with nodules measuring < 25 mm in diameter (0.71; 95% CI, 0.66-0.75). Studies were limited by low scores for study quality, inconsistent results, and the indirectness that characterizes most studies of diagnostic accuracy (Fig 5, Tables S14-S16 (895KB, doc) ).
Figure 5.
[Section 4.6.2] EBUS-TBB compared with TBB guided by fluoroscopy for patients with peripheral lung nodules.
Two small randomized trials compared EBUS-TBB with conventional TBB (Fig 5). In one study, the sensitivity of EBUS-TBB for identifying malignant nodules measuring ≤ 20 mm was markedly greater than that for conventional TBB (71% vs 23%).103 However, a subsequent study reported a sensitivity of only 11% for EBUS-TBB compared with 31% for conventional TBB102 perhaps because few of the participating bronchoscopists had experience with EBUS. A pooled analysis of complication rates from both trials neither confirmed nor excluded differences between the two tests (relative risk, 0.49; 95% CI, 0.02-14.7) (Fig S1 (8.6KB, png) ).
ENB shows promise as another tool for guiding biopsy of peripheral nodules.104,105 Our literature review identified 10 studies that reported the sensitivity of ENB-guided TBB for the identification of malignancy in peripheral lung lesions, including four studies that described results for nodules measuring < 2 cm (Table S17-S19 (895KB, doc) ). Among the latter studies, diagnostic yield ranged from 44% to 75% (median, 68.5%). Across all 10 studies, the risk of pneumothorax ranged from 0% to 7.5% (median, 2.2%). Studies were limited by small sample sizes, uncertain representativeness of the study populations, and retrospective uncontrolled design.
A small (n = 118) randomized controlled trial compared EBUS-TBB, ENB, and EBUS-TBB plus ENB for diagnostic yield in the absence of fluoroscopic guidance.106 For peripheral lesions of any size, diagnostic yield was higher for the combined procedure (88%) than for EBUS-TBB (69%) or ENB (59%) alone. Results were similar when the analysis was restricted to nodules measuring 20 to 30 mm in diameter or nodules measuring < 20 mm in diameter.
More recently, a randomized controlled trial from three centers in Japan compared VBN-assisted EBUS with nonassisted EBUS for diagnostic yield among 199 individuals with nodules measuring up to 30 mm in diameter.107 In this trial, diagnostic yield was higher for the VBN-assisted procedure (80%) than for the unassisted procedure (67%).
A recent meta-analysis identified 39 studies of bronchoscopy with biopsy guided by radial EBUS (20 studies), ENB (11 studies), guide sheath (10 studies), ultrathin bronchoscopy (11 studies), or VBN (10 studies).108 Most studies were prospective but limited by small samples. Across all studies, the pooled diagnostic yield was 70% (95% CI, 67%-73%), with slightly more favorable results for guide sheath (73%) and slightly less favorable results for ENB (67%). Heterogeneity in study results was identified but not entirely explained, although diagnostic yield for nodules measuring ≤ 20 mm in diameter (61%; 95% CI, 54%-68%) was substantially lower than that for nodules measuring > 20 mm in diameter (82%; 95% CI, 78%-86%). Across 24 studies that reported adverse events, the pooled risk of pneumothorax was 1.6%, and the risk of pneumothorax requiring chest tube placement was 0.7%.
For individuals who choose to pursue nonsurgical biopsy, the decision to perform CT scan-guided TTNB; conventional bronchoscopy; or bronchoscopy guided by EBUS, ENB, or VBN depends on multiple factors. CT scan-guided TTNB is preferred for nodules located in proximity to the chest wall or for deeper lesions provided that fissures do not need to be traversed and there is no surrounding emphysema. Bronchoscopic techniques are favored for nodules located in proximity to a patent bronchus and in individuals who are at high risk for pneumothorax following TTNB. In most other situations, operator experience should guide the decision.
4.6.2.1 Recommendation
4.6.2.1.1. In the individual with a solid, indeterminate nodule that measures > 8 mm in diameter, we suggest nonsurgical biopsy in the following circumstances (Grade 2C):
When clinical pretest probability and findings on imaging tests are discordant
When the probability of malignancy is low to moderate (~10% to 60%)
When a benign diagnosis requiring specific medical treatment is suspected
When a fully informed patient desires proof of a malignant diagnosis prior to surgery, especially when the risk of surgical complications is high.
Remark: The type of biopsy should be selected based on nodule size, location, and relation to a patent airway; the risk of complications in the individual patient; and available expertise.
4.6.3 Surgical Diagnosis:
Surgical resection is both the gold standard for diagnosis and the definitive treatment of a malignant nodule. The decision to pursue surgical diagnosis must take into account the benefits of definitive diagnosis and treatment when compared with the surgical risk. Video-assisted thoracic surgery (VATS), thoracotomy, and mediastinoscopy may be used alone or in combination, depending on the clinical circumstances. If the nodule proves to be a primary lung cancer, diagnosis, staging, and therapeutic resection often are completed in a single operative procedure.
Thoracoscopic wedge resection is the strongly preferred diagnostic approach for nodules. Although less invasive and almost certainly less morbid than open thoracotomy, data on complications of VATS diagnostic wedge resection are sparse. In two small older studies, there were no fatal complications; nonfatal complications occurred in about 5% of patients.109,110 More recent reports are difficult to interpret because they combine results for diagnostic VATS with VATS lobectomy, often in patients with severe comorbid conditions.
Nodules that are small in size (< 1 cm), deep in location, and subsolid in attenuation can pose a technical challenge because it may be difficult to find such nodules by digital palpation. Localization techniques to increase diagnostic yield during thoracoscopy include hook and wire, radioguidance, methylene blue, percutaneous microcoils, ultrasound, and fluoroscopy. In a recent review of methods to localize small nodules, the sensitivity of finger palpation was very poor in one study (< 30%) but excellent in another (88%).111 Hook-and-wire techniques had a sensitivity of 58% to 97%, with wire dislodgment being the largest source of failure. Technetium-99 radioguidance and fluoroscopic guidance with contrast material had high sensitivity with few complications. Ultrasonography had a sensitivity of 93% to 100% but is believed to be operator dependent.
The diagnosis is most often established by intraoperative consultation with pathology. Frozen section analysis is sensitive and specific for diagnosis of malignancy; however, the technique has limitations the surgeon should understand. In one study, the sensitivity for identifying malignancy was 87% for nodules that measured < 1.1 cm in diameter and 94% for nodules that measured between 1.1 and 1.5 cm.112 The technique has limitations in distinguishing minimally invasive adenocarcinoma or adenocarcinoma in situ (AIS) from atypical adenomatous hyperplasia (AAH) and in establishing a specific cell type in non-small cell carcinoma. It is limited in recognizing small peripheral carcinoid tumors. Lesions measuring < 5 mm should probably not be used for frozen section analysis unless there is other material available for permanent studies.112
For the surgical candidate with a nodule shown to be non-small cell lung cancer, lobectomy and systematic sampling of mediastinal lymph nodes is the standard of care for complete oncologic resection and staging.113 Minimally invasive techniques are increasingly preferred for lobectomy. Several large contemporary studies reported risks of fatal and nonfatal complications. Among nearly 6,000 individuals from the Society of Thoracic Surgeons database who underwent lobectomy between 1999 and 2006, of whom 30% underwent thorascopic procedures, the reported 30-day mortality was about 2%.114 Among smaller numbers of individuals who underwent bilobectomy and pneumonectomy in this study, the risks of fatal complications were 4% and 6.2%, respectively. More recently, data from the Nationwide Inpatient Sample showed in-hospital mortality to be 3.1% among 12,860 individuals who underwent open lobectomy and 3.4% among 759 individuals who underwent VATS lobectomy.115 An observational study of > 2,500 propensity-matched patients from the Society of Thoracic Surgeons database reported a higher percentage of patients who were free of complications among those who underwent thorascopic lobectomy compared with open lobectomy (74% vs 65%).116
For individuals with marginal cardiac performance or limited pulmonary reserve, sublobar resection can be considered acceptable treatment, although lobectomy has been the standard of care for medically fit populations. In the only randomized trial comparing lobectomy with lesser resection, there was an increase in the risk of locoregional recurrence with sublobar resection.117,118 That trial completed accrual in 1988, and advances in radiologic detection of small nodules and increased understanding of varied tumor biology have led to a resurgence of interest in limited resection for stage I non-small cell lung cancer. Accordingly, a randomized trial of lobectomy vs sublobar resection for biopsy specimen-proven, node-negative tumors measuring < 1 cm is ongoing.
An oncologic resection is not complete without staging the mediastinum. Recommendations for intraoperative staging can be found elsewhere.65
4.6.3.1 Recommendations
4.6.3.1.1. In the individual with a solid, indeterminate nodule that measures > 8 mm in diameter, we suggest surgical diagnosis in the following circumstances (Grade 2C):
When the clinical probability of malignancy is high (> 65%)
When the nodule is intensely hypermetabolic by PET or markedly positive by another functional imaging test
When nonsurgical biopsy is suspicious for malignancy
When a fully informed patient prefers undergoing a definitive diagnostic procedure.
4.6.3.1.2. In the individual with a solid, indeterminate nodule measuring ≥ 8 mm in diameter who chooses surgical diagnosis, we recommend thoracoscopy to obtain a diagnostic wedge resection (Grade 1C).
Remark: Use of advanced localization techniques or open thoracotomy may be necessary when resecting small or deep nodules.
5.0 Solid Nodules Measuring ≤ 8 mm in Diameter
On the basis of observations from lung cancer screening trials, the attenuation of nodules may be characterized as solid or subsolid. Subsolid nodules can be further classified as part-solid or pure ground glass (defined as focal densities in which underlying lung morphology is preserved). Part-solid and ground glass nodules are discussed subsequently. Solid nodules are the most frequently encountered type but least likely to be malignant among the three types.119,120
Small, solid nodules can be solitary or nonsolitary and are usually detected incidentally on a CT scan that has been ordered for some other reason. As is true for larger nodules, the likelihood of malignancy depends on patient risk factors, nodule size, and morphology.
5.1 Predictors of Malignancy
Patient characteristics have been incompletely studied as predictors of malignancy among individuals with solid nodules measuring ≤ 8 mm in diameter. In the Lung Screening Study, abnormal findings on a single low-dose CT screening examination were more common in current smokers and individuals who were aged at least 65 years.121 The likelihood of malignancy is probably highest in current smokers and lowest in nonsmokers who have nodules that are comparable in size. Extrapolation from studies in patients with larger nodules suggests that the risk of malignancy probably increases with age.26‐28
5.1.1 Size:
Studies of CT screening in volunteers at risk for lung cancer confirmed a strong association between nodule diameter and the likelihood of malignancy.39 Data from baseline screening in US trials of low-dose CT imaging showed that the probability of malignancy is extremely low (< 1%) in prevalent nodules that measure < 5 mm in diameter.121‐123 For nodules that measure 5 to 9 mm in diameter, the prevalence of malignancy ranges from 2.3% to 6%.121,123 In one Japanese study, the prevalence of malignancy in subcentimeter nodules was > 20%, which is considerably higher than in the US studies.124
Similar results have been reported in nonscreened populations evaluated by CT imaging. One retrospective review of 3,446 consecutive chest CT scans at a single institution identified 87 patients with incidentally detected lung nodules measuring < 10 mm in diameter and definitive 2-year follow-up. Although 10 (11%) of these nodules were malignant, nine proved to be metastases in patients with known extrathoracic malignancies (who comprised 56% of the study population).125 In a retrospective review of 414 patients with no history of neoplasm, infection, fibrosis, or immune deficiency and one or more noncalcified lung nodules measuring < 5 mm, none of the nodules were observed to grow over 3 to 24 months of follow-up.126 The upper boundaries of the 95% CIs for the probability of growth in these small nodules were 0.9%, 1.0%, and 1.3% at 3, 6, and 12 months, respectively.
5.2 Management Strategies
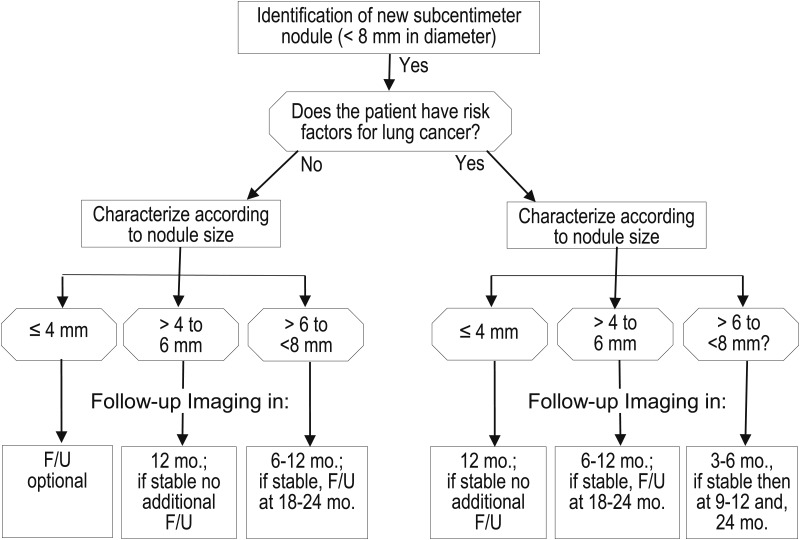
The optimal approach to the evaluation and management of solid nodules measuring ≤ 8 mm remains problematic. Small nodules are difficult to biopsy, and although evidence from a few small studies is decidedly mixed,127‐129 consensus holds that they are not reliably characterized by PET scan. Given the relatively low prevalence of malignancy, the risks of surgical diagnosis usually outweigh the benefits. Accordingly, solid, subcentimeter nodules are typically followed with serial CT scans. The frequency and duration of follow-up is guided by consensus-based recommendations first published by the Fleischner Society (Fig 6) and subsequently endorsed in the second edition of these guidelines.6,73 Decisions about the frequency and duration of follow-up for patients with small solid nodules need to weigh multiple considerations, including clinical risk factors; nodule size; the variable rate of nodule growth; the limited accuracy of available techniques for establishing growth by cross-sectional and volumetric measurements, especially for nodules that measure < 5 mm in size81,83,130; concerns regarding radiation dose24,131,132; risk factors for surgical complications; and cost.
Figure 6.
[Section 5.2] Management algorithm for individuals with solid nodules measuring < 8 mm in diameter. F/U =follow-up.
To date, recommendations from the Fleischner Society have not been subjected to formal validation, and limited data suggest that adherence may be suboptimal in some settings.133,134 Given the absence of new, high-quality evidence, our recommendations for follow-up of solid nodules that measure < 8 mm are unchanged. Recommendations specifically pertain to asymptomatic individuals with no history of extrathoracic malignancy. Once again, we reiterate that follow-up studies should be performed with the lowest possible radiation dose to minimize cumulative radiation exposure in individuals who require multiple follow-up CT examinations.
5.3 Recommendations
5.3.1. In the individual with a solid nodule that measures ≤ 8 mm in diameter and no risk factors for lung cancer, we suggest that the frequency and duration of CT surveillance be chosen according to the size of the nodule (Grade 2C):
Nodules measuring ≤ 4 mm in diameter need not be followed, but the patient should be informed about the potential benefits and harms of this approach
Nodules measuring > 4 mm to 6 mm should be reevaluated at 12 months without the need for additional follow-up if unchanged
Nodules measuring > 6 mm to 8 mm should be followed sometime between 6 and 12 months and then again at between 18 and 24 months if unchanged.
Remark: For the individual with multiple small, solid nodules, the frequency and duration of follow-up should be based on the size of the largest nodule.
Remark: CT surveillance of solid nodules ≤ 8 mm should use low-dose, noncontrast techniques.
5.3.2. In the individual with a solid nodule that measures ≤ 8 mm in diameter who has one or more risk factors for lung cancer, we suggest that the frequency and duration of CT surveillance be chosen according to the size of the nodule (Grade 2C):
Nodules measuring ≤ 4 mm in diameter should be reevaluated at 12 months without the need for additional follow-up if unchanged
Nodules measuring > 4 mm to 6 mm should be followed sometime between 6 and 12 months and then again between 18 and 24 months if unchanged
Nodules measuring > 6 mm to 8 mm should be followed initially sometime between 3 and 6 months, then subsequently between 9 and 12 months, and again at 24 months if unchanged.
Remark: For the individual with multiple small, solid nodules, the frequency and duration of follow-up should be based on the size of the largest nodule.
Remark: CT surveillance of solid nodules ≤ 8 mm should use low-dose, noncontrast techniques.
6.0 Subsolid Nodules
In this section, we make recommendations for evaluation and management of asymptomatic individuals with focal, rounded opacities that are subsolid, that is, either nonsolid (pure ground glass) or part solid (with a solid component but > 50% ground glass). Recommendations are predicated on several competing considerations, including the relatively high prevalence of premalignant and malignant disease, uncertainty about the sensitivity of PET scan and needle biopsy, challenges associated with measuring and identifying growth on serial CT scans, and the uncertain prognosis of untreated premalignant disease and AIS.
Among individuals with resected subsolid nodules, the prevalence of premalignant or malignant disease is high, although surgical series may be biased by selection of individuals with greater suspicion for malignancy. As summarized by Detterbeck and Homer,135 the frequency of AAH, AIS, and invasive adenocarcinoma vary by attenuation characteristics and size. Small (≤ 10 mm), pure ground glass nodules usually represent AAH or AIS; invasive adenocarcinoma is rare.136‐140 The frequency of invasive adenocarcinoma is greater for pure ground glass nodules measuring > 10 mm, reportedly varying from 10% to 50%.136‐139
AIS and invasive adenocarcinoma are especially prevalent in subsolid nodules that have a large (> 50%) solid component, and development of a solid component in a previously nonsolid nodule is strong presumptive evidence of invasive malignancy.119,137,141‐144 As is true for pure ground glass nodules, larger part-solid nodules are more likely to be malignant and invasive than smaller part-solid nodules.138‐140,143,145‐147 Clinical, pathologic, radiographic, and molecular features of pulmonary adenocarcinoma and its precursor lesions have been described in a recently revised classification scheme.148
Although attenuation characteristics and size are potentially helpful guides to predicting malignancy, some studies have reported counterintuitive results. One small study reported that factors associated with resolution of subsolid nodules detected by screening included larger size, a lobular border, polygonal shape, and partly solid (mixed) attenuation.19 Another study of nonresolving ground glass nodules, 75% of which were adenocarcinoma (invasive or in situ), found no differences among the size, shape, margin contour, and attenuation characteristics of malignant and benign nodules, although the study was probably underpowered to detect such differences.136
6.1 Functional Imaging With PET Scan
Many experts believe that subsolid nodules are not reliably characterized by PET scan,135,149 but only a few small studies addressed this question. In one study that included 15 nonsolid nodules, PET scan correctly identified only one of 10 malignant nodules and one of five benign nodules.150 A later study of 68 subsolid nodules reported that the sensitivity of a standardized uptake value of > 1.2 for identifying malignancy was 62%, whereas specificity at this threshold was 80%.151 In other studies of patients with lepidic growth-predominant adenocarcinoma (bronchioloalveolar cell carcinoma), the sensitivity of PET scan for identifying malignancy ranged from 47%152 to 60%153 to 89%.154 In these studies, sensitivity was higher for mixed and multifocal bronchioloalveolar cell carcinoma. Other studies reported that FDG uptake was inversely correlated with the extent of the lepidic component on pathologic analysis, highlighting the limitations of PET imaging in lepidic-predominant tumors.155‐157
Although false-negative PET scan results may be more common among individuals with subsolid nodules, absence of FDG avidity portends a favorable prognosis following surgical resection.152,157‐159 However, it is not certain whether this favorable prognosis extends to patients with nonavid nodules in whom resection is delayed by a period of observation.
6.2 Role of Nonsurgical Biopsy
Few studies have examined the accuracy of nonsurgical biopsy among individuals with subsolid nodules. In a study of 28 such individuals, CT scan fluoroscopy-guided needle biopsy had a sensitivity of 67% for identifying malignancy, although sensitivity was lower for pure ground glass nodules.160 Similarly, the diagnostic yield of CT scan-guided fine needle aspiration in another study was only 51% for 43 ground glass-predominant nodules compared with 76% for 53 solid-predominant nodules.161 However, in another study of 50 individuals with subsolid nodules, the sensitivity of CT scan-guided core biopsy was > 90%, regardless of nodule size or extent of the ground glass component.162 In another small study of 40 individuals with subsolid nodules, the diagnostic yield of CT scan-guided core needle biopsy was 84% (16 of 19), but two of three individuals with nondiagnostic results were subsequently found to have cancer.163 Hence, although TTNB appears to be less sensitive for subsolid than for solid nodules, it is still potentially useful, particularly for individuals who are at higher risk for surgical complications and those who wish to confirm malignancy before undergoing surgical resection. Although nonsurgical biopsy specimens can confirm the diagnosis of malignancy preoperatively in some cases, it should not be used to exclude malignancy in view of its imperfect sensitivity and limited negative predictive value.
6.3 CT Scan Surveillance
Measurement of subsolid nodules is challenging, and CT scan surveillance is confounded by measurement error, indistinct margins, and long VDTs. Although measurement techniques are improving, both manual and computer-assisted methods remain limited by poor reliability and lack of large-scale validation. Other sources of measurement error include breathing artifact and variable patient positioning.
The slow growth rates of most subsolid malignant nodules have implications for both the frequency and the duration of follow-up. In most cases, observed growth rates for subsolid malignant nodules range between 400 and 800 days, but doubling times as long as 1,500 days have been reported.164‐167 In a small study of 13 subsolid malignant nodules detected by screening, mean times for growth to be detectable (defined as the upper limit of agreement between readers) were 715, 673, and 425 days for measurements of diameter, volume, and mass, respectively.79 Although development of a solid component often is associated with progression to invasive adenocarcinoma, one study showed that both interreader and intrareader agreement for detection of a solid component were only modest.79
Controversy persists regarding how long follow-up should be continued for both part-solid and, especially, pure ground glass nodules.145,165,168 On the one hand, prognosis appears to be excellent for malignant nodules that are either pure ground glass138,169,170 or part solid,171‐175 even when treated by sublobar resection, raising the question of whether at least some of these nodules represent indolent cases of lung cancer that may not require treatment (ie, overdiagnosis). On the other hand, measurement challenges and the potential to take on a more-aggressive phenotype argue for greater caution. As a consequence, longer follow-up extending over several years may be appropriate, particularly when there is a history of lung cancer.176
6.4 Recommendations for Management
Informal recommendations for the management of subsolid nodules have been published previously,135,149 and guidelines from the Fleischner Society are forthcoming.177 The Fleischner Society recommends no follow-up for small (≤ 5 mm) pure ground glass nodules. For larger nonsolid lesions, it recommends an initial follow-up CT scan at 3 months followed by annual follow-up of at least 3 to 5 years. The Fleischner Society views part-solid nodules that persist over 3 months as malignant until proven otherwise, especially when the solid component measures > 5 mm in diameter. All three groups recommend against the use of either PET scan or needle biopsy in most cases, although the Fleischner Society recommends PET scan for part-solid nodules measuring at least 8 mm in diameter.
6.5 Recommendations
6.5.1. In the individual with a nonsolid (pure ground glass) nodule measuring ≤ 5 mm in diameter, we suggest no further evaluation (Grade 2C).
6.5.2. In the individual with a nonsolid (pure ground glass) nodule measuring > 5 mm in diameter, we suggest annual surveillance with chest CT for at least 3 years (Grade 2C).
Remark: CT surveillance of nonsolid nodules should use noncontrast techniques with thin sections through the nodule of interest.
Remark: Nonsolid nodules that grow or develop a solid component are often malignant, prompting further evaluation and/or consideration of resection.
Remark: Early follow-up at 3 months may be indicated for nonsolid nodules measuring > 10 mm (followed by nonsurgical biopsy and/or surgical resection for nodules that persist).
Remark: Limited duration or no follow-up may be preferred by individuals with life-limiting comorbidities in whom a low-grade malignancy would be of little consequence or by others who place a high value on avoiding treatment of possibly indolent lung cancer.
6.5.3. In the individual with a part-solid nodule measuring ≤ 8 mm in diameter, we suggest CT surveillance at approximately 3, 12, and 24 months, followed by annual CT surveillance for an additional 1 to 3 years (Grade 2C).
Remark: CT surveillance of part-solid nodules should use noncontrast techniques with thin sections through the nodule of interest.
Remark: Part-solid nodules that grow or develop a solid component are often malignant, prompting further evaluation and/or consideration of resection.
Remark: Limited duration or no follow-up may be preferred by individuals with life-limiting comorbidities in whom a low-grade malignancy would be of little consequence or by others who place a high value on avoiding treatment of possibly indolent lung cancer.
6.5.4. In the individual with a part-solid nodule measuring > 8 mm in diameter, we suggest repeat chest CT at 3 months, followed by further evaluation with PET, nonsurgical biopsy, and/or surgical resection for nodules that persist (Grade 2C).
Remark: PET should not be used to characterize part-solid lesions in which the solid component measures ≤ 8 mm.
Remark: Nonsurgical biopsy can be used to establish the diagnosis and/or be combined with wire, radioactive seed, or dye localization to facilitate subsequent resection. A nondiagnostic biopsy result does not exclude the possibility of malignancy.
Remark: Part-solid nodules measuring > 15 mm in diameter should proceed directly to further evaluation with PET, nonsurgical biopsy, and/or surgical resection.
7.0 Individuals With One or More Additional Nodules Detected During Nodule Evaluation
In individuals with known or suspected lung cancer, CT scan will frequently identify one or more additional nodules. Most of these additional nodules are benign. A study from Japan showed that 10% of patients with suspected lung cancer had a second nodule detected during subsequent evaluation, and 60% of these were benign at surgery.178 In another study, CT scan detected a second indeterminate nodule in 16% of patients with clinically operable stage I to IIIA non-small cell lung cancer.179 The nodules ranged in size from 4 to 12 mm, and although many of the nondominant nodules were lost to follow-up, > 85% of those with a definite diagnosis were benign.
Screening studies provide additional evidence that patients with a malignant nodule commonly will have additional benign nodules. In an uncontrolled study of CT scan screening in New York, 30% of the participants with cancer identified during baseline (prevalence) screening had one or more additional nodules at the time of detection.180 None of these were reported to be malignant after follow-up.181 In the Mayo Clinic screening study, > 50% of the 31 participants with cancer had other nodules detected, and all but one (a carcinoid tumor) were proven to be benign by absence of growth during follow-up.182 In these studies, the majority of secondary nodules measured < 4 mm, which implies a very low risk of malignancy. Therefore, although the likelihood of finding one or more additional nodules increases with the use of smaller slice thickness on CT scan, the majority of additional nodules will be benign.
When confronted with one or more additional nodules during nodule evaluation, it is prudent to consider each nodule individually rather than assuming that the additional nodules are either metastatic or benign. Preoperative PET scanning may help in the decision of whether more than one nodule is likely malignant and guide further evaluation, although many of these nodules will be too small to be reliably characterized by PET scan. Above all, candidates for curative treatment with known or suspected malignant nodules who have one or more additional nodules present should not be denied curative therapy unless metastasis is confirmed by histopathology. A more-detailed discussion is provided by Kozower et al183 in the in “Special Treatment Issues in Non-small Cell Lung Cancer” article of the ACCP Lung Cancer Guidelines.
7.1 Recommendation
7.1.1. In the individual with a dominant nodule and one or more additional small nodules, we suggest that each nodule be evaluated individually and curative treatment not be denied unless there is histopathological confirmation of metastasis (Grade 2C).
Remark: The classification and appropriate treatment of patients with more than one pulmonary focus of lung cancer is difficult and requires multidisciplinary consideration.
8.0 Conclusions and Recommendations for Research
The pulmonary nodule is increasingly common and remains a vexing problem. Individuals with solid nodules measuring > 8 mm should be managed by reviewing old imaging studies; estimating the probability of malignancy; performing imaging tests to better characterize the nodule; evaluating the risks associated with various management alternatives; and eliciting patient preferences for CT scan surveillance, nonsurgical biopsy, or surgical diagnosis. Solid nodules measuring ≤ 8 mm are infrequently malignant, difficult to biopsy, risky to resect, and not reliably characterized by PET scan or other functional imaging tests, leaving CT scan surveillance as the most appropriate option. At this time, the frequency and duration of surveillance are guided by expert consensus-based recommendations from the Fleischner Society. Subsolid nodules often are premalignant or malignant and may require extended-duration surveillance for growth or development of a solid component. Further research is needed to weigh the benefits and harms of alternative methods for evaluating both solid and subsolid nodules. Research priorities include developing and validating risk assessment models to estimate the probability of cancer among individuals with small nodules or subsolid nodules, performing studies that compare the benefits and harms of alternative management strategies among individuals stratified by cancer risk, determining the safety of CT scan surveillance by examining outcomes among individuals who choose this strategy, and developing and validating novel noninvasive biomarkers to facilitate diagnosis and determine prognosis.
Supplementary Material
Online Supplement 1
Online Supplement 2
Online Supplement 3
Online Supplement 4
Acknowledgments
Author contributions: Dr Gould had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Gould: contributed to the conception and design, acquisition of data, analysis and interpretation of data, formulation and approval of the recommendations, drafting of the manuscript, revision of the manuscript for important intellectual content, and final approval of the article.
Dr Donington: contributed to the conception and design, analysis and interpretation of data, formulation and approval of the recommendations, revision of the manuscript for important intellectual content, and final approval of the article.
Dr Lynch: contributed to the conception and design, analysis and interpretation of data, formulation and approval of the recommendations, revision of the manuscript for important intellectual content, and final approval of the article.
Dr Mazzone: contributed to the conception and design, acquisition of data, analysis and interpretation of data, formulation and approval of the recommendations, revision of the manuscript for important intellectual content, and final approval of the article.
Dr Midthun: contributed to the conception and design, analysis and interpretation of data, formulation and approval of the recommendations, revision of the manuscript for important intellectual content, and final approval of the article.
Dr Naidich: contributed to the conception and design, analysis and interpretation of data, formulation and approval of the recommendations, revision of the manuscript for important intellectual content, and final approval of the article.
Dr Wiener: contributed to the conception and design, acquisition of data, analysis and interpretation of data, formulation and approval of the recommendations, revision of the manuscript for important intellectual content, and final approval of the article.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Mazzone participated in an advisory board meeting organized by Oncimmune (USA) LLC and received research funding from Metabolomx. Dr Naidich has served as a consultant for law firms and for Siemens Medical Solutions in the development of methods for computer-assisted detection of lung nodules. Drs Gould, Donington, Lynch, Midthun, and Wiener have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of Sponsors: The American College of Chest Physicians was solely responsible for the development of these guidelines. The remaining supporters played no role in the development process. External supporting organizations cannot recommend panelists or topics, nor are they allowed prepublication access to the manuscripts and recommendations. Further details on the Conflict of Interest Policy are available online at http://chestnet.org.
Endorsements: This guideline is endorsed by the European Society of Thoracic Surgeons, Oncology Nursing Society, American Association for Bronchology and Interventional Pulmonology, and the Society of Thoracic Surgeons.
Other contributions: The authors thank the authors of the first and second editions of the ACCP lung cancer guidelines for their contributions to this article. They also thank Rebecca Diekemper, MPH; Joseph Ornelas, PhD(c); Belinda Ireland, MD; Peter Mestaz, MS; and Jackie Porcel for reviewing studies and creating evidence tables and Cynthia Bishop for downloading articles and assisting with manuscript preparation. This guideline is dedicated to the memory of Glen Lillington, MD, who inspired by virtue of his keen intelligence, perceptive wit, and abundant good nature.
Additional information: The supplement appendixes, figures, and tables can be found in the “Supplemental Materials” area of the online article.
Abbreviations
- AAH
atypical adenomatous hyperplasia
- ACCP
American College of Chest Physicians
- AIS
adenocarcinoma in situ
- EBUS
endobronchial ultrasound
- ENB
electromagnetic navigation bronchoscopy
- FDG
fluorodeoxyglucose
- HU
Hounsfield unit
- LR
likelihood ratio
- SPECT
single-photon emission CT
- TBB
transbronchial biopsy
- TTNB
transthoracic needle biopsy
- VATS
video-assisted thoracic surgery
- VBN
virtual bronchoscopy navigation
- VDT
volume doubling time
Footnotes
Funding/Sponsors: The overall process for the development of these guidelines, including matters pertaining to funding and conflicts of interest, are described in the methodology article.1 The development of this guideline was supported primarily by the American College of Chest Physicians. The lung cancer guidelines conference was supported in part by a grant from the Lung Cancer Research Foundation. The publication and dissemination of the guidelines was supported in part by a 2009 independent educational grant from Boehringer Ingelheim Pharmaceuticals, Inc.
COI grids reflecting the conflicts of interest that were current as of the date of the conference and voting are posted in the online supplementary materials.
Disclaimer: American College of Chest Physicians guidelines are intended for general information only, are not medical advice, and do not replace professional medical care and physician advice, which always should be sought for any medical condition. The complete disclaimer for this guideline can be accessed at http://dx.doi.org/10.1378/chest.1435S1.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
VATS = video-assisted thoracoscopic surgery.
FDG = fluorodeoxyglucose; NA = not applicable; TTNA = transthoracic needle aspiration.
aIn three studies, independent risk factors for malignancy included older age, current or former smoking, history of extrathoracic cancer > 5 y prior to nodule detection, larger nodule diameter, spiculated margins, and upper-lobe location26; older age, current or former smoking, shorter time since quitting smoking, and larger nodule diameter27; and high serum C-reactive protein level, high serum carcinoembryonic antigen level, absence of calcification, spiculation, and CT scan bronchus sign.28 In another study, the combination of smooth or lobulated borders, irregular shape, and solid attenuation had a negative predictive value of 86%.29
bApproximately 20% of observed cancers have decreased in size at least at some point during the observation period.
EBUS = endobronchial ultrasound; ENB = electromagnetic navigation bronchoscopy; VBN = virtual bronchoscopy navigation.
GRADE Working Group grades of evidence: High quality indicates that further research is very unlikely to change our confidence in the estimate of effect; moderate quality, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; and very-low quality, we are very uncertain about the estimate.
From Steinfort et al,l01 Roth et al,102 and Paone et al.103
GRADE = working group grades of evidence; RCT = randomized controlled trial; RR = relative risk; TBB = transbronchial biopsy. See Figure 3 legend for expansion of other abbreviation.
aApproximately 10% of patients were excluded from postrandomization.
bStatistically significant unexplained heterogeneity between studies.
cSurrogate outcome.
dPooled effect imprecise and consistent with either benefit or no effect.
eSeven of 16 studies in systematic review provided data for nodules measuring ≤ 25 mm.
fMean QUADAS (quality assessment of studies of diagnostic accuracy) score 3.3 (maximum, 6-14).
References
- 1.Lewis SZ, Diekemper R, Addrizzo-Harris DJ.Methodology for development of guidelines for lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest.2013;143(5)(suppl):41S-50S [DOI] [PubMed] [Google Scholar]
- 2.Tuddenham WJ.Glossary of terms for thoracic radiology: recommendations of the Nomenclature Committee of the Fleischner Society.AJR Am J Roentgenol.1984;143(3):509-517 [DOI] [PubMed] [Google Scholar]
- 3.Tuddenham WJ.Glossary of terms for thoracic radiology: recommendations of the Nomenclature Committee of the Fleischner Society.AJR Am J Roentgenol.1984;143(3):509-517 [DOI] [PubMed] [Google Scholar]
- 4.Rivera MP, Mehta AC, Wahidi MM.Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest.2013;143(5)(suppl):e142S-e165S [DOI] [PubMed] [Google Scholar]
- 5.Schünemann HJ, Oxman AD, Brozek J, et al. GRADE Working Group Grading quality of evidence and strength of recommendations for diagnostic tests and strategies [published correction in BMJ. 2008;336(7654)].BMJ.2008;336(7653):1106-1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gould MK, Fletcher J, Iannettoni MD, et al. American College of Chest Physicians. Evaluation of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132(suppl 3):108S-130S [DOI] [PubMed] [Google Scholar]
- 7.Good CA.Management of patient with solitary mass in lung.Chic Med Soc Bull.1953;55:893-896 [Google Scholar]
- 8.Zwirewich CV, Vedal S, Miller RR, Müller NL.Solitary pulmonary nodule: high-resolution CT and radiologic-pathologic correlation.Radiology.1991;179(2):469-476 [DOI] [PubMed] [Google Scholar]
- 9.Siegelman SS, Khouri NF, Scott WW, Jr, et al. Pulmonary hamartoma: CT findings.Radiology.1986;160(2):313-317 [DOI] [PubMed] [Google Scholar]
- 10.Siegelman SS, Khouri NF, Leo FP, Fishman EK, Braverman RM, Zerhouni EA.Solitary pulmonary nodules: CT assessment.Radiology.1986;160(2):307-312 [DOI] [PubMed] [Google Scholar]
- 11.Zerhouni EA, Stitik FP, Siegelman SS, et al. CT of the pulmonary nodule: a cooperative study.Radiology.1986;160(2):319-327 [DOI] [PubMed] [Google Scholar]
- 12.Zwirewich CV, Vedal S, Miller RR, Müller NL.Solitary pulmonary nodule: high-resolution CT and radiologic-pathologic correlation.Radiology.1991;179(2):469-476 [DOI] [PubMed] [Google Scholar]
- 13.Kishi K, Homma S, Kurosaki A, et al. Small lung tumors with the size of 1cm or less in diameter: clinical, radiological, and histopathological characteristics.Lung Cancer.2004;44(1):43-51 [DOI] [PubMed] [Google Scholar]
- 14.Woodring JH, Fried AM.Significance of wall thickness in solitary cavities of the lung: a follow-up study.AJR Am J Roentgenol.1983;140(3):473-474 [DOI] [PubMed] [Google Scholar]
- 15.Woodring JH, Fried AM.Significance of wall thickness in solitary cavities of the lung: a follow-up study.AJR Am J Roentgenol.1983;140(3):473-474 [DOI] [PubMed] [Google Scholar]
- 16.Harders SW, Madsen HH, Rasmussen TR, Hager H, Rasmussen F.High resolution spiral CT for determining the malignant potential of solitary pulmonary nodules: refining and testing the test.Acta Radiol.2011;52(4):401-409 [DOI] [PubMed] [Google Scholar]
- 17.Xu DM, van Klaveren RJ, de Bock GH, et al. Limited value of shape, margin and CT density in the discrimination between benign and malignant screen detected solid pulmonary nodules of the NELSON trial.Eur J Radiol.2008;68(2):347-352 [DOI] [PubMed] [Google Scholar]
- 18.Diederich S, Hansen J, Wormanns D.Resolving small pulmonary nodules: CT features.Eur Radiol.2005;15(10):2064-2069 [DOI] [PubMed] [Google Scholar]
- 19.Felix L, Serra-Tosio G, Lantuejoul S, et al. CT characteristics of resolving ground-glass opacities in a lung cancer screening programme.Eur J Radiol.2011;77(3):410-416 [DOI] [PubMed] [Google Scholar]
- 20.Kamiya H, Murayama S, Kakinohana Y, Miyara T.Pulmonary nodules: a quantitative method of diagnosis by evaluating nodule perimeter difference to approximate oval using three-dimensional CT images.Clin Imaging.2011;35(2):123-126 [DOI] [PubMed] [Google Scholar]
- 21.Mori K, Niki N, Kondo T, et al. Development of a novel computer-aided diagnosis system for automatic discrimination of malignant from benign solitary pulmonary nodules on thin-section dynamic computed tomography.J Comput Assist Tomogr.2005;29(2):215-222 [DOI] [PubMed] [Google Scholar]
- 22.Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007.Arch Intern Med.2009;169(22):2071-2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer.Arch Intern Med.2009;169(22):2078-2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayo JR, Aldrich J, Müller NL.Fleischner Society Radiation exposure at chest CT: a statement of the Fleischner Society.Radiology.2003;228(1):15-21 [DOI] [PubMed] [Google Scholar]
- 25.Brunelli A, Kim AW, Berger KI, Addrizzo-Harris DJ.Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest.2013;143(5)(suppl):e166S-e190S [DOI] [PubMed] [Google Scholar]
- 26.Swensen SJ, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES.The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules.Arch Intern Med.1997;157(8):849-855 [PubMed] [Google Scholar]
- 27.Gould MK, Ananth L, Barnett PG.Veterans Affairs SNAP Cooperative Study Group A clinical model to estimate the pretest probability of lung cancer in patients with solitary pulmonary nodules.Chest.2007;131(2):383-388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yonemori K, Tateishi U, Uno H, et al. Development and validation of diagnostic prediction model for solitary pulmonary nodules.Respirology.2007;12(6):856-862 [DOI] [PubMed] [Google Scholar]
- 29.Gurney JW, Lyddon DM, McKay JA.Determining the likelihood of malignancy in solitary pulmonary nodules with Bayesian analysis. Part II. Application.Radiology.1993;186(2):415-422 [DOI] [PubMed] [Google Scholar]
- 30.Cummings SR, Lillington GA, Richard RJ.Estimating the probability of malignancy in solitary pulmonary nodules. A Bayesian approach.Am Rev Respir Dis.1986;134(3):449-452 [DOI] [PubMed] [Google Scholar]
- 31.Gurney JW, Lyddon DM, McKay JA.Determining the likelihood of malignancy in solitary pulmonary nodules with Bayesian analysis. Part II. Application.Radiology.1993;186(2):415-422 [DOI] [PubMed] [Google Scholar]
- 32.Copp DH, Godwin JD, Kirby KA, Limaye AP.Clinical and radiologic factors associated with pulmonary nodule etiology in organ transplant recipients.Am J Transplant.2006;6(11):2759-2764 [DOI] [PubMed] [Google Scholar]
- 33.Herder GJ, van Tinteren H, Golding RP, et al. Clinical prediction model to characterize pulmonary nodules: validation and added value of 18F-fluorodeoxyglucose positron emission tomography.Chest.2005;128(4):2490-2496 [DOI] [PubMed] [Google Scholar]
- 34.Isbell JM, Deppen S, Putnam JB, Jr, et al. Existing general population models inaccurately predict lung cancer risk in patients referred for surgical evaluation.Ann Thorac Surg.2011;91(1):227-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schultz EM, Sanders GD, Trotter PR, et al. Validation of two models to estimate the probability of malignancy in patients with solitary pulmonary nodules.Thorax.2008;63(4):335-341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tammemagi MC, Freedman MT, Pinsky PF, et al. Prediction of true positive lung cancers in individuals with abnormal suspicious chest radiographs: a prostate, lung, colorectal, and ovarian cancer screening trial study. J Thorac Oncol. 2009;4(6):710-721 [DOI] [PubMed] [Google Scholar]
- 37.Yamashita K, Matsunobe S, Tsuda T, et al. Solitary pulmonary nodule: preliminary study of evaluation with incremental dynamic CT.Radiology.1995;194(2):399-405 [DOI] [PubMed] [Google Scholar]
- 38.Gould MK, Simkovich SM, Mestaz PJ, et al. Predicting the probability of malignancy in patients with pulmonary nodules: comparison of clinical judgment with two validated models.Am J Respir Crit Care Med.2012;185:A4425 [Google Scholar]
- 39.Wahidi MM, Govert JA, Goudar RK.Gould MK, McCrory DC; American College of Chest Physicians. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132(suppl 3):94S-107S. [DOI] [PubMed] [Google Scholar]
- 40.Swensen SJ, Viggiano RW, Midthun DE, et al. Lung nodule enhancement at CT: multicenter study.Radiology.2000;214(1):73-80 [DOI] [PubMed] [Google Scholar]
- 41.Yamashita K, Matsunobe S, Takahashi R, et al. Small peripheral lung carcinoma evaluated with incremental dynamic CT: radiologic-pathologic correlation.Radiology.1995;196(2):401-408 [DOI] [PubMed] [Google Scholar]
- 42.Yamashita K, Matsunobe S, Tsuda T, et al. Solitary pulmonary nodule: preliminary study of evaluation with incremental dynamic CT.Radiology.1995;194(2):399-405 [DOI] [PubMed] [Google Scholar]
- 43.Yamashita K, Matsunobe S, Tsuda T, et al. Intratumoral necrosis of lung carcinoma: a potential diagnostic pitfall in incremental dynamic computed tomography analysis of solitary pulmonary nodules?.J Thorac Imaging.1997;12(3):181-187 [PubMed] [Google Scholar]
- 44.Bai RJ, Cheng XG, Qu H, Shen BZ, Han MJ, Wu ZH.Solitary pulmonary nodules: comparison of multi-slice computed tomography perfusion study with vascular endothelial growth factor and microvessel density.Chin Med J (Engl).2009;122(5):541-547 [PubMed] [Google Scholar]
- 45.Zhang M, Kono M.Solitary pulmonary nodules: evaluation of blood flow patterns with dynamic CT.Radiology.1997;205(2):471-478 [DOI] [PubMed] [Google Scholar]
- 46.Bai RJ, Cheng XG, Qu H, Shen BZ, Han MJ, Wu ZH.Solitary pulmonary nodules: comparison of multi-slice computed tomography perfusion study with vascular endothelial growth factor and microvessel density.Chin Med J (Engl).2009;122(5):541-547 [PubMed] [Google Scholar]
- 47.Li Y, Yang ZG, Chen TW, Yu JQ, Sun JY, Chen HJ.First-pass perfusion imaging of solitary pulmonary nodules with 64-detector row CT: comparison of perfusion parameters of malignant and benign lesions.Br J Radiol.2010;83(993):785-790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cronin P, Dwamena BA, Kelly AM, Carlos RC.Solitary pulmonary nodules: meta-analytic comparison of cross-sectional imaging modalities for diagnosis of malignancy.Radiology.2008;246(3):772-782 [DOI] [PubMed] [Google Scholar]
- 49.Grgic A, Yüksel Y, Gröschel A, et al. Risk stratification of solitary pulmonary nodules by means of PET using (18)F-fluorodeoxyglucose and SUV quantification.Eur J Nucl Med Mol Imaging.2010;37(6):1087-1094 [DOI] [PubMed] [Google Scholar]
- 50.Kagna O, Solomonov A, Keidar Z, et al. The value of FDG-PET/CT in assessing single pulmonary nodules in patients at high risk of lung cancer.Eur J Nucl Med Mol Imaging.2009;36(6):997-1004 [DOI] [PubMed] [Google Scholar]
- 51.Mizugaki H, Shinagawa N, Kanegae K, et al. Combining transbronchial biopsy using endobronchial ultrasonography with a guide sheath and positron emission tomography for the diagnosis of small peripheral pulmonary lesions.Lung Cancer.2010;68(2):211-215 [DOI] [PubMed] [Google Scholar]
- 52.Mori T, Nomori H, Ikeda K, et al. Diffusion-weighted magnetic resonance imaging for diagnosing malignant pulmonary nodules/masses: comparison with positron emission tomography.J Thorac Oncol.2008;3(4):358-364 [DOI] [PubMed] [Google Scholar]
- 53.Ohba Y, Nomori H, Mori T, Shiraishi K, Namimoto T, Katahira K.Diffusion-weighted magnetic resonance for pulmonary nodules: 1.5 vs. 3 Tesla.Asian Cardiovasc Thorac Ann.2011;19(2):108-114 [DOI] [PubMed] [Google Scholar]
- 54.Ohba Y, Nomori H, Mori T, Shiraishi K, Namimoto T, Katahira K.Diffusion-weighted magnetic resonance for pulmonary nodules: 1.5 vs. 3 Tesla.Asian Cardiovasc Thorac Ann.2011;19(2):108-114 [DOI] [PubMed] [Google Scholar]
- 55.Fletcher JW, Kymes SM, Gould M, et al. VA SNAP Cooperative Studies Group. A comparison of the diagnostic accuracy of 18F-FDG PET and CT in the characterization of solitary pulmonary nodules. J Nucl Med. 2008;49(2):179-185 [DOI] [PubMed] [Google Scholar]
- 56.Gould MK, Sanders GD, Barnett PG, et al. Cost-effectiveness of alternative management strategies for patients with solitary pulmonary nodules.Ann Intern Med.2003;138(9):724-735 [DOI] [PubMed] [Google Scholar]
- 57.Gould MK, Sanders GD, Barnett PG, et al. Cost-effectiveness of alternative management strategies for patients with solitary pulmonary nodules.Ann Intern Med.2003;138(9):724-735 [DOI] [PubMed] [Google Scholar]
- 58.Berghmans T, Dusart M, Paesmans M, et al. European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol. 2008;3(1):6-12 [DOI] [PubMed] [Google Scholar]
- 59.Cheran SK, Nielsen ND, Patz EF., Jr False-negative findings for primary lung tumors on FDG positron emission tomography: staging and prognostic implications.AJR Am J Roentgenol.2004;182(5):1129-1132 [DOI] [PubMed] [Google Scholar]
- 60.Cheran SK, Nielsen ND, Patz EF., Jr False-negative findings for primary lung tumors on FDG positron emission tomography: staging and prognostic implications.AJR Am J Roentgenol.2004;182(5):1129-1132 [DOI] [PubMed] [Google Scholar]
- 61.Marom EM, Sarvis S, Herndon JE, II, Patz EF., Jr T1 lung cancers: sensitivity of diagnosis with fluorodeoxyglucose PET.Radiology.2002;223(2):453-459 [DOI] [PubMed] [Google Scholar]
- 62.Chang C-Y, Tzao C, Lee S-C, et al. Incremental value of integrated FDG-PET/CT in evaluating indeterminate solitary pulmonary nodule for malignancy.Mol Imaging Biol.2010;12(2):204-209 [DOI] [PubMed] [Google Scholar]
- 63.Wiener RS, Gould MK, Woloshin S, Schwartz LM, Clark JA.“What do you mean, a spot?”: A qualitative analysis of patients’ reactions to discussions with their doctors about pulmonary nodules.Chest.2013;143(3):672-677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim SK, Allen-Auerbach M, Goldin J, et al. Accuracy of PET/CT in characterization of solitary pulmonary lesions.J Nucl Med.2007;48(2):214-220 [PubMed] [Google Scholar]
- 65.Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest.2013;143(5)(suppl):e211S-e250S [DOI] [PubMed] [Google Scholar]
- 66.Brix G, Lechel U, Glatting G, et al. Radiation exposure of patients undergoing whole-body dual-modality 18F-FDG PET/CT examinations. J Nucl Med 2005;46(4):608-613. [PubMed] [Google Scholar]
- 67.Cummings SR, Lillington GA, Richard RJ.Managing solitary pulmonary nodules. The choice of strategy is a “close call”.Am Rev Respir Dis.1986;134(3):453-460 [DOI] [PubMed] [Google Scholar]
- 68.Wiener RS, Gould MK, Woloshin S, Schwartz LM, Clark JA.“What do you mean, a spot?”: A qualitative analysis of patients’ reactions to discussions with their doctors about pulmonary nodules.Chest.2013;143(3):672-677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaplan SH, Gandek B, Greenfield S, Rogers W, Ware JE.Patient and visit characteristics related to physicians’ participatory decision-making style. Results from the Medical Outcomes Study.Med Care.1995;33(12):1176-1187 [DOI] [PubMed] [Google Scholar]
- 70.Geddes DM.The natural history of lung cancer: a review based on rates of tumour growth.Br J Dis Chest.1979;73(1):1-17 [PubMed] [Google Scholar]
- 71.Nathan MH, Collins VP, Adams RA.Differentiation of benign and malignant pulmonary nodules by growth rate.Radiology.1962;79:221-232 [DOI] [PubMed] [Google Scholar]
- 72.Nathan MH, Collins VP, Adams RA.Differentiation of benign and malignant pulmonary nodules by growth rate.Radiology.1962;79:221-232 [DOI] [PubMed] [Google Scholar]
- 73.MacMahon H, Austin JH, Gamsu G, et al. Fleischner Society Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society.Radiology.2005;237(2):395-400 [DOI] [PubMed] [Google Scholar]
- 74.O’Rourke N, Edwards R.Lung cancer treatment waiting times and tumour growth.Clin Oncol (R Coll Radiol).2000;12(3):141-144 [DOI] [PubMed] [Google Scholar]
- 75.Ost DE, Yeung S-J, Tanoue LT, Gould MK.Clinical and organizational factors in the initial evaluation of patients with lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest.2013;143(5)(suppl):e121S-e141S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Olsson JK, Schultz EM, Gould MK.Timeliness of care in patients with lung cancer: a systematic review.Thorax.2009;64(9):749-756 [DOI] [PubMed] [Google Scholar]
- 77.Jennings SG, Winer-Muram HT, Tann M, Ying J, Dowdeswell I.Distribution of stage I lung cancer growth rates determined with serial volumetric CT measurements.Radiology.2006;241(2):554-563 [DOI] [PubMed] [Google Scholar]
- 78.Revel MP, Merlin A, Peyrard S, et al. Software volumetric evaluation of doubling times for differentiating benign versus malignant pulmonary nodules.AJR Am J Roentgenol.2006;187(1):135-142 [DOI] [PubMed] [Google Scholar]
- 79.de Hoop B, Gietema H, van de Vorst S, Murphy K, van Klaveren RJ, Prokop M.Pulmonary ground-glass nodules: increase in mass as an early indicator of growth.Radiology.2010;255(1):199-206 [DOI] [PubMed] [Google Scholar]
- 80.Korst RJ, Lee BE, Krinsky GA, Rutledge JR.The utility of automated volumetric growth analysis in a dedicated pulmonary nodule clinic.J Thorac Cardiovasc Surg.2011;142(2):372-377 [DOI] [PubMed] [Google Scholar]
- 81.Jennings SG, Winer-Muram HT, Tarver RD, Farber MO.Lung tumor growth: assessment with CT—comparison of diameter and cross-sectional area with volume measurements.Radiology.2004;231(3):866-871 [DOI] [PubMed] [Google Scholar]
- 82.Nietert PJ, Ravenel JG, Leue WM, et al. Imprecision in automated volume measurements of pulmonary nodules and its effect on the level of uncertainty in volume doubling time estimation.Chest.2009;135(6):1580-1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Revel MP, Bissery A, Bienvenu M, Aycard L, Lefort C, Frija G.Are two-dimensional CT measurements of small noncalcified pulmonary nodules reliable?.Radiology.2004;231(2):453-458 [DOI] [PubMed] [Google Scholar]
- 84.Marchianò A, Calabrò E, Civelli E, et al. Pulmonary nodules: volume repeatability at multidetector CT lung cancer screening.Radiology.2009;251(3):919-925 [DOI] [PubMed] [Google Scholar]
- 85.Lindell RM, Hartman TE, Swensen SJ, Jett JR, Midthun DE, Mandrekar JN.5-year lung cancer screening experience: growth curves of 18 lung cancers compared to histologic type, CT attenuation, stage, survival, and size.Chest.2009;136(6):1586-1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kothary N, Lock L, Sze DY, Hofmann LV.Computed tomography-guided percutaneous needle biopsy of pulmonary nodules: impact of nodule size on diagnostic accuracy.Clin Lung Cancer.2009;10(5):360-363 [DOI] [PubMed] [Google Scholar]
- 87.Ng YL, Patsios D, Roberts H, et al. CT-guided percutaneous fine-needle aspiration biopsy of pulmonary nodules measuring 10 mm or less.Clin Radiol.2008;63(3):272-277 [DOI] [PubMed] [Google Scholar]
- 88.Mazza E, Maddau C, Ricciardi A, Falchini M, Matucci M, Ciarpallini T.On-site evaluation of percutaneous CT-guided fine needle aspiration of pulmonary lesions. A study of 321 cases.Radiol Med (Torino).2005;110(3):141-148 [PubMed] [Google Scholar]
- 89.Wiener RS, Schwartz LM, Woloshin S, Welch HG.Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records.Ann Intern Med.2011;155(3):137-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mazza E, Maddau C, Ricciardi A, Falchini M, Matucci M, Ciarpallini T.On-site evaluation of percutaneous CT-guided fine needle aspiration of pulmonary lesions. A study of 321 cases.Radiol Med (Torino).2005;110(3):141-148 [PubMed] [Google Scholar]
- 91.Rizzo S, Preda L, Raimondi S, et al. Risk factors for complications of CT-guided lung biopsies.Radiol Med (Torino).2011;116(4):548-563 [DOI] [PubMed] [Google Scholar]
- 92.Chakrabarti B, Earis JE, Pandey R, et al. Risk assessment of pneumothorax and pulmonary haemorrhage complicating percutaneous co-axial cutting needle lung biopsy.Respir Med.2009;103(3):449-455 [DOI] [PubMed] [Google Scholar]
- 93.Wang HJ, Leung TK, Lee CM, Chen YY.Three-step needle withdrawal method: a modified technique for reducing the rate of pneumothorax after CT-guided lung biopsy.Chang Gung Med J.2009;32(4):432-437 [PubMed] [Google Scholar]
- 94.Wang HJ, Leung TK, Lee CM, Chen YY.Three-step needle withdrawal method: a modified technique for reducing the rate of pneumothorax after CT-guided lung biopsy.Chang Gung Med J.2009;32(4):432-437 [PubMed] [Google Scholar]
- 95.Guimarães MD, Andrade MQ, Fonte AC, Benevides G, Chojniak R, Gross JL.Predictive complication factors for CT-guided fine needle aspiration biopsy of pulmonary lesions.Clinics (Sao Paulo).2010;65(9):847-850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yamagami T, Terayama K, Yoshimatsu R, Matsumoto T, Miura H, Nishimura T.Role of manual aspiration in treating pneumothorax after computed tomography-guided lung biopsy.Acta Radiol.2009;50(10):1126-1133 [DOI] [PubMed] [Google Scholar]
- 97.Ayyappan AP, Souza CA, Seely J, Peterson R, Dennie C, Matzinger F.Ultrathin fine-needle aspiration biopsy of the lung with transfissural approach: does it increase the risk of pneumothorax?.AJR Am J Roentgenol.2008;191(6):1725-1729 [DOI] [PubMed] [Google Scholar]
- 98.Rivera MP, Mehta AC.American College of Chest Physicians. Initial diagnosis of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132(suppl 3):131S-148S [DOI] [PubMed] [Google Scholar]
- 99.Naidich DP, Sussman R, Kutcher WL, Aranda CP, Garay SM, Ettenger NA.Solitary pulmonary nodules. CT-bronchoscopic correlation.Chest.1988;93(3):595-598 [DOI] [PubMed] [Google Scholar]
- 100.Bandoh S, Fujita J, Tojo Y, et al. Diagnostic accuracy and safety of flexible bronchoscopy with multiplanar reconstruction images and ultrafast Papanicolaou stain: evaluating solitary pulmonary nodules.Chest.2003;124(5):1985-1992 [DOI] [PubMed] [Google Scholar]
- 101.Steinfort DP, Khor YH, Manser RL.Irving LB. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: systematic review and meta-analysis. Eur Respir J. 2011;37(4):902-910 [DOI] [PubMed] [Google Scholar]
- 102.Roth K, Eagan TM, Andreassen AH, Leh F, Hardie JA.A randomised trial of endobronchial ultrasound guided sampling in peripheral lung lesions.Lung Cancer.2011;74(2):219-225 [DOI] [PubMed] [Google Scholar]
- 103.Paone G, Nicastri E, Lucantoni G, et al. Endobronchial ultrasound-driven biopsy in the diagnosis of peripheral lung lesions.Chest.2005;128(5):3551-3557 [DOI] [PubMed] [Google Scholar]
- 104.Schwarz Y, Greif J, Becker HD, Ernst A, Mehta A.Real-time electromagnetic navigation bronchoscopy to peripheral lung lesions using overlaid CT images: the first human study.Chest.2006;129(4):988-994 [DOI] [PubMed] [Google Scholar]
- 105.Gildea TR, Mazzone PJ, Karnak D, Meziane M, Mehta AC.Electromagnetic navigation diagnostic bronchoscopy: a prospective study.Am J Respir Crit Care Med.2006;174(9):982-989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Eberhardt R, Anantham D, Ernst A, Feller-Kopman D, Herth F.Multimodality bronchoscopic diagnosis of peripheral lung lesions: a randomized controlled trial.Am J Respir Crit Care Med.2007;176(1):36-41 [DOI] [PubMed] [Google Scholar]
- 107.Allen MS, Deschamps C, Lee RE, Trastek VF, Daly RC, Pairolero PC.Video-assisted thoracoscopic stapled wedge excision for indeterminate pulmonary nodules.J Thorac Cardiovasc Surg.1993;106(6):1048-1052 [PubMed] [Google Scholar]
- 108.Wang Memoli JS, Nietert PJ, Silvestri GA.Meta-Analysis of guided bronchoscopy for the evaluation of the pulmonary nodule.Chest.2012;142(2):385-393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Allen MS, Deschamps C, Lee RE, Trastek VF, Daly RC, Pairolero PC.Video-assisted thoracoscopic stapled wedge excision for indeterminate pulmonary nodules.J Thorac Cardiovasc Surg.1993;106(6):1048-1052 [PubMed] [Google Scholar]
- 110.Mack MJ, Hazelrigg SR, Landreneau RJ.Acuff TE. Thoracoscopy for the diagnosis of the indeterminate solitary pulmonary nodule. Ann Thorac Surg. 1993;56(4):825-830 [DOI] [PubMed] [Google Scholar]
- 111.Zaman M, Bilal H, Woo CY, Tang A.In patients undergoing video-assisted thoracoscopic surgery excision, what is the best way to locate a subcentimetre solitary pulmonary nodule in order to achieve successful excision?.Interact Cardiovasc Thorac Surg.2012;15(2):266-272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Marchevsky AM, Changsri C, Gupta I, Fuller C, Houck W, McKenna RJ., Jr Frozen section diagnoses of small pulmonary nodules: accuracy and clinical implications.Ann Thorac Surg.2004;78(5):1755-1759 [DOI] [PubMed] [Google Scholar]
- 113.Martini N, Ginsberg RJ.Aisner R, Arriga R, Green N.Treatment of stage I and stage II disease. Chapter 14.In.eds. The Comprehensive Textbook of Thoracic Oncology Baltimore, MD: Williams and Wilkens; 1996;338-350 [Google Scholar]
- 114.Boffa DJ, Allen MS, Grab JD, Gaissert HA, Harpole DH, Wright CD.Data from The Society of Thoracic Surgeons General Thoracic Surgery database: the surgical management of primary lung tumors.J Thorac Cardiovasc Surg.2008;135(2):247-254 [DOI] [PubMed] [Google Scholar]
- 115.Gopaldas RR, Bakaeen FG, Dao TK, Walsh GL, Swisher SG, Chu D.Video-assisted thoracoscopic versus open thoracotomy lobectomy in a cohort of 13,619 patients.[published correction appears in Ann Thorac Surg. 2011;92(3):1162] Ann Thorac Surg.2010;89(5):1563-1570 [DOI] [PubMed] [Google Scholar]
- 116.Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database.J Thorac Cardiovasc Surg.2010;139(2):366-378 [DOI] [PubMed] [Google Scholar]
- 117.Henschke CI, Yankelevitz DF, Mirtcheva R, McGuinness G, McCauley D, Miettinen OS.ELCAP Group CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules.AJR Am J Roentgenol.2002;178(5):1053-1057 [DOI] [PubMed] [Google Scholar]
- 118.Warren WH, Faber LP.Segmentectomy versus lobectomy in patients with stage I pulmonary carcinoma. Five-year survival and patterns of intrathoracic recurrence.J Thorac Cardiovasc Surg.1994;107(4):1087-1093 [PubMed] [Google Scholar]
- 119.Henschke CI, Yankelevitz DF, Mirtcheva R, McGuinness G, McCauley D, Miettinen OS.ELCAP Group CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules.AJR Am J Roentgenol.2002;178(5):1053-1057 [DOI] [PubMed] [Google Scholar]
- 120.Li F, Sone S, Abe H, Macmahon H, Doi K.Malignant versus benign nodules at CT screening for lung cancer: comparison of thin-section CT findings.Radiology.2004;233(3):793-798 [DOI] [PubMed] [Google Scholar]
- 121.Swensen SJ, Jett JR, Sloan JA, et al. Screening for lung cancer with low-dose spiral computed tomography.Am J Respir Crit Care Med.2002;165(4):508-513 [DOI] [PubMed] [Google Scholar]
- 122.Swensen SJ, Jett JR, Sloan JA, et al. Screening for lung cancer with low-dose spiral computed tomography.Am J Respir Crit Care Med.2002;165(4):508-513 [DOI] [PubMed] [Google Scholar]
- 123.Henschke CI, Yankelevitz DF, Naidich DP, et al. CT screening for lung cancer: suspiciousness of nodules according to size on baseline scans.Radiology.2004;231(1):164-168 [DOI] [PubMed] [Google Scholar]
- 124.Suzuki K, Nagai K, Yoshida J, et al. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: indications for preoperative marking.Chest.1999;115(2):563-568 [DOI] [PubMed] [Google Scholar]
- 125.Benjamin MS, Drucker EA, McLoud TC, Shepard JA.Small pulmonary nodules: detection at chest CT and outcome.Radiology.2003;226(2):489-493 [DOI] [PubMed] [Google Scholar]
- 126.Piyavisetpat N, Aquino SL, Hahn PF, Halpern EF, Thrall JH.Small incidental pulmonary nodules: how useful is short-term interval CT follow-up?.J Thorac Imaging.2005;20(1):5-9 [DOI] [PubMed] [Google Scholar]
- 127.Divisi D, Di Tommaso S, Di Leonardo G, Brianzoni E, De Vico A, Crisci R.18-fluorine fluorodeoxyglucose positron emission tomography with computerized tomography versus computerized tomography alone for the management of solitary lung nodules with diameters inferior to 1.5 cm.Thorac Cardiovasc Surg.2010;58(7):422-426 [DOI] [PubMed] [Google Scholar]
- 128.Herder GJ, Golding RP, Hoekstra OS, et al. The performance of (18)F-fluorodeoxyglucose positron emission tomography in small solitary pulmonary nodules.Eur J Nucl Med Mol Imaging.2004;31(9):1231-1236 [DOI] [PubMed] [Google Scholar]
- 129.Nomori H, Watanabe K, Ohtsuka T, et al. Fluorine 18-tagged fluorodeoxyglucose positron emission tomographic scanning to predict lymph node metastasis, invasiveness, or both, in clinical T1 N0 M0 lung adenocarcinoma.J Thorac Cardiovasc Surg.2004;128(3):396-401 [DOI] [PubMed] [Google Scholar]
- 130.Rusinek H, Naidich DP, McGuinness G, et al. Pulmonary nodule detection: low-dose versus conventional CT.Radiology.1998;209(1):243-249 [DOI] [PubMed] [Google Scholar]
- 131.Rusinek H, Naidich DP, McGuinness G, et al. Pulmonary nodule detection: low-dose versus conventional CT.Radiology.1998;209(1):243-249 [DOI] [PubMed] [Google Scholar]
- 132.Diederich S, Lenzen H.Radiation exposure associated with imaging of the chest: comparison of different radiographic and computed tomography techniques.Cancer.2000;8911):2457-2460 [DOI] [PubMed] [Google Scholar]
- 133.Eisenberg RL, Bankier AA, Boiselle PM.Compliance with Fleischner Society guidelines for management of small lung nodules: a survey of 834 radiologists.Radiology.2010;255(1):218-224 [DOI] [PubMed] [Google Scholar]
- 134.Wiener RS, Gould MK, Slatore CG, et al. Evaluation of potentially malignant pulmonary nodules in the VA system: resource use and adherence to guideline recommendations.Poster session presented at: VA HSR&D/QUERI National Meeting; July 18, 2012;National Harbor, MD [Google Scholar]
- 135.Detterbeck FC, Homer RJ.Approach to the ground-glass nodule.Clin Chest Med.2011;32(4):799-810 [DOI] [PubMed] [Google Scholar]
- 136.Kim HY, Shim YM, Lee KS, Han J, Yi CA, Kim YK.Persistent pulmonary nodular ground-glass opacity at thin-section CT: histopathologic comparisons.Radiology.2007;245(1):267-275 [DOI] [PubMed] [Google Scholar]
- 137.Kodama K, Higashiyama M, Takami K, et al. Treatment strategy for patients with small peripheral lung lesion(s): intermediate-term results of prospective study. Eur J Cardiothorac Surg. 2008;34(5):1068-1074 [DOI] [PubMed] [Google Scholar]
- 138.Mun M, Kohno T.Efficacy of thoracoscopic resection for multifocal bronchioloalveolar carcinoma showing pure ground-glass opacities of 20 mm or less in diameter.J Thorac Cardiovasc Surg.2007;134(4):877-882 [DOI] [PubMed] [Google Scholar]
- 139.Nakata M, Sawada S, Saeki H, et al. Prospective study of thoracoscopic limited resection for ground-glass opacity selected by computed tomography.Ann Thorac Surg.2003;75(5):1601-1605 [DOI] [PubMed] [Google Scholar]
- 140.Ohtsuka T, Watanabe K, Kaji M, Naruke T, Suemasu K. A clinicopathological study of resected pulmonary nodules with focal pure ground-glass opacity. Eur J Cardiothorac Sur. 2006;30(1):160-163 [DOI] [PubMed] [Google Scholar]
- 141.Carretta A, Ciriaco P, Melloni G, et al. Surgical treatment of multiple primary adenocarcinomas of the lung.Thorac Cardiovasc Surg.2009;57(1):30-34 [DOI] [PubMed] [Google Scholar]
- 142.Ikeda K, Awai K, Mori T, Kawanaka K, Yamashita Y, Nomori H.Differential diagnosis of ground-glass opacity nodules: CT number analysis by three-dimensional computerized quantification.Chest.2007;132(3):984-990 [DOI] [PubMed] [Google Scholar]
- 143.Takashima S, Maruyama Y, Hasegawa M, et al. CT findings and progression of small peripheral lung neoplasms having a replacement growth pattern.AJR Am J Roentgenol.2003;180(3):817-826 [DOI] [PubMed] [Google Scholar]
- 144.Takashima S, Maruyama Y, Hasegawa M, et al. CT findings and progression of small peripheral lung neoplasms having a replacement growth pattern.AJR Am J Roentgenol.2003;180(3):817-826 [DOI] [PubMed] [Google Scholar]
- 145.Kodama K, Higashiyama M, Yokouchi H, et al. Natural history of pure ground-glass opacity after long-term follow-up of more than 2 years.Ann Thorac Surg.2002;73(2):386-392 [DOI] [PubMed] [Google Scholar]
- 146.Nakata M, Saeki H, Takata I, et al. Focal ground-glass opacity detected by low-dose helical CT.Chest.2002;121(5):1464-1467 [DOI] [PubMed] [Google Scholar]
- 147.Park CM, Goo JM, Kim TJ, et al. Pulmonary nodular ground-glass opacities in patients with extrapulmonary cancers: what is their clinical significance and how can we determine whether they are malignant or benign lesions?.Chest.2008;133(6):1402-1409 [DOI] [PubMed] [Google Scholar]
- 148.Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6(2):244-285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Godoy MC, Naidich DP.Subsolid pulmonary nodules and the spectrum of peripheral adenocarcinomas of the lung: recommended interim guidelines for assessment and management.Radiology.2009;253(3):606-622 [DOI] [PubMed] [Google Scholar]
- 150.Nomori H, Watanabe K, Ohtsuka T, Naruke T, Suemasu K, Uno K.Evaluation of F-18 fluorodeoxyglucose (FDG) PET scanning for pulmonary nodules less than 3 cm in diameter, with special reference to the CT images.Lung Cancer.2004;45(1):19-27 [DOI] [PubMed] [Google Scholar]
- 151.Chun EJ, Lee HJ, Kang WJ, et al. Differentiation between malignancy and inflammation in pulmonary ground-glass nodules: The feasibility of integrated (18)F-FDG PET/CT.Lung Cancer.2009;65(2):180-186 [DOI] [PubMed] [Google Scholar]
- 152.Raz DJ, Odisho AY, Franc BL, Jablons DM.Tumor fluoro-2-deoxy-D-glucose avidity on positron emission tomographic scan predicts mortality in patients with early-stage pure and mixed bronchioloalveolar carcinoma.J Thorac Cardiovasc Surg.2006;132(5):1189-1195 [DOI] [PubMed] [Google Scholar]
- 153.Heyneman LE, Patz EF.PET imaging in patients with bronchioloalveolar cell carcinoma.Lung Cancer.2002;38(3):261-266 [DOI] [PubMed] [Google Scholar]
- 154.Yap CS, Schiepers C, Fishbein MC, Phelps ME, Czernin J.FDG-PET imaging in lung cancer: how sensitive is it for bronchioloalveolar carcinoma?.Eur J Nucl Med Mol Imaging.2002;29(9):1166-1173 [DOI] [PubMed] [Google Scholar]
- 155.Casali C, Cucca M, Rossi G, et al. The variation of prognostic significance of Maximum Standardized Uptake Value of [18F]-fluoro-2-deoxy-glucose positron emission tomography in different histological subtypes and pathological stages of surgically resected Non-Small Cell Lung Carcinoma.Lung Cancer.2010;69(2):187-193 [DOI] [PubMed] [Google Scholar]
- 156.Maeda R, Isowa N, Onuma H, et al. The maximum standardized uptake values on positron emission tomography to predict the Noguchi classification and invasiveness in clinical stage IA adenocarcinoma measuring 2 cm or less in size.Interact Cardiovasc Thorac Surg.2009;9(1):70-73 [DOI] [PubMed] [Google Scholar]
- 157.Okada M, Tauchi S, Iwanaga K, et al. Associations among bronchioloalveolar carcinoma components, positron emission tomographic and computed tomographic findings, and malignant behavior in small lung adenocarcinomas.J Thorac Cardiovasc Surg.2007;133(6):1448-1454 [DOI] [PubMed] [Google Scholar]
- 158.Lee HY, Han J, Lee KS, et al. Lung adenocarcinoma as a solitary pulmonary nodule: prognostic determinants of CT, PET, and histopathologic findings.Lung Cancer.2009;66(3):379-385 [DOI] [PubMed] [Google Scholar]
- 159.Watanabe K, Nomori H, Ohtsuka T, et al. [F-18]Fluorodeoxyglucose positron emission tomography can predict pathological tumor stage and proliferative activity determined by Ki-67 in clinical stage IA lung adenocarcinomas.Jpn J Clin Oncol.2006;36(7):403-409 [DOI] [PubMed] [Google Scholar]
- 160.Hur J, Lee HJ, Nam JE, et al. Diagnostic accuracy of CT fluoroscopy-guided needle aspiration biopsy of ground-glass opacity pulmonary lesions.AJR Am J Roentgenol.2009;192(3):629-634 [DOI] [PubMed] [Google Scholar]
- 161.Shimizu K, Ikeda N, Tsuboi M, Hirano T, Kato H.Percutaneous CT-guided fine needle aspiration for lung cancer smaller than 2 cm and revealed by ground-glass opacity at CT.Lung Cancer.2006;51(2):173-179 [DOI] [PubMed] [Google Scholar]
- 162.Hasegawa M, Sone S, Takashima S, et al. Growth rate of small lung cancers detected on mass CT screening.Br J Radiol.2000;73(876):1252-1259 [DOI] [PubMed] [Google Scholar]
- 163.Infante M, Lutman RF, Imparato S, et al. Differential diagnosis and management of focal ground-glass opacities. Eur Respir J 2009;33(4):821-827. [DOI] [PubMed] [Google Scholar]
- 164.Aoki T, Nakata H, Watanabe H, et al. Evolution of peripheral lung adenocarcinomas: CT findings correlated with histology and tumor doubling time.AJR Am J Roentgenol.2000;174(3):763-768 [DOI] [PubMed] [Google Scholar]
- 165.Hasegawa M, Sone S, Takashima S, et al. Growth rate of small lung cancers detected on mass CT screening.Br J Radiol.2000;73(876):1252-1259 [DOI] [PubMed] [Google Scholar]
- 166.Lindell RM, Hartman TE, Swensen SJ, et al. Five-year lung cancer screening experience: CT appearance, growth rate, location, and histologic features of 61 lung cancers.Radiology.2007;242(2):555-562 [DOI] [PubMed] [Google Scholar]
- 167.Sone S, Nakayama T, Honda T, et al. CT findings of early-stage small cell lung cancer in a low-dose CT screening programme.Lung Cancer.2007;56(2):207-215 [DOI] [PubMed] [Google Scholar]
- 168.Kakinuma R, Ohmatsu H, Kaneko M, et al. Progression of focal pure ground-glass opacity detected by low-dose helical computed tomography screening for lung cancer.J Comput Assist Tomogr.2004;28(1):17-23 [DOI] [PubMed] [Google Scholar]
- 169.Nakamura H, Saji H, Ogata A, Saijo T, Okada S, Kato H.Lung cancer patients showing pure ground-glass opacity on computed tomography are good candidates for wedge resection.Lung Cancer.2004;44(1):61-68 [DOI] [PubMed] [Google Scholar]
- 170.Yoshida J, Nagai K, Yokose T, et al. Limited resection trial for pulmonary ground-glass opacity nodules: fifty-case experience.J Thorac Cardiovasc Surg.2005;129(5):991-996 [DOI] [PubMed] [Google Scholar]
- 171.Ichiki Y, Hanagiri T, Baba T, et al. Limited pulmonary resection for peripheral small-sized adenocarcinoma of the lung.Int J Surg.2011;9(2):155-159 [DOI] [PubMed] [Google Scholar]
- 172.Ikeda N, Maeda J, Yashima K, et al. A clinicopathological study of resected adenocarcinoma 2 cm or less in diameter.Ann Thorac Surg.2004;78(3):1011-1016 [DOI] [PubMed] [Google Scholar]
- 173.Koike T, Togashi K, Shirato T, et al. Limited resection for noninvasive bronchioloalveolar carcinoma diagnosed by intraoperative pathologic examination.Ann Thorac Surg.2009;88(4):1106-1111 [DOI] [PubMed] [Google Scholar]
- 174.Kondo T, Yamada K, Noda K, Nakayama H, Kameda Y.Radiologic-prognostic correlation in patients with small pulmonary adenocarcinomas.Lung Cancer.2002;36(1):49-57 [DOI] [PubMed] [Google Scholar]
- 175.Okada M, Nishio W, Sakamoto T, Uchino K, Tsubota N.Discrepancy of computed tomographic image between lung and mediastinal windows as a prognostic implication in small lung adenocarcinoma. Ann Thorac Surg. 2003;76(6):1828-1832 [DOI] [PubMed] [Google Scholar]
- 176.Hiramatsu M, Inagaki T, Matsui Y, et al. Pulmonary ground-glass opacity (GGO) lesions-large size and a history of lung cancer are risk factors for growth. J Thorac Oncol. 2008;3(11):1245-1250 [DOI] [PubMed] [Google Scholar]
- 177.Naidich DP.Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology. 2013;266(1):304-317 [DOI] [PubMed] [Google Scholar]
- 178.Kunitoh H, Eguchi K, Yamada K, et al. Intrapulmonary sublesions detected before surgery in patients with lung cancer.Cancer.1992;70(7):1876-1879 [DOI] [PubMed] [Google Scholar]
- 179.Keogan MT, Tung KT, Kaplan DK, Goldstraw PJ, Hansell DM.The significance of pulmonary nodules detected on CT staging for lung cancer.Clin Radiol.1993;48(2):94-96 [DOI] [PubMed] [Google Scholar]
- 180.Henschke CI, McCauley DI, Yankelevitz DF, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening.Lancet.1999;354(9173):99-105 [DOI] [PubMed] [Google Scholar]
- 181.Henschke CI, Naidich DP, Yankelevitz DF, et al. Early lung cancer action project: initial findings on repeat screenings.Cancer.2001;92(1):153-159 [DOI] [PubMed] [Google Scholar]
- 182.Swensen SJ, Jett JR, Hartman TE, et al. CT screening for lung cancer: five-year prospective experience.Radiology.2005;235(1):259-265 [DOI] [PubMed] [Google Scholar]
- 183.Kozower BD, Larner JM, Detterbeck FC, Jones DR.Special treatment issues in non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest.2013;143(5)(suppl):e369S-e399S [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement 1
Online Supplement 2
Online Supplement 3
Online Supplement 4