Abstract
Significance: Aging leads to a loss of skeletal muscle mass and function that causes instability, increased risk of falls, and need for residential care. This is due to a reduction in the muscle mass and strength that is primarily due caused by a decrease in the number of muscle fibers, particularly, type II fibers, and atrophy and weakening of those remaining. Recent Advances: Although increased oxidative damage was originally thought to be the key to the aging process, data now indicate that reactive oxygen species (ROS) may be one of the several components of the degenerative processes in aging. The skeletal muscle shows important rapid adaptations to the ROS generated by contractions that are attenuated in aged organisms and transgenic studies have indicated that overcoming these attenuated responses can prevent the age-related loss of muscle mass and function. Critical Issues: Elucidation of the mechanisms by which the skeletal muscle adapts to the ROS generated to contractions and the way in which these processes are attenuated by aging is critical to the development of logical approaches to prevent age-related loss of muscle mass and function. Future Directions: Future studies are likely to focus on the redox regulation of adaptive pathways and their maintenance during aging as an approach to maintain and improve muscle function. Antioxid. Redox Signal. 19, 804–812.
Introduction
Effects of aging on skeletal muscle
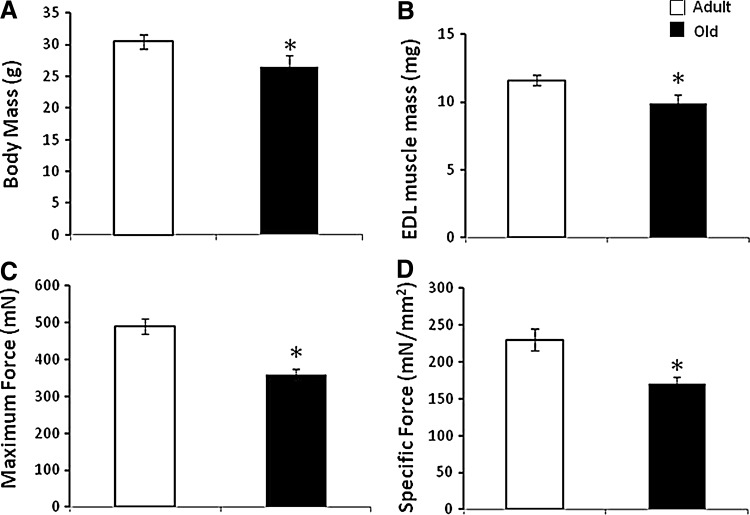
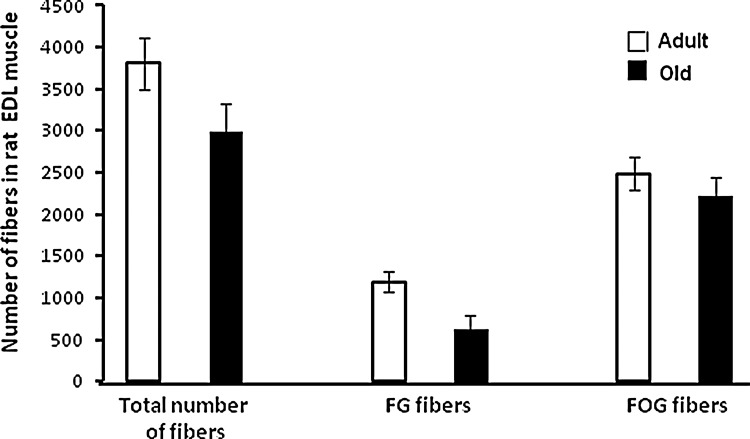
In older people, declining muscle mass and function lead to instability, increased risk of falls, and need for residential care. By the age of 70, the cross-sectional area of skeletal muscle is reduced by 25%–30% and muscle strength is reduced by 30%–40% (40). The reduction in muscle mass and function with age in humans and rodents is primarily due to a decrease in the number of muscle fibers, particularly, type II fibers, and atrophy and weakening of those fibers that remain (8, 29). The loss of muscle fibers and other changes in muscles during aging shows considerable similarities between man and rodents (35). Figures 1 and 2 show typical data from rodent models of the changes that occur in muscles with aging. These data show a reduction in muscle mass in addition to reduced maximum force generation and maximum specific force generation (Fig. 1). This latter measure provides an indication of the reduced strength of the fibers remaining in muscles of these old mice. Figure 2 illustrates the shift in fiber types that occurs in rodent muscle with aging. There are also a number of other functional changes in the remaining muscle fibers in old rodents and humans, including a slowing of the muscle phenotype (11), an attenuation of important responses to contractions that include acute stress responses (49), mitochondrial biogenesis (30), and anabolic responses (10). These functional changes in the remaining fibers appear to be important in the limitations of muscle function that affect the elderly. Thus, for instance, changes in neuromuscular control lead to slowed reactions and a lack of ability to fine tune movements, while correction of specific attenuated responses to contractions is associated with preservation of muscle force generation (9, 25).
FIG. 1.
Age-related changes in skeletal muscle. Data showing the age-related fall in body mass (A), mass of the extensor digitorum longus (EDL) muscle (B), maximum force generation by the EDL muscle (C), and specific force generation by the EDL muscle (D) in mice. *p<0.05 compared with adult group. Data derived from McArdle et al. (32).
FIG. 2.
Example of the relatively larger effect of aging on the number of fast glycolytic (FG) compared with fast oxidative glycolytic (FOG) fibers in the rat EDL muscle. Data derived from Alnaqeeb and Goldspink (1).
Aging Is Associated with Loss of Whole Motor Units
In man, a 25% reduction in the number of α-motor neurons occurs with aging. The causes of this loss are unknown, but small motor neurons (which tend to innervate type I fibers) are preserved relative to large motor neurons. Over time, the loss of large motor neurons is partially compensated by a sprouting phenomenon through which small motor neurons innervate those type II fibers that have become temporarily denervated, and hence, these fibers acquire a slower phenotype. This process is incomplete and eventually, the new giant motor units are lost (11). Studies to determine whether the age-related loss of muscle fibers is associated with loss of motor units in man and rodents indicate that substantial net loss of whole motor units occurs with increasing age in both species (28). Data indicate that in both species, net motor neuron loss occurs in parallel with muscle fiber loss and loss of muscle function (29), but it is unclear which of these is the primary event (14, 28, 52).
Aberrant Reactive Oxygen Species Generation Appears to Play an Important Role in Processes of Muscle Aging
In all species, skeletal muscle of aged organisms contains oxidative damage to macromolecules in comparison with levels found in young organisms (50), and the hypothesis that increased oxidative damage plays a key role in age-related tissue dysfunction has been extensively examined. In nonmammalian models, some interventions designed to reduce the activities of reactive oxygen species (ROS), such as overexpression of CuZn, superoxide dismutase (SOD1), catalase, or both in Drosophila (38) or treatment with a MnSOD and catalase mimetic in Caenorhabditis elegans (33) extended lifespan and thus support the hypothesis, but these effects are not universally observed and are controversial (15). In mammals, only few genetic manipulations designed to reduce ROS activities have resulted in an increased lifespan [e.g., see Refs. (46, 54)]. A consensus has therefore recently developed that increased ROS generation is not the fundamental cause of aging (or more precisely, the fundamental determinant of lifespan) (38). Many studies have reported that mitochondrial ROS generation is increased in skeletal muscle during aging and that this occurs in association with impaired mitochondrial function and oxidative damage to mitochondrial components (23). There are some studies that indicate manipulation of ROS activities can preserve muscle function during aging (9, 46) and increased mitochondrial ROS generation has also been proposed to play a key mediating role in pathological changes, in muscle, in conditions such as disuse atrophy (41).
ROS Play a Crucial Role in Muscle Physiology Through Redox-Signaling Pathways and Are Mediators of Adaptations to Contractions
Early studies indicated that ROS were deleterious to cells causing oxidative damage to lipids, DNA, and proteins (16), but it is now recognized that, in normal physiology, ROS mediate many adaptive processes. ROS are important physiological signaling molecules with regulatory functions that modulate changes in cell and tissue homeostasis and gene expression (13, 19). Signaling by these reactive molecules appears to be mainly achieved by targeted modifications of specific residues in proteins (23). Skeletal muscle fibers respond to contractile activity by an increase in the intracellular generation of superoxide and nitric oxide (NO) with the formation of secondary ROS and reactive nitrogen species (41). This leads to activation of a number of redox-regulated transcription factors, including nuclear factor kappa-B (NFκB), activator protein 1 (AP-1) and HSF-1 (19, 24, 51), and an increased expression of regulatory enzymes and cytoprotective proteins (17, 31). ROS also stimulate the expression of genes associated with myogenesis (3), catabolism-related genes (5), and mitochondrial biogenesis (18).
Does Defective Redox Signaling Play a Role in Muscle Aging?
Skeletal muscle from old mice shows an inability to activate redox-sensitive transcription factors, such as NFκB or AP-1 in response to contractile activity (49). This is characterized by chronic activation of transcription factors at rest and an inability to further activate these factors following an acute nondamaging muscle contraction protocol (49). This lack of activation of redox-sensitive transcription factors with contractile activity is associated with an inability to increase the expression of various cytoprotective proteins (49) and influences the susceptibility of skeletal muscle from old animals to oxidative damage. There is also evidence that this attenuation is also seen in other redox-regulated systems such as mitochondrial biogenesis (30) and some neurotrophic factors (BDNF, GDNF) are also subject to transcriptional regulation by NFκB and other redox-sensitive transcription factors. Hence, defective activation of redox-regulated transcription factors may also underlie diminished expression of these proteins in aging muscle.
Redox-dependent processes occur in discrete cellular compartments, such as cytosol, mitochondria, and nuclei, and antioxidant systems are not distributed uniformly throughout the cell. Understanding the processes of redox signaling and how this is modified during aging is important since it may help facilitate the development of novel targeted therapeutic interventions. Cellular reactive thiol pools play a crucial role in the regulation of redox signaling and recent attention has focused on proteins that encompass thiol–disulfide regulation, where the redox status depends on a redox-active site that has an amino acid sequence containing one or two active thiols. The major intracellular thiol/disulfide systems include reduced glutathione/oxidized glutathione (GSH/GSSG) and the thioredoxin (Trx) systems. These control diverse cellular events through discrete redox pathways, which influence redox signaling, are responsive to oxidative stress, and help maintain intracellular redox homeostasis (53). Trx are a class of small multifunctional proteins that are present in all prokaryotic and eukaryotic organisms and are characterized by the invariant redox-active site sequence (Trp-Cys-Gly-Pro-Cys-Lys) (42). The oxidized (inactive) form of Trx (Trx-S2) has two cysteines at its active site forming a disulfide bond that is reduced by thioredoxin reductase and reduced nicotinamide adenine dinucleotide phosphate (NADPH) to a di-thiol [Trx(SH)2]. Thioredoxins appear to be key intracellular regulators of redox signaling and Trx1 isoform is found in the nucleus and cytosol. Distinct pools are evident, since Trx1 can be imported into the nucleus from the cytoplasm during various forms of oxidative stress and Trx2 is localized in mitochondria. Trx2 is encoded by a nuclear gene and localized to the mitochondrial matrix by a mitochondrial leader sequence (47).
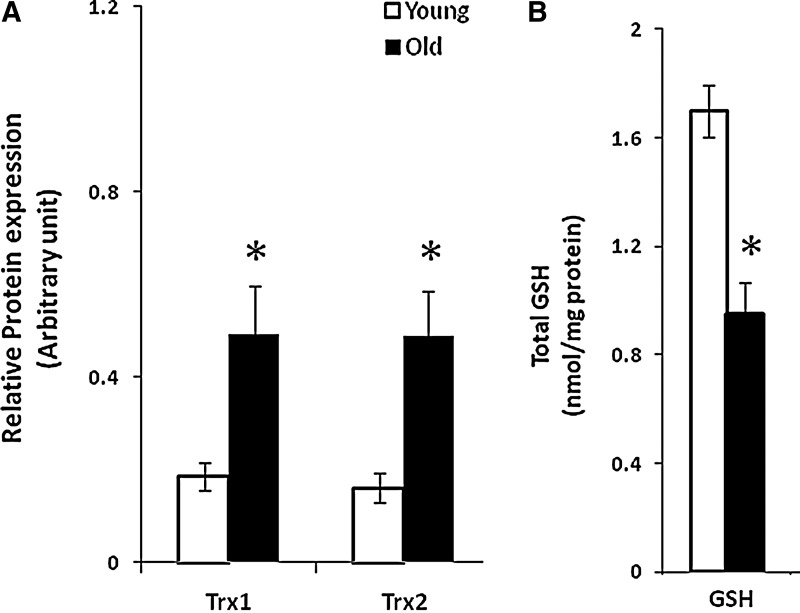
In recent studies, we have examined the effects of aging and contractile activity on the contents of Trx1, Trx2, and glutathione in skeletal muscle in vivo (12). Aging was found to increase the muscle Trx1 and Trx2 contents and to decrease the glutathione content (Fig. 3), but contractions had no effect on the content or redox status of Trx proteins. In contrast, the contractile activity induced a decrease in the muscle glutathione content in young mice, but had no effect on the muscles of old mice. Thus, there is evidence of a discrepant effect of aging on the two major cellular thiol proteins that regulate cellular redox signaling pathways although the functional effects of this pattern of changes in these proteins is unclear.
FIG. 3.
Age-related changes in major muscle thiol proteins. Comparison of the contents of glutathione (B) and Trx1 and Trx2 (A) in skeletal muscle from aged mice. *p<0.05 compared with young group. Data derived from Dimauro et al. (12). GSH, reduced glutathione; Trx1, thioredoxin 1; Trx2, thioredoxin 2.
Is Aging Associated with Increased ROS Generation During Contractile Activity?
Few studies have attempted to monitor ROS activities in intact skeletal muscle during aging although the tissue from aged rodents contains increased amounts of oxidative damage [e.g., see Refs. (9, 50)]. There are a number of techniques that may potentially address this issue and provide a nonspecific measure of ROS activities and Bejma and Ji (7) studied ROS activities in homogenates of skeletal muscle and found these to be elevated in tissues from old rats. They observed increased 2′, 7′-dichlorodihydrofluorescin (DCFH) oxidation for tissue from old rats, but the data from such studies are potentially influenced by the differential effects of the homogenization procedure on old compared with young tissue and the probe used is nonspecific since DCFH is acknowledged to react with a variety of ROS (e.g., hydrogen peroxide, organic peroxides, and hydroxyl radical), NO, and peroxynitrite (37).
Since there is a lack of a reliable approach to generally monitor ROS activities in skeletal muscle during contractions and the effects of aging on this, the question has been focused on whether the major sources for ROS generation in muscle are modified by aging. The sources of ROS that are activated by contractions have been the subject of considerable discussion and will not be considered in detail here [for a review, see Ref. (20)], but in essence, there is still no clear agreement on the prime intracellular sources for superoxide production in muscle during contractions. Although early studies suggested that 2%–5% of the total oxygen consumed by mitochondria may undergo one electron reduction with the generation of superoxide, recent data argue against such a substantial formation of superoxide within mitochondria and recent assessments of the rate of superoxide production by mitochondria indicate that ∼0.15% of the total O2 consumed is reduced to superoxide (47), a value that is an order of magnitude lower than the original estimates. Recent studies have also examined superoxide in the mitochondrial matrix (2) or mitochondrial redox potential (34) and indicated that mitochondria are unlikely to be the major source for the increased fiber ROS generation observed during contractile activity.
Additional extramitochondrial sites for superoxide production within skeletal muscle have been proposed, including NADPH oxidase enzymes (45), enzymes of the phospholipase A2 family, and xanthine oxidase (41), but their contribution during contractile activity has not been fully evaluated.
As previously discussed, there are extensive data indicating that mitochondria from skeletal muscle of old rodents have increased release of hydrogen peroxide [see Ref. (22)] and we have previously reported an ∼30% increase in mitochondrial hydrogen peroxide generation in state 1 (48). Thus, if mitochondria were the prime site of ROS generation in muscle during contractions, these data are consistent with an increased overall leak of ROS by mitochondria from aged muscle tissue. The alternative possibility that contraction-induced activation of NADPH oxidase(s) or xanthine oxidase are exacerbated by aging does not appear to have been evaluated.
Can Manipulation of Muscle ROS Activities Affect Muscle Aging?
An alternative approach to examine the role of ROS and contractile activity in skeletal muscle aging has been to determine the effect of modification of regulatory proteins for ROS and our previous studies have examined the effects of deletion of a number of regulatory enzymes for ROS. Despite frequent observation of increased oxidative damage in these models, no clear effects on skeletal muscle aging were seen, with the exception being mice with a whole body deletion of SOD1, which show neuromuscular changes with aging that appear to reflect an accelerated skeletal muscle aging process (36). Adult Sod1−/− mice show a decline in the skeletal muscle mass, loss of muscle fibers, and a decline in the number of motor units, loss of motor function and contractility, partial denervation, mitochondrial dysfunction, and a failure of adaptive responses to contractions by 8 months of age (21, 27, 51). The fiber loss in Sod1−/− mice is accompanied by degeneration of neuromuscular junctions, (21). Such changes are all seen in old wild-type (WT) mice, but not until 28–30 months of age.
SOD1 Knockout as a Model of Accelerated Muscle Aging
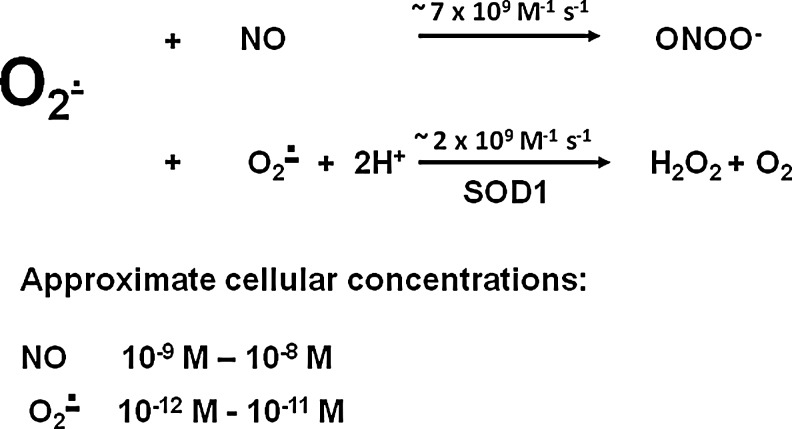
Although classic texts report that Cu,Zn SOD (SOD1) is expressed in the cytosol of cells [e.g., Ref. (16)], there is now considerable evidence that SOD1 is also localized to the mitochondrial inter-membrane space (IMS), where it is likely to be present at high concentration compared with cytosolic SOD1. The implication of this is that lack of SOD1 may influence redox homeostasis in the mitochondrial IMS in addition to the cytosol, and hence that disturbances in mitochondrial integrity or metabolism may underlie the changes observed. In our studies, we have examined the nature of the species that are generated in excess in mice lacking SOD1. Superoxide and NO are the primary radical species generated by skeletal muscle and their activities increase during contractile activity (4, 26, 43). Superoxide and NO are the precursors for the generation of a number of secondary species and muscle and other tissues have sophisticated enzymatic systems that control the cellular activities of these species. Intracellular superoxide is regulated by the activities of the SODs. When superoxide and NO are both present, their chemical reaction to form peroxynitrite is likely and competes with the dismutation of superoxide to hydrogen peroxide by SOD (6), (Fig. 4). In adult mice lacking SOD1, the loss of muscle fibers that contributes to the accelerated muscle aging phenotype may therefore be associated with excess superoxide within the muscle cells, but it is possible that alternative species play important roles in the initiation of degeneration. Thus, peroxynitrite or a change in NO bioavailability due to reaction with excess superoxide might both affect tissue function in mice lacking SOD1. We demonstrated that muscle fibers from adult SOD1 null mice showed an increase in oxidation of the nonspecific intracellular ROS probe, DCFH, at rest compared with fibers from WT mice (51), but surprisingly showed no further increase in oxidation of the probe following contractile activity, whereas an increase in DCFH oxidation was seen in fibers from adult WT mice following contractile activity. DCFH is reported to be relatively insensitive to oxidation by superoxide, but to be oxidized by hydrogen peroxide, hydroxyl radicals, peroxynitrite, and NO (37).
FIG. 4.
Potential key intracellular reactions of superoxide. In the presence of the usual very high content of SOD1, enzymatic dismutation of superoxide to hydrogen peroxide predominates over the chemical reaction of superoxide with nitric oxide (NO) despite the slower reaction rate. In the SOD1 knockout mice and the presence of excess NO, the formation of peroxynitrite (ONOO−) is facilitated. See Sakellariou et al. (44), for details. SOD, superoxide dismutase.
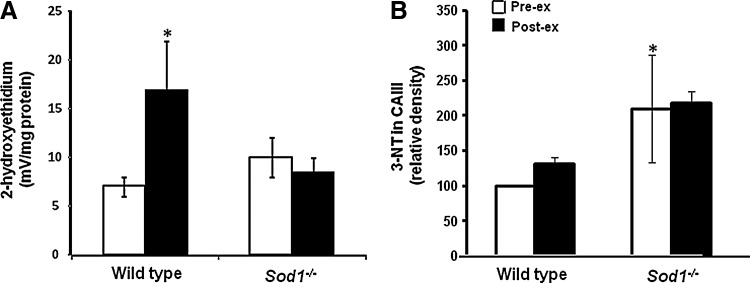
Monitoring of the amounts or activities of specific ROS in cells is inherently difficult due to the labile and reactive nature of these species. Using dihydroethidium (DHE) as a probe for superoxide and monitoring hydroxyethidium (the specific product of reaction between DHE and superoxide) and ethidium fluorescence (nonspecific product of DHE and ROS), we demonstrated that muscle fibers from adult Sod1−/− mice did not show the anticipated increase in cytosolic superoxide availability at rest, but in a similar manner to muscles of old mice, muscles from adult Sod1−/− mice demonstrated evidence for an increase in muscle peroxynitrite (Fig. 5). In Sod1−/− mice, this was modifiable by manipulation of muscle NO synthase activity (44) and indicated by an increased 3-nitrotyrosine content of muscle proteins and increased expression of the peroxynitrite reductase, peroxyredoxin 5. Thus, we concluded that peroxynitrite may play an important role in the phenotypic changes seen in SOD1 null mice and to be an important mediator of the accelerated muscle aging phenotype in this model.
FIG. 5.
Changes in muscle superoxide and peroxynitrite during contractions. Comparison of the 2-hydroxyethidium (A) and 3-NT (B) contents of muscle from SOD1 null and wild-type mice before and following exposure to an isometric contraction protocol. *p<0.05 compared with data from wild-type mice at rest. Redrawn from Sakellariou et al. (44).
Does the SOD1 Knockout Mouse Show Defective Redox Signaling in Muscle?
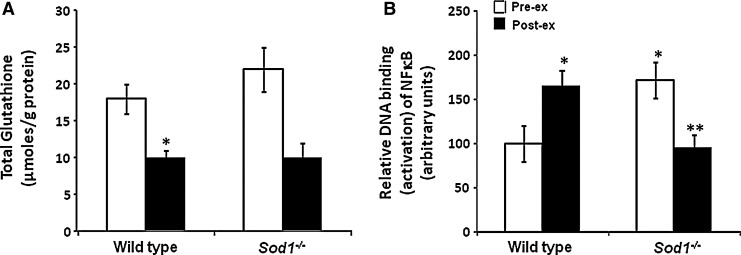
We have also examined whether mice lacking SOD1 show the lack of adaptation to contractile responses that is seen in old WT mice. Data indicate that, in contrast to adult WT mice, but in common with old WT mice, Sod1−/− mice failed to activate cytoprotective adaptive responses to contractile activity (51) (Fig. 6). This results in diminished expression of heat shock proteins (HSPs) and other cytoprotective proteins following contractile activity. This could potentially occur through a lack of induction of additional superoxide and/or hydrogen peroxide during contractile activity (43, 51). This lack of contraction-induced changes was also associated with constitutive activation of NFκB with increased production of proinflammatory cytokines and constitutive increase in the content of HSPs in muscle from adult Sod1−/− that are also seen in muscle from old WT mice at rest (51). Thus, a further effect of the lack of SOD1 that mimics that seen in old WT mice is a failure of redox-mediated signaling of adaptive responses to contractile activity and an increased production of cytokines.
FIG. 6.
Changes in the glutathione content (A) and NFκB activation (B) in muscles from SOD1 null and wild-type mice before and following exposure to an isometric contraction protocol. NFκB activation was assessed by DNA binding techniques. *p<0.05 compared with wild-type mice at rest, **p<0.05 compared with SOD1 null mice at rest. Data derived from Vasilaki et al. (51). NFκB, nuclear factor kappa-B.
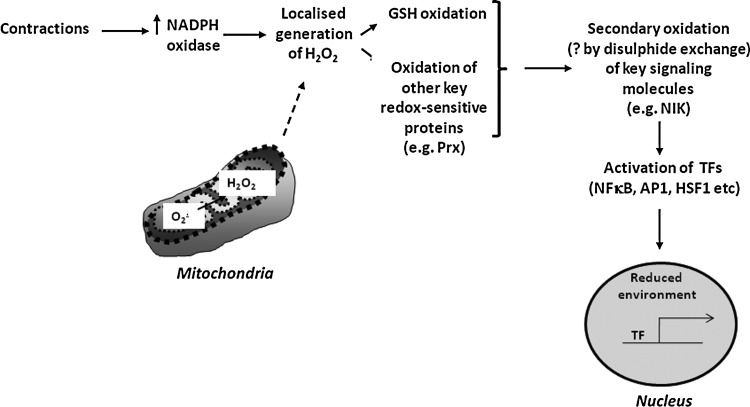
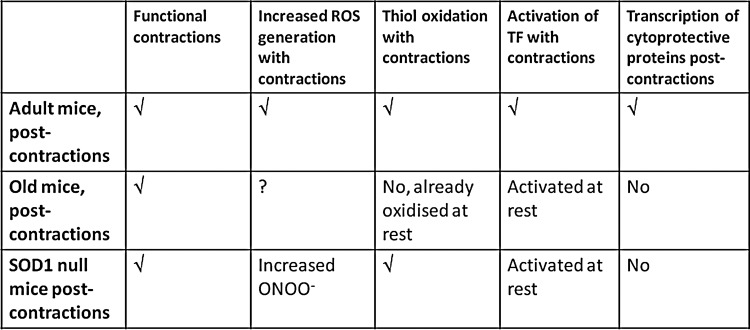
There is therefore evidence that manipulation of the activity of specific ROS through modified expression of SOD1 may produce a phenotype that resembles accelerated aging in skeletal muscle. Figure 7 shows our current concept of the mechanisms by which contraction-induced ROS lead to an increased expression of cytoprotective proteins through redox-regulated signaling pathways and Figure 8 illustrates how the key steps in this scheme are modified in muscle from aged mice or from the SOD1 null mouse. Clearly, although both aging and/or the lack of SOD1 lead to some similar phenotypic changes and to a failure of contraction-induced upregulation of cytoprotective proteins, there are differences in how they affect the proposed pathway.
FIG. 7.
Schematic representation of the putative steps in the redox signaling pathway leading to adaptive activation of transcription factors and upregulation of the expression of cytoprotective proteins following contractile activity in skeletal muscle. Prx, peroxiredoxins; NIK, NFκB inducing kinase; TF, transcription factor; ?, not demonstrated.
FIG. 8.
Comparison of key markers of the redox signaling pathway leading to adaptations to contractions in adult wild-type, old wild-type and SOD1 null mice. Both SOD1 null mice and aged wild-type mice show attenuated transcriptional responses to contractions that may play a key role in the aging phenotype seen in each model, but these comparisons indicate that different mechanisms are likely to play a role in these two situations. √, process established; ?, studies not undertaken.
Conclusions and Future Directions
Transgenic studies have identified that specific modification of pathways of ROS regulation can lead to accelerated aging of muscles that appear to be mediated by peroxynitrite and acting, in part, through inducing a failure of adaptations to contractile activity. Future studies will further examine the relevance of this model to normal aging and the potential for the model to identify sites for interventions targeted to maintain adaptive responses to contractions. Understanding of the role of redox regulation of adaptations to contractile activity has the potential to lead to improvements in the maintenance of muscle in aging and other situations where loss of muscle mass occurs.
Abbreviations Used
- AP-1
activator protein 1
- CuZnSOD
copper, zinc superoxide dismutase (SOD1)
- DCFH
2′, 7′ dichlorodihydrofluorescein
- DHE
dihydroethidium
- HSP
heat shock protein
- NADPH
reduced nicotinamide adenine dinucleotide phosphate
- NFκB
nuclear factor kappa-B
- NIK
NFκB inducing kinase
- NO
nitric oxide
- Prx
peroxiredoxin
- ROS
reactive oxygen species.
- SOD
superoxide dismutase
- TF
transcription factor
- Trx1
thioredoxin 1
- Trx2
thioredoxin 2
- WT
wild type
Acknowledgments
The author would like to acknowledge the multiple funders whose generous support has enabled this work to develop, including the Medical Research Council, Biotechnology and Biological sciences Research Council, US National Institute on Aging, Research into Ageing, Wellcome Trust and Arthritis Research UK. He would also like to acknowledge the enormous input of numerous collaborators, colleagues, and students who have undertaken this work.
References
- 1.Alnaqeeb MA. Goldspink G. Changes in fibre type, number and diameter in developing and ageing skeletal muscle. J Anat. 1987;153:31–45. [PMC free article] [PubMed] [Google Scholar]
- 2.Aydin J. Andersson DC. Hanninen SL. Wredenberg A. Tavi P. Park CB. Larsson NG. Bruton JD. Westerblad H. Increased mitochondrial Ca2+ and decreased sarcoplasmic reticulum Ca2+ in mitochondrial myopathy. Hum Mol Genet. 2009;18:278–288. doi: 10.1093/hmg/ddn355. [DOI] [PubMed] [Google Scholar]
- 3.Bakkar N. Wang J. Ladner K J. Wang H. Dahlman JM. Carathers M. Acharyya S. Rudnicki MA. Hollenbach AD. Guttridge DC. IKK/NF-kappaB regulates skeletal myogenesis via a signaling switch to inhibit differentiation and promote mitochondrial biogenesis. J Cell Biol. 2008;180:787–802. doi: 10.1083/jcb.200707179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balon TW. Nadler JL. Nitric oxide release is present from incubated skeletal muscle preparations. J Appl Physiol. 1994;77:2519–2521. doi: 10.1152/jappl.1994.77.6.2519. [DOI] [PubMed] [Google Scholar]
- 5.Bar-Shai M. Carmeli E. Reznick AZ. The role of NF-kappaB in protein breakdown in immobilization, aging, and exercise: from basic processes to promotion of health. Ann N Y Acad Sci. 2005;1057:431–447. doi: 10.1196/annals.1356.034. [DOI] [PubMed] [Google Scholar]
- 6.Beckman JS. Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 7.Bejma J. Ji LL. Aging and acute exercise enhance free radical generation in rat skeletal muscle. J Appl Physiol. 1999;87:465–470. doi: 10.1152/jappl.1999.87.1.465. [DOI] [PubMed] [Google Scholar]
- 8.Brooks SV. Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol. 1988;404:71–82. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broome CS. Kayani AC. Palomero J. Dillmann WH. Mestril R. Jackson MJ. Mcardle A. Effect of lifelong overexpression of HSP70 in skeletal muscle on age-related oxidative stress and adaptation after nondamaging contractile activity. FASEB J. 2006;20:1549–1551. doi: 10.1096/fj.05-4935fje. [DOI] [PubMed] [Google Scholar]
- 10.Cuthbertson D. Smith K. Babraj J. Leese G. Waddell T. Atherton P. Wackerhage H. Taylor PM. Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 11.Delbono O. Neural control of aging skeletal muscle. Aging Cell. 2003;2:21–29. doi: 10.1046/j.1474-9728.2003.00011.x. [DOI] [PubMed] [Google Scholar]
- 12.Dimauro I. Pearson T. Caporossi D. Jackson MJ. In vitro susceptibility of thioredoxins and glutathione to redox modification and aging-related changes in skeletal muscle. Free Rad Biol Med. 2012;53:2017–2027. doi: 10.1016/j.freeradbiomed.2012.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 14.Einsiedel LJ. Luff AR. Effect of partial denervation on motor units in the ageing rat medial gastrocnemius. J Neurol Sci. 1992;112:178–184. doi: 10.1016/0022-510x(92)90148-e. [DOI] [PubMed] [Google Scholar]
- 15.Gems D. Doonan R. Antioxidant defense and aging in C. elegans: is the oxidative damage theory of aging wrong? Cell Cycle. 2009;8:1681–1687. doi: 10.4161/cc.8.11.8595. [DOI] [PubMed] [Google Scholar]
- 16.Halliwell B. Gutteridge JM. Free Radical Biology and Medicine. Oxford University Press; Oxford: 1989. [Google Scholar]
- 17.Hollander JM. Lin KM. Scott BT. Dillmann WH. Overexpression of PHGPx and HSP60/10 protects against ischemia/reoxygenation injury. Free Radic Biol Med. 2003;35:742–751. doi: 10.1016/s0891-5849(03)00400-3. [DOI] [PubMed] [Google Scholar]
- 18.Irrcher I. Ljubicic V. Hood DA. Interactions between ROS and AMP kinase activity in the regulation of PGC-1alpha transcription in skeletal muscle cells. Am J Physiol Cell Physiol. 2009;296:C116–C123. doi: 10.1152/ajpcell.00267.2007. [DOI] [PubMed] [Google Scholar]
- 19.Jackson MJ. Papa S. Bolanos J. Bruckdorfer R. Carlsen H. Elliott RM. Flier J. Griffiths HR. Heales S. Holst B. Lorusso M. Lund E. Oivind Moskaug J. Moser U. Di Paola M. Polidori MC. Signorile A. Stahl W. Vina-Ribes J. Astley SB. Antioxidants, reactive oxygen and nitrogen species, gene induction and mitochondrial function. Mol Aspects Med. 2002;23:209–285. doi: 10.1016/s0098-2997(02)00018-3. [DOI] [PubMed] [Google Scholar]
- 20.Jackson MJ. Control of reactive oxygen species production in contracting skeletal muscle. Antioxid Redox Signal. 2011;15:2477–2486. doi: 10.1089/ars.2011.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jang YC. Lustgarten MS. Liu Y. Muller FL. Bhattacharya A. Liang H. Salmon AB. Brooks SV. Larkin L. Hayworth CR. Richardson A. Van Remmen H. Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J. 2010;24:1376–1390. doi: 10.1096/fj.09-146308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jang YC. Remmen VH. The mitochondrial theory of aging: insight from transgenic and knockout mouse models. Exp Gerontol. 2009;44:256–260. doi: 10.1016/j.exger.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Janssen-Heininger YM. Mossman BT. Heintz NH. Forman HJ. Kalyanaraman B. Finkel T. Stamler JS. Rhee SG. Van Der Vliet A. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med. 2008;45:1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ji LL. Gomez-Cabrera MC. Steinhafel N. Vina J. Acute exercise activates nuclear factor (NF)-kappaB signaling pathway in rat skeletal muscle. FASEB J. 2004;18:1499–1450. doi: 10.1096/fj.04-1846com. [DOI] [PubMed] [Google Scholar]
- 25.Kayani AC. Close GL. Dillmann WH. Mestril R. Jackson MJ. Mcardle A. Overexpression of HSP10 in skeletal muscle of transgenic mice prevents the age-related fall in maximum tetanic force generation and muscle Cross-Sectional Area. Am J Physiol Regul Integr Comp Physiol. 2010;299:R268–R276. doi: 10.1152/ajpregu.00334.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobzik L. Reid MB. Bredt DS. Stamler JS. Nitric oxide in skeletal muscle. Nature. 1994;372:546–548. doi: 10.1038/372546a0. [DOI] [PubMed] [Google Scholar]
- 27.Larkin LM. Davis CS. Sims-Robinson C. Kostrominova TY. Remmen HV. Richardson A. Feldman EL. Brooks SV. Skeletal muscle weakness due to deficiency of CuZn-superoxide dismutase is associated with loss of functional innervation. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1400–R1407. doi: 10.1152/ajpregu.00093.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larsson L. Ansved T. Effects of ageing on the motor unit. Prog Neurobiol. 1995;45:397–458. doi: 10.1016/0301-0082(95)98601-z. [DOI] [PubMed] [Google Scholar]
- 29.Lexell J. Taylor CC. Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 30.Ljubicic V. Hood DA. Kinase-specific responsiveness to incremental contractile activity in skeletal muscle with low and high mitochondrial content. Am J Physiol Endocrinol Metab. 2008;295:E195–E204. doi: 10.1152/ajpendo.90276.2008. [DOI] [PubMed] [Google Scholar]
- 31.McArdle A. Pattwell D. Vasilaki A. Griffiths RD. Jackson MJ. Contractile activity-induced oxidative stress: cellular origin and adaptive responses. Am J Physiol Cell Physiol. 2001;280:C621–C627. doi: 10.1152/ajpcell.2001.280.3.C621. [DOI] [PubMed] [Google Scholar]
- 32.McArdle A. Dillman WH. Mestril R. Faulkner JA. Jackson MJ. Overexpression of HSP70 in mouse skeletal muscle protects against muscle damage and age-related muscle dysfunction. FASEB J. 2004;18:355–357. doi: 10.1096/fj.03-0395fje. [DOI] [PubMed] [Google Scholar]
- 33.Melov S. Mitochondrial oxidative stress. Physiologic consequences and potential for a role in aging. Ann N Y Acad Sci. 2000;908:219–225. doi: 10.1111/j.1749-6632.2000.tb06649.x. [DOI] [PubMed] [Google Scholar]
- 34.Michaelson LP. Shi G. Ward CW. Rodney GG. Mitochondrial redox potential during contraction in single intact muscle fibers. Muscle Nerve. 2010;42:522–529. doi: 10.1002/mus.21724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller RA. ‘Accelerated aging’: a primrose path to insight? Aging Cell. 2004;3:47–51. doi: 10.1111/j.1474-9728.2004.00081.x. [DOI] [PubMed] [Google Scholar]
- 36.Muller FL. Song W. Liu Y. Chaudhuri A. Pieke-Dahl S. Strong R. Huang TT. Epstein CJ. Roberts LJ., 2nd Csete M. Faulkner JA. Van Remmen H. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic Biol Med. 2006;40:1993–2004. doi: 10.1016/j.freeradbiomed.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 37.Murrant CL. Reid MB. Detection of reactive oxygen and reactive nitrogen species in skeletal muscle. Microsc Res Tech. 2001;55:236–248. doi: 10.1002/jemt.1173. [DOI] [PubMed] [Google Scholar]
- 38.Orr WC. Sohal RS. Does overexpression of Cu,Zn-SOD extend life span in Drosophila melanogaster? Exp Gerontol. 2003;38:227–230. doi: 10.1016/s0531-5565(02)00263-2. [DOI] [PubMed] [Google Scholar]
- 39.Pérez VI. Bokov A. Van Remmen H. Mele J. Ran Q. Ikeno Y. Richardson A. Is the oxidative stress theory of aging dead? Biochim Biophys Acta. 2009;1790:1005–1014. doi: 10.1016/j.bbagen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Porter MM. Vandervoort AA. Lexell J. Aging of human muscle: structure, function and adaptability. Scand J Med Sci Sports. 1995;5:129–142. doi: 10.1111/j.1600-0838.1995.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 41.Powers SK. Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Powis G. Montfort WR. Properties and biological activities of thioredoxins. Ann Rev Biophys Biomol Struct. 2001;30:421–455. doi: 10.1146/annurev.biophys.30.1.421. [DOI] [PubMed] [Google Scholar]
- 43.Reid MB. Shoji T. Moody MR. Entman ML. Reactive oxygen in skeletal muscle. II. Extracellular release of free radicals. J Appl Physiol. 1992;73:1805–1809. doi: 10.1152/jappl.1992.73.5.1805. [DOI] [PubMed] [Google Scholar]
- 44.Sakellariou GK. Pye D. Vasilaki A. Zibrik L. Palomero J. Kabayo T. Mcardle F. Van Remmen H. Richardson A. Tidball JG. Mcardle A. Jackson MJ. Role of superoxide-nitric oxide interactions in the accelerated age-related loss of muscle mass in mice lacking Cu,Zn superoxide dismutase. Aging Cell. 2011;10:749–760. doi: 10.1111/j.1474-9726.2011.00709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakellariou GK. Vasilaki A. Palomero J. Kayani A. Zibrik L. McArdle A. Jackson MJ. Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxid Redox Signal. 2013;18:603–621. doi: 10.1089/ars.2012.4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schriner SE. Linford NJ. Martin GM. Treuting P. Ogburn CE. Emond M. Coskun PE. Ladiges W. Wolf N. Van Remmen H. Wallace DC. Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 47.Spyrou G. Enmark E. Miranda-Vizuete A. Gustafsson J. Cloning and expression of a novel mammalian thioredoxin. J Biol Chem. 1997;272:2936–2941. doi: 10.1074/jbc.272.5.2936. [DOI] [PubMed] [Google Scholar]
- 48.St-Pierre J. Buckingham JA. Roebuck SJ. Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem. 2002;277:44784–4490. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 49.Vasilaki A. McArdle F. Iwanejko LM. McArdle A. Adaptive responses of mouse skeletal muscle to contractile activity: the effect of age. Mech Ageing Dev. 2006;127:830–839. doi: 10.1016/j.mad.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 50.Vasilaki A. Mansouri A. Remmen H. Van Der Meulen JH. Larkin L. Richardson AG. McArdle A. Faulkner JA. Jackson MJ. Free radical generation by skeletal muscle of adult and old mice: effect of contractile activity. Aging Cell. 2006;5:109–117. doi: 10.1111/j.1474-9726.2006.00198.x. [DOI] [PubMed] [Google Scholar]
- 51.Vasilaki A. Van Der Meulen JH. Larkin L. Harrison DC. Pearson T. Van Remmen H. Richardson A. Brooks SV. Jackson MJ. Mcardle A. The age-related failure of adaptive responses to contractile activity in skeletal muscle is mimicked in young mice by deletion of Cu,Zn superoxide dismutase. Aging Cell. 2010;9:979–990. doi: 10.1111/j.1474-9726.2010.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang ZM. Zheng Z. Messi ML. Delbono O. Extension and magnitude of denervation in skeletal muscle from ageing mice. J Physiol. 2005;565:757–764. doi: 10.1113/jphysiol.2005.087601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watson WH. Yang X. Choi YE. Jones DP. Kehrer JP. Thioredoxin and its role in toxicology. Toxicol Sci. 2004;78:3–14. doi: 10.1093/toxsci/kfh050. [DOI] [PubMed] [Google Scholar]
- 54.Yoshida T. Nakamura H. Masutanim H. Yodoi J. The involvement of thioredoxin and thioredoxin binding protein-2 on cellular proliferation and aging process. Ann N Y Acad Sci. 2005;1055:1–12. doi: 10.1196/annals.1323.002. [DOI] [PubMed] [Google Scholar]