Abstract
BACKGROUND
We hypothesized that for obese patients, abdominal-based free flap, rather than implant-based, and delayed, rather than immediate, breast reconstruction would result in fewer overall complications and reconstruction losses.
METHODS
We retrospectively analyzed consecutive implant- and abdominal-based free-flap breast reconstructions performed in obese patients between 2005 and 2010 utilizing the World Health Organization obesity classifications: class I (30.0–34.9 kg/m2), class II (35.0–39.9 kg/m2), and class III (≥40 kg/m2). Primary outcome measures included flap failures and overall complications. Logistic regression analysis identified associations between patient, defect, and reconstructive characteristics and surgical outcomes.
RESULTS
The analysis included 990 breast reconstructions (548 flaps vs. 442 implants) in 700 patients. Mean follow-up was 17 months. Age (p<0.01), smoking (p=0.02), medical illness (p=0.01), and BMI>37 (p=0.01) predicted overall complications on regression analysis. Implants demonstrated a higher failure rate (15.8%) than flaps (1.5%; p<0.001). While failure rates were similar for immediate and delayed flap reconstructions overall (1.3% vs. 1.9%; p=0.7) and among obesity classifications, there was a trend toward more implant failures in immediate rather than delayed reconstructions (16.8% vs. 5.3%; p=0.06). Differences between immediate implant versus flap reconstruction failure rates were highest among more obese patients (class II [24.7% vs. 1.3%, respectively; p<0.01] and class III [25.4% vs. 0%, respectively; p<0.01] compared to class I [11.7% vs. 1.4%, respectively; p<0.01]).
CONCLUSIONS
Obese patients, particularly patients with class II and III obesity, experience higher failure rates with implant-based breast reconstruction, particularly immediate reconstruction. Free flap techniques or delayed implant reconstruction may be warranted in this population.
INTRODUCTION
Obese patients experience higher rates of wound-related complications and reconstructive failure than nonobese patients following breast reconstruction.1–10 Given the increasing prevalence of obesity in the Western population, plastic surgeons are more frequently asked to provide breast reconstruction for this challenging population.11 Studies have compared outcomes in breast reconstruction between obese and non-obese patients based on patients’ body mass index (BMI), but few studies have addressed differences in outcomes according to increasing obesity severity.7,8,12 To better stratify patients’ medical risks according to the degree of obesity, the World Health Organization (WHO) has sub-classified the definition of obesity: class I/obese (30.0–34.9 kg/m2), class II/morbidly obese (35.0–39.9 kg/m2), and class III/superobese (≥40 kg/m2).13–15 The effects of increasing obesity on both patient health and the outcomes of other surgical procedures suggest that outcomes in breast reconstruction need to be similarly stratified.7,12–19
Few studies have evaluated breast reconstruction outcomes according to obesity classification or compared the outcomes of implant and flap reconstruction in the obese.7–9,12,20,21 Our experience with breast reconstruction in the obese patient population led us to hypothesize that, for obese patients, abdominal-based free flap breast reconstruction, rather than implant-based reconstruction, and delayed, rather than immediate, breast reconstruction would result in fewer overall complications and reconstruction losses following skin-sparing mastectomy.
PATIENTS AND METHODS
We retrospectively reviewed a prospectively maintained database of consecutive implant and abdominal-based free flap breast reconstructions performed in obese patients (BMI≥30 kg/m2) at a single center between 2005 and 2010. Implant reconstructions (saline and silicone) included direct-to-implant reconstructions and 2-stage tissue expander plus implant reconstructions. Flaps were classified in a standard fashion as muscle-sparing free transverse rectus abdominis musculocutaneous (MS FTRAM), deep inferior epigastric artery perforator (DIEP), or superficial inferior epigastric artery (SIEA) flaps.22–24 We excluded patients who were not obese (BMI<30 kg/m2), in whom the reconstruction was performed with a technique other than implant only or abdomen-based free flap, who had a “delayed-delayed” or “delayed-immediate” breast reconstruction, and who underwent latissimus dorsi– or gluteus-based flap reconstructions.25–28
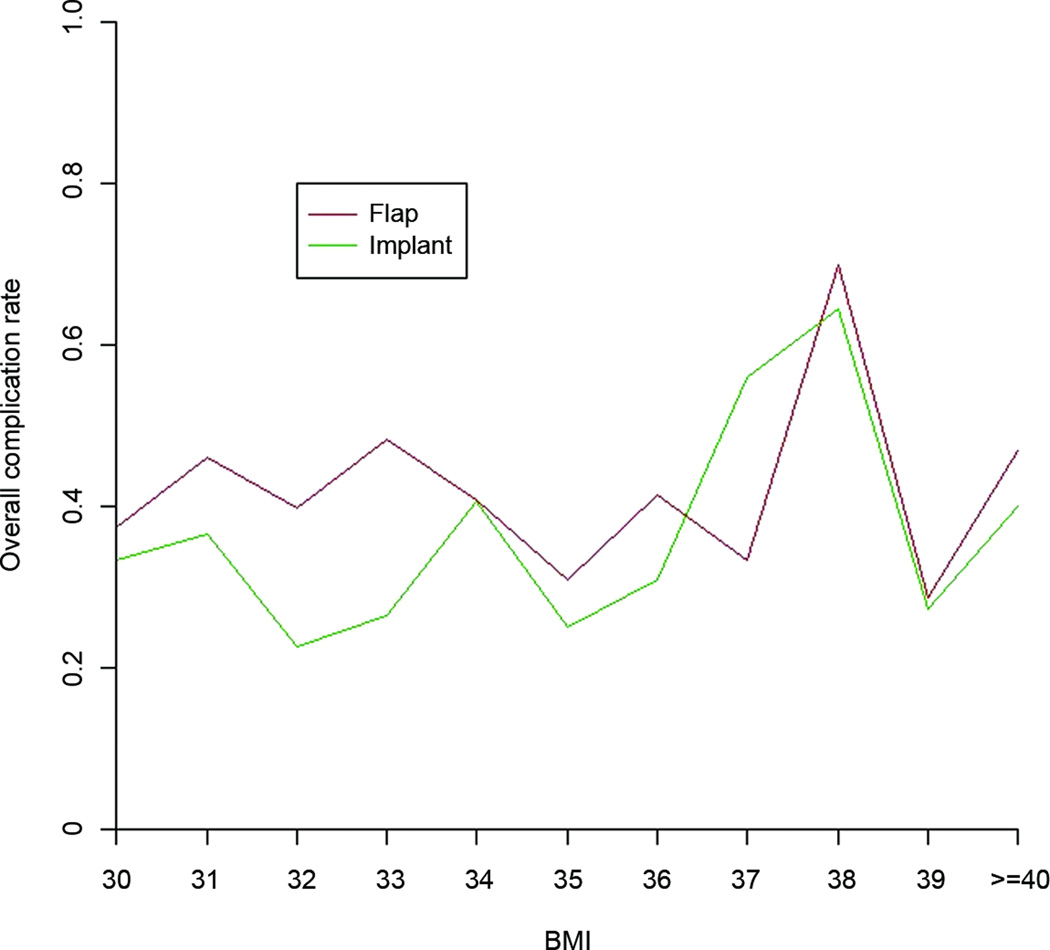
Differences in outcomes were evaluated for implant versus flap reconstructions and immediate versus delayed reconstructions. Subset analysis evaluated patients subclassified into three groups according to the WHO obesity classifications (Figure 1).13–15,18 Patient, treatment, defect, and reconstruction outcome data were analyzed and directly compared between patient groups. Outcome measures included the effect of BMI on overall and specific surgical complications. We also plotted each patient’s BMI relative to the overall complication rate to identify any infection points along the curve where the complication rates increased.
Complications evaluated included loss of the reconstruction (defined as the need to remove the reconstruction)29, as well as the following specific complications: breast wound complications (infection, delayed healing, skin dehiscence, hematoma/seroma), mastectomy skin necrosis, perfusion-related complications (fat necrosis, partial flap necrosis), implant-related complications (malposition, rippling), microvascular complications (arterial thrombosis, venous thrombosis), compromised integrity of the abdominal wall (bulge, hernia, umbilical necrosis), and abdominal wound complications (infection, delayed healing, skin dehiscence, hematoma/seroma).
Skin dehiscence was defined as a separation of the incision ≥0.5 cm. Delayed healing was a wound requiring debridement and healing by secondary intention. Infection was an infectious process (cellulitis/abscess) treated with intravenous or oral antibiotics with/without surgery. Hematoma and seroma were subcutaneous collections of blood or serous fluid, respectively, which required percutaneous or operative drainage. Abdominal hernia and bulging were contour deformities noted on physical examination with or without a fascial defect, respectively.30 Fat necrosis was a palpable firmness ≥1 cm in diameter that persisted beyond 3 months postoperatively. Partial flap necrosis was necrosis of the flap skin island and underlying fat.31 For the purpose of this evaluation, fat necrosis and partial flap necrosis, as well as hernia and bulging, were considered mutually exclusive complications. The presence of fat necrosis or partial flap necrosis was determined by clinical examination and radiographic and/or pathologic confirmation. The decision to image and/or biopsy a palpable firmness was made at the surgeons’ and/or oncologists’ clinical discretion. Patients were followed postoperatively at least monthly after discharge for 6 months, every 3–6 months until 1 year, and then at least yearly thereafter.
Statistical Analysis
Means and standard deviations were used to summarize continuous variables. Frequencies and proportions were used to present the categorical clinical characteristics. A two-tailed Fisher’s exact test was used to test associations between categorical variables. The Wilcoxon rank sum test or Kruskal-Wallis test was used to compare ordinal variables among patient groups. A correlated logistic regression model was used to determine the association between the overall complication rate and demographic or clinical characteristics. A stepwise model selection method was used to fit a multivariate regression model. All tests were two-sided. A p-value of <0.05 was considered significant.32 The analyses were performed in SAS 9.2 (SAS Institute Inc., Cary, NC) and R (The R Foundation for Statistical Computing). A senior staff biostatistician (J.L.) performed all statistical analyses.
RESULTS
A total of 990 breast reconstructions in 700 obese patients were analyzed. Abdominal-based free flaps were used in 548 reconstructions and implants were used in 442 reconstructions. Mean follow-up was 17 months (range, 3–118 months). Mean age was 50 years (range, 26–78 years). The average BMI was 34.5 kg/m2 (range, 30.0–58.6 kg/m2). The majority of the patients had class I obesity (N=483, 69%), followed by class II obesity (N=154, 22%) and class III obesity (N=63, 9%).
Patients’ characteristics were noted to vary by reconstruction type (Table 1). More of the patients in the implant group had class II or class III obesity compared to the flap group (p<0.01). Patients in the implant group were older, more obese, and had higher incidences of coronary artery disease, diabetes mellitus, and hypertension than patients in the flap group. A higher percentage of patients in the implant group received postoperative chemotherapy and a lower percentage received preoperative radiation therapy and chemotherapy compared with the flap group.
Table 1.
Patient Characteristics by Reconstruction Type
| Characteristics | Implant N= 442 |
Flap N=548 |
P-value |
|---|---|---|---|
| Age (years) | 52.2 ± 10.3 | 48.9 ± 8.9 | <0.01 |
| BMI | 35.3 ± 4.9 | 33.9 ± 3.8 | <0.001 |
| Length of Follow-up (months) | 17.4 ± 19.7 | 17.8 ± 18.7 | 0.74 |
| Obesity Classification | |||
| Class I (Obese) | 275 (62.2%) | 406 (74.1%) | |
| Class II (Morbidly Obese) | 107 (24.2%) | 110 (20.1%) | |
| Class III (Superobese) | 60 (13.6%) | 32 (5.8%) | <0.01 |
| Pre-op Chemo | 130 (29.4%) | 287 (52.4%) | <0.01 |
| Pre-op XRT | 16 (3.6%) | 170 (31%) | <0.01 |
| Post-op Chemo | 58 (13.1%) | 39 (7.1%) | <0.01 |
| Post-op XRT | 49 (11.1%) | 34 (6.2%) | 0.01 |
| Co-morbidity | |||
| Coronary Artery Disease | 18 (4.1%) | 7 (1.3%) | 0.01 |
| Diabetes Mellitus | 66 (14.9%) | 33 (6%) | <0.01 |
| Alcohol Abuse | 0 (0%) | 27 (7.5%) | <0.01 |
| HTN | 161 (36.4%) | 161 (29.4%) | 0.02 |
| Pulmonary Disease | 18 (4.1%) | 12 (2.2%) | 0.10 |
| Smoking | |||
| Non-smoker | 417 (94.3%) | 519 (94.7%) | |
| Active Smoker | 25 (5.7%) | 29 (5.3%) | 0.89 |
| Medical Illnesses | |||
| None | 181 (41%) | 287 (52.4%) | |
| One | 152 (34.4%) | 177 (32.3%) | |
| Two or more | 109 (24.7%) | 84 (15.3%) | <0.01 |
BMI, body mass index; XRT, radiation therapy; HTN, hypertension
Overall and Obesity Group Differences in Surgical Technique for Implants vs. Flaps
The majority of the reconstructions performed were immediate (793, 80.1%). More of the delayed reconstructions were flap, compared with implant, reconstructions (29.0% vs. 8.6%; p>0.01) (Table 2). More of the bilateral reconstructions were implant rather than flap reconstructions (63.1% vs. 56.0%; p=0.03).
Table 2.
Surgical Characteristics by Reconstruction Type
| Characteristics | Implant N=442 |
Flap N=548 |
P value |
|---|---|---|---|
| Timing | |||
| Immediate | 404 (91.4%) | 389 (71.0%) | |
| Delayed | 38 (8.6%) | 159 (29.0%) | <0.01 |
| Laterality | |||
| Unilateral | 163 (36.9%) | 241 (44.0%) | |
| Bilateral | 279 (63.1%) | 307 (56.0%) | 0.03 |
| Side | |||
| Right | 215 (48.6%) | 274 (50.0%) | |
| Left | 227 (51.4%) | 274 (50.0%) | 0.70 |
The different types of flap procedures (DIEP, SIEA, and MS FTRAM) were performed with similar frequency among the obesity classifications, except that class I obesity patients underwent MS1 FTRAM reconstruction more often than the other two obesity groups (Table 3). There were no differences in the choice of recipient vessels or the use of mesh reinforcement of the abdominal wall closure among the obesity classification groups.
Table 3.
Flap Reconstruction Characteristics by Obesity Classification
| Characteristics | Total N=548 |
Class I N=406 |
Class II N=110 |
Class III N=32 |
P value |
|---|---|---|---|---|---|
| Flap Type | |||||
| DIEP | 162 (29.6%) | 120 (29.6%) | 35 (31.8%) | 7 (21.9%) | |
| SIEA | 34 (6.2%) | 25 (6.2%) | 4 (3.6%) | 5 (15.6%) | |
| MS FTRAM | 352 (64.2%) | 261 (64.2) | 71 (64.6%) | 20 (62.5%) | 0.20 |
| MS0 FTRAM | 33 (9.4%) | 23 (8.8%) | 8 (11.3%) | 2 (10.0%) | |
| MS1 FTRAM | 69 (19.6%) | 61 (23.4%) | 5 (7.0%) | 3 (15.0%) | |
| MS2 FTRAM | 181 (51.4%) | 126 (48.3%) | 43 (60.6%) | 12 (60.0%) | 0.02 |
| Unknown | 69 (19.6%) | 51 (19.5%) | 15 (21.2%) | 3 (15.0%) | |
| Recipient Vessels | |||||
| Internal Mammary | 490 (89.4%) | 355 (87.4%) | 103 (93.6%) | 32 (100%) | |
| Thoracodorsal | 52 (9.5%) | 46 (11.3%) | 6 (5.5%) | 0 (0%) | |
| Other Vessels | 6 (1.1%) | 5 (1.2%) | 1 (0.9%) | 0 (0%) | 0.86 |
| Donor Site Closure | |||||
| Primary Closure | 451 (82.3%) | 332 (81.8%) | 92 (83.6%) | 27 (84.4%) | |
| Mesh Closure | 97 (17.7%) | 74 (18.2%) | 18 (16.4%) | 5 (15.6%) | 0.92 |
| Prosthetic | 89 (91.7%) | 69 (93.2%) | 17 (94.4%) | 3 (60.0%) | |
| Bio-prosthetic | 8 (8.3%) | 5 (6.8%) | 1 (5.6%) | 2 (40.0%) | 0.06 |
DIEP, deep inferior epigastric artery perforator; SIEA, superficial inferior epigastric artery; MS FTRAM, muscle-sparing free transverse rectus abdominis musculocutaneous
In the implant reconstruction patients, there did not appear to be any obesity group differences with respect to reconstructive strategy (i.e. tissue expanders vs. direct-to-implant), reconstructive technique (i.e. total submuscular vs. dual plane vs. acellular dermal matrix [ADM]), type of ADM used, or time to tissue expander exchange for a permanent implant (Table 4). There were no differences in the initial tissue expander fill volume among the groups, but the initial expander size and final size of the permanent implant did increase with increasing obesity classification. The time to removal of the last surgical drain was shorter for the class I obesity patients (21 ± 9 days) than for the class II (25 ± 17 days) and class III (25 ± 14 days) obesity patients (p=0.02).
Table 4.
Implant Reconstruction Characteristics by Obesity Classification
| Variable | Total N =442 |
Class I N=275 |
Class II N=107 |
Class III N=60 |
P value |
|---|---|---|---|---|---|
| Strategy | |||||
| Direct to Implant | 15 (3.4%) | 9 (3.3%) | 6 (5.6%) | 0 (0%) | |
| Staged TE Implant | 427 (96.6%) | 266 (96.7%) | 101 (94.4%) | 60 (100%) | 0.16 |
| Reconstruction Technique | |||||
| Total Submuscular | 206 (46.6%) | 134 (48.7%) | 44 (41.1%) | 28 (46.7%) | |
| Dual Plane | 130 (29.4%) | 74 (26.9%) | 32 (29.9%) | 24 (40%) | |
| Acellular Dermal Matrix | 106 (24%) | 67 (24.4%) | 31 (29%) | 8 (13.3%) | 0.10 |
| Human | 69 (15.6%) | 40 (14.5%) | 22 (20.6%) | 7 (11.7%) | |
| Bovine | 19 (4.3%) | 12 (4.4%) | 7 (6.5%) | 0 (0%) | |
| Porcine | 14 (3.2%) | 12 (4.4%) | 1 (0.9%) | 1 (1.7%) | |
| Other | 4 (0.9%) | 3 (1.1%) | 1 (0.9%) | 0 (0%) | 0.17 |
| Size of Expander | 570 ± 137 | 530 ± 126 | 612 ± 133 | 677 ± 112 | <0.01 |
| Initial Fill Volume of Expander | 236 ± 179 | 218 ± 162 | 262 ± 200 | 276 ± 205 | 0.09 |
| Final Fill volume of Expander | 665 ± 251 | 633 ± 220 | 731 ± 294 | 700 ± 285 | <0.001 |
| Size of Final Implant | 702 ± 130 | 676 ± 120 | 721 ± 127 | 812 ± 135 | <0.01 |
| Time to TE Exchange for Implant | 257 ± 209 | 246 ± 163 | 274 ± 292 | 285 ± 269 | 0.70 |
| Time to Removal of Last Drain | 22 ± 12.8 | 21 ± 9 | 25 ± 17 | 25 ± 14 | 0.02 |
TE, tissue expander
Overall Complication Rates for Implants vs. Flaps in Obese Patients
The overall complication rate for breast reconstruction in our study was 39.5%. There were more complications in the flap reconstruction group (42.3%) than in the implant reconstruction group (35.9%; p=0.04) (Table 5). Reconstruction loss was significantly more frequent in the implant (15.8%) compared to the flap (1.5%; p<0.001) reconstructions. Implants demonstrated higher rates of infection (11.3% vs. 3.8%; p<0.001) and hematoma/seroma (13.8% vs. 4.9%; p<0.001) compared to flaps. The flap group demonstrated higher rates of delayed wound healing than did the implant group (7.5% vs. 4.3%; p=0.01).
Table 5.
Complication Rates by Reconstruction Type
| Complications | Implant N=442 |
Flap N=548 |
P value |
|---|---|---|---|
| Overall Complications | 159 (35.9%) | 232 (42.3%) | 0.04 |
| Loss of Reconstruction | 70 (15.8%) | 8 (1.5%) | <0.001 |
| Breast Wound–Related Complications | |||
| Infection | 50 (11.3%) | 21 (3.8%) | <0.001 |
| Delayed Healing | 19 (4.3%) | 41 (7.5%) | 0.01 |
| Skin Dehiscence | 28 (6.3%) | 25 (4.6%) | 0.25 |
| Hematoma/Seroma | 61 (13.8%) | 27 (4.9%) | <0.001 |
| Mastectomy Skin Necrosis | 58 (13.1%) | 90 (16.4%) | 0.15 |
| Perfusion-related Complications | |||
| Fat Necrosis | -- | 38 (6.9%) | -- |
| Partial Flap Necrosis | -- | 21 (3.8%) | -- |
| Implant-related Complications | |||
| Implant Malposition | 8 (1.8%) | -- | -- |
| Implant Rippling | 4 (0.9%) | -- | -- |
| Microvascular Complications | |||
| Arterial Thrombosis | -- | 6 (1.1%) | -- |
| Venous Thrombosis | -- | 4 (0.7%) | -- |
| Donor Site Complications | |||
| Bulge | -- | 19 (3.5%) | -- |
| Hernia | -- | 10 (1.8%) | -- |
| Umbilical Necrosis | -- | 25 (4.6%) | -- |
| Donor Site Wound-related Complications | |||
| Delayed Wound Healing | -- | 34 (6.2%) | -- |
| Skin Dehiscence | -- | 31 (5.7%) | -- |
| Skin Necrosis | -- | 6 (1.1%) | -- |
| Hematoma/Seroma | -- | 23 (4.2%) | -- |
The implant and flap reconstruction groups required additional surgery for complications (25.3% vs. 21.7%; p=0.20, respectively) and to improve symmetry (54.3% vs. 60.2%; p=0.07, respectively) at similar rates. More of the flap reconstructions underwent nipple reconstruction than did the implant reconstructions (39.2% vs. 11.3%; p<0.001). Sixty-three percent of the implant reconstructions underwent a tissue expander exchange for an implant. Of the seven total flap losses in the study, six (85.7%) of the patients eventually underwent a second reconstruction attempt (3 tissue expander/implants, 2 latissimus dorsi/implant, and 1 latissimus dorsi alone). However, only 10 (14.3%) of the 70 patients with implant reconstruction losses eventually underwent a second reconstruction attempt (9 tissue expander/implants and 1 latissimus dorsi alone). The difference in second breast reconstruction attempts between flap and implant reconstructions was statistically significant (p=0.0002).
Complications for Implants vs. Flaps by Obesity Classification
Patients with class I obesity demonstrated overall complication rates that were significantly higher for flaps (42.3%) than for implants (31.6%; p<0.01) (Table 6). Flap and implant reconstructions demonstrated similar rates of overall complications in class II (40.9% vs. 44.8%; p=0.59) and class III (46.8% vs. 40.0%; p=0.68) patients. Fewer flap than implant reconstructions were lost for all three obesity classifications.
Table 6.
Complications for Implants vs. Flaps by Obesity Classification
| Class I Obesity (BMI 30–34.9 kg/m2) | |||
|---|---|---|---|
| Implant N=275 |
Flap N=406 |
P value | |
| Overall Complications | 87 (31.6%) | 172 (42.3%) | <0.01 |
| Loss of Reconstruction | 31 (11.3%) | 6 (1.5%) | <0.01 |
| Class II Obesity (BMI 35-39.9 kg/m2) | |||
| Implant N=107 |
Flap N=110 |
P value | |
| Overall Complications | 48 (44.8%) | 65 (40.9%) | 0.59 |
| Loss of Reconstruction | 25 (23.4%) | 2 (1.8%) | <0.01 |
| Class III Obesity (BMI ≥ 40 kg/m2) | |||
| Implant N=60 |
Flap N=32 |
P value | |
| Overall Complications | 25 (40%) | 15 (46.8%) | 0.68 |
| Loss of Reconstruction | 14 (23.3%) | 0 (0%) | <0.01 |
BMI, body mass index
Immediate and delayed flap reconstructions experienced similar rates of overall complications and flap losses (Table 7). While we did see higher rates of implant losses among the immediate vs. delayed implant reconstructions for all obesity classifications, these differences were not statistically significant.
Table 7.
Complication Rates for Immediate vs. Delayed Flap vs. Implant Reconstruction in Obese Patients
| Class I | Class II | Class III | |
|---|---|---|---|
| Overall Complications | |||
| Flap | |||
| Immediate | 125/284 (44.0%) | 28/78 (35.9%) | 13/27 (48.2%) |
| Delayed | 47/122 (38.5%) | 17/32 (53.1%) | 2/5 (40%) |
| P-value | 0.32 | 0.13 | >0.99 |
| Implant | |||
| Immediate | 81/248 (32.6%) | 46/101 (45.5%) | 24/55 (43.6%) |
| Delayed | 6/27 (22.2%) | 2/6 (33.3%) | 0/5 (0%) |
| P-value | 0.38 | 0.69 | 0.05 |
|
Loss of Reconstruction> | |||
|
Flap | |||
| Immediate | 4/284 (1.4%) | 1/78 (1.3%) | 0/27 (0%) |
| Delayed | 2/122 (1.6%) | 1/32 (3.1%) | 0/5 (0%) |
| P-value | >0.99 | 0.45 | >0.99 |
|
Implant | |||
| Immediate | 29/248 (11.7%) | 25/101 (24.7%) | 14/55 (25.4%) |
| Delayed | 2/27 (7.4%) | 0/6 (0%) | 0/5 (0%) |
| P-value | 0.75 | 0.33 | 0.34 |
Complications for Implants vs. Flaps by BMI
When the overall complication rate was analyzed with respect to BMI, inflection points for both the implant and flap groups were demonstrated at BMI≥37 kg/m2 (Figure 2). When patients were then grouped into two cohorts, BMI<37 kg/m2 and BMI≥37 kg/m2, the overall complication rate was significantly higher for the population with BMI≥37 kg/m2 (47.1% vs. 37.4%, respectively; p=0.01).
Among the BMI<37 kg/m2 patients, the implant reconstruction group had a significantly lower overall complication rate compared with the flap reconstruction group (31.2% vs. 41.3%, respectively; p<0.01). However, among the BMI≥37 kg/m2 patients, the overall complication rates were equivalent between the implant and flap reconstructions (46.4% vs. 48.6%, respectively; p=0.77). Implant loss rates were significantly higher than flap loss rates for both of these BMI groups, with the difference being more pronounced in the BMI≥37 kg/m2 group (24.6% vs. 2.7%; p<0.001) than in the BMI<37 kg/m2 group (11.8% vs. 1.3%; p<0.01) (Table 8).
Table 8.
Complications Rates for BMI < 37 kg/m2 vs. BMI ≥ 37 kg/m2
| Implant | Flap | P-value | |
|---|---|---|---|
| Overall Complications | |||
| BMI < 37 kg/m2 N=778 |
95/304 (31.3%) | 196/474 (41.4%) | <0.01 |
| BMI ≥ 37 kg/m2 N=212 |
64/138 (46.4%) | 36/74 (48.9%) | 0.77 |
| Loss of Reconstruction | |||
| BMI < 37 kg/m2 N=778 |
36/304 (11.8%) | 6/474 (1.3%) | <0.01 |
| BMI ≥ 37 kg/m2 N=212 |
34/138 (24.6%) | 2/74 (2.7%) | <0.001 |
BMI, body mass index
Factors Predictive of Complications
Regression analysis demonstrated age (p<0.01), active smoking (p=0.02), BMI>37 (p=0.01), and the presence of at least one medical co-morbidity (p=0.01) to be risk factors for overall complications among all patients, irrespective of type of reconstruction. Subgroup analysis of the implant patients demonstrated greater expander size and final expander fill volume to be significantly associated with complications (p<0.01 for both factors). Subset regression analysis of the flap reconstruction group failed to demonstrate additional factors associated with overall complications.
DISCUSSION
In this largest study to date evaluating outcomes following breast reconstruction in obese patients, we had hypothesized higher complication rates for implant reconstructions in comparison to abdominal flap reconstructions. However, what we actually observed was a higher overall complication rate for flap reconstructions (p=0.04) and a higher reconstruction loss rate for implant reconstructions (p<0.001). This observed difference in the reconstruction loss rate was more pronounced for class II and class III obesity patients than for class I obesity patients. In contradistinction to implant loss rates, flap loss rates remained similar irrespective of increasing obesity classification (p=0.803). The timing of the reconstruction also played an important role in the outcomes of our obese patients, although not exactly as we had hypothesized. Whereas the rates of overall complications and reconstruction losses were similar for the immediate vs. delayed flap reconstruction patients overall and by obesity classification, the majority of the implant reconstruction losses occurred in the immediate tissue expander plus implant reconstructions.
Previous studies have demonstrated a higher risk of reconstruction failure for both abdominal-based free flaps and implants in obese patients compared to nonobese patients.2,8,10 A 2008 study evaluating 1170 tissue expander plus implant reconstructions at a single center demonstrated that the 108 obese patients (BMI>30 kg/m2) in the study had more complications and reconstructive failures than the nonobese patients.10 A 2000 study compared the outcomes of free TRAM flaps in 64 obese patients (BMI≥30 kg/m2) to those in 442 normal weight (BMI=18.5–24.9 kg/m2) and 212 overweight (BMI=25–29.9 kg/m2) patients and found the obese patients had higher rates of overall flap and donor site complications and total flap loss.2 Neither of these studies performed subgroup comparisons to try to identify whether any particular class of obesity or BMI level experienced more complications, nor did they directly compare outcomes between implant and flap reconstruction in the obese.
A 2011 study retrospectively reviewed the outcomes of abdominal-based free flap breast reconstruction performed in 25 patients with class III obesity (BMI≥40 kg/m2) compared to 379 patients whose BMI was less than 40 kg/m2.8. Two (8%) of the 25 class III obesity patients suffered total flap loss, compared to the 0.5% flap loss rate for the control group (p=0.02); the authors concluded that flap failure is significantly more common among patients with class III obesity. However, to reach this conclusion, superobese patients were compared to a control group comprised of not only obese and morbidly obese patients but also normal weight (BMI=18.5–24.9 kg/m2) and overweight (BMI=25–29.9 kg/m2) patients. When this 2011 study analyzed flap failures by BMI category, the authors found no significant difference in flap loss rates between any of the BMI groups (i.e., normal weight, overweight, obese, morbidly obese, or superobese), which is similar to what we observed in our study. As in our study, delayed wound healing of the donor site incision was also more common among the superobese, whereas the abdominal hernia rate was similar. This study also had fewer obese patients than our study and did not evaluate outcomes for implant reconstruction.
For our study, we directly compared implant to flap reconstruction in the obese; however, direct comparison between specific complications in these two groups is not possible. As such, we selected overall complications and loss of the reconstruction as our primary outcome measures for comparison. The metric of overall complications includes both donor and recipient site complications for flaps, yet only includes recipient site complications for implants. Multiple studies have demonstrated patients’ quality of life and satisfaction with their breast reconstruction to be less favorable if they have a complication, irrespective of whether it is at the recipient or donor site.33–37 Interestingly, among the very obese (class II and III obesity) in our study, the overall complication rate for one surgical site in the implant patients was equivalent to the overall complication rate for two surgical sites in the flap patients.
Previous studies have reported rates of premature tissue expander removal ranging between 1.8% and 5.7% for all patients, regardless of obesity status.10,20,38–41 Such studies have demonstrated conflicting conclusions regarding the effects of obesity (BMI>30 kg/m2) on implant failure rates and have not analyzed differences in outcomes according to increasing BMI.10,39 Implant loss rates for non-obese patients have been reported to be similar for both immediate and delayed implant breast reconstructions,20 but no study, to our knowledge, has evaluated the effect of timing on reconstruction outcomes among obese patients. While implant loss may be perceived as a less significant complication than the loss of a free flap, any reconstructive loss is a tremendous psychological setback.34,36 This is highlighted by our finding that only 14.3% of the implant patients vs. 85.7% of the flap patients who lost their reconstruction ultimately chose to undergo a second attempt at breast reconstruction. Unfortunately, the retrospective design of our study prevents our discovering whether this discrepancy reflects differences in patient- or surgeon-driven decision-making, as the patients who elected to undergo the added initial morbidity of free flap breast reconstruction may simply have represented a self-selected group that was more motivated with regard to breast reconstruction in general. Given the known psychological implant of a reconstruction failure, our surgeons do go to great lengths to try to salvage both failing free flaps and implants. For implants, this may entail intravenous antibiotics, operative washouts, or implant replacement.42
We believe our data support flap reconstruction over implant reconstruction for class II and III obesity patients; however, some patients simply either refuse flap reconstruction, are not healthy enough for the prolonged anesthesia times associated with flap reconstruction, or have an overhanging abdominal pannus sizable enough to preclude an abdominal flap reconstruction. Our findings suggest that such patients with class II and III obesity who seek implant reconstruction may be better served with a standard mastectomy followed by a delayed, rather than immediate, implant reconstruction, ideally after successful completion of a medically supervised weight reduction program. Alternatively, latissimus dorsi flap reconstruction or a staged implant-based reconstruction after initial reduction mammoplasty may prove to be more attractive strategies to traditional delayed reconstruction in these patients, but our data can neither support nor refute these approaches, as we lacked sufficient numbers of such patients to include them in this study and produce meaningful data.43,44
The strengths of our study include the large number of breast reconstructions performed by multiple surgeons using similar techniques at a single center, careful study design to compare morbidity among obese patients stratified by BMI, data obtained from a prospectively entered patient database, and regression analyses. Study limitations include its retrospective design, potential surgeon selection bias affecting the reconstruction choice, exclusion of other forms of breast reconstruction such as latissimus dorsi– or gluteal-based flap reconstruction, lack of comparative aesthetic outcomes data, and inability to analyze for patients’ fat distribution and body composition.45
CONCLUSIONS
We hope that the data presented will enable surgeons to optimally guide the reconstructive choices (flap vs. implant and immediate vs. delayed breast reconstruction) of obese patients. Although obese patients represent a higher surgical risk group than normal weight patients, obesity is not an absolute contraindication to breast reconstruction.1,2,4,7,8 However, our data emphasize the importance of choosing the correct reconstructive strategy for this elevated-risk patient population, especially when also presenting with advanced age, active smoking, BMI>37, or medical co-morbidities. The greater failure rates for implants versus flaps in this population and the consequences of reconstruction failure with respect to patients choosing to undergo a second attempt at breast reconstruction suggest that strong consideration should be given to performing flap- rather than implant-based breast reconstruction in the obese, particularly patients with class II and III obesity, and that a delayed rather than immediate reconstruction be considered an option for obese patients seeking implant-based breast reconstruction.
ACKNOWLEGEMENTS
Financial Support: This research was supported in part by the National Institutes of Health through MD Anderson's Cancer Center Support Grant CA016672.
The authors wish to recognize former and current members of the Department of Plastic Surgery at The University of Texas MD Anderson Cancer Center for their support and/or contribution of patients to this series: Drs. David M. Adelman, Donald P. Baumann, Charles E. Butler, David W. Chang, Melissa A. Crosby, Matthew M. Hanasono, Steven J. Kronowitz, Scott D. Oates, Gregory P. Reece, Jesse C. Selber, and Roman J. Skoracki, and former colleagues Drs. Bonnie J. Baldwin, Pierre M. Chevray, Mennen T. Gallas, Lior Heller, Stephen S. Kroll, Howard N. Langstein, Michael J. Miller, and Justin M. Sacks. The authors also thank Dawn Chalaire from The University of Texas MD Anderson Cancer Center, Department of Scientific Publications for assistance with scientific editing. Lastly, the authors would like to acknowledge the hard work and dedication of our fellows and residents who helped with these cases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: None of the authors has a financial interest associated with this publication.
REFERENCES
- 1.Kroll SS, Netscher DT. Complications of TRAM flap breast reconstruction in obese patients. Plast Reconstr Surg. 1989;84:886–893. doi: 10.1097/00006534-198912000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Chang DW, Wang B, Robb GL, et al. Effect of Obesity on Flap and Donor-Site Complications in Free Transverse Rectus Abdominis Myocutaneous Flap Breast Reconstruction. Plast Reconstr Surg. 2000;105:1640–1649. doi: 10.1097/00006534-200004050-00007. [DOI] [PubMed] [Google Scholar]
- 3.Moran SL, Serletti JM. Outcome comparison between free and pedicled TRAM flap breast reconstruction in the obese patient. Plast Reconstr Surg. 2001;108:1954–1960. doi: 10.1097/00006534-200112000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Garvey PB, Buchel EW, Pocakj BA, Gray RJ, Samson TD. The deep inferior epigastric perforator flap for breast reconstruction in overweight and obese patients. Plast Reconstr Surg. 2005;115:447–457. doi: 10.1097/01.prs.0000149588.09148.53. [DOI] [PubMed] [Google Scholar]
- 5.Spear SL, Ducic ID, Cuoco F, Taylor N. Effect of obesity on flap and donor-site complications in pedicled TRAM flap breast reconstruction. Plast Reconstr Surg. 2007;119:788–795. doi: 10.1097/01.prs.0000252003.14537.d2. [DOI] [PubMed] [Google Scholar]
- 6.Vyas RM, Dickman BP, Fastekjian JH, Watson JP, DaLio AL, Crisera CA. Risk factors for abdominal donor-site morbidity in free flap breast reconstruction. Plast Reconstr Surg. 2008;121:1519–1526. doi: 10.1097/PRS.0b013e31816b1458. [DOI] [PubMed] [Google Scholar]
- 7.Chen CL, Shore AD, Johns R, Clark JM, Manahan M, Makary MA. The Impact of Obesity on Breast Surgery Complications. Plast Reconstr Surg. 2011;128:395e–403e. doi: 10.1097/PRS.0b013e3182284c05. [DOI] [PubMed] [Google Scholar]
- 8.Jandali S, Nelson JA, Sonnad SS, et al. Breast reconstruction with free tissue transfer from the abdomen in the morbidly obese. Plast Reconstr Surg. 2011;127:2206–2213. doi: 10.1097/PRS.0b013e3182131c93. [DOI] [PubMed] [Google Scholar]
- 9.Alderman AK, Wilkins EG, Kim HM, Lowery JC. Complications in postmastectomy breast reconstruction: Two-year results of the Michigan breast reconstruction outcome study. Plast Reconstr Surg. 2002;109:2265–2275. doi: 10.1097/00006534-200206000-00015. [DOI] [PubMed] [Google Scholar]
- 10.McCarthy CM, Mehrara BJ, Riedel E, et al. Predicting complications following expander / implant breast reconstruction: an outcomes analysis based on preoperative clinical risk. Plast Reconstr Surg. 2008;121:1886–1893. doi: 10.1097/PRS.0b013e31817151c4. [DOI] [PubMed] [Google Scholar]
- 11.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 12.Chun YS, Schwartz MA, Gu X, Lipsitz SR, Carty MJ. Body mass index as a predictor of postoperative complications in reduction mammaplasty. Plast Reconstr Surg. 2012;129:228e–234e. doi: 10.1097/PRS.0b013e31823ae949. [DOI] [PubMed] [Google Scholar]
- 13.WHO. WHO Technical Report Series 854. Geneva: World Health Organization; 1995. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. [PubMed] [Google Scholar]
- 14.WHO. WHO Technical Report Series 894. Geneva: World Health Organization; 2000. Obesity: preventing and managing the global epidemic. Report of a WHO Consultation. [PubMed] [Google Scholar]
- 15.WHO. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 16.Prasad US, Walker WS, Sang CTM, Campanella C, Cameron EWJ. Influence of obesity on the early and long term results of surgery for coronary artery disease. Eur J Cardiothorac Surg. 1991;5:67–73. doi: 10.1016/1010-7940(91)90003-3. [DOI] [PubMed] [Google Scholar]
- 17.Choban PS, Flancbaum L. The impact of obesity on surgical outcomes: a review. JACS. 1997;185:593–603. doi: 10.1016/s1072-7515(97)00109-9. [DOI] [PubMed] [Google Scholar]
- 18.Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Arch Intern Med. 1998;158:1855–1867. doi: 10.1001/archinte.158.17.1855. [DOI] [PubMed] [Google Scholar]
- 19.Benoist S, Panis Y, Alves A, Valleur P. Impact of obesity on surgical outcomes after colorectal resection. Am J Surg. 2000;179:275–281. doi: 10.1016/s0002-9610(00)00337-8. [DOI] [PubMed] [Google Scholar]
- 20.Francel TJ, Ryan JJ, Manson PN. Breast reconstruction utilizing implants: a local experience and comparison of three techniques. Plast Reconstr Surg. 1993;92:786–795. [PubMed] [Google Scholar]
- 21.Kroll SS, Baldwin B. A comparison of outcomes using three different methods of breast reconstruction. Plast Reconstr Surg. 1992;90:455–463. doi: 10.1097/00006534-199209000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Nahabedian MY, Dooley W, Singh N, Manson PM. Contour abnormalities of the abdomen after breast reconstruction with abdominal flaps: The role of muscle preservation. Plast Reconstr Surg. 2002;109:91–101. doi: 10.1097/00006534-200201000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Nahabedian MY, Momen B, Galdino G, Manson PN. Breast reconstruction with the free TRAM or DIEP flap: patient selection, choice of flap, and outcome. Plast Reconstr Surg. 2002;110:466–475. doi: 10.1097/00006534-200208000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Nahabedian MY, Tsangaris T, Momen B. Breast reconstruction with the DIEP flap or the muscle-sparing (MS-2) free TRAM flap: Is there a difference? Plast Reconstr Surg. 2005;115:436–444. doi: 10.1097/01.prs.0000149404.57087.8e. [DOI] [PubMed] [Google Scholar]
- 25.Kronowitz SJ, Hunt KK, Kuerer HM, et al. Delayed-immediate breast reconstruction. Plast Reconstr Surg. 2004;113:1617–1628. doi: 10.1097/01.prs.0000117192.54945.88. [DOI] [PubMed] [Google Scholar]
- 26.Kronowitz SJ. Immediate versus delayed reconstruction. Clin Plast Surg. 2007;34:39–50. doi: 10.1016/j.cps.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Kronowitz SJ. Delayed-immediate breast reconstruction: technical and timing considerations. Plast Reconstr Surg. 2010;125:463–474. doi: 10.1097/PRS.0b013e3181c82d58. [DOI] [PubMed] [Google Scholar]
- 28.Kronowitz SJ, Lam C, Terefe W, et al. A multidisciplinary protocol for planned skin-preserving delayed breast reconstruction for patients with locally advanced breast cancer requiring postmastectomy radiation therapy: 3-year follow-up. Plast Reconstr Surg. 2011;127:2154–2166. doi: 10.1097/PRS.0b013e3182131b8e. [DOI] [PubMed] [Google Scholar]
- 29.Chang DW, Barnea Y, Robb GL. Effects of an autologous flap combined with an implant for breast reconstruction: an evaluation of 1000 consecutive reconstructions of previously irradiated breasts. Plast Reconstr Surg. 2008;122:356–363. doi: 10.1097/PRS.0b013e31817d6303. [DOI] [PubMed] [Google Scholar]
- 30.Garvey PB, Salavati S, Feng L, Butler CE. Abdominal donor-site outcomes for medial versus lateral deep inferior epigastric artery branch perforator harvest. Plast Reconstr Surg. 2011;127:2198–2206. doi: 10.1097/PRS.0b013e3182131caf. [DOI] [PubMed] [Google Scholar]
- 31.Garvey PB, Salavait S, Feng L, Butler CE. Perfusion-related complications are similar for DIEP and muscle-sparing free TRAM flaps harvested on medial or lateral deep inferior epigastric artery branch perforators for breast reconstruction. Plast Reconstr Surg. 2011;128:581e–589e. doi: 10.1097/PRS.0b013e318230c122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Belle G. Biostatistics: a methodology for the health sciences. 2nd edition Ed. Hoboken: Wiley-Interscience; 2004. [Google Scholar]
- 33.Edsander-Nord A, Brandberg Y, Wickman M. Quality of life, patients' satisfaction, and aesthetic outcome after pedicled or free TRAM flap breast surgery. Plast Reconstr Surg. 2001;107:1142–1154. doi: 10.1097/00006534-200104150-00007. [DOI] [PubMed] [Google Scholar]
- 34.Gopie JP, Timman R, Hilhorst MT, Hofer SOP, Mureau MAM, Tibben A. The short-term psychological impact of complications after breast reconstruction. Psycho-Oncology. doi: 10.1002/pon.2089. Epub 2011 Oct 28. [DOI] [PubMed] [Google Scholar]
- 35.Potter S, Thompson RJ, Hopwood P, Winters ZE. Health-related quality of life assessment after breast reconstruction. Br J Surg. 2009;96:613–620. doi: 10.1002/bjs.6605. [DOI] [PubMed] [Google Scholar]
- 36.Spector DJ, Mayer DK, Knafl K, Pusic A. Women's recovery experiences after breast cancer reconstruction surgery. J Psychosoc Oncol. 2011;29:664–676. doi: 10.1080/07347332.2011.615384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhong T, McCarthy C, Sandar Min, et al. Patient satisfaction and health-related quality of life after autologous tissue breast reconstruction. Cancer. doi: 10.1002/cncr.26417. Epub 2011 Oct 24. [DOI] [PubMed] [Google Scholar]
- 38.Slavin SA, Schnitt SJ, Duda RB, et al. Skin-sparing mastectomy and immediate reconstruction: oncologic risks and aesthetic results in patients with early-stage breast cancer. Plast Reconstr Surg. 1998;102:49–62. doi: 10.1097/00006534-199807000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Disa JJ, Ad-El DD, Cohen SM, Cordeiro PG, Hidalgo DA. The premature removal of tissue expanders in breast reconstruction. Plast Reconstr Surg. 1999;104:1662–1665. doi: 10.1097/00006534-199911000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Cunningham B. The Mentor Core study on silicone MemoryGel breast implants. Plast Reconstr Surg. 2007;120:19S–30S. doi: 10.1097/01.prs.0000286574.88752.04. [DOI] [PubMed] [Google Scholar]
- 41.Cunningham B. The Mentor study on Contour Profile Gel silicone MemoryGel breast implants. Plast Reconstr Surg. 2007;120:33S–39S. doi: 10.1097/01.prs.0000286665.91043.bc. [DOI] [PubMed] [Google Scholar]
- 42.Spear SL, Howard MA, Boehmler JH, Ducic I, Low M, Abbruzzesse MR. The infected or exposed breast implant: management and treatment strategies. Plast Reconstr Surg. 2004;113:1634–1645. doi: 10.1097/01.prs.0000117194.21748.02. [DOI] [PubMed] [Google Scholar]
- 43.Spear SL, Rottman SJ, Seiboth LA, Hannan CM. Breast reconstruction using a staged nipple-sparing mastectomy following mastopexy or reduction. Plast Reconstr Surg. 2012;129:572–581. doi: 10.1097/PRS.0b013e318241285c. [DOI] [PubMed] [Google Scholar]
- 44.Bonomi S, Salval A, Settembrini F, Gregorelli C, Musumarra G. Autologous latissimus dorsi flap as an alternative to free abdomen-based flap for breast reconstruction in the morbidly obese. Plast Reconstr Surg. 2012;129:357e–358e. doi: 10.1097/PRS.0b013e31823af0e5. [DOI] [PubMed] [Google Scholar]
- 45.Waisbren E, Rosen H, Bader AM, Lipsitz SR, Rogers SO, Eriksson E. Percent body fat and prediction of surgical site infection. JACS. 2010;210:381–389. doi: 10.1016/j.jamcollsurg.2010.01.004. [DOI] [PubMed] [Google Scholar]