Abstract
Mitochondrial abnormalities may lead to metabolic complications in HIV-infected children who have been receiving long-term antiretroviral treatment. We conducted a matched, case-control study comparing 21 HIV-infected children with insulin resistance (cases) to 21 HIV-infected children without insulin resistance (controls) to assess differences in mitochondrial DNA (mtDNA) copies/cell and oxidative phosphorylation NADH dehydrogenase (C1) and cytochrome c oxidase (C4) enzyme activities in peripheral blood mononuclear cells. MtDNA copies/cell tended to be lower in cases, and fasting serum glucose levels were inversely and significantly correlated with C1 enzyme activity, more so in cases. Larger pediatric studies should evaluate mitochondrial etiologies of insulin resistance and determine the role of antiretroviral therapies or HIV infection on mitochondrial dysfunction.
Introduction
Effective combination antiretroviral therapy (cART) has extended life in individuals with human immunodeficiency virus (HIV), yet long-term complications, such as lipodystrophy and cardiometabolic disease, have become a great medical challenge.1,2 The etiology of these abnormalities includes antiretroviral medications (ARVs), which are known to be associated with mitochondrial toxicity.3–8 The proposed mechanisms of mitochondrial dysfunction include impairment of mitochondrial DNA (mtDNA) replication and/or transcription, resulting in impaired oxidative phosphorylation (OXPHOS).5–10 Studies have also shown an association between ARVs and mitochondrial abnormalities in HIV-infected children.11,12 Little is known, however, regarding mitochondrial alterations in HIV-infected children with clinical evidence of insulin resistance or other metabolic complications, and whether these changes can be attributed to ARV-associated mitochondrial toxicity.
Impaired OXPHOS is a potential mechanism for type 2 diabetes mellitus in uninfected adults,13 and a potential link between cART and metabolic dysfunction through cART-induced mitochondrial toxicity in HIV-infected adults has been suggested.7,14 MtDNA levels in peripheral blood mononuclear cells (PBMCs) have been shown to be decreased in adult patients with type II diabetes by 25–35% compared to controls.15 Perinatally HIV-infected children represent a unique population because they have been exposed to HIV since birth and many have been exposed to ARVs since infancy or early childhood. Many of these children exhibit metabolic changes at an early age.16 A direct relationship between mitochondrial toxicity and metabolic changes has not yet been demonstrated in these children. Approximately 15% of children in the Pediatric HIV/AIDS Cohort Study (PHACS) have insulin resistance, as determined by abnormal homeostatic model assessment of insulin resistance (HOMA-IR) scores.17 In this population, insulin resistance has been associated with obesity, higher nadir CD4+ T cell counts, and amprenavir use, suggesting that HIV treatment may have some effect on the development of insulin resistance in HIV-infected children.17
The primary objective of our study was to evaluate differences in mtDNA copy number and mitochondrial OXPHOS NADH dehydrogenase [Complex 1 (C1)] and cytochrome c oxidase [Complex 4 (C4)] enzyme activities among HIV-infected children with and without insulin resistance. We chose to measure OXPHOS C1 and C4 activity since they are encoded for in mtDNA and are representative of both the beginning and end of the electron transport chain.
Materials and Methods
Study design and population
We conducted a matched, case-control study utilizing stored repository specimens from HIV-infected children who were enrolled in the multicenter U.S.-based Adolescent Master Protocol (AMP) of PHACS (https://phacs.nichdclinicalstudies.org/overview.asp). PHACS is an ongoing, prospective study evaluating long-term effects of HIV infection and ARVs in children with perinatally acquired HIV infection. Children enrolled in AMP have clinical, laboratory, and body composition assessments at predetermined intervals to monitor metabolic abnormalities, neurodevelopmental parameters, growth, HIV disease status, and other outcomes. Sociodemographic information, clinical history, and family history are routinely collected for all AMP participants, as well as detailed ARV history (past, current, lifetime duration). Blood samples including PBMCs are also collected annually and stored for future studies in the PHACS central repository.
From the 451 total HIV-infected AMP participants, the PHACS data coordinating center identified those participants with insulin resistance who also had available PBMCs stored in the PHACS repository from the AMP entry visit. Insulin resistance was determined by the HOMA-IR score {[fasting insulin (μU/ml)×fasting glucose (mmol/liter)]/22.5}18 and defined as a score of ≥2.5 in patients who were Tanner stage 1 and >4.0 in Tanner stage 2 or higher.19 Of 65 participants with abnormal HOMA-IR scores and with a stored repository specimen, 47 had an available aliquot with 2×106 PBMCs, the minimum required PBMC volume needed for the proposed mitochondrial assays. We confirmed that the 47 participants with sufficient PBMC volume also had available clinical data, including height, weight, and body mass index (BMI) as defined by [weight (kg)/height2 (m2)] and expressed as z-scores, ARV history, HIV viral load, and T cell subsets.
Matching
The PHACS Data Coordinating center also identified all HIV-infected study participants without insulin resistance who had a sufficient quantity of PBMCs from the AMP entry visit stored in the repository. Samples from participants with insulin resistance (cases) were matched with an equal number of samples of participants without insulin resistance (controls) based on age at the time of sample collection (±12 months), sex, race, and BMI z-score (±0.5 z-score difference). Participants with available Tanner stage information were also matched on this measure (±one Tanner stage difference in patients with Tanner stage >1) since HOMA-IR cutoffs are determined using Tanner stage and initiation of puberty could be different in children of the same age.
For both cases and controls, concurrent Centers for Disease Control (CDC) pediatric HIV disease stage, absolute CD4+ T-lymphocyte cell (CD4) count, plasma HIV-1 RNA by quantitative polymerase chain reaction (PCR) (viral load), and ARV regimens were recorded, but the samples were not matched on these measures.
Laboratory methods
PBMCs
PBMCs were obtained using EDTA vacutainers within 8 h of phlebotomy. PBMCs were isolated using the Ficoll-Hypaque Overlay Method and cell preparation tubes. Platelets were removed by two phosphate-buffered saline (PBS) washes and centrifugation at 300 RCF. PBMCs were stored as frozen pellets.
Mitochondrial assays
PBMC mtDNA copies/cell was assayed by absolute quantitative real time PCR as previously published.20 Each sample and standard were run in duplicate and the results were analyzed with Version 1.5.0 LightCycler 480 software.
PBMC OXPHOS C1 and C4 enzyme activities were performed in duplicate by thin-layer chromatography and immunoassays as described previously.20 Activity is measured as optical density (OD)/μg of protein×103.
Statistical analysis
Characteristics of cases and controls, including sociodemographics, CD4+ T cell percent, HIV viral load (copies/ml), ARVs, and CDC disease staging were summarized by the frequency or median (interquartile ranges, IQR). Comparisons between the two groups on these characteristics were performed in an unmatched analysis using Chi-square or Fisher's exact test for categorical variables and Wilcoxon test for continuous variables. For each mitochondrial marker, the difference between each matched case and control was calculated and then the mean (95% confidence intervals) of the differences across matched pairs was determined. A one sample t-test was performed to test if the mean was significantly different from zero (zero being no difference between matched cases and controls).21,22 Due to the small sample size, we did not perform multivariable analyses to adjust for other potential confounding factors, nor did we perform power calculations. Correlations of mitochondrial markers with fasting glucose, insulin, and HOMA-IR score were determined by Spearman correlations in cases and controls separately.
Results
Of the 47 cases that were initially identified from the available samples stored in the PHACS repository, 21 were able to be matched to 21 HIV-infected controls without insulin resistance based on all of the matching characteristics outlined in our methods. Two case-control pairs were matched using all matching criteria (including age) except for Tanner stage, because the two cases did not have Tanner stage documented on clinical data collection forms stored at the PHACS data coordinating center. It was clear based on their age alone that both of these cases were expected to have completed puberty. Therefore the higher cutoff for HOMA-IR score was used to determine insulin resistance without the need for Tanner stage information.
Sociodemographic and clinical characteristics in the cases and controls are described in Table 1 and the p values are shown for unmatched comparisons between the two groups. Since cases and controls were matched on age, sex, race, Tanner stage, and BMI z-score, it was expected that these characteristics would be similar between the two groups. All study participants were receiving cART at the time of the PBMC collection and there were no significant differences in HIV disease status/severity between cases and controls. We specifically looked for differences between cases and controls in the use of individual ARVs that have been reported to be associated with metabolic changes or cardiovascular disease risk in HIV-infected individuals, including zidovudine, stavudine, lamivudine, abacavir, ritonavir, and ritonavir-boosted protease inhibitors.23 No significant differences in the current or past use of these ARVs were noted in cases versus controls (data not shown).
Table 1.
Comparison of Clinical Characteristics Between Cases and Controls (Unmatched)
| |
Case-control status |
|
|
|---|---|---|---|
| Characteristic | Control (N=21) | Case (N=21) | p-valuea |
| Sex | |||
| Female | 11 (52%) | 11 (52%) | 1.000 |
| Male | 10 (48%) | 10 (48%) | |
| Age (years) | |||
| Median (Min, Max) | 13.54 (8.72, 16.20) | 13.45 (8.50, 16.02) | 0.706 |
| Q1, Q3 | 11.46, 14.35 | 11.38, 14.04 | |
| Race | |||
| Black or African-American | 17 (81%) | 17 (81%) | 0.451 |
| White | 3 (14%) | 1 (5%) | |
| Unknown | 1 (5%) | 3 (14%) | |
| Ethnicity | |||
| Hispanic or Latino | 3 (14%) | 3 (14%) | 1.000 |
| Not Hispanic or Latino | 18 (86%) | 18 (86%) | |
| BMI z-score | |||
| Median (Min, Max) | 1.24 (−1.16, 2.21) | 1.28 (−1.37, 2.47) | 0.880 |
| Q1, Q3 | 0.06, 1.58 | 0.06, 1.67 | |
| Tanner stage | |||
| 1 | 3 (14%) | 5 (24%) | 0.864 |
| 2 | 4 (19%) | 2 (10%) | |
| 3 | 4 (19%) | 4 (19%) | |
| 4 | 6 (29%) | 4 (19%) | |
| 5 | 4 (19%) | 4 (19%) | |
| Unknown | 0 (0%) | 2 (10%) | |
| First degree relative with diabetes | |||
| Yes | 6 (29%) | 2 (10%) | 0.24 |
| No | 15 (71%) | 19 (90%) | |
| HOMA-IR | |||
| Median (Min, Max) | 1.80 (0.20, 4.00) | 5.95 (2.60, 24.80) | <0.001 |
| Q1, Q3 | 1.20, 3.00 | 4.95, 8.05 | |
| Fasting insulin value (mcU/ml) | |||
| Median (Min, Max) | 8.60 (1.20, 21.70) | 29.70 (10, 88) | <0.001 |
| Q1, Q3 | 5.50, 13.00 | 23.45, 40.20 | |
| Fasting glucose value (mg/dl) | |||
| Median (Min, Max) | 82 (69, 96) | 87 (57, 114) | 0.062 |
| Q1, Q3 | 75, 90 | 82.50, 94.50 | |
| CD4 percent | |||
| Median (Min, Max) | 34.50 (13, 51.4) | 33.1 (14.35, 46.00) | 0.910 |
| Q1, Q3 | 31, 36.2 | 26.85, 40.5 | |
| CD4 percent <15% | |||
| No | 20 (95%) | 20 (95%) | 1.000 |
| Yes | 1 (5%) | 1 (5%) | |
| HIV RNA <400 copies/ml | |||
| No | 8 (38%) | 8 (38%) | 1.000 |
| Yes | 13 (62%) | 13 (62%) | |
| CDC class | |||
| A—Mildly symptomatic | 6 (29%) | 10 (48%) | 0.237 |
| B—Moderately symptomatic | 6 (29%) | 4 (19%) | |
| C—Severely symptomatic | 4 (19%) | 6 (29%) | |
| N—Not symptomatic | 5 (24%) | 1 (5%) | |
| PI current use | |||
| No | 6 (29%) | 8 (38%) | 0.744 |
| Yes | 15 (71%) | 13 (62%) | |
| PI ever use | |||
| No | 1 (5%) | 3 (14%) | 0.606 |
| Yes | 20 (95%) | 18 (86%) | |
| PI lifetime use (months) | |||
| Median (Min, Max) | 78.25 (22.36, 141.67) | 98.39 (43.99, 128.98) | 0.183 |
| Q1, Q3 | 54.10, 103.79 | 78.64, 119.31 | |
| NNRTI current use | |||
| No | 15 (71%) | 17 (81%) | 0.719 |
| Yes | 6 (29%) | 4 (19%) | |
| NNRTI ever use | |||
| No | 11 (52%) | 8 (38%) | 0.536 |
| Yes | 10 (48%) | 13 (62%) | |
| NNRTI lifetime use (months) | |||
| Median (Min, Max) | 60.20 (9.07, 113.92) | 44.32 (14.24, 111.36) | 0.515 |
| Q1, Q3 | 30.67, 86.37 | 38.10, 64.34 | |
| NRTI current use | |||
| No | 2 (10%) | 3 (14%) | 1.000 |
| Yes | 19 (90%) | 18 (86%) | |
| NRTI ever use | |||
| Yes | 21 (100%) | 21 (100%) | b |
| NRTI lifetime use (months) | |||
| Median (Min, Max) | 117.11 (22.36, 164.26) | 128.98 (98.07, 173.49) | 0.339 |
| Q1, Q3 | 101.49, 153.04 | 112.67, 140.16 | |
| Current ARV regimen | |||
| HAART with PI | 15 (71%) | 13 (62%) | 0.911 |
| HAART without PI | 3 (14%) | 3 (14%) | |
| Non-HAART ARV | 1 (5%) | 2 (10%) | |
| Not on ARV | 2 (10%) | 3 (14%) | |
| Zidovudine (AZT) current use | |||
| Yes | 4 (19%) | 6 (29%) | 0.72 |
| Zidovudine (AZT) ever use | |||
| Yes | 17 (81%) | 20 (95%) | 0.34 |
| Lamivudine (3TC) current use | |||
| Yes | 11 (52%) | 12 (57%) | 1.0 |
| Lamivudine (3TC) ever use | |||
| Yes | 17 (81%) | 20 (95%) | 0.34 |
| Stavudine (d4T) current use | |||
| Yes | 5 (24%) | 4 (19%) | 1.0 |
| Stavudine (d4T) ever use | |||
| Yes | 16 (76%) | 17 (81%) | 1.0 |
| Abacavir current use | |||
| Yes | 5 (24%) | 8 (38%) | 0.50 |
| Abacavir ever use | |||
| Yes | 6 (29%) | 8 (38%) | 0.74 |
| Ritonavir current use | |||
| Yes | 12 (57%) | 11 (52%) | 1.0 |
| Ritonavir ever use | |||
| Yes | 16 (76%) | 16 (76%) | 1.0 |
Fisher's exact test or Wilcoxon test was used as appropriate.
p-value could not be calculated—all of the children have ever used NRTI.
BMI, body mass index; HOMA-IR, homeostatic model assessment of insulin resistance; CDC, Centers for Disease Control and Prevention; PI, protease inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside/nucleotide reverse transcriptase inhibitor; characteristics in bold indicate characteristics on which cases and controls were matched.
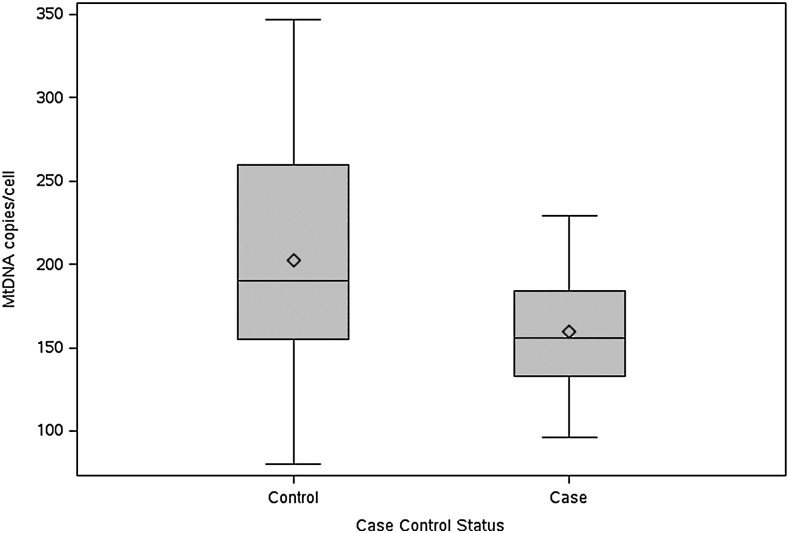
Table 2 shows the median (IQR) values of the mitochondrial markers in cases and controls. The median mtDNA copy number was 158 copies/cell in cases and 199 copies/cell in controls with a difference of −39.9 (p=0.098 in a matched analysis). There was one case and one control with mtDNA copies/cell of >400, 421, and 443, respectively. Using a boxplot, we plotted the distribution of the mtDNA values in the cases and the controls separately. The value >400 was >3 IQR from the box in the cases and >1.5 IQR in the controls. Therefore, we also conducted the analysis without these two outliers. When these outliers and their matched case and control were excluded, the mean difference between matched cases and controls was −40.5 (−79.8, −1.3; p=0.044 in a matched analysis, Fig. 1). No differences were observed in OXPHOS C1 and C4 enzyme activities between the matched cases and controls.
Table 2.
Mitochondrial Markers in Matched Cases and Controls
| |
Median (25th, 75th) |
|
|
|
|---|---|---|---|---|
| Mitochondrial markers | Controls | Cases | Mean difference (95% CL) | p-value |
| MtDNA copies/cell | ||||
| With outlier | 199 (80, 421) | 158 (96, 443) | −39.9 (−87.81, 8.0) | 0.098 |
| Without outlier | 182 (80, 347) | 153 (96, 229) | −40.5 (−79.8, −1.3) | 0.044 |
| C1 enzyme activity (×1,000 optical density units) | 86.10 (13.70, 17.4) | 94.80 (11.4, 172) | 10.4 (−15.16, 35.9) | 0.41 |
| C4 enzyme activity (×1,000 optical density units) | 65.40 (18.00, 134.4) | 72.90 (7.6, 128.2) | 1.3 (−17.8, 20.4) | 0.89 |
MtDNA, mitochondrial DNA; CL, confidence limit; C1, Complex I NADH dehydrogenase; C4, Complex IV cytochrome c oxidase; outlier value was >3 IQR in cases and >1.5 IQR in controls.
FIG. 1.
Difference in mitochondrial DNA (mtDNA) copy number between cases and controls.
We determined the association between fasting glucose, fasting insulin, HOMA-IR score, and each mitochondrial marker. Serum glucose was inversely related to C1 enzyme activity in both cases and controls, with a greater correlation noted in the cases, which by definition had insulin resistance (C1 enzyme activity cases: −0.72, p=0.0003; controls: −0.47, p=0.03). When the case and control with unusually high mtDNA copy numbers >400 and their matched pairs were excluded, these correlations remained significant (C1 enzyme activity cases: −0.70, p=0.0008; controls: −0.44, p=0.05). Serum glucose was not associated with mtDNA copies/cell or C4 enzyme activity. Fasting insulin level and HOMA-IR were highly correlated with each other, as expected (cases: 0.93, p<0.0001, controls: 0.98, p<0.0001), but insulin and glucose were not significantly correlated in cases or controls and there was no association between insulin or HOMA-IR score and mtDNA copies/cell or C1 or C4 enzyme activity (data not shown).
Discussion
There are few studies evaluating mitochondrial genetic and functional markers in HIV-infected children with insulin resistance. In this matched case-control study we provide evidence for differences in mtDNA copy number in HIV-infected children with and without insulin resistance as measured by HOMA-IR score, and also describe a significant inverse relationship between fasting glucose levels and mitochondrial OXPHOS C1 enzyme activity. These findings were present despite similar HIV disease status and severity in both cases and controls, suggesting that the mitochondrial changes were not due solely to the effect of HIV infection itself on mitochondria.
In this study we did not find a reduction in OXPHOS C1 and C4 enzyme activity in cases compared to controls; in fact the median levels of both were higher in the cases than in the controls, although the results were not statistically significant. Higher, rather than lower, OXPHOS C1 and C4 enzyme activity in cases could represent an initial compensatory response to elevated levels of circulating insulin, but additional studies that confirm these findings are needed to determine whether this might indeed be an important observation. We did find a significant negative correlation between fasting glucose levels and PBMC C1 enzyme activity and this has also been observed in adult type II diabetic skeletal muscle C1 activity.24 All of the cases in our study had glucose levels within the normal range, as would be expected in patients with insulin resistance who have not yet developed frank diabetes mellitus. We hypothesize that the negative correlation between glucose and C1 activity reflects the expected pathophysiological pathway in which ongoing impairment of OXPHOS is associated with progression to diabetes mellitus. As glucose levels rise, the initial compensation of C1 activity in response to high levels of insulin begins to fail. A similar matched study in HIV-infected children with and without type II diabetes may demonstrate more dramatic findings that better illustrate this process.
The role of ARVs in altering the function of glucose transporter GLUT4 has been established in various studies25 and mitochondrial OXPHOS impairment leading to the development of insulin resistance is also established.13 A clear link between ARV-associated mitochondrial dysfunction and insulin resistance, although demonstrated in adults, has yet to be identified in HIV-infected children.26 Larger studies are needed to better evaluate whether there is indeed an association.
While our study was limited by the small sample size, our matched design is a strength in that we were better able to control for potential confounders. Small PBMC volumes in the repository samples limited our ability to perform a broader array of mitochondrial assays, and more comprehensive studies will be needed to better characterize the range of mitochondrial effects. Although PBMCs have not been considered the ideal tissue with which to conduct mitochondrial studies, other types of cells, such as tissue from muscle biopsies, would be extremely difficult to obtain in children. It has been previously demonstrated that mitochondrial OXPHOS protein levels in PBMCs do correlate with levels in subcutaneous adipose tissue and may also correlate with other cells.14 We were unable to include HIV-negative children with evidence of insulin resistance in this pilot study; however, such a group would be a potentially important added control to determine whether mitochondrial changes in our patient population could indeed be linked to cART. We hypothesized that insulin resistance and mitochondrial abnormalities could be associated with ARV use in HIV-infected children; however, we did not see a difference in ARV use between cases and controls. Though a small sample size could limit the power to detect differences in individual ARVs between the two groups, other factors such as chronic inflammation due to HIV disease, family history of diabetes mellitus, and other risk factors for cardiometabolic disease could also influence the progression to insulin resistance in some children and not in others. Further studies are needed to better understand the interplay between ARVs and other clinical features leading to the development of insulin resistance in HIV-infected children.
Our findings suggest that there might be changes in mitochondrial parameters in HIV-infected children with insulin resistance, and there is a need to further evaluate the association of mitochondrial abnormalities and metabolic changes. In addition, the role of cART in the development of mitochondrial abnormalities must be more closely scrutinized. Ultimately, prospective studies in which HIV-infected children with insulin resistance are followed over time will allow us to better understand the dynamics of mitochondrial parameters as these children progress through puberty and have continued exposure to cART.
Acknowledgments
We thank the children and families for their participation in PHACS and the individuals and institutions involved in the conduct of PHACS. The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with cofunding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard University School of Public Health (HD052102, 3 U01 HD052102-05S1, 3 U01 HD052102-06S3) (Principal Investigator: George Seage; Project Director: Julie Alperen) and the Tulane University School of Medicine (HD052104, 3U01 HD052104-06S1) (Principal Investigator: Russell Van Dyke; Co-Principal Investigator: Kenneth Rich; Project Director: Patrick Davis). Data management services were provided by Frontier Science and Technology Research Foundation (PI: Suzanne Siminski) and regulatory services and logistical support were provided by Westat, Inc. (PI: Julie Davidson).
Tanvi Sharma is supported by a Eunice Kennedy Shriver National Institute of Child Health and Human Development K23 grant award, National Institutes of Health (NIH): 1K23HD055100. Tracie Miller, Mariana Gerschenson, and Denise Jacobson are supported by a National Institute of Nursing Research, NIH Grant: 1R01NR012885.
The following institutions, clinical site investigators, and staff participated in conducting PHACS AMP in 2011, in alphabetical order: Baylor College of Medicine: William Shearer, Mary Paul, Norma Cooper, Lynette Harris; Bronx Lebanon Hospital Center: Murli Purswani, Mahboobullah Baig, Anna Cintron; Children's Diagnostic and Treatment Center: Ana Puga, Sandra Navarro, Doyle Patton, Deyana Leon; Children's Hospital, Boston: Sandra Burchett, Nancy Karthas, Betsy Kammerer; Children's Memorial Hospital: Ram Yogev, Margaret Ann Sanders, Kathleen Malee, Scott Hunter; Jacobi Medical Center: Andrew Wiznia, Marlene Burey, Molly Nozyce; St. Christopher's Hospital for Children: Janet Chen, Latreca Ivey, Maria Garcia Bulkley, Mitzie Grant; St. Jude Children's Research Hospital: Katherine Knapp, Kim Allison, Megan Wilkins; San Juan Hospital/Department of Pediatrics: Midnela Acevedo-Flores, Heida Rios, Vivian Olivera; Tulane University Health Sciences Center: Margarita Silio, Medea Jones, Patricia Sirois; University of California, San Diego: Stephen Spector, Kim Norris, Sharon Nichols; University of Colorado Denver Health Sciences Center: Emily Barr, Carrie Chambers, Alisa Katai; University of Medicine and Dentistry of New Jersey: Arry Dieudonne, Linda Bettica, Susan Adubato; University of Miami: Gwendolyn Scott, Patricia Bryan, Elizabeth Willen.
The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health or U.S. Department of Health and Human Services.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Marin B. Thiebaut R. Bucher HC, et al. Non-AIDS-defining deaths and immunodeficiency in the era of combination antiretroviral therapy. AIDS. 2009;23:1743–1753. doi: 10.1097/QAD.0b013e32832e9b78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neuhaus J. Angus B. Kowalska JD, et al. Risk of all-cause mortality associated with nonfatal AIDS and serious non-AIDS events among adults infected with HIV. Acquir Immune Defic Syndr. 2010;24:697–706. doi: 10.1097/QAD.0b013e3283365356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flint OP. Noor MA. Hruz PW, et al. The role of protease inhibitors in the pathogenesis of HIV-associated lipodystrophy: Cellular mechanisms and clinical implications. Toxicol Pathol. 2009;37:65–77. doi: 10.1177/0192623308327119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perez-Molina JA. Domingo P. Martinez E, et al. The role of efavirenz compared with protease inhibitors in the body fat changes associated with highly active antiretroviral therapy. J Antimicrob Chemother. 2008;62:234–245. doi: 10.1093/jac/dkn191. [DOI] [PubMed] [Google Scholar]
- 5.Poirier M. Divi R. Al-Harthi L, et al. Women and Infants Transmission Study (WITS) Study Group. Long-term mitochondrial toxicity in HIV-uninfected infants born to HIV-infected mothers. JAIDS. 2003;33:175–183. doi: 10.1097/00126334-200306010-00010. [DOI] [PubMed] [Google Scholar]
- 6.Poirier M. Olivero O. Walker D, et al. Perinatal genotoxicity and carcinogenicity of anti-retroviral nucleoside analog drugs. Toxicol Appl Pharmacol. 2004;199:151–161. doi: 10.1016/j.taap.2003.11.034. [DOI] [PubMed] [Google Scholar]
- 7.Shikuma CM. Day LJ. Gerschenson M. Insulin resistance in the HIV-infected population: The potential role of mitochondrial dysfunction. Curr Drug Targets Infect Disord. 2005;5:255–262. doi: 10.2174/1568005054880163. [DOI] [PubMed] [Google Scholar]
- 8.McComsey GA. Libutti DE. O'Riordan M, et al. Mitochondrial RNA and DNA alterations in HIV lipoatrophy are linked to ART therapy and not HIV infection. Antivir Ther. 2008;13(5):715–722. [PMC free article] [PubMed] [Google Scholar]
- 9.Shikuma CM. Gerschenson M. Chow D, et al. Mitochondrial oxidative phosphorylation protein levels in peripheral blood mononuclear cells correlate with levels in subcutaneous adipose tissue within samples differing by HIV and lipoatrophy status. AIDS Res Hum Retroviruses. 2008;24:1255–1262. doi: 10.1089/aid.2007.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster C. Lyall H. HIV and mitochondrial toxicity in children. J Antimicrob Chemother. 2008;61:8–12. doi: 10.1093/jac/dkm411. [DOI] [PubMed] [Google Scholar]
- 11.Crain MJ. Chernoff MC. Oleske JM, et al. Possible mitochondrial dysfunction and its association with antiretroviral therapy use in children perinatally infected with HIV. J Infect Dis. 2010;202(2):291–301. doi: 10.1086/653497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saitoh A. Fenton T. Alvero C. Fletcher CV. Spector SA. Impact of nucleoside reverse transcriptase inhibitors on mitochondria in human immunodeficiency virus type 1-infected children receiving highly active antiretroviral therapy. Antimicrob Agents Chemother. 2007;51:4236–4242. doi: 10.1128/AAC.00893-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morino K. Petersen KF. Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 2006;55(Suppl 2):S9–S15. doi: 10.2337/db06-S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerschenson M. Shiramizu B. LiButti DE. Shikuma CM. Mitochondrial DNA levels of peripheral blood mononuclear cells and subcutaneous adipose tissue from thigh, fat and abdomen of HIV-1 seropositive and negative individuals. Antivir Ther. 2005;10(Suppl 2):M83–89. [PubMed] [Google Scholar]
- 15.Lee HK. Song JH. Shin CS, et al. Decreased mitochondrial DNA content in peripheral blood precedes the development of non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract. 1998;42:161–167. doi: 10.1016/s0168-8227(98)00110-7. [DOI] [PubMed] [Google Scholar]
- 16.Miller TL. Borkowsky W. DiMeglio LA, et al. for the Pediatric HIV/AIDS Cohort Study (PHACS) Metabolic abnormalities and viral replication are associated with biomarkers of vascular dysfunction in HIV-infected children. HIV Med. 2012;13(5):264–275. doi: 10.1111/j.1468-1293.2011.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geffner ME. Patel K. Miller TL, et al. Factors associated with insulin resistance among children and adolescents perinatally infected with HIV-1 in the Pediatric HIV/AIDS Cohort Study. Hormone Res Paediatr. 2011;76:386–391. doi: 10.1159/000332957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthews DR. Hosker JP. Rudenski AS, et al. Homeostasis model assessment: Insulin resistance and B cell function from fasting plasma glucose and insulin concentration in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 19.Kurtoğlu S. Hatipoğlu N. Mazıcıoğlu M, et al. Insulin resistance in obese children and adolescents: HOMA-IR cutoff levels in the prepubertal and pubertal periods. J Clin Res Pediatr Endocrinol. 2010;2(3):100–106. doi: 10.4274/jcrpe.v2i3.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brogly SB. DiMauro S. Van Dyke RB, et al. Transplacental nucleoside analog exposure and mitochondrial parameters in HIV-uninfected children. AIDS Res Hum Retroviruses. 2011;27(7):777–783. doi: 10.1089/aid.2010.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosner B. Fundamentals of Biostatistics. Duxbury Press; Pacific Grove, CA: 1995. p. 252. Sect. 8.4. [Google Scholar]
- 22.Selvin S. Statistical Analysis of Epidemiologic Data. Oxford University Press; New York: 1996. p. 278. [Google Scholar]
- 23.Hester EK. HIV medications: An update and review of metabolic complications. Nutr Clin Pract. 2012;27:51–64. doi: 10.1177/0884533611431985. [DOI] [PubMed] [Google Scholar]
- 24.Kelley DE. He J. Menshikova EV. Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 25.Hruz PW. Molecular mechanisms for altered glucose homeostasis in HIV infection. Am J Infect Dis. 2006;2(3):187–192. doi: 10.3844/ajidsp.2006.187.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fleischman A. Johnsen S. Systrom DM, et al. Effects of a nucleoside reverse transcriptase inhibitor, stavudine, on glucose disposal and mitochondrial function in muscle of healthy adults. Am J Physiol Endocrinol Metab. 2007;292:E1666–E1673. doi: 10.1152/ajpendo.00550.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]