Abstract
Pericytes play a crucial role in angiogenesis and vascular maintenance. They can be readily identified in vivo and isolated as CD146+CD34− cells from various tissues. Whether these and other markers reliably identify pericytes in vitro is unclear. CD146+CD34− selected cells exhibit multilineage potential. Thus, their perivascular location might represent a stem cell niche. This has spurred assumptions that not only all pericytes are mesenchymal stromal cells (MSCs), but also that all MSCs can act as pericytes. Considering this hypothesis, we developed functional assays by confronting test cells with endothelial cultures based on matrigel assay, spheroid sprouting, and cord formation. We calibrated these assays first with commercial cell lines [CD146+CD34− placenta-derived pericytes (Pl-Prc), bone marrow (bm)MSCs and fibroblasts]. We then functionally compared the angiogenic abilities of CD146+CD34−selected bmMSCs with CD146− selected bmMSCs from fresh human bm aspirates. We show here that only CD146+CD34− selected Pl-Prc and CD146+CD34− selected bmMSCs maintain endothelial tubular networks on matrigel and improve endothelial sprout morphology. CD146− selected bmMSCs neither showed these abilities, nor did they attain pericyte function despite progressive CD146 expression once passaged. Thus, cell culture conditions appear to influence expression of this and other reported pericyte markers significantly without correlation to function. The newly developed assays, therefore, promise to close a gap in the in vitro identification of pericytes via function. Indeed, our functional data suggest that pericytes represent a subpopulation of MSCs in bm with a specialized role in vascular biology. However, these functions are not inherent to all MSCs.
Introduction
Pericytes of the microvasculature are attached to the abluminal side of endothelial cell tubules and are located within the surrounding basement membrane [1]. Identification of this morphological feature requires electron microscopy, a less practical approach for studying developing microvasculature, in particular at angiogenic sprouts where the basement membrane is discontinuous. Therefore, the perivascular location in combination with immunocytochemical markers to identify pericytes histologically in vivo is preferred [2]. As an in vitro proof for the pericytic nature of cells, the expression of pericyte-related markers and colocalization of cells with the tubular network formed by endothelial cells on matrigel is deemed sufficient [3–5]. To the best of our knowledge, no in vitro assay currently can identify pericytes functionally, demonstrating their angiogenic behavior and distinguishing them from other mesenchymal cells. Such functional identification of pericytes and pericyte-specific behavior in vitro would open avenues to study the role of this elusive cell type in greater detail. Although pericytes do not seem to be necessary in the initial formation of vasculature during development, they induce its maturation and regulate microvessel integrity, structure, and function [6]. Furthermore, they were shown to support angiogenesis in tumors [3,7] and wound healing [8]. For the last two decades pericytes were speculated to be mesenchymal progenitors with the potential to differentiate into osteoblasts [9], chondrocytes [10], and adipocytes [11]. After it was demonstrated that pericytes can be identified by expression of CD146 in vivo [12], Crisan and colleagues were the first to systematically isolate them from various tissues using this marker and the simultaneous absence of the endothelial marker CD34. The isolated cells proved to be multipotent on a clonal level and demonstrated regenerative potential in vivo [13].
These findings paved the path for the hypothesis that all mesenchymal stromal cells (MSCs) can serve as pericytes [14]. Indeed, during tumor angiogenesis [3,15,16] and wound healing [17], pericytes are recruited from both the nonlesional vicinity of tissue and the bone marrow (bm) to facilitate blood vessel formation. However, MSCs are a notoriously heterogeneous cell population [18]. With respect to vasculature, they also have been found in the tunica adventitia of larger vessels. Although these CD34+CD146− cells from the tunica adventitia were phenotypically distinct from pericytes, these cells could be induced to differentiate into pericyte-like cells [19]. Therefore, the question remains whether all MSCs can act as pericytes, or whether pericytes are a subpopulation of MSCs with specialized functions.
Current literature suggests that ex vivo pericytes can be only distinguished from other MSCs on the basis of their isolation procedure. Pericytes are selected by their CD146+CD34− marker profile [13], while MSCs in general are isolated via their adherence to plastic [20] yielding a heterogeneous population [18]. Here we tested the hypothesis that MSCs may have a general pericyte functionality by comparing pericytes and nonpericytic MSCs in terms of marker expression and functional behavior. Various angiogenic in vitro assays were adapted to demonstrate pericyte features like tube stabilization and sprouting improvement.
Materials and Methods
Cell culture
Human placenta-derived pericytes (Pl-Prc) were purchased from Promocell, who isolated pericytes by positive selection for CD146 and absence of CD34 to avoid endothelial cells (EC). Pericytes were propagated in pericyte growth media (PGM, Promocell). Human bmMSCs (Lonza) and fetal lung fibroblast cell line IMR-90 were cultured in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% FBS, respectively. We used three different lots of Pl-Prc and three different lots of bmMSCs. Human umbilical vein endothelial cells (HUVECs) were grown in EGM-2 (Lonza). Bm cells were cultured from excess in three separate patients undergoing Institutional Review Board (IRB) approved trials. Bm aspiration from patients was approved IRB. Bm cells were isolated over a Ficoll density-gradient (GE Healthcare) following manufacturers protocol. Whole Bm mononucleated cells were stained with antibodies for CD146 and CD34 (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/scd) and CD146+CD34− cells were separated from remaining CD146− cells by cell sorting (Supplementary Fig. S1) and isolated further as previously described [21]. Remaining bmMSCs were isolated from CD146− cells by plastic adherence [20]. Outgrown colonies from both cultures were further cultured in PGM and used for experiments at passage 3 to 4.
Immunostaining
All cells were grown both in DMEM and PGM to confluency. Cells were methanol-fixed in 24 well-plates and stained with antibodies (Supplementary Table S2) as described [22]. Pictures were taken with an epifluorescence microscope (Olympus; IX71). For flow cytometry, cells were propagated in three separate T75 flasks resuspended in PBS containing 1% FBS and stained with monoclonal FITC- or PE-tagged antibodies (Supplementary Table S2). They were then washed once with PBS 1% FBS, fixed with 1% formaldehyde and analyzed by flow cytometry (Cyan; Dako cytomation).
Differentiation
Cells were adipogenically and osteogenically induced according to standard protocols [13] under conditions of macromolecular crowding [23]. Adipogenic differentiated cultures were stained with nile red and osteogenic differentiated cultures with alizarin red. To induce chondrogenesis in Pl-Prc, bmMSCs, and IMR90s a standard protocol employing TGFβ1 was used [24]. Pl-Prc were also induced using a protocol optimized for the differentiation of pericytes employing TGFβ3 [10]. Chondrogenic pellets were sectioned and stained for proteoglycans with alcian blue.
Functional assays
All cells were fluorescently prelabeled with PKH26 (red) or PKH67 (green) (Sigma) following manufacturer's instructions. For tube formation assay [3–5] cells were coseeded on presolidified matrigel™ (Becton and Dickinson) in EGM-2 or DMEM with 0.5% FBS for tube length quantitation. A minimum of triplicates per condition was used. Cumulative tube length was measured using the simple neurite tracer plugin in the Fiji software (http://fiji.sc/). Significance was calculated using Student's t-test. The spheroid sprouting assay protocol was adapted from [25,26]. Five hundred HUVECs per well were coseeded at a ratio of 10:1 with one of the mesenchymal cell types (pericytes, MSCs, IMR90s) in nonadherence round-bottom 96-well plates in EGM-2 containing 2.5 μg/mL methylcellulose, overnight to form spheroids. Formed spheroids were placed into solidifying collagen I gels (1 mg/mL) in EGM-2 and 2.5 μg/mL methylcellulose. Twenty-four spheroids were produced for each condition. For monolayer cocultures [22], mesenchymal cells were seeded at 30,000 cells per cm2 and grown to confluency for 4 days in DMEM/10% FBS/antibiotics. HUVEC were seeded at a ratio of 1:4 on top and cultured in EBM-2 containing 0.5% FBS with 5 ng/mL of VEGF for further 3 days. Cocultures were immunostained for vWF and α-smooth muscle actin (α-SMA) and were viewed using an Apotome (Zeiss) and confocal laser scanning microscope (Olympus). For assay optimization using commercial cell sources at least triplicates were used. Duplicates were used for bm samples. All experiments were repeated at least three times.
Results and Discussion
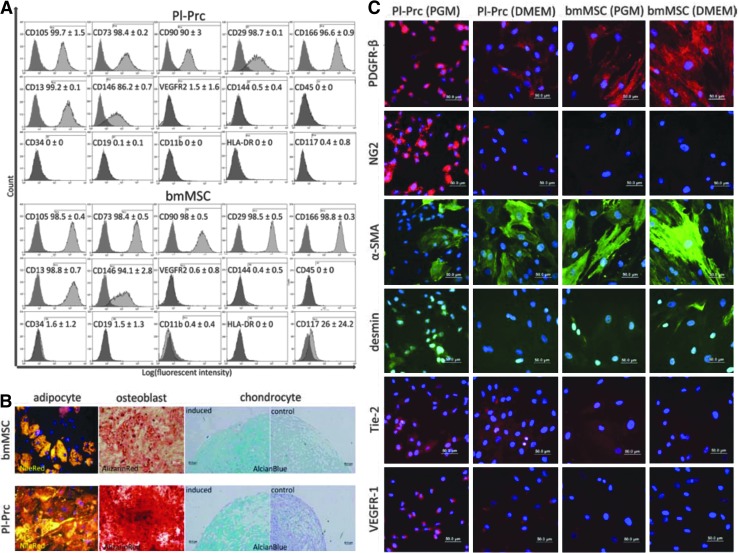
Pericytes were originally described to be indistinguishable in culture from MSCs [27]. Accordingly, flow cytometry analysis of Pl-Prc, which were isolated as CD146+CD34− cells showed a cell surface marker profile highly similar to those of the tested bmMSCs, which is the same as generally expected for MSCs [28] (Fig. 1A). Pl-Prc and bmMSC expressed the MSC markers CD105, CD73, CD90, CD29, CD166, and CD13 and lacked hematopoietic and endothelial markers CD45, CD19, CD11b, and HLA-DR, CD34, CD144, and VEGFR-2 (Fig. 1A). Therefore, the Pl-Prc used in this study had a stromal cell phenotype and were clearly distinguishable from endothelial and hematopoietic cells. When a common fibroblast cell line (IMR-90) was used as a non-MSC control the same marker profile was observed (Supplementary Fig. S2), indicating that in general marker expression alone might not be sufficient to distinguish MSCs from other mesenchymal cells. Surprisingly, CD146 as the marker chosen to isolate pericytes from various tissues [13] and chosen in this study was present on bmMSCs (Fig. 1A) and IMR90s (Supplementary Fig. S2) as well.
FIG. 1.
Pl-Prc are MSCs but express additional markers when propagated in PGM. (A) Pl-Prc and bmMSCs (n=3) share a common MSC marker profile as indicated by MSC marker (CD105 through CD13), also CD146 (used for pericyte isolation), and absence of endothelial/hematopoietic markers (CD144 through CD117). (B) Pl-Prc and bmMSCs (n=3) were induced to differentiate into adipocytes, osteoblasts, and chondrocytes. Lipid droplets (adipocyte) were stained with nile red (gold), calcification (osteoblast) with alizarin red (red) and proteoglycans (chondrocyte) with alcian blue (blue), respectively. (C) Immunocytochemical detection of pericytic markers. Presence of PDGFR-β and α-SMA is independent from culture medium; however, NG2, desmin, Tie-2 and VEGFR-1 are more strongly expressed in Pl-Prc cultured in PGM. (A–C) Results are representatives of three independent studies. α-SMA, α-smooth muscle actin; bmMSC, bone marrow mesenchymal stromal cells; MSCs, mesenchymal stromal cells; PDGFR-β, platelet derived growth factor β; PGM, pericyte growth media; Pl-Prc, placenta-derived pericytes.
Pl-Prc and bmMSCs were tested for their differentiation potential. Both were found to differentiate into the three mesenchymal lineages: adipocytes, osteoblasts, and chondrocytes (Fig. 1B). The multipotency of Pl-Prc confirms earlier findings [13]. IMR90s failed to produce lipid droplets and did not exhibit significant staining for chondrogenic proteoglycans (Supplementary Fig. S3). However, they exhibited a substantial calcification as well (Supplementary Fig. S3). The ability of fibroblasts to deposit Ca2+ under standard induction conditions is well documented and does not necessarily indicate their ability to differentiate into osteoblasts but rather points to their implication in pathological calcification [29].
Since it is well established that there is no single selective marker for pericytes [2] we sought to identify markers that could distinguish pericytes from other MSCs. Therefore, we tested Pl-Prc and bmMSCs other pericyte-related markers (Fig. 1C). As these markers might be sensitive to culture conditions, we tested cells grown in different media (PGM and DMEM). The commonly used pericyte markers, platelet-derived growth factor β (PDGFR-β) and α-SMA, were shared by both cell types independent of culture conditions. Pl-Prc grown in PGM showed a strong perinuclear PDGFR-β staining, whereas cells in all other conditions showed a homogenous cytoplasmic distribution. α-SMA staining was enhanced in both cell types when cultured in DMEM (Fig. 1C). In contrast, NG2 was strongly expressed only in Pl-Prc pericytes cultured in PGM, but staining intensity decreased after transfer to DMEM. NG2 staining was mostly absent in any bmMSC culture (Fig. 1C). We observed a high variability in desmin staining. Generally, in Pl-Prc grown in PGM desmin occurred as weak, granular staining around the nucleus or as fibers with a high fluorescent intensity. bmMSCs cultured in DMEM sometimes also displayed desmin staining, but with high variability. The marker panel was then extended to receptors involved in angiogenesis Tie-2 and VEGFR-1. Staining for both receptors was strongest in Pl-Prc cultured in PGM with a decrease in signal intensity when transferred to DMEM. BmMSCs showed no or a very low signal for both markers. Tie-2 has recently been found to be expressed by pericyte progenitors in addition to endothelial cells and macrophages in vivo [30] and on retinal pericytes in vitro [31]. Ang-Tie-2 signaling was found to be crucial for endothelial cell- pericyte communication and Tie-2 knock-outs resulted in the lack of pericytes and a similar phenotype of microvasculature as PDGFR-β knock-outs [32,33]. VEGFR-1 was detected on retinal pericytes in vitro [34] and in vivo [35,36]. We conclude that NG2, Tie-2, and VEGFR-1 in coexpression with PDGFR-β or α-SMA can indicate pericytes in vitro. However, it should be noted that the expression of these markers by pericytes is variable and depends not only on the tissue of origin [2], but also on culture conditions.
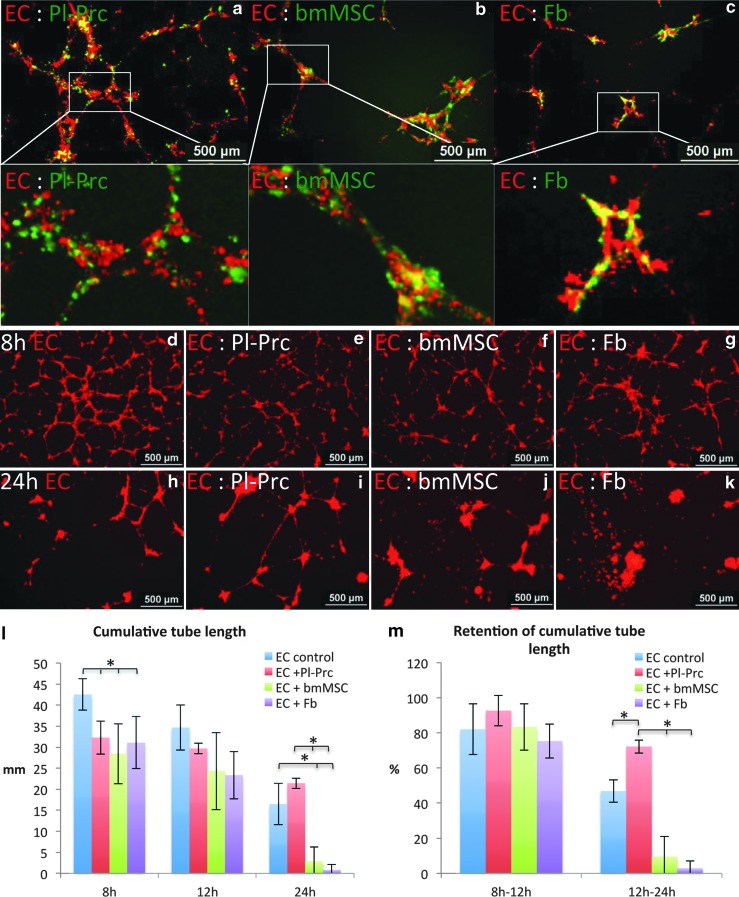
We therefore, sought to distinguish pericytes from other MSCs by their functionality in vitro. For the initial set-up of the assays, we employed commercially and therefore, standardized cells (Pl-Prc expanded in PGM and bmMSCs and IMR90s expanded in DMEM). These cell types do not form tubular networks in matrigel as opposed to endothelial cells (Supplementary Fig. S4, 1st row). Surprisingly, the colocalization of cells with such a network in a coseeding setting was not restricted to Pl-Prc (Fig. 2a), also bmMSCs (and IMR-90s) exhibited this feature in the assay (Fig. 2b, c). However, it became apparent that the different coseeded cell types had distinct capabilities to maintain and stabilize the formed networks. Depending on cell-ratios in endothelial cell- Pl-Prc cocultures, thicker but less tubes formed within the first 12 h as compared to endothelial controls. In contrast, bmMSCs and IMR-90s collapsed the network in a dose-dependent manner (Supplementary Fig. S4). At an optimized endothelial- mesenchymal cell ratio of 20 to 1 in starving media, we observed that, although in endothelial cell controls a denser network was formed (Fig. 2d–g), more tubules remained intact in endothelial cell- Pl-Prc cocultures after 24 h (Fig. 2h, i). Again cocultures with bmMSCs or IMR-90s resulted in single detached cells and cell aggregates (Fig. 2j, k). Quantifications of the cumulative tube length confirmed that these observed differences in tube length were significant (Fig. 2l). Analysis of the retention of the cumulative tube length demonstrated that only Pl-Prc were able to significantly maintain a larger portion of the tubular network than measured in endothelial cell controls (Fig. 2m). In accordance to the function of pericytes to maintain microvessel integrity in vivo [6], the ability to stabilize endothelial networks was inherent to Pl-Prc in this assay.
FIG. 2.
Only Pl-Prc stabilize endothelial networks. Coculture of HUVEC with Pl-Prc, bmMSCs or IMR90s (Fb) on matrigel (n≥3). (a–c) In mixed cultures all mesenchymal cells (green label) colocalized with the endothelial network (red label). Coculture at a ratio of 20:1 at 8 h (d–g) and 24 h (h–k) demonstrate formation of EC network (only red labeled EC shown) and decay at 24 h. In the presence of Pl-Prc, the decay is slowed. (l) Quantitation of cumulative tube length (*P<0.02) and (m) retention of cumulative tube length at various time intervals (*P≤0.01) demonstrate that only Pl-Prc can significantly retain integrity of endothelial tubules over 24 h. Results are displayed as average±standard deviation. Results are representative of three independent studies. EC, endothelial cells; HUVECs, human umbilical vein endothelial cells.
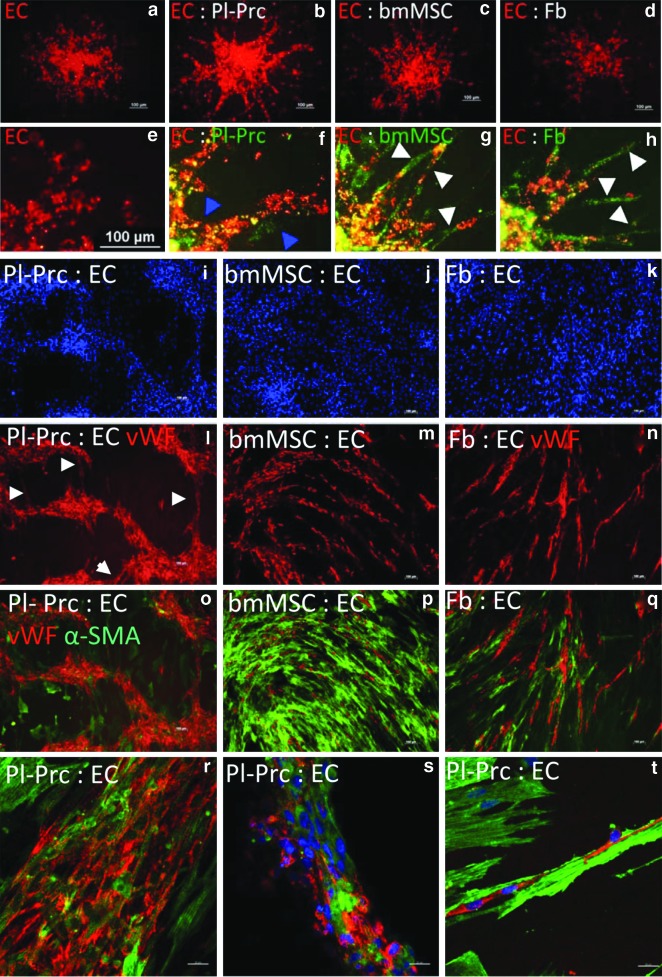
We then modified established protocols [25,26] to study the effect of mesenchymal cells on sprout formation in mixed cell spheroids. Although, even optimized endothelial cell to mesenchymal cell ratios did not affect the total length of forming sprouts per spheroid (Fig. 3a–d), change in sprout morphology was observed in cocultures (Fig. 3e–h). Sprouts in endothelial cell- Pl-Prc cocultures had a smooth and compact morphology (Fig. 3f), while sprouts formed by endothelial cells alone (Fig. 3e) exhibited loosely attached cells with irregular shapes and margins. We found that Pl-Prc colocalized with formed sprouts (Fig. 3f, blue arrowheads) as expected of pericytes. In contrast, bmMSCs and IMR90s migrated larger distances away from endothelial cells and thus, segregated from sprouts (Fig. 3g, h white arrowheads). Sprouts formed in these cocultures often disintegrated (Fig. 3g, h), forming pseudosprouts composed of loosely arranged endothelial cells bridged by bmMSCs or IMR90s (Fig. 3g, h white arrowheads). In accordance with findings that pericyte recruitment supports blood vessel formation in vivo [3,7,8], only Pl-Prc were able to improve the integrity of the sprouts formed from mixed spheroids.
FIG. 3.
Only Pl-Prc improve sprout integrity and move with endothelial cells to form cords in coculture. EC (red label) were cocultured with Pl-Prc, bmMSCs, IMR90s (Fb) (green label) to form spheroids (n=24), which were introduced into collagen I gels to sprout. (a–d) only red labeled EC are shown. (a) EC cultures show spontaneous formation of loosely arranged sprouts. (b) In the presence of Pl-Prc, formed sprouts are broader and have a smooth compact morphology. (c, d) In the presence of bmMSCs and Fb, sprouts appear thin and discontinuous. (e–h) Close-ups reveal that Pl-Prc colocalize with EC (blue arrowheads), while bmMSCs and Fb segregate from EC or bridge detached ECs (white arrowheads). (i–t) In a cord forming coculture assay EC were seeded on top of confluent Pl-Prc, bmMSCs, or Fb (n≥3). While bmMSCs and Fb support cord formation of single-cell thickness (m, n), only Pl-Prc show formation of cords of various thicknesses with interconnections (l, white arrowheads). (r–t) Confocal analyses reveal that Pl-Prc incorporate into formed cords and are aligned parallel to cord direction. (t) α-SMA fibers are more pronounced in Pl-Prc contributing to cords and are aligned in the same direction. Results are representatives of three independent studies.
Pl-Prc were then subjected to a cord-forming assay [22], in which they demonstrated a unique behavior by actively contributing to the formation of cord-like structures of varying thicknesses, leaving spaces free of cells, as evident by the lack of DAPI and α-SMA, in between the cords (Fig. 3i, l, o). The cords were further interconnected to form a network (Fig. 3l, white arrowheads). Both endothelial cells and Pl-Prc constituted the cord and were aligned along the cord direction (Fig 3r–t), also evident from the alignment of α-SMA fibers that were strongest expressed in Pl-Prc within the cords (Fig. 3t). Smaller cords constituted of endothelial cells surrounded by Pl-Prc (Fig. 3t), whereas larger cords were composed of a mixed arrangement of both cell types (Fig. 3r, s). In contrast, bmMSCs and IMR90s served merely as a feeder layer, on top of which endothelial cells arranged parallel to mesenchymal cell direction in a cord-like pattern. No major movement of mesenchymal cells could be observed towards endothelial cords (Fig. 3j, k, m, n, p, q).
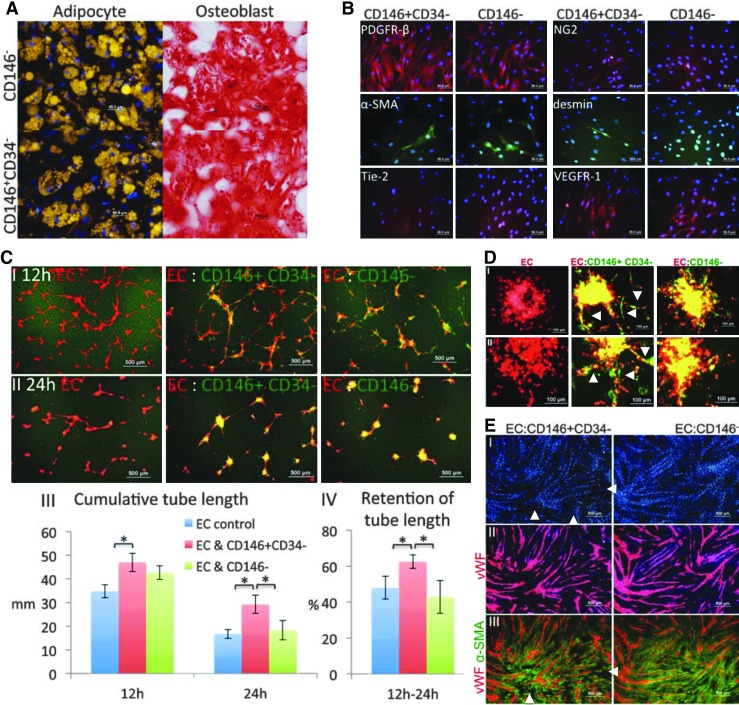
Although derived from different tissues, bmMSCs and IMR90s behaved alike in all in vitro assays. In contrast, only Pl-Prc exhibited functions resembling those observed in vivo [3,6–8]. However, Pl-Prc, bmMSCs and IMR90s are derived from different commercial sources; therefore, handled differently, and different media were used to propagate them. Therefore, different culture conditions before functional assays might have had an effect on their performance. To confirm that the observed behavior by Pl-Prc was pericyte- and not tissue-specific or culture medium-dependent, CD146+CD34− selected cells and CD146− selected cells from the same bm aspirates were assessed in parallel on the established assay platform. We observed an outgrowth of spindle-shaped cell colonies from both cell pools; however, colonies formed in CD146+CD34− selected cell cultures appeared smaller and cells from these cultures reached senescence earlier than cells from CD146− selected bm cells. Since we had found earlier that in DMEM expression of pericyte-related markers might be downregulated (Fig. 1c), outgrown cells from both cell pools were further cultured in PGM. Differentiation of both cell preparations into adipocytes and osteoblasts confirmed their multipotency and identified them as MSCs (Fig. 4A).
FIG. 4.
CD146+CD34− selected but not CD146− selected bmMSCs stabilize endothelial networks and improve endothelial sprout integrity. CD146+CD34− cells and CD146− cells from the same bm specimens were subjected to: (A) Induction into adipocytes and osteoblasts. Lipid droplets were stained with nile red (gold) and calcification was stained with alizarin red (red) in the respective cultures. Cells were confirmed to be MSCs. (B) Immunocytochemistry of pericyte-related markers. Cells share a very similar marker profile of PDGFR-β, α-SMA, NG2, desmin, Tie-2, and VEGFR-1. (C) Coculture with HUVEC (1:20) on matrigel (n≥3). (I, II) Superimposition of signals for EC (red) and cocultured bm-derived cells (green) at 12 and 24 h. (III) Quantitation of cumulative tube length (*P<0.01). (IV) Quantitation of retention of cumulative tube length (*P<0.01). Quantitative results are displayed as average value±standard deviation. CD146+CD34− cells stabilize endothelial network significantly. (D) Mixed spheroid sprouting assay. EC (red label) were introduced as spheroids (n=24) with bm derived cells (green label) into collagen I gels to sprout. (I, II) Results using cells from two independent samples are displayed. (E) Mono-layer coculture assay. (I) EC were seeded on top of CD146+CD34− cells and CD146− cells at 1:4 in media containing VEGF and cultured for 3 days. Methanol fixed cocultures were immunostained for vWF (red, EC maker) (II) and α-SMA (green, mesenchymal cell marker) (III), and nuclei were stained with DAPI (blue) (n=2). CD146+CD34− cells move slightly more towards formed endothelial cords resulting in areas in between cords with less DAPI and no α-SMA (white arrowheads). Results are representatives of studies performed with three different bm samples.
Interestingly, both CD146+CD34− and CD146− selected cells from bm showed a very similar marker profile (Fig. 4B). Both cell types expressed PDGFR-β strongly, had a weaker staining of NG2 and in both cultures a proportion of cells showed stained fibers of α-SMA. Desmin was seen only and rarely in CD146+CD34− selected cells. Both cell cultures lacked Tie-2, but were positive for VEGFR-1 (Fig. 4B). Furthermore, CD146− selected MSCs were tested for CD146 expression after culture in DMEM and PGM. Surprisingly, the cells became CD146-positive regardless of culture conditions (Supplementary Fig. S5. Therefore, it appears that CD146 is suitable to select functional pericytes from various tissues in a cell sorting approach, but cannot identify functional pericytes after in vitro expansion.
Similarly, expression of other pericyte-related markers in vitro is dependent on culture conditions and does not allow to distinguish pericytes from other MSCs. To this point CD146+CD34− selected pericytes and CD146− selected MSCs were not distinguishable from each other, indicating the necessity of functional assays.
As expected both cell preparations colocalized with the endothelial network, when included in the tube formation assay on matrigel (Fig. 4CI, II). Cocultures with CD146+CD34− selected cells resulted in the formation of networks with significantly higher cumulative tube length (Fig. 4CI, III) and it remained highest in cocultures with CD146+CD34− selected cells (Fig. 4CII, III). Quantitation of the percentage of retained endothelial tubules over time demonstrated that CD146+CD34− selected cells were able to significantly enhance the stability of the networks (Fig. 4CIV). In contrast, networks formed in the presence of CD146− selected MSCs disintegrated at the same rate as pure endothelial cell networks. Interestingly, CD146− selected cells did not collapse the network as it was observed with nonselected bmMSC expanded in DMEM, indicating that culture conditions will also have an effect on functionality. We concluded that CD146+CD34− selected cells derived from bm were able to stabilize endothelial networks, whereas CD146− selected cells were not.
When cocultured with endothelial cells in mixed spheroids, only CD146+CD34− selected cells induced the formation of defined and continuous sprouts with smooth margins (Fig. 4DI, II white arrowheads). In cocultures with CD146− selected cells, sprouts were composed of loosely arranged endothelial cells with endothelial cells disconnecting from formed sprouts and migrating as single cells (Fig. 4DI, II).
The seeding of endothelial cells on confluent monolayers (cord formation assay) of CD146+CD34− selected cells or CD146− selected cells resulted in the formation of endothelial cords of similar morphology (Fig. 4EII). Cocultures with CD146+CD34− selected cells showed slight differences to cocultures with CD146− selected cells. Cord structures, as indicated by DAPI staining (Fig. 4EI) were better defined in cocultures with CD146+CD34− selected cells than CD146− selected cells. Further, small spaces almost free of DAPI and free of α-SMA in between cords were mostly present in cocultures with CD146+CD34− selected cells (Fig. 4EI, III white arrowheads). In comparison, the adapted tube stabilization assay on matrigel and the spheroid sprouting assay were most meaningful to identify functional pericytes in vitro.
Conclusion
Markers used in vivo to identify pericytes are not reliable in vitro. Even the upregulation of CD146 in vitro does not correlate with a gain of function. Therefore, we have adapted a combination of assays to assess pericytic functions in vitro and allow to distinguish pericytes from other stromal cells independent of the tissue source. These assays represent a platform to better capture the functional characteristics of pericytes and differences to other stromal cells. The pericyte-specific functions manifest as active interactions with endothelial cells comprising of stabilization of endothelial tubules and improvement of sprout integrity. The data obtained suggest that pericytes represent a subpopulation of MSC; they are multipotent, but pericytic behavior is not an intrinsic ability of all multipotent stromal cells.
Supplementary Material
Acknowledgments
The authors thank Flow Cytometry Lab (Life Sciences Institute, National University of Singapore) for their experienced help with cell sorting. This work was supported by the National Research Foundation of Singapore through the Singapore MIT Alliance for Research and Technology (SMART) ignition grant (ING11024-BIO), the research funds associated to the SMART Scholars Postdoctoral Fellowship and NUS Faculty Research Committee Grant (Engineering in Medicine).
Author Disclosure Statement
The authors declare no conflict of interest.
References
- 1.Sims DE. The pericyte—a review. Tissue Cell. 1986;18:153–174. doi: 10.1016/0040-8166(86)90026-1. [DOI] [PubMed] [Google Scholar]
- 2.Armulik A. Genové G. Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Song S. Ewald AJ. Stallcup W. Werb Z. Bergers G. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005;7:870–879. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darland DC. D'Amore PA. TGF beta is required for the formation of capillary-like structures in three-dimensional cocultures of 10T1/2 and endothelial cells. Angiogenesis. 2001;4:11–20. doi: 10.1023/a:1016611824696. [DOI] [PubMed] [Google Scholar]
- 5.Dar A. Domev H. Ben-Yosef O. Tzukerman M. Zeevi-Levin N. Novak A, et al. Multipotent vasculogenic pericytes from human pluripotent stem cells promote recovery of murine ischemic limb. Circulation. 2012;125:87–99. doi: 10.1161/CIRCULATIONAHA.111.048264. [DOI] [PubMed] [Google Scholar]
- 6.Hellström M. Gerhardt H. Kalén M. Li X. Eriksson U. Wolburg H, et al. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abramsson A. Analysis of mural cell recruitment to tumor vessels. Circulation. 2002;105:112–117. doi: 10.1161/hc0102.101437. [DOI] [PubMed] [Google Scholar]
- 8.Rajkumar VS. Shiwen X. Bostrom M. Leoni P. Muddle J. Ivarsson M, et al. Platelet-derived growth factor-β receptor activation is essential for fibroblast and pericyte recruitment during cutaneous wound healing. Am J Pathol. 2006;169:2254–2265. doi: 10.2353/ajpath.2006.060196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brighton CT. Lorich DG. Kupcha R. Reilly TM. Jones AR. Woodbury RA, et al. The pericyte as a possible osteoblast progenitor cell. Clin Orthop Relat Res. 1992:287–299. [PubMed] [Google Scholar]
- 10.Farrington-Rock C. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004;110:2226–2232. doi: 10.1161/01.CIR.0000144457.55518.E5. [DOI] [PubMed] [Google Scholar]
- 11.Tang W. Zeve D. Suh JM. Bosnakovski D. Kyba M. Hammer RE, et al. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Q. Yu Y. Bischoff J. Mulliken JB. Olsen BR. Differential expression of CD146 in tissues and endothelial cells derived from infantile haemangioma and normal human skin. J Pathol. 2003;201:296–302. doi: 10.1002/path.1443. [DOI] [PubMed] [Google Scholar]
- 13.Crisan M. Yap S. Casteilla L. Chen CW. Corselli M. Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Caplan AI. All MSCs are pericytes? Cell Stem Cell. 2008;3:229–230. doi: 10.1016/j.stem.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Rajantie I. Ilmonen M. Alminaite A. Ozerdem U. Alitalo K. Salven P, et al. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood. 2004;104:2084–2086. doi: 10.1182/blood-2004-01-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kidd S. Spaeth E. Watson K. Burks J. Lu H. Klopp A, et al. Origins of the tumor microenvironment: quantitative assessment of adipose-derived and bone marrow-derived stroma. PLoS One. 2012;7:e30563. doi: 10.1371/journal.pone.0030563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kokovay E. Li L. Cunningham LA. Angiogenic recruitment of pericytes from bone marrow after stroke. J Cereb Blood Flow Metab. 2005;26:545–555. doi: 10.1038/sj.jcbfm.9600214. [DOI] [PubMed] [Google Scholar]
- 18.Horwitz E. Le Blanc K. Dominici M. Mueller I. Slaper-Cortenbach I. Marini F, et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 19.Corselli M. Chen CW. Sun B. Yap S. Rubin JP. Péault B, et al. The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev. 2012;21:1299–1308. doi: 10.1089/scd.2011.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lennon DP. Caplan AI. Isolation of human marrow-derived mesenchymal stem cells. Exp Hematol. 2006;34:1604–1605. doi: 10.1016/j.exphem.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Crisan M. Deasy B. Gavina M. Zheng B. Huard J. Lazzari L, et al. Purification and long-term culture of multipotent progenitor cells affiliated with the walls of human blood vessels: myoendothelial cells and pericytes. Methods Cell Biol. 2008;86:295–309. doi: 10.1016/S0091-679X(08)00013-7. [DOI] [PubMed] [Google Scholar]
- 22.Raghunath M. Sy Wong Y. Farooq M. Ge R. Pharmacologically induced angiogenesis in transgenic zebrafish. Biochem Biophys Res Commun. 2009;378:766–771. doi: 10.1016/j.bbrc.2008.11.127. [DOI] [PubMed] [Google Scholar]
- 23.Chen C. Loe F. Blocki A. Peng Y. Raghunath M. Applying macromolecular crowding to enhance extracellular matrix deposition and its remodeling in vitro for tissue engineering and cell-based therapies. Adv Drug Deliv Rev. 2011;63:277–290. doi: 10.1016/j.addr.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Afizah H. Yang Z. Hui JHP. Ouyang HW. Lee EH. A comparison between the chondrogenic potential of human bone marrow stem cells (BMSCs) and adipose-derived stem cells (ADSCs) taken from the same donors. Tissue Eng. 2007;13:659–666. doi: 10.1089/ten.2006.0118. [DOI] [PubMed] [Google Scholar]
- 25.Korff T. Augustin HG. Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation. J Cell Biol. 1998;143:1341–1352. doi: 10.1083/jcb.143.5.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winnik S. Klinkert M. Kurz H. Zoeller C. Heinke J. Wu Y, et al. HoxB5 induces endothelial sprouting in vitro and modifies intussusceptive angiogenesis in vivo involving angiopoietin-2. Cardiovasc Res. 2009;83:558–565. doi: 10.1093/cvr/cvp133. [DOI] [PubMed] [Google Scholar]
- 27.Peault B. Are mural cells guardians of stemness?.: From pluri- to multipotency via vascular pericytes. Circulation. 2012;125:12–13. doi: 10.1161/CIRCULATIONAHA.111.073445. [DOI] [PubMed] [Google Scholar]
- 28.Dominici M. Le Blanc K. Mueller I. Slaper-Cortenbach I. Marini F. Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 29.Guzman RJ. Clinical, cellular, and molecular aspects of arterial calcification. J Vasc Surg 45 Suppl. 2007;A:A57–A63. doi: 10.1016/j.jvs.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Palma M. Venneri MA. Galli R. Sergi LS. Politi LS. Sampaolesi M, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Cai J. Kehoe O. Smith GM. Hykin P. Boulton ME. The angiopoietin/Tie-2 system regulates pericyte survival and recruitment in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008;49:2163–2171. doi: 10.1167/iovs.07-1206. [DOI] [PubMed] [Google Scholar]
- 32.Gaengel K. Genove G. Armulik A. Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:630–638. doi: 10.1161/ATVBAHA.107.161521. [DOI] [PubMed] [Google Scholar]
- 33.Patan S. TIE1 and TIE2 receptor tyrosine kinases inversely regulate embryonic angiogenesis by the mechanism of intussusceptive microvascular growth. Microvasc Res. 1998;56:1–21. doi: 10.1006/mvre.1998.2081. [DOI] [PubMed] [Google Scholar]
- 34.Nomura M. Yamagishi S. Harada S. Hayashi Y. Yamashima T. Yamashita J, et al. Possible participation of autocrine and paracrine vascular endothelial growth factors in hypoxia-induced proliferation of endothelial cells and pericytes. J Biol Chem. 1995;270:28316–28324. doi: 10.1074/jbc.270.47.28316. [DOI] [PubMed] [Google Scholar]
- 35.Takagi H. King GL. Aiello LP. Identification and characterization of vascular endothelial growth factor receptor (Flt) in bovine retinal pericytes. Diabetes. 1996;45:1016–1023. doi: 10.2337/diab.45.8.1016. [DOI] [PubMed] [Google Scholar]
- 36.Witmer AN. van Blijswijk BC. van Noorden CJF. Vrensen GFJM. Schlingemann RO. In vivo angiogenic phenotype of endothelial cells and pericytes induced by vascular endothelial growth factor-A. J Histochem Cytochem. 2004;52:39–52. doi: 10.1177/002215540405200105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.