Abstract
Background
In designing an osteocutaneous fibula flap, poor planning, aberrant anatomy, or inadequate perforators may necessitate modification of the flap design, exploration of the contralateral leg, or additional flap harvest. The purpose of this study was to determine the predictive power of computed tomographic angiography (CTA) in osteocutaneous fibula flap planning and execution.
Methods
We studied a prospective cohort of 40 consecutive patients who underwent preoperative CTA mapping of the peroneal artery and its perforators and subsequent free fibula flap reconstruction of mandibular or maxillary defects. We compared CTA analysis of perforator anatomy, peroneal artery origin, and fibula length with intraoperative clinical findings.
Results
Overall, CTA identified 94.9% of the cutaneous perforators found intraoperatively. Clinically, perforators were located an average of 8.7 mm from their CTA-predicted locations. The peroneal artery origin from the tibioperoneal trunk averaged 6.0 mm from its CTA-predicted location. The average length of the fibula differed from the CTA-predicted length by 8.0 mm. CTA accurately predicted perforators as either septocutaneous or musculocutaneous 93.0% of the time. Perforator size was accurately predicted 66.7% of the time. Skin islands and osteotomies were modified in 25.0% of the cases on the basis of CTA findings. Two patients had hypoplastic posterior tibial arteries, prompting selection of the contralateral leg. There were no total flap or skin paddle losses.
Conclusions
CTA accurately predicted the course and location of the peroneal artery and perforators; perforator size was less accurately estimated. CTA provides valuable information to facilitate osteocutaneous fibula flap harvest.
Level of Evidence
Diagnostic, II.
INTRODUCTION
The free fibula osteocutaneous flap has become the workhorse flap for reconstruction of complex defects requiring vascularized bone.1–3 Since its original description by Taylor et al. in 1975 as a bone-only flap, the design has been modified to include a skin island based on peroneal artery perforators for the reconstruction of composite defects.1,2,4,5 Early experience with the fibula osteocutaneous flap resulted in high rates of skin paddle loss.2,6 Greater familiarity with this flap and more detailed anatomic studies of the infrapopliteal vasculature have led to increased reliability of the cutaneous skin island.2,6–13 Nevertheless, the variable anatomy of the peroneal artery and its perforators still make fibula osteocutaneous flap harvest challenging.
Preoperative imaging of flap vasculature using computed tomographic angiography (CTA) facilitates abdominal- and thigh-based free flap design and harvest.14–26 However, the clinical utility of preoperative CTA for fibula flaps has not been adequately demonstrated.27,28 The purpose of this study was to evaluate the clinical utility of preoperative CTA for free fibula flap harvest by comparing CTA to intraoperative findings and evaluating how CTA data affect reconstructive decision-making.
PATIENTS AND METHODS
We studied a prospective cohort of 40 consecutive patients who underwent preoperative CTA mapping of the fibula and peroneal artery and subsequent free fibula flap reconstruction for composite head and neck defects at a single center over a 14-month period (5/11/10–8/8/11). We compared patient anatomic characteristics demonstrated on CTA to intraoperative anatomic findings. Institutional Review Board approval was obtained prior to conducting this study.
CTA Protocol
Scans were performed in an antegrade direction from above the knee to below the ankle. Following intravenous injection of contrast medium (OptiRay; Mallinckrodt-Covidien, Hazelwood, MO), helical CT scanning (120 kVp, 290 mA max, 0.8-second exposure, 2.5-mm collimation, 39.37 cm/second speed, 0.984:1 pitch, 64 channels) was performed on a GE LightSpeed VCT (General Electric HealthCare, Waukesha, WI) in two phases (30 seconds and 60 seconds, designated as arterial and venous phases, respectively). For each phase, axial source images were reconstructed with a soft tissue kernel at 2.5-mm thickness and spacing for standard radiological review. The section chief of Musculoskeletal Diagnostic Radiology (J.E.M.), the reconstructing surgeons, and the principal investigator (P.B.G.) reviewed all CTA images preoperatively.
Comparison of CTA and Intraoperative Findings
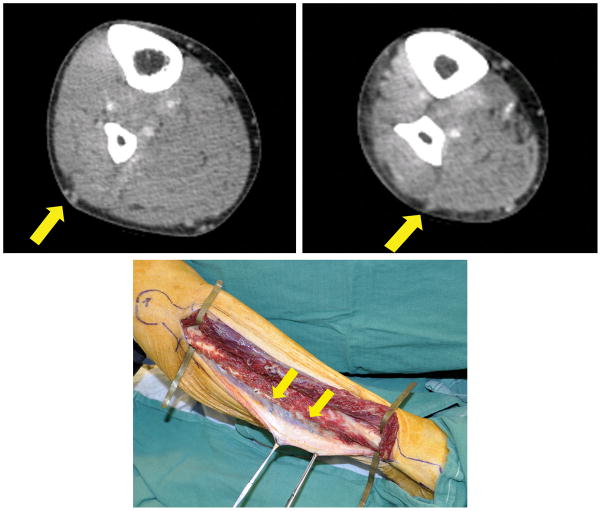
CTA images were calibrated to the surface anatomy to compare them with intraoperative findings. The fibular head and lateral malleolus served as fiduciary landmarks because they were readily identifiable on both CTA and clinical examination. A virtual line drawn between these two bony landmarks served as the y-axis for assigning longitudinal coordinates to perforators where they penetrated the deep fascia on both CTA and intraoperative examination. We also compared anatomic details of the fibula and peroneal artery demonstrated by CTA to intraoperative findings. (Figure 1)
Figure 1.
Example of CTA and intraoperative images of peroneal artery perforators: (a) proximal perforator (yellow arrow), (b) distal perforator (yellow arrow), (c) intraoperative appearance of perforators seen in preoperative CTA (yellow arrows).
Fibula length
The length of the fibula, defined as the distance between the fibular head and the lateral malleolus, estimated by CTA was compared to the actual length measured on clinical examination.
Peroneal artery and perforator characteristics
Anatomic details of the peroneal artery and its perforators included the location of the artery’s origin from the tibioperoneal trunk and the following perforator characteristics: number, location of origin, course (i.e., musculocutaneous vs. septocutaneous), and size. To account for inter-patient differences in fibula length, perforator and peroneal artery origins were reported as a ratio of the distance from the fibular head to the perforator/artery location over the length of the fibula. Perforator size on CTA and intraoperatively was categorized as small (<0.5 mm), medium (0.5–1.0 mm), or large (>1.0 mm).26,29 We compared the CTA findings with the intraoperative findings to define the sensitivity, specificity, and accuracy ([true positives + true negatives]/total) of CTA for each measurement.
Surgical Planning and Optimization
Surgeons recorded whether the CTA findings affected decision-making and, if so, how the plan was modified. We also evaluated the association between an abnormal clinical vascular examination, defined as the absence of or disparity between the dorsalis pedis or posterior tibial artery pulses in each leg, and changes in the reconstructive plan.
Statistical Analysis
A two-tailed paired t-test compared the differences between CTA and intraoperative fibula lengths and peroneal artery origins, as well as perforator location, course, and size. Cohen’s kappa measured the agreement between categories of perforator sizes and courses predicted by CTA and measured intraoperatively (0.4 = fair agreement, 0.4 – 0.6 = moderate agreement, 0.6 – 0.8 = good agreement, and >0.8 = excellent agreement). The sensitivity and accuracy of CTA for detecting perforators were calculated. A histogram showed perforator locations. The kernel density of the distribution of perforator location was estimated by using the “S3” methodology in R (version 12.2; The R Foundation for Statistical Computing). Descriptive statistics summarized fibula length, peroneal artery origin, and perforator location, as well as the differences between CTA and intraoperative measurements of these values. A biostatistician (J.L.) performed all analyses using SAS 9.2 (SAS Institute Inc., Cary, NC) and R software.
RESULTS
Patient Characteristics
Average age of the 40 patients (25 men and 15 women) was 57±17 years. Average BMI was 26.5±4.4 kg/m2. Twenty-two patients (55.0%) underwent reconstruction with a right fibula flap, and 18 (45.0%) with a left fibula flap. Twenty-nine patients (72.5%) had a normal clinical vascular examination; of the remaining 11 patients, 8 had abnormal pulses in the planned donor leg and 3 in the contralateral leg. All flaps harvested in this study were fibula osteocutaneous flaps. No patients developed signs or symptoms of extremity ischemia following free fibula flap harvest, and all flaps survived.
Fibula Measurements
Fibula length was clinically documented in 37 patients and averaged 35.1±2.0 cm (male = 35.6±2.1 cm; female = 34.2±1.6 cm), compared with an average length of 34.6±1.8 cm based on CTA, which was significantly different (p=0.019). The mean absolute difference in fibula length between the CTA and operative findings was 8.0±7.6 mm.
Peroneal Artery Origin
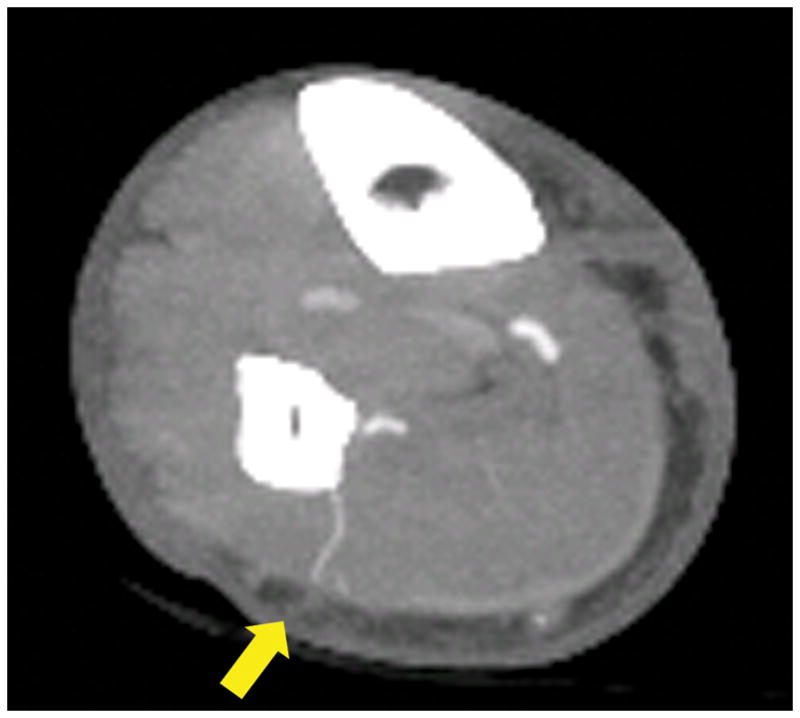
The location of the origin of the peroneal vessels from the tibial-peroneal trunk was clinically documented in 36 patients. (Figure 2) The average location was 6.1±1.3 cm distal to the fibular head on intraoperative exploration and 5.5±1.6 cm distal on CTA, which was significantly different (p=0.02). The mean absolute difference between the CTA and operative measurements was 6.0±5.9 mm. In 2 patients (5.0%), CTA evaluation found hypoplastic posterior tibial arteries, with the peroneal artery appearing to be the dominant blood supply to the foot. One patient appeared to have hypoplastic/aplastic posterior tibial and anterior tibial arteries, with only a single peroneal artery supplying the foot (i.e., “peronea magna” or Type IIIC popliteal artery branching), while the other patient had a hypoplastic/aplastic posterior tibial artery, with both anterior tibial and posterior tibial arteries supplying the distal foot (Type IIIA popliteal branching).9,12,13
Figure 2.
CTA three-dimensional reconstruction demonstrating the distances between fibular heads and peroneal artery origins from tibioperoneal trunks (yellow arrows).
Perforator Characteristics
Detection
CTA detected 93 of the 98 perforators found intraoperatively, resulting in a sensitivity of 94.9% and accuracy of 94.9%. All perforators seen on CTA were present intraoperatively (i.e., no false positives). Since we were unable to define the number of true negatives (i.e., perforators seen neither on CTA nor intraoperatively), we could not calculate the specificity or the negative predictive value. Four perforators that appeared on CTA to take a direct course from the peroneal artery to the overlying skin instead appeared intraoperatively to originate from the more proximal peroneal artery and take a long intramuscular course through the soleus muscle. However, our surgeons did not confirm these four perforators’ proximal origin with surgical dissection through the soleus muscle; rather, the surgical plan was modified to include other, more direct septocutaneous perforators that had also been visualized on CTA.
Location, Course, and Size
The average distance of a perforator from the fibula head was similar intraoperatively (19.1±5.9 cm) and on CTA (18.8±5.7 cm) and differed by an average of 8.7 mm from the predicted location (p=0.67). CTA predicted whether a perforator was musculocutaneous or septocutaneous with an accuracy of 93.0%. (Figure 3) The Kappa was 0.75 (95%CI=0.55–0.94). CTA correctly identified the courses of 11/13 musculocutaneous perforators (85.0% accuracy) and 73/77 septocutaneous perforators (95.0% accuracy) that were mapped intraoperatively.
Figure 3.
Septocutaneous course of peroneal artery demonstrated by preoperative CTA.
CTA less accurately predicted perforator size: 62/93 perforators detected by CTA were correctly predicted, yielding an overall accuracy of 66.7% and a Kappa of 0.477 (95%CI=0.330–0.623). CTA more accurately predicted the sizes of large and small perforators (73.0% and 75.0% accuracy, respectively) than of medium-sized perforators (58.0% accuracy).
Distribution
Histogram analysis suggested a bimodal perforator distribution, (Figure 4) with one group of perforators appearing more proximal and a second cluster found more distal. The ratio of the distance from the fibular head to the lateral malleolus was 0.33 intraoperatively and 0.35 on CTA in the proximal group and 0.60 intraoperatively and 0.57 on CTA in the distal group. (Figure 5) To verify our results, we changed the histogram bins from 0.01 to 0.1, used various smoothing kernels, and again demonstrated this bimodal distribution.
Figure 4.
Histogram presentation of the perforator distribution based on CTA analysis and intraoperative exploration.
Figure 5.
Frequency of perforator locations along the length of the fibula by CTA (blue line) and by intraoperative observation (red line).
Surgical Decision-Making
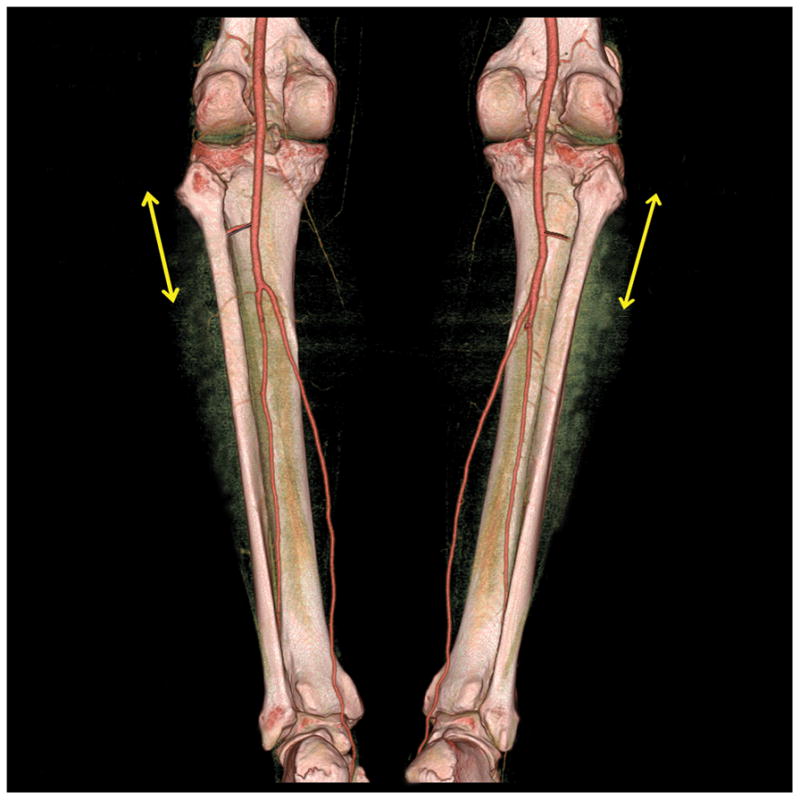
In our series, the operative plan was modified on the basis of CTA findings in 10 patients (25.0%). As mentioned, 2 patients were found on CTA to have hypoplastic posterior tibial arteries in the planned donor leg, which prompted selection of the contralateral leg with normal infrapopliteal vascular anatomy.9–13,28,30–32 (Figure 6) Four patients had a very proximal origin of the peroneal vessels from the tibioperoneal trunk, prompting the surgeons to create more proximal osteotomies than usual in order to facilitate pedicle exposure.1–5,33 In one patient, the fibula wedge osteotomies were repositioned on the basis of CTA to position the perforator to the skin island in a more optimal relationship to the bone segments.34–36 All CTA studies in this series were adequate to determine the suitability of the infrapopliteal vessels and perforators for free fibula osteocutaneous flap harvest.
Figure 6.
CTA appearance of hypoplastic right posterior tibial artery. The peroneal artery (yellow arrow) appears to be the dominant vascular supply to the distal right lower leg. Normal infrapopliteal arterial trifurcation is demonstrated in the contralateral left leg.
Three patients had proximal musculocutaneous perforators that did not directly originate from the peroneal artery. In these patients, cutaneous skin islands were harvested based on distal perforators also visualized on preoperative CTA. Finally, one patient with an extensive soft tissue defect was scheduled to undergo a two-skin-island fibula flap reconstruction for extra- and intraoral resurfacing. Instead, reconstruction was performed with a folded, single-island fibula flap because CTA visualized only one septocutaneous perforator per leg, which was confirmed ipsilaterally intraoperatively.
Although 11 patients (27.5%) had abnormal pulse examinations preoperatively, the surgical plan was changed in only 4 of those patients (36.4%) because CTA demonstrated anterior tibial and posterior tibial artery patency in the remaining 7 patients. Physical examination was not able to reliably identify the two patients in our study with hypoplastic posterior tibial arteries, as one of these patients had normal palpable pulses bilaterally, whereas the other (peronea magna) patient did not.
DISCUSSION
In this study, CTA accurately predicted the dimensions of the fibula, the origin of the peroneal artery, and the location and course of visible perforators, but less accurately predicted perforator size. Although CTA fibula length and peroneal artery origin measurements were significantly different in comparison to intraoperative findings by the t-test, our surgeons found that the <1 cm mean absolute value differences for these measurements were still accurate enough to facilitate execution of the fibula flap harvest. The anatomic information provided by CTA agrees with previous studies in regards to the distribution of peroneal artery perforators along the lower leg.8,27,37 Most importantly, the information provided by CTA influenced our surgeons to modify the reconstructive strategy in 25.0% of the cases, resulting in more precise skin paddle placement and avoidance of potentially compromised extremity perfusion in two patients.
In one of the earliest descriptions of the use of a free fibula osteocutaneous flap for mandible reconstruction, Hidalgo judged the skin island perfusion to be poor enough to justify excision of the skin island in 3 of 4 patients.2 Therefore, Hidalgo cautioned against reconstructing mandibles that had significant soft tissue loss with osteocutaneous free fibula flaps. He also stated that “angiography does not allow adequate preoperative study of skin blood supply, although it may be useful to confirm that the peroneal artery is present, free of disease, and not the dominant source of blood supply to the distal leg.”
The current recommendations for use of preoperative angiography prior to free fibula flap harvest have their origins in Hidalgo’s original study. More recent studies have specifically explored the utility of preoperative angiography in free fibula flap reconstruction, with contradictory conclusions, but all of these studies focused on the macrovascular patency of the infrapopliteal trifurcation rather than the status of the cutaneous perforators.10,30,31 Studies by Lutz et al.30 and Disa et al.31 discouraged the routine use of preoperative angiography and recommended clinical vascular examination as the primary means of evaluating the fibula donor site, reserving preoperative angiography for patients with abnormal pulse examinations. A study by Blackwell,10 however, reported that the surgical plan was changed in 21% of patients who underwent preoperative angiography prior to oromandibular reconstruction and, thus, recommended the routine use of preoperative vascular imaging to avoid “a potentially catastrophic complication.” It is important to note, however, that all three of these studies were based on the results of standard digital subtraction angiography, not CTA.
The advent of CTA has drastically decreased the indications for traditional digital subtraction angiography, an invasive technique that requires intravascular contrast media injection via arterial catheterization, which can result in arterial laceration, pseudoaneurysm, or arterovenous fistula. Compared with digital subtraction angiography, CTA has not only been shown to be effective in the diagnosis of peripheral arterial disease but also avoids the potential complications associated with invasive arterial catheterization, costs less, requires less time, and may expose patients to less ionizing radiation.38 Therefore, CTA has become the preferred modality for imaging the infrapopliteal vessels in patients with peripheral arterial disease, and yet, studies describing the clinical utility of CTA for fibula flap planning are lacking, in that they provide inadequate data regarding the perforators or no validating intraoperative data.11,27,39,40
On the basis of our findings and previous studies, we find it difficult to definitively recommend that all patients undergo CTA prior to fibula flap harvest for the sole purpose of verifying adequate perfusion to the foot.10–12,28,39 We agree with previous authors’ recommendations to selectively perform preoperative angiography on any patient with an abnormal clinical vascular exam or a history suggestive of arterial insufficiency.8,30,31 We do prefer CTA to standard digital subtraction angiography for identifying peripheral arterial disease, not only for the reasons already discussed but also because it provides useful information about perforator and fibula anatomy.41 However, our data suggest that CTA, like conventional angiography, does occasionally contradict the physical examination, findings that agree with previous studies cautioning against relying solely on clinical vascular examination as a means of screening for variant anatomy.11,12,27,39 Almost a third of our patients had abnormal clinical vascular examinations, yet we found that the clinical examination alone accurately predicted a clinically relevant vascular anomaly that would have affected surgical decision-making in only one of the 40 patients in our study. Following our experience with CTA, some of our surgeons now routinely order preoperative CTAs for all patients prior to fibula flap harvest, but most selectively order CTAs in patients with abnormal pulse exams or clinical signs of peripheral arterial disease or when knowledge of the vascular or bony anatomy is crucial to the reconstructive plan (e.g., when multiple skin paddles are needed or for precise planning of osteotomies for computer-assisted design/medical modeling).
Even in patients with a low index of suspicion for vascular insufficiency by clinical examination, we found that our surgeons changed their surgical plans based on CTA results not to avoid distal ischemia or to improve flap survival but to more precisely design their flaps. For example, knowing the peroneal arterial anatomy facilitated accurate location of the proximal osteotomy, such that optimal exposure of the peroneal artery origin was achieved without excising more bone than necessary or making multiple cuts for exposure. Knowledge of the perforator anatomy was useful in flap design, helping us to determine ahead of time if an additional free or locoregional flap might be needed. Rather than spending excessive time dissecting proximal musculocutaneous perforators that took an indirect course through the soleus muscle, we utilized CTA-visualized distal perforators that took a more direct septocutaneous course to the overlying skin.6 However, since our study lacked a control group of patients who did not receive CTA, we were unable to determine whether or not CTA actually saves time in the OR, as has been claimed for abdominal-based free flaps.23
This study’s findings corroborate our prior clinical study8 demonstrating that most patients have at least two useful perforators in a bimodal distribution (proximal and distal), although a subset of patients’ legs have only one perforator and some have none. For situations in which precise anatomic information is critical, CTA enables an even higher level of patient-specific flap design than can be realized by population-based anatomic mapping. While CTA was not particularly accurate in classifying the size of the perforators, precise size determination is probably not critical, as fibula flap perforator selection is primarily based on perforator location, and even small perforators are usually adequate to supply most skin paddles used in head and neck reconstruction.
Critics have raised questions regarding the cost and safety of CTA mapping and suggested alternative modalities to avoid the negative characteristics associated with CTA.42–46 The current Medicare reimbursement for a CTA of the lower extremities in Texas is $451.69. Laser-assisted indocyanine green (ICGN) imaging avoids radiation exposure, demonstrates cutaneous perforasomes, facilitates optimal skin island position, and requires a minimal volume of contrast media.43–45,47,48 However, ICGN cannot visualize the macrovascular (tibio-peroneal) and fibula bony anatomy and is only available in the operating room, precluding preoperative planning. Although magnetic resonance angiography (MRA) avoids radiation exposure, CTA has been shown to be superior to MRA for perforator mapping.46
CONCLUSIONS
The anatomical detail provided preoperatively by CTA facilitated informed decision-making in planning free fibula osteocutaneous flaps for complex head and neck defects. Such information can be used to not only verify vascular sufficiency to the distal leg but also more precisely plan the flap harvest. CTA appears to be indicated for any case where preoperative knowledge of the perforator anatomy might facilitate complex flap design or when lower leg vascular sufficiency is in question. In such cases, CTA is preferable to conventional angiography because it costs less, has fewer risks, and may provide additional useful information for surgical planning.
Acknowledgments
Financial Support: This research is supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant CA016672.
The authors thank Dawn Chalaire from The University of Texas MD Anderson Cancer Center, Department of Scientific Publications for assistance with scientific editing.
Footnotes
Financial Disclosure: None of the authors has a financial interest associated with this publication.
Products Mentioned: There are no products mentioned in this manuscript.
References
- 1.Wei FC, Chen HC, Chuang CC, Noordhoff MS. Fibular osteoseptocutaneous flap: anatomic study and clinical application. Plast Reconstr Surg. 1986;78:191–200. doi: 10.1097/00006534-198608000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Hidalgo DA. Fibula free flap: a new method of mandible reconstruction. Plast Reconstr Surg. 1989;84:71–80. [PubMed] [Google Scholar]
- 3.Cordeiro PG, Disa JJ, Hidalgo DA, Hu QY. Reconstruction of the mandible with osseous free flaps: a 10-year experience with 150 consecutive patients. Plast Reconstr Surg. 1999;104:1314–1320. doi: 10.1097/00006534-199910000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Taylor GI, Miller GD, Ham FJ. The free vascularized bone graft: a clinical extension of microvascular techniques. Plast Reconstr Surg. 1975;55:533–545. doi: 10.1097/00006534-197505000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Chen ZW, Yan W. The study and clinical application of the osteocutaneous flap of fibula. Microsurgery. 1983;4:11–16. doi: 10.1002/micr.1920040107. [DOI] [PubMed] [Google Scholar]
- 6.Winters HA, de Jongh GJ. Reliability of the proximal skin paddle of the osteocutaneous free fibula flap: a prospective clinical study. Plast Reconstr Surg. 1999;103:846–849. doi: 10.1097/00006534-199903000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Anthony JP, Ritter EF, Young DM, Singer MI. Enhancing fibula free flap skin island reliability and versatility for mandibular reconstruction. Ann Plast Surg. 1993;31:106–111. doi: 10.1097/00000637-199308000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Yu P, Chang EI, Hanasono MM. Design of a reliable skin paddle for the fibula osteocutaneous flap: perforator anatomy revisited. Plast Reconstr Surg. 2011;128:440–447. doi: 10.1097/PRS.0b013e31821e7058. [DOI] [PubMed] [Google Scholar]
- 9.Kim D, Orron DE, Skillman JJ. Surgical significance of popliteal arterial variants: a unified angiographic classification. Ann Surg. 1989;210:776–782. doi: 10.1097/00000658-198912000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blackwell KE. Donor site evaluation for fibula free flap transfer. Am J Otolaryngol. 1998;19:89–95. doi: 10.1016/s0196-0709(98)90101-6. [DOI] [PubMed] [Google Scholar]
- 11.Chow LC, Napoli A, Klein MB, Chang J, Rubin GD. Vascular mapping of the leg with multi-detector row CT angiography prior to free-flap transplantation. Radiology. 2005;237:353–360. doi: 10.1148/radiol.2371040675. [DOI] [PubMed] [Google Scholar]
- 12.Rosson GD, Singh NK. Devascularizing complications of free fibula harvest: peronea arteria magna. J Reconstr Microsurg. 2005;21:553–538. doi: 10.1055/s-2005-922432. [DOI] [PubMed] [Google Scholar]
- 13.Day CP, Orme R. Popliteal artery branching patterns - an angiographic study. Clin Radiol. 2006;61:696–699. doi: 10.1016/j.crad.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Saint-Cyr M. Assessing perforator architecture. Clin Plast Surg. 2011;38:175–202. doi: 10.1016/j.cps.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Mathes DW, Neligan PC. Preoperative imaging techniques for perforator selection in abdomen-based microsurgical breast reconstruction. Clin Plast Surg. 2010;7:581–591. doi: 10.1016/j.cps.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Rosson GD, Williams CG, Fishman EK, Singh NK. 3D CT angiography of abdominal wall vascular perforators to plan DIEAP flaps. Microsurgery. 2007;27:641–646. doi: 10.1002/micr.20423. [DOI] [PubMed] [Google Scholar]
- 17.Phillips TJ, Stella DL, Rozen WM, Ashton M, Taylor GI. Abdominal wall CT angiography: a detailed account of a newly established preoperative imaging technique. Radiology. 2008;249:32–47. doi: 10.1148/radiol.2483072054. [DOI] [PubMed] [Google Scholar]
- 18.Rozen WM, Ashton MW, Grinsell D, Stella DL, Phillips TJ, Taylor GI. Establishing the case for CT angiography in the preoperative imaging of abdominal wall perforators. Microsurgery. 2008;28:306–313. doi: 10.1002/micr.20496. [DOI] [PubMed] [Google Scholar]
- 19.Rozen WM, Palmer KP, Suami H, et al. The DIEA branching pattern and its relationship to perforators: the importance of preoperative computed tomographic angiography for DIEA perforator flaps. Plast Reconstr Surg. 2008;121:367–374. doi: 10.1097/01.prs.0000298313.28983.f4. [DOI] [PubMed] [Google Scholar]
- 20.Rozen WM, Ashton MW, Stella DL, Phillips TJ, Grinsell D, Taylor GI. The accuracty of computed tomographic angiography for mapping the perforators of the deep inferior epigastric artery: A blinded, prospective cohort study. Plast Reconstr Surg. 2008;122:1003–1010. doi: 10.1097/PRS.0b013e3181845994. [DOI] [PubMed] [Google Scholar]
- 21.Rozen WM, Anavekar NS, Ashton MW, et al. Does the preoperative imaging of perforators with CT angiography improve operative outcomes in breast reconstruction? Microsurgery. 2008;28:516–523. doi: 10.1002/micr.20526. [DOI] [PubMed] [Google Scholar]
- 22.Rozen WM, Phillips TJ, Ashton MW, Stella DL, Gibson RN, Taylor GI. Preoperative imaging for DIEA perforator flaps: a comparative study of computed tomographic angiography and doppler ultrasound. Plast Reconstr Surg. 2008;121:121–129. doi: 10.1097/01.prs.0000293874.71269.c9. [DOI] [PubMed] [Google Scholar]
- 23.Casey WJ, Chew RT, Rebecca AM, Smith AA, Collins JM, Pockaj BA. Advantages of preoperative computed tomography in deep inferior epigastric artery perforator flap breast reconstruction. Plast Reconstr Surg. 2009;23:1148–1156. doi: 10.1097/PRS.0b013e31819e23e1. [DOI] [PubMed] [Google Scholar]
- 24.Scott JR, Liu D, Said H, Neligan PC, Mathes DW. Computed tomographic angiography in planning abdomen-based microsurgical breast reconstruction: a comparison with color duplex ultrasound. Plast Reconstr Surg. 2010;125:446–454. doi: 10.1097/PRS.0b013e3181c82d24. [DOI] [PubMed] [Google Scholar]
- 25.Mathes DW, Neligan PC. Current techniques in preoperative imaging for abdomen-based perforator flap microsurgical breast reconstruction. J Reconst Microsurg. 2010;26:3–11. doi: 10.1055/s-0029-1244806. [DOI] [PubMed] [Google Scholar]
- 26.Garvey PB, Selber JC, Madewell JE, Bidaut L, Feng L, Yu P. A prospective study of preoperative computed tomographic angiography for head and neck reconstruction with anterolateral thigh flaps. Plast Reconstr Surg. 2011;127:1505–1515. doi: 10.1097/PRS.0b013e318208d23e. [DOI] [PubMed] [Google Scholar]
- 27.Ribuffo D, Matteo A, Saba L, et al. Clinical study of peroneal artery perforators with computed tomographic angiography: implications for fibular flap harvest. Surg Radiol Anat. 2010;32:329–334. doi: 10.1007/s00276-009-0559-y. [DOI] [PubMed] [Google Scholar]
- 28.Karanas YL, Antony A, Rubin G, Chang J. Preoperative CT angiography for free fibula transfer. Microsurgery. 2004;24:125–127. doi: 10.1002/micr.20009. [DOI] [PubMed] [Google Scholar]
- 29.Yu P. Characteristics of the anterolateral thigh flap in a western population and its application in head and neck reconstruction. Head Neck. 2004;26:759–769. doi: 10.1002/hed.20050. [DOI] [PubMed] [Google Scholar]
- 30.Lutz BS, Wei FC, Ng SH, Chen IH, Chen SH. Routine donor leg angiography before vascualized free fibula transplantation is not necessary: a prospective study in 120 clinical cases. Plast Reconstr Surg. 1999;103:121–127. doi: 10.1097/00006534-199901000-00019. [DOI] [PubMed] [Google Scholar]
- 31.Disa JJ, Cordeiro PG. The current role of preoperative arteriography in free fibula flaps. Plast Reconstr Surg. 1998;102:1083–1088. doi: 10.1097/00006534-199809040-00025. [DOI] [PubMed] [Google Scholar]
- 32.Young DM, Trabulsy PP, Anthony JP. The need for preoperative leg angiography in fibula free flaps. J Reconstr Microsurg. 1994;10:287–289. doi: 10.1055/s-2007-1006596. [DOI] [PubMed] [Google Scholar]
- 33.Mathes SJ, Nahai F. Reconstructive Surgery: Principles, Anatomy, & Technique. 2. Vol. 2. Philadelphia Churchill Livingstone; 1997. [Google Scholar]
- 34.Hanasono MM, Jacob RF, Bidaut L, Robb GL, Skoracki RJ. Midfacial reconstruction using virtual planning, rapid prototype modeling, and stereotactic navigation. Plast Reconstr Surg. 2010;126:2002–2007. doi: 10.1097/PRS.0b013e3181f447e1. [DOI] [PubMed] [Google Scholar]
- 35.Hirsch DL, Garfein ES, Christensen AM, Weimer KA, Saddeh PB, Levine JP. Use of computer-aided design and computer-aided manufacturing to produce orthognathically ideal surgical outcomes: a paradigm shift in head and neck reconstruction. J Oral Maxillofac Surg. 2009;67:2115–2122. doi: 10.1016/j.joms.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Thankappan K, Trivedi NP, Subash P, et al. Three-dimensional computed tomography-based contouring of a free fibula bone graft for mandibular reconstruction. J Oral Maxillofac Surg. 2008;66:2185–2192. doi: 10.1016/j.joms.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 37.Lykoudis EG, Koutsouris M, Lykissas MG. Vascular anatomy of hte integument of the lateral lower leg: an anatomical study focused on cutaneous perforators and their clinical importance. Plast Reconstr Surg. 2011;128:188–199. doi: 10.1097/PRS.0b013e3182174303. [DOI] [PubMed] [Google Scholar]
- 38.Napoli A, Andidei M, Zaccagna F, et al. Peripheral arterial occlusive disease: diagnostic performance and effect on therapeutic management of 64-section CT angiography. Radiology. 2011;261:976–986. doi: 10.1148/radiol.11103564. [DOI] [PubMed] [Google Scholar]
- 38.Kwang NJ, Lee W, Yin YH, et al. Preoperative evaluation of lower extremity arteries for free fibula transfer using MDCT angiography. J Comput Assist Tomogr. 2007;31:820–825. doi: 10.1097/RCT.0b013e318033defd. [DOI] [PubMed] [Google Scholar]
- 40.Satoh T, Kimata Y, Hasegawa K, Namba Y. The utility of multidetector-row computed tomography angiography for evaluating perforators of fibular osteocutaneous flaps. J Reconstr Microsurg. 2011;27:29–36. doi: 10.1055/s-0030-1267380. [DOI] [PubMed] [Google Scholar]
- 41.Chang EI, Clemens M, Garvey PB, Skoracki RJ, Hanasono MM. Cephalometric analysis for microvascular head and neck reconstruction. Head Neck. doi: 10.1002/hed.21967. Epub 2012 Jan 31. [DOI] [PubMed] [Google Scholar]
- 42.Brenner DJ, Hall EJ. Computed tomography - An increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 43.Pestana IA, Coan B, Erdmann D, Marcus J, Levin LS, Zenn MR. Early experience wiht fluorescent angiography in free-tissue transfer reconstruction. Plast Reconstr Surg. 2009;123:1239–1245. doi: 10.1097/PRS.0b013e31819e67c1. [DOI] [PubMed] [Google Scholar]
- 44.Komorowska-Timek E, Gurtner GC. Intraoperative perfusion mapping with laser-assisted indocyanine green imaging can predict and prevent complications in immediate breast reconstruction. Plast Reconstr Surg. 2010;125:1065–1074. doi: 10.1097/PRS.0b013e3181d17f80. [DOI] [PubMed] [Google Scholar]
- 45.Sacks JM, Nguyen AT, Yu P, Baumann DP. Laser-assisted indocyanine green imaging optimizes the design of the anterolateral thigh flap. Plast Reconstr Surg. 2011;127:S72. [PMC free article] [PubMed] [Google Scholar]
- 46.Rozen WM, Stella DL, Bowden J, Taylor GI, Ashton MW. Advances in the pre-operative planning of deep inferior epigastric artery perforator flaps: magnetic resonance angiography. Microsurgery. 2008;29:119–123. doi: 10.1002/micr.20590. [DOI] [PubMed] [Google Scholar]
- 47.Holm C, Mayr M, Hofter E, Ninkovic M. Perfusion zones of the DIEP flap revisited: A clinical study. Plast Reconstr Surg. 2006;117:37–44. doi: 10.1097/01.prs.0000185867.84172.c0. [DOI] [PubMed] [Google Scholar]
- 48.Holm C, Tegeler J, Mayr M, et al. Monitoring free flaps using laser-induced fluorescence of indocyanine green: A preliminary experience. Microsurgery. 2002;22:278–287. doi: 10.1002/micr.10052. [DOI] [PubMed] [Google Scholar]