Abstract
HIV elite controllers are able to control HIV-1 infection spontaneously to undetectable levels in the absence of antiretroviral therapy, but the mechanisms leading to this phenotype are poorly understood. Although, low frequencies of HIV infected peripheral CD4+ T-cells have been reported in this group, it remains unclear to what extent this is due to viral attenuation, active immune containment, or intracellular host factors that restrict virus replication. Here we assessed proviral DNA levels, autologous viral growth from and infectability of in-vitro activated, CD8+ T-cell depleted CD4+ T cells from HIV elite controllers (mean VL<50cp/ml), viremic controllers (mean VL<2000cp/ml), chronic progressors and HAART treated individuals. Although we successfully detected autologous virus production in ex-vivo activated CD4+ T-cells from all chronic progressors and most of the viremic controllers we were only able to measure robust autologous viral replication in only 2 of 14 elite controllers subjected to the same protocol. In vitro activated autologous CD4+ T-cells from elite controllers, however, supported infection with both X4 and R5 tropic HIV strains at comparable levels to CD4+ T-cells from HIV negative subjects. Proviral DNA levels were the lowest in elite controllers, suggesting that extremely low frequencies of infected cells contributes to difficulty in isolation of virus. These data indicate that elite control is not due to inability of activated CD4+ T-cells to support HIV infection, but the relative contribution of host and viral factors that account for maintenance of low level infection remain to be determined.
Keywords: HIV elite controllers, autologous viral replication, viral reservoir, in-vitro infectability
Introduction
A small proportion of HIV-1 infected individuals, elite and viremic controllers (EC and VC), spontaneously control plasma HIV RNA between undetectable (EC) to <2000 copies per ml (VC) in the absence of antiretroviral therapy. Some have postulated that ECs exhibit control predominantly as a consequence of infection with replication-defective virus, resulting in poor viral outgrowth [1–3]. Although impaired in-vitro viral replication capacity has been documented in some elite controllers [4], studies thus far show that replication competent viruses can be isolated from these individuals as well, suggesting that not all ECs are infected with defective viruses [5, 6]. Host genetic analyses suggest that immune mechanisms, associated with the major-histocompatibility complex (MHC), contribute to the extraordinary control of viral replication observed in this unique patient population [7–12]. The overrepresentation of certain HLA class I alleles [10, 13], CD8+ T-cell depletion studies [14], evidence of selection for CTL epitope mutations [12, 15], and in-vitro studies showing strong antiviral activity of CD8+ T-cells [11, 16] suggest that cellular immune mechanisms are involved in this remarkable antiviral control.
Interestingly, long-term non-progressors exhibit not only reduced levels of plasma viral replication, but also have lower numbers of CD4+ T-cells with integrated HIV provirus compared to individuals with fast-progressive disease and AIDS [17, 18]. This finding suggests that either: a) these individuals have a unique capacity to control viral replication actively over time, b) they are infected with viruses that do not replicate, and/or c) these individuals possess CD4+ T-cells with a unique ability to resist HIV-infection. To begin to explore the 2 latter possibilities, we sought to determine whether ECs are infected with replication competent virus compared to a group of normal HIV-infected progressors and whether their CD4+ T-cells, upon vigorous in vitro activation, are differentially susceptible to HIV-1 infection compared to seronegative controls.
Materials and Methods
Study Subjects
25 HIV elite controllers (EC) with plasma HIV RNA below 50 copies of RNA/ml plasma were randomly selected from The International HIV Controllers Study. Also included were 10 viremic controllers (VC) with plasma HIV RNA levels between 50 and 2000 copies/ml [mean HIV plasma RNA 1256 copies/ml]. Both groups were antiretroviral therapy naïve and a minimum of 3 qualifying determinations of plasma HIV RNA spanning at least a 12-month period was required for inclusion into the study. Eleven untreated viremic progressors with plasma HIV RNA levels above 10,000 copies/mL [mean HIV plasma RNA 125.158 copies/ml] and 9 subjects on successful highly active antiretroviral therapy (HAART) [mean HIV plasma RNA <75 copies/ml] were recruited from outpatient clinics at local Boston hospitals. HAART was defined as treatment with ≥3 antiretroviral drugs, including two nucleoside reverse transcriptase inhibitors and a non-nucleoside reverse-transcriptase inhibitor or a protease inhibitor. Additionally, we obtained blood from 12 healthy controls. All subjects gave written informed consent.
Assessment of autologous virus production
To detect autologous virus growth CD4+ cells were purified from freshly isolated peripheral blood mononuclear cells (PBMC) by negative selection using the Rosette Sep™ CD4+ cell enrichment cocktail (Stemcell Technologies) depleting CD8+ T-cells, NK-cells, B-cells, macrophages, monocytes and dendritic. CD4+ cells were then stimulated in IL-2 (50units/ml) containing T-cell medium in the presence of a bispecific anti-CD3:anti-CD8 monoclonal antibody, which selectively activates CD4+ T-lymphocytes, while simultaneously depleting all remaining CD8+ T-cells [19]. CD4+ T-cell blasts, generated from HIV negative donors, were added every 7 days to maintain the cultures and provide additional targets for viral outgrowth.
In parallel, an aliquot of CD4+ T-cells from elite controllers was infected with a clinical HIV-1 (X4) isolate at multiplicity of infections (MOI) of 0.01 as described [20] and maintained under the same culture conditions. Every 2–3 days, p24 was measured in culture supernatants using a p24 based ELISA (PerkinElmer Life Sciences, Inc., following the manufacturer’s protocol).
Infectability assays
In order to determine in-vitro infectability of CD4+ T-cells and their intrinsic ability to support viral replication comparing elite controllers versus HIV negative individuals whole PBMCs were depleted from CD8+ T-cells using anti-CD8 magnetic beads (DYNAL™) according to the manufacturer’s protocol and CD4+ T-cell blasts were generated using the bispecific anti-CD3:anti-CD8 antibody in the presence of IL-2 (50units/ml) for 3 days. CD4+ T-cells were then infected with X4 and R5 HIV lab strains (NL4-3 and JRCSF) at a MOI of 0.01 for 4 hours. Cells were washed and then cultured in IL-2 (50units/ml) containing medium for 7 days without the addition of further CD4 blasts and p24 was measured in culture supernatants every 2–3 days.
Real-time PCR-based quantification of proviral HIV-1 DNA
HIV-1 DNA purification from 10 × 106 PBMCs was isolated with a standard protocol (QIAamp DNA Blood Kit, Qiagen). A single-step real-time PCR was used to quantify proviral HIV-1 DNA in a 50µl PCR reaction mix containing 25µl of TaqMan® Universal PCR Master Mix (Applied Biosystems), 20µl of HIV-1 DNA and primers and probe that anneal in the 5’ and 3’ end of the R and U5 region of the LTR respectively, as has previously been described [21] using a forward primer; 5’ GG CTA ACT AGG GAA CCC ACT G 3’ and a reverse primer; 5´GCT AGA GAT TTT CCA CAC TGA CTA A 3´. The fluorescence TaqMan® probe was 5´GGA TCT CTA GTT ACC AGA GTC A 3´. Amplification reactions were performed with an Applied Biosystems 7000 Real-time PCR system. The thermocycling conditions were: 95°C 10 min, 50 cycles at 95°C 15 sec and 60°C 1 min and a final cycle of 72°C 5 min. Copy number estimation of proviral HIV-1 DNA was performed in duplicate and determined by extrapolation from a standard curve generated with a plasmid that harbors the sequence of the HIV-1 LTR and CCR5 gene. Proviral HIV-1 DNA copy number was calculated relative to CCR5 gene copy number previously quantified with the standard curve.
HLA typing
HLA class I typing was performed as previously described [22].
Statistical Analyses
All values throughout the text are expressed as means ± SD. P values were calculated using Kruskal Wallis test followed by Dunn's method for multiple comparison and Wilcoxon ranksum tests for pair-wise comparisons.
Results
Autologous virus production
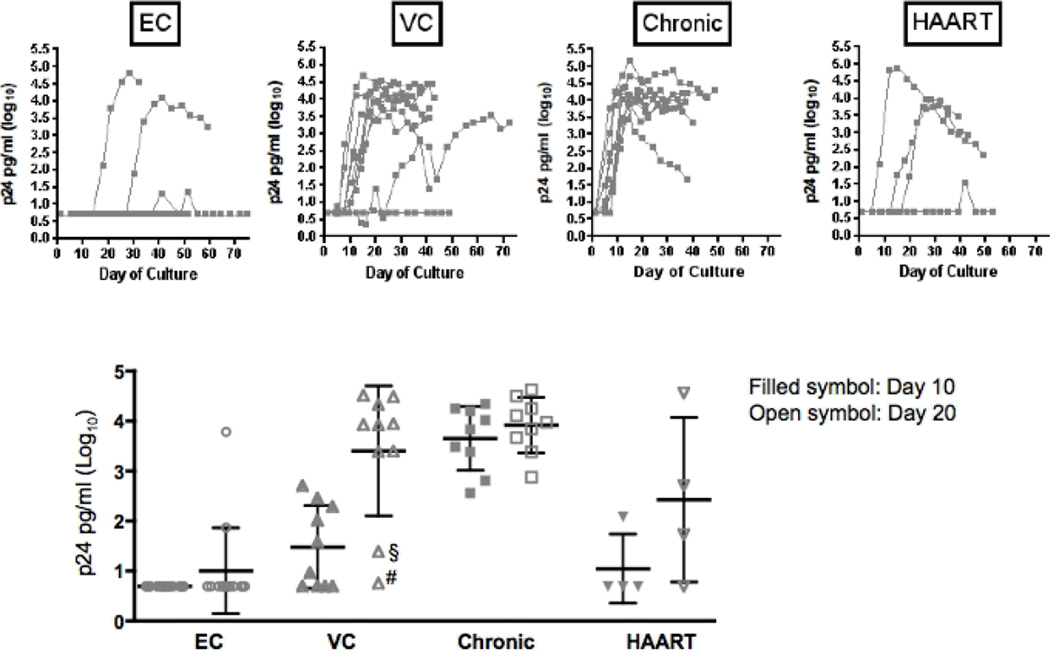
We first evaluated whether ex-vivo activation of CD4+ T-cells from ECs and VCs resulted in outgrowth of autologous virus, comparing these results to those obtained from chronically HIV infected patient subpopulations. PBMC were stimulated with a bispecific monoclonal antibody that results in the selective expansion of CD4+ T-cells and elimination of CD8+ T-cells [19], and uninfected activated donor CD4 cells were added to the cultures weekly such that viral outgrowth was being examined in non-autologous cells. Of 14 ECs examined, virus was detected in-vitro in only 3, and in 1 subject detection by p24 ELISA was transient and could not be confirmed by repeated RT-PCR. In contrast, 9 of 9 untreated HIV progressors and 3 of 4 HAART treated subjects demonstrated p24 antigen production in the stimulated cultures (Fig 1A). Although virus was significantly less frequently detected in the EC, in both individuals in whom sustained virus production was observed, p24 levels of Log10 4.08–4.82 pg/ml could be detected, comparable to the p24 antigen levels observed in HIV progressor cultures (Log10 3.91±0.5 pg/m), suggesting that the viruses that did grow out were as replication competent as viruses infecting progressors (Fig 1B). Viral replication was not detectable in CD4+ cultures from the remaining 11 ECs, despite maintaining these cultures a median of 37±12 days and some as long as 78 days. Interestingly, 8 of 10 HIV viremic controllers displayed robust viral replication in-vitro after 20 days despite their low HIV RNA plasma loads in-vivo, with levels lower but comparable to those observed in progressors (p24 of Log10 3.4±1.3 pg/ml in viremic controllers, 3.91±0.5 pg/m in progressors) (Fig 1B). Of the remaining 2 VCs one showed a delayed viral growth (first virus detected after >30 days) while no autologous virus was detectable in the other subject’s cultures up to 50 days. Interestingly, both individuals were homozygous for protective HLA B alleles (B2705/B5701 and B5701/B5703, respectively) suggesting that a strong immune response may have led to the reduced viral reservoir in these individuals suggesting the possible role of active immune containment of viral replication (Fig 1B). These data indicate furthermore that replication competent HIV can be isolated from a minority of ECs, suggesting that either the majority of ECs are infected with replication defective virus or that the frequency of HIV-1 infected CD4+ T-cells is extremely low in the peripheral blood of ECs, resulting in a reduced chance of capturing an infected cell in a given PBMC sample.
Fig 1.
Autologous virus replication in activated autologous CD4+ T-cells
A. Kinetics of autologous virus replication (log10 p24 in pg/ml) among EC, VC, progressors and HAART treated individuals
B. Mean log10 p24 levels (pg/ml) after 10 and 20 days among EC, VC (§ HLA B5701/B5703; # HLA B2705/B5701), progressors and HAART treated individuals
Proviral HIV-1 DNA
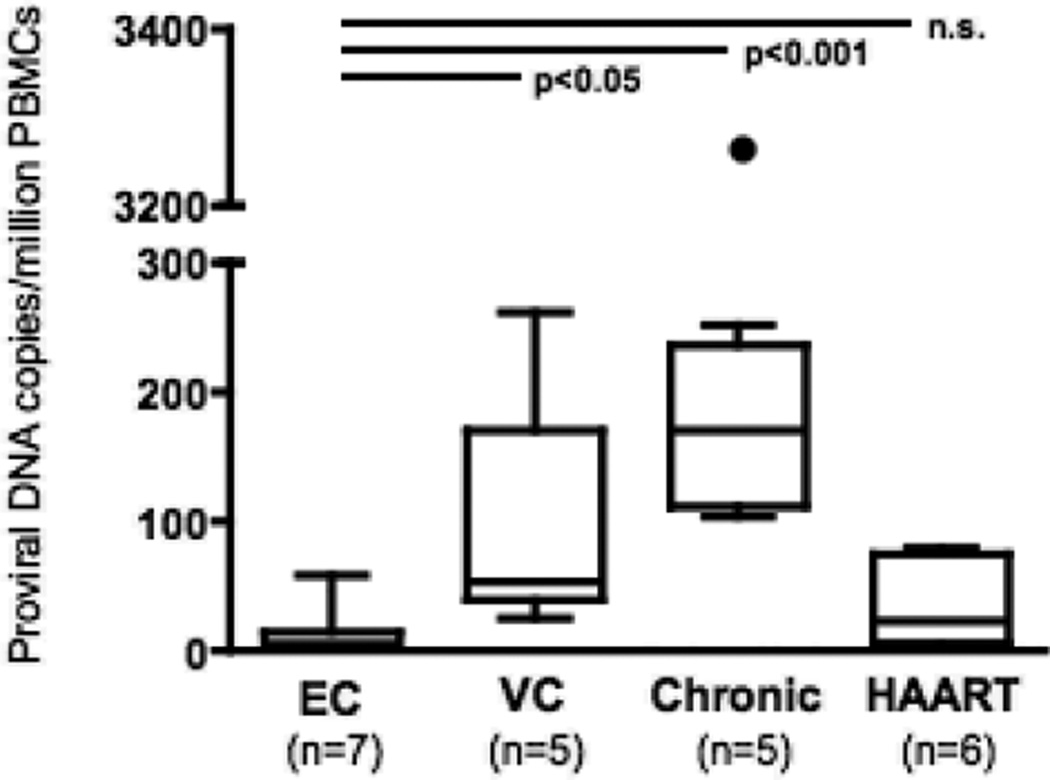
To test the hypothesis that the reduced outgrowth of autologous virus from optimally activated HIV controller CD4+ T-cells is due to the low frequency of infected CD4+ cells in the peripheral blood we measured HIV proviral DNA in total PBMCs of 7 EC and 5 VC and compared these to levels of 5 chronic progressive patients and 6 virally suppressed individuals on HAART. ECs showed significantly lower levels of provirus compared to VCs and chronic progressors (proviral DNA copies/106 PBMC: (EC) 12.79±20.92 vs. (VC) 94.80±95.47 and (chronic progressors) 792.0±1383; p<0.05 and p<0.001 respectively). In contrast we did not detect any significant difference between ECs and patients on HAART (proviral DNA copies/106 PBMC: 34.76±32.74; n.s.) (Fig 2). This observation suggests that the low viral reservoir in the EC CD4+ compartment might lead to limited outgrowth of autologous virus in the EC rather than general replication incompetence of their infecting virus. This assumption is supported by the observation that the elite controller, who demonstrated the strongest outgrowth of autologous virus in the long-term cultures (peak Log10 p24 levels: 4.82 pg/ml at day 28) showed the highest proviral DNA loads of the EC group (proviral DNA copies/106 PBMC: 58.33).
Fig 2.
Proviral loads (DNA copies/106 PBMCs) among EC, VC, progressors and HAART treated individual
Susceptibility to HIV infection
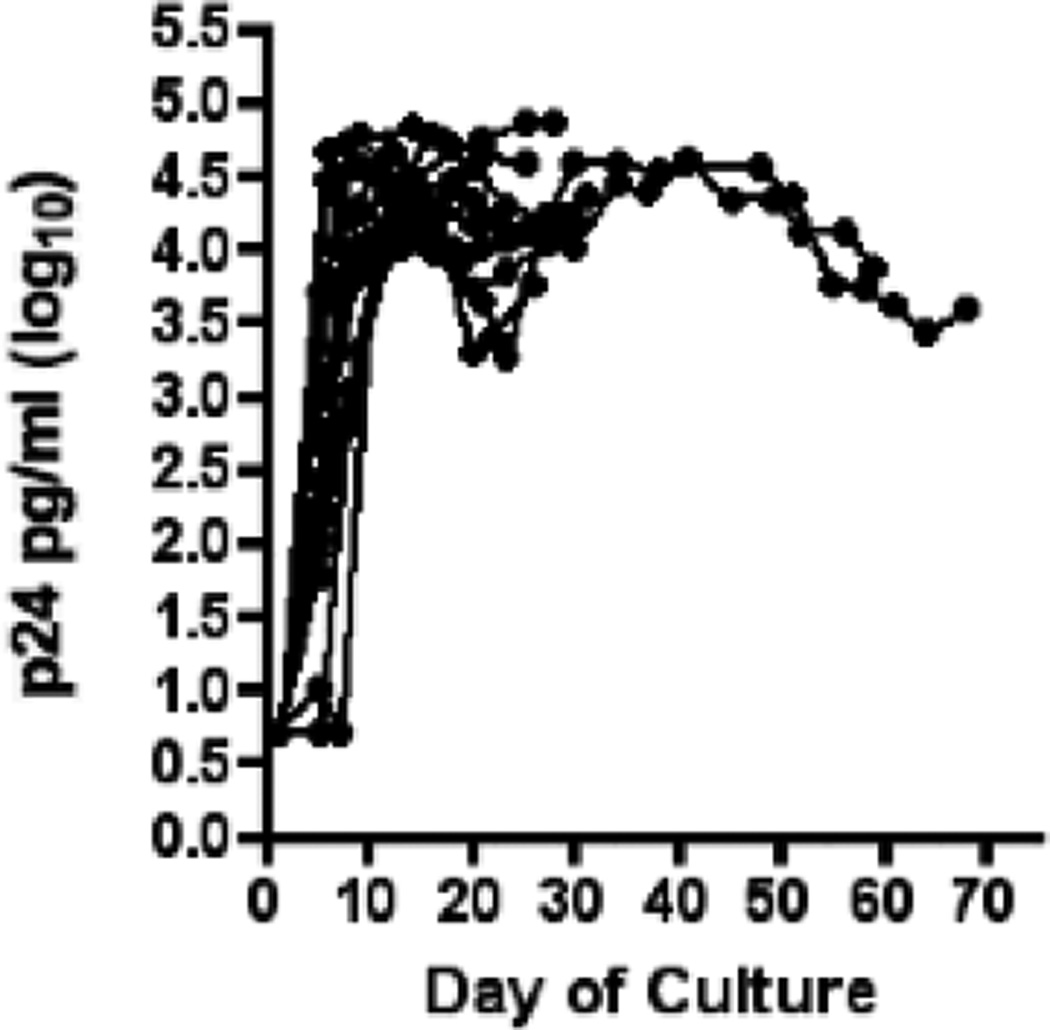
The reduced outgrowth of autologous virus from in vitro activated CD4+ T-cells in ECs could also indicate that these cells may be less permissive to HIV virus replication. To test this hypothesis, we determined whether the same EC CD4+ T-cells used for the outgrowth cultures were susceptible to in vitro infection with an X4 tropic HIV-1 clinical isolate (F716). We therefore infected CD4+ T-cells with exogenous virus in parallel while setting up the outgrowth cultures. CD4+ T-cells from all ECs were readily infectable and exhibited peak Log10 p24 levels of 4.29±0.3 after 10 days (Fig 3).
Fig 3.
Replication of an HIV-1 clinical isolate in activated CD4+ T-cells of 14 elite controllers.
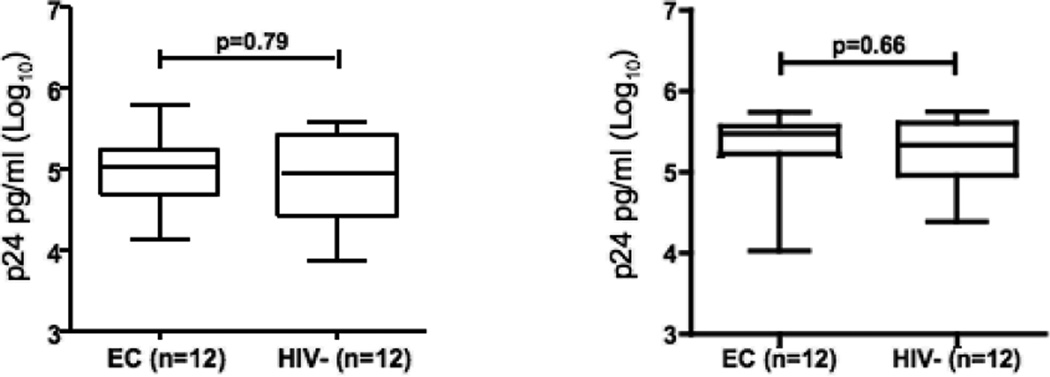
We next ascertained whether there were differences in the level of superinfecting viral production in in vitro stimulated CD4+ T-cells from ECs to non-EC controls. Due to the confounding issue of the outgrowth of autologous virus from cells from viremic individuals, we compared CD4+ T-cell infectability in ECs to a group of matched HIV-seronegative controls. By infecting with X4 and R5 tropic (NL4-3 and JRCSF) HIV-1 strains, we were able to observe peak levels of viral replication in CD4+ T-cells from ECs on day 7, reaching a mean of 4.98±0.45 pg/ml (Log10 p24) for NL4-3 infection and 5.28±0.49 pg/ml (Log10 p24) for JRCSF infection, respectively (Fig. 4 A and B). Similar levels and kinetics of viral replication were seen in CD4+ cell cultures from HIV negative individuals (log10 4.88±0.59 pg/ml for NL4-3 infection and 5.26±0.42 pg/ml for JRCSF infection, respectively). These data suggest that CD4+ T-cells from ECs, after exogenous activation, are not resistant to HIV-1 in-vitro infection.
Fig 4.
Replication of (A) the X4 tropic HIV-1 strain NL4-3 and (B) the R5 tropic HIV-1 strain JRCSF in activated CD4+ T-cells of 12 elite controllers and 12 HIV negative individuals (MOI 0.01). Shown are mean log10 p24 levels (pg/ml) at day 7.
Discussion
In this study we investigated whether low plasma HIV RNA levels in elite and viremic controllers are associated with infection by replication-incompetent viruses or that their CD4+ T-cells are not able to produce virus or become infected. To address these possibilities, purified CD4+ T-cells from ECs and VCs were isolated, stimulated in vitro, and outgrowth of autologous virus was assessed. Of 14 elite controllers tested, autologous virus only grew robustly from samples derived from 2 individuals CD4+ T-cell cultures. In contrast, in vitro activated CD4+ T-cells from all elite controllers were readily infectable with exogenous virus following in-vitro stimulation, suggesting that the elite control observed is not associated with an inability of activated CD4+ cells to support HIV replication. However, we cannot exclude, that a distinct interaction between EC CD4+ T-cells and their infecting viral strain might exists which would not allow viral outgrowth in these individuals. Due to the limitation of retrieving viral strains from ECs, we were unable to test this hypothesis.
Our results are consistent with those of recent reports, in which difficulty in growing autologous virus was observed in the vast majority of ECs [5, 6]. However, as all EC CD4+ T-cells were infectable and produced large quantities of virus upon superinfection with laboratory or primary HIV strains, our data suggest that low levels of virally infected cells in the peripheral circulation rather than an intrinsic inability of their activated CD4+ T-cells to support robust virus replication may explain the difficulty in isolating autologous virus in-vitro. This was supported by the reduced levels of HIV proviral DNA we found in the ECs compared to chronic progressors and even VCs or individuals on suppressive antiretroviral therapy. Consistent with this observation we detected the strongest outgrowth of autologous virus in the EC with the highest proviral DNA levels.
As autologous virus replicated readily in most of the VC despite their low plasma viral loads, the exclusive explanation of HIV control by a defective infecting virus is questionable. Ours and other groups have shown that elite control is associated with persistent low but fluctuating levels of viremia, suggesting individual differences in host immune responses [23, 24]. Our observation that the 2 VCs, which showed either a delayed or no autologous viral outgrowth, express the protective HLA B alleles B27 and B57 implies that the reduction of the viral reservoir in the peripheral CD4+ compartment may be mediated by a potent and persistent immune response. These findings are supported by a recent report from Saez-Cirion et al who demonstrated that among ECs with weak antiviral CD8 T-cell responses highly in vitro replicative viruses were detectable [25].
It has been postulated that CD4+ T-cells from ECs may be less susceptible to infection as it has been shown for individuals, who are highly exposed to HIV-1 and yet remain uninfected [26, 27]. However, detectable low level viremia in the majority of elite controllers [24] points to a small persistent cellular reservoir for ongoing viral replication. Here we artificially activated CD4+ T-cells from all donors, and upon maximal activation superinfected the cells with either an X4 or R5 lab strain. In this setting, we did not observe any differences among the ECs or HIV-negative controls to support viral replication. This observation argues against but does not disprove the hypothesis that host factors may render activated CD4+ T-cells less infectable as a cause for the reduced frequencies of infected peripheral CD4+ T-cells in HIV controllers. However it is still plausible that other factors, such as potential in-vivo differences in CD4+ T-cell activation levels or more subtle differences in the ability to support autologous viral replication may differentiate the infectability of CD4+ cells from ECs compared to other individuals. This said, it is still plausible that added intrinsic differences in activation potentials among CD4+ T-cells from ECs and controls may account for differences in susceptibility to HIV-1 infection., or in the burst size of viruses produced per infected cell.
Overall, these data suggest that in vitro activated CD4 cells from elite controllers can readily accommodate HIV replication and that elite controller can harbour replication competent virus. The poor autologous virus outgrowth from ECs is likely related to the low frequency of HIV infected cells in the peripheral circulation of elite controllers. Low levels of HIV+ CD4+ T-cells in-vivo are likely due to highly antiviral immune pressure unique to HIV controllers but further studies examining subtle differences in immune effector mechanisms that might be detectable with more sensitive assays are warranted.
Acknowledgement
BDW: RO1 AI030914, Bill and Melinda Gates Foundation, CFAR.
References
- 1.Alexander L, Weiskopf E, Greenough TC, et al. Unusual polymorphisms in human immunodeficiency virus type 1 associated with nonprogressive infection. J Virol. 2000 May;74(9):4361–4376. doi: 10.1128/jvi.74.9.4361-4376.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deacon NJ, Tsykin A, Solomon A, et al. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 1995 Nov 10;270(5238):988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 3.Kirchhoff F, Greenough TC, Brettler DB, Sullivan JL, Desrosiers RC. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N Engl J Med. 1995 Jan 26;332(4):228–232. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- 4.Miura T, Brockman MA, Brumme ZL, et al. HLA-associated alterations in replication capacity of chimeric NL4-3 viruses carrying gag-protease from elite controllers of human immunodeficiency virus type 1. J Virol. 2009 Jan;83(1):140–149. doi: 10.1128/JVI.01471-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamine A, Caumont-Sarcos A, Chaix ML, et al. Replication-competent HIV strains infect HIV controllers despite undetectable viremia (ANRS EP36 study) Aids. 2007 May 11;21(8):1043–1045. doi: 10.1097/QAD.0b013e3280d5a7ac. [DOI] [PubMed] [Google Scholar]
- 6.Blankson JN, Bailey JR, Thayil S, et al. Isolation and characterization of replication-competent human immunodeficiency virus type 1 from a subset of elite suppressors. J Virol. 2007 Mar;81(5):2508–2518. doi: 10.1128/JVI.02165-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao Y, Qin L, Zhang L, Safrit J, Ho DD. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N Engl J Med. 1995 Jan 26;332(4):201–208. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 8.Bailey JR, Williams TM, Siliciano RF, Blankson JN. Maintenance of viral suppression in HIV-1-infected HLA-B*57+ elite suppressors despite CTL escape mutations. J Exp Med. 2006 May 15;203(5):1357–1369. doi: 10.1084/jem.20052319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Migueles SA, Osborne CM, Royce C, et al. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity. 2008 Dec;29(6):1009–1021. doi: 10.1016/j.immuni.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pereyra F, Addo MM, Kaufmann DE, et al. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J Infect Dis. 2008 Feb 15;197(4):563–571. doi: 10.1086/526786. [DOI] [PubMed] [Google Scholar]
- 11.Saez-Cirion A, Lacabaratz C, Lambotte O, et al. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci U S A. 2007 Apr 17;104(16):6776–6781. doi: 10.1073/pnas.0611244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miura T, Brumme CJ, Brockman MA, et al. HLA-associated viral mutations are common in human immunodeficiency virus type 1 elite controllers. J Virol. 2009 Apr;83(7):3407–3412. doi: 10.1128/JVI.02459-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deeks SG, Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. 2007 Sep;27(3):406–416. doi: 10.1016/j.immuni.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Schmitz JE, Kuroda MJ, Santra S, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999 Feb 5;283(5403):857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 15.Miura T, Brockman MA, Schneidewind A, et al. Hla-B57/B*5801 Hiv-1 Elite Controllers Select for Rare Gag Variants Associated with Reduced Viral Replication Capacity and Strong Ctl Recognition. J Virol. 2008 Dec 30; doi: 10.1128/JVI.02265-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang OO, Kalams SA, Trocha A, et al. Suppression of human immunodeficiency virus type 1 replication by CD8+ cells: evidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J Virol. 1997 Apr;71(4):3120–3128. doi: 10.1128/jvi.71.4.3120-3128.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garbuglia AR, Salvi R, Di Caro A, et al. Peripheral lymphocytes of clinically non-progressor patients harbor inactive and uninducible HIV proviruses. J Med Virol. 1995 Jun;46(2):116–121. doi: 10.1002/jmv.1890460206. [DOI] [PubMed] [Google Scholar]
- 18.Lambotte O, Boufassa F, Madec Y, et al. HIV controllers: a homogeneous group of HIV-1-infected patients with spontaneous control of viral replication. Clin Infect Dis. 2005 Oct 1;41(7):1053–1056. doi: 10.1086/433188. [DOI] [PubMed] [Google Scholar]
- 19.Wilson CC, Wong JT, Girard DD, et al. Ex vivo expansion of CD4 lymphocytes from human immunodeficiency virus type 1-infected persons in the presence of combination antiretroviral agents. J Infect Dis. 1995 Jul;172(1):88–96. doi: 10.1093/infdis/172.1.88. [DOI] [PubMed] [Google Scholar]
- 20.Chen H, Piechocka-Trocha A, Miura T, et al. Differential neutralization of HIV replication in autologous CD4 T cells by HIV-specific CTL. J Virol. 2009 Jan 21; doi: 10.1128/JVI.02073-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brussel A, Sonigo P. Analysis of early human immunodeficiency virus type 1 DNA synthesis by use of a new sensitive assay for quantifying integrated provirus. J Virol. 2003 Sep;77(18):10119–10124. doi: 10.1128/JVI.77.18.10119-10124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frahm N, Korber BT, Adams CM, et al. Consistent cytotoxic-T-lymphocyte targeting of immunodominant regions in human immunodeficiency virus across multiple ethnicities. J Virol. 2004 Mar;78(5):2187–2200. doi: 10.1128/JVI.78.5.2187-2200.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dinoso JB, Kim SY, Siliciano RF, Blankson JN. A comparison of viral loads between HIV-1-infected elite suppressors and individuals who receive suppressive highly active antiretroviral therapy. Clin Infect Dis. 2008 Jul 1;47(1):102–104. doi: 10.1086/588791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pereyra F, Palmer S, Miura T, et al. Persistent low-level viremia in HIV-1 elite controllers and relationship to immunologic parameters. J Infect Dis. 2009 Sep 15;200(6):984–990. doi: 10.1086/605446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saez-Cirion A, Sinet M, Shin SY, et al. Heterogeneity in HIV suppression by CD8 T cells from HIV controllers: association with Gag-specific CD8 T cell responses. J Immunol. 2009 Jun 15;182(12):7828–7837. doi: 10.4049/jimmunol.0803928. [DOI] [PubMed] [Google Scholar]
- 26.Paxton WA, Martin SR, Tse D, et al. Relative resistance to HIV-1 infection of CD4 lymphocytes from persons who remain uninfected despite multiple high-risk sexual exposure. Nat Med. 1996 Apr;2(4):412–417. doi: 10.1038/nm0496-412. [DOI] [PubMed] [Google Scholar]
- 27.Saez-Cirion A, Versmisse P, Truong LX, et al. Persistent resistance to HIV-1 infection in CD4 T cells from exposed uninfected Vietnamese individuals is mediated by entry and post-entry blocks. Retrovirology. 2006;3:81. doi: 10.1186/1742-4690-3-81. [DOI] [PMC free article] [PubMed] [Google Scholar]