Abstract
OBJECTIVE
To test the idea that a pause (~3-min) in the delivery of shock waves (SW) soon after the initiation of treatment is unnecessary for achieving a reduction in renal injury, if treatment is begun at a low power setting that generates low-amplitude SWs.
MATERIALS AND METHODS
Anesthetized female pigs were assigned to one of three SWL treatment protocols that did not involve a pause in SW delivery of more than 10 seconds (2000 SWs at 24 kV; 100 SWs at 12 kV + ~10-sec pause + 2000 SWs at 24 kV; 500 SWs at 12 kV + ~10-sec pause + 2000 SWs at 24 kV; all SWs delivered at 120 SWs/min using an unmodified Dornier HM3 lithotripter).
Renal function was measured before and after SWL.
The kidneys were then processed for quantification of the SWL-induced hemorrhagic lesion. Values for lesion size were compared to previous data collected from pigs in which treatment included a 3-min pause in SW delivery.
RESULTS
All SWL treatment protocols produced a similar degree of vasoconstriction (23–41% reduction in GFR and ERPF) in the SW-treated kidney.
The mean renal lesion in pigs treated with 100 low-amplitude SWs delivered before the main dose of 2000 high-amplitude SWs (2.27% FRV) was statistically similar to that measured for pigs treated with 2000 SWs all at high-amplitude (3.29% FRV). However, pigs treated with 500 low-amplitude SWs before the main SW dose had a significantly smaller lesion (0.44% FRV) that was comparable to the lesion in pigs from a previous study in which there was a 3-min pause in treatment separating a smaller initial dose of 100 low-amplitude SWs from the main dose of 2000 high-amplitude SWs (0.46% FRV). Time between the initiation of the low- and high-amplitude SWs was ~4-min for these latter two groups compared to ~1-min when there was negligible pause after the initial 100 low-amplitude SWs in the protocol.
CONCLUSIONS
Pig kidneys treated by SWL using a 2-step low-to-high power ramping protocol were protected from injury with negligible pause between steps, provided the time between the initiation of low-amplitude SWs and switching to high-amplitude SWs was ~4-min.
Comparison with results from previous studies shows that protection can be achieved using various step-wise treatment scenarios in which either the initial dose of SWs is delivered at low-amplitude for ~4-min, or there is a definitive pause before resuming SW treatment at higher amplitude.
Thus, we conclude that renal protection can be achieved without instituting a pause in SWL treatment. It remains prudent to consider that renal protection depends on the acoustic and temporal properties of SWs administered at the beginning stages of a SWL ramping protocol, and that this may differ according to the lithotripter at hand.
Keywords: kidney, lithotripsy, swine, tissue injury
INTRODUCTION
An undesirable side effect of SWL treatment is that SWs can injure renal and surrounding tissue [1]. The primary acute lesion is vascular trauma with breakage of blood vessels and pooling of blood within the parenchyma, which if extending to the kidney surface will result in subcapsular or perirenal hematomas [1]. Along with the vascular insult, there is damage to tubules and the production and release of proinflammatory cytokines and injurious agents (e.g. iron/reactive oxygen metabolites; vasoconstrictor peptides/ischemia; metabolic toxins) that can result in fibrosis and the loss of functional tissue [1,2]. Such SW-induced injury has been linked to adverse outcomes such as hypertension, diabetes and exacerbation of kidney stone disease [3–5]. This raises concern about the long-term safety of SWL, and developing SWL treatment strategies that reduce or prevent tissue injury would certainly help mitigate such concerns.
One approach to reduce SWL-induced tissue injury has been to alter the manner in which SWs are delivered to the kidney [2,6], and in this regard we have reported that treatment of the pig kidney with low-amplitude SWs followed by a 3-min pause in treatment prior to applying high-amplitude SWs will reduce SWL-induced hemorrhagic lesion sizes by as much as 20-fold [7]. In fact, similar protocols in which low-amplitude SWs were substituted with a relatively small number of higher-amplitude SWs were also shown to reduce SW-induced tissue damage, implicating the 3-min pause in treatment to be a critical factor in the development of the renal protective response [8].
On the other hand, some clinical centers begin SWL treatment at a low power setting to condition the patient to treatment-related discomfort and then gradually ramp up to higher levels with continuous delivery of SWs. That is, there is typically no pause in treatment during the lithotripsy session [9–12]. It is unclear even with power ramping if continuous delivery of SWs can be used to protect the kidney from injury. Therefore, we sought to determine in our pig model, using a 2-step ramping protocol, whether a definitive pause in SW delivery is needed in order to protect the kidney from SWL-related tissue damage.
METHODS AND MATERIALS
All animal studies were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committees of Indiana University School of Medicine and Methodist Hospital. Juvenile female farm pigs at 7–8 weeks of age (12–18 kg body wt., Hardin Farms, Danville, IN) were anesthetized [induction with ketamine (20 mg/kg) and xylazine (2 mg/kg); maintenance with 1–3% isoflurane] and prepared for renal function assessment as previously described [13]. In brief, inulin and para-amino hippuric acid were intravenously infused into the pig and blood samples taken from a catheter inserted into the femoral artery, and timed urine collections from catheters inserted into both ureters. Colorimetric assays were employed to measure the renal clearance of inulin and para-amino hippuric acid in order to calculate GFR and ERPF, respectively [13]. Renal function measurements were taken before SWL and at 1 hour and 4.5 hours after SW treatment. At the conclusion of the post-SWL blood and urine sampling, the kidneys were perfusion fixed in situ with 2.5% gluteraldehyde, harvested and processed for quantification of tissue hemorrhagic lesion size and for light microscopy [13].
SWL treatment was carried out using an unmodified Dornier HM3 lithotripter (Dornier Medical Systems, Kennesaw, GA, USA) delivering SWs (120 SWs/min) targeted to the lower pole of the left kidney. Pigs were assigned to receive either 100 SWs or 500 SWs at 12 kV followed by 2000 SWs at 24 kV. For these animals there was a very short pause (~10-sec) in treatment while adjusting the lithotripter from 12 kV to 24 kV. A third group of pigs was treated with 2000 SWs all at 24 kV. This latter group (n=9) builds upon a core set of seven animals from a previously published study [8]. Renal lesion size data for these three groups are compared to previously reported lesion data for pigs treated in two steps (12 kV to 24 kV, 18 kV to 24 kV, 24 kV to 24 kV) in which there was a 3-min pause between the steps [7,8]. In all groups, treatment with 2000 SWs at 24 kV was paused every 500 SWs for X-ray verification of targeting on the kidney lower pole (~30-sec) and after 1000 SWs to replace the electrode and check targeting (~1-min).
STATISTICS
Comparisons of cardiovascular/renal function within (change from baseline at 1 hour and 4.5 hours post-SWL) and across groups were done using paired and two-sample t-tests. Kruskal-Wallis ANOVA (a nonparametric method for non-normally distributed data) was used to compare the renal lesion sizes of all groups. Given the significant result from Kruskal-Wallis ANOVA, an independent two-sample t-test was used to compare the mean lesion sizes and Mood’s median test was used to compare the median lesion sizes between each pair of groups. Differences were considered significant if two-tailed P<0.05, and data were presented as the mean ± SD. All analyses were performed using SAS 9.2 (SAS Institute, Cary, NC).
RESULTS
Body and kidney weights were comparable for all groups and averaged 14.5 kg and 68.5 g, respectively (Table 1).
TABLE 1.
Weights, cardiovascular and renal function.
| Pre-SWL | 1 h post-SWL | 4.5 h post-SWL | |
|---|---|---|---|
| Body Weight (kg) | |||
| 2000 SWs at 24 kV (n=9) | 14.3 ± 1.6 | ||
| 100 SWs at 12 kV + 2000 SWs at 24 kV (n=8) | 14.8 ± 1.9 | ||
| 500 SWs at 12 kV + 2000 SWs at 24 kV (n=10) | 14.5 ± 1.6 | ||
| P value | 0.83 | ||
| Kidney Weight (g) | |||
| 2000 SWs at 24 kV (n=9) | 67.8 ± 9.9 | ||
| 100 SWs at 12 kV + 2000 SWs at 24 kV (n=8) | 73.1 ± 8.3 | ||
| 500 SWs at 12 kV + 2000 SWs at 24 kV (n=10) | 65.6 ± 10.2 | ||
| P value | 0.27 | ||
| MAP (mm Hg) | |||
| 2000 SWs at 24 kV (n=9) | 71.9 ± 7.5 | 66.9 ± 8.6 | 56.2 ± 7.3* |
| 100 SWs at 12 kV + 2000 SWs at 24 kV (n=8) | 69.8 ± 4.8 | 66.1 ± 4.1 | 62.2 ± 5.6* |
| 500 SWs at 12 kV + 2000 SWs at 24 kV (n=10) | 71.8 ± 13.3 | 68.4 ± 7.1 | 64.5 ± 8.8* |
| P value | 0.88 | 0.78 | 0.07 |
| GFR (ml/min) | |||
| 2000 SWs at 24 kV (n=9) | 9.1 ± 2.9 | 5.8 ± 2.5* | 7.9 ± 3.3 |
| 100 SWs at 12 kV + 2000 SWs at 24 kV (n=8) | 9.4 ± 1.4 | 7.1 ± 1.7* | 7.4 ± 1.2* |
| 500 SWs at 12 kV + 2000 SWs at 24 kV (n=10) | 8.8 ± 1.7 | 6.2 ± 1.9* | 7.6 ± 2.3 |
| P value | 0.84 | 0.43 | 0.91 |
| ERPF (ml/min) | |||
| 2000 SWs at 24 kV (n=9) | 47.6 ± 19.5 | 28.5 ± 16.5* | 31.9 ± 16.8* |
| 100 SWs at 12 kV + 2000 SWs at 24 kV (n=8) | 52.3 ± 13.2 | 36.1 ± 13.8* | 36.9 ± 10.8* |
| 500 SWs at 12 kV + 2000 SWs at 24 kV (n=10) | 43.1 ± 9.0 | 29.7 ± 10.3* | 39.2 ± 20.4 |
| P value | 0.42 | 0.48 | 0.64 |
Data are shown as mean ± SD. The P value was derived from group analysis;
P<0.05 from pre-SWL value within a group.
Function
Blood pressure and renal function were similar in all groups at baseline and following SWL (Table 1). Although there was a significant fall in MAP (6–16 mmHg) within each group of animals throughout the time course of the experiment, similar changes in MAP have been observed in sham (time control) animals and, consequently, are not related to SWL [13]. The SW-treated kidneys of all groups demonstrated a similar fall in GFR (~30%) and ERPF (~35%) at 1 hour after SWL, with variable degrees of recovery at 4.5 hours post-treatment (Table 1 and Figure 1).
Figure 1.
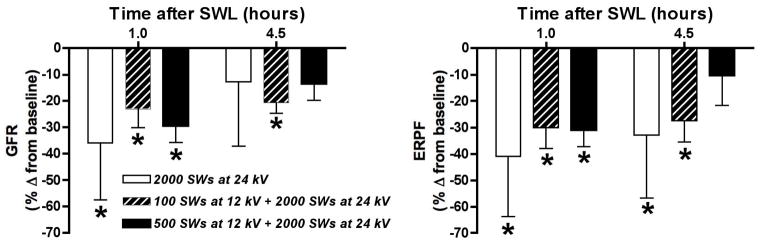
Shown are renal filtration (GFR, left panel) and perfusion (ERPF, right panel) responses to SWL in pigs treated with only the main dose of 2000 high-amplitude (24 kV) SWs, 100 low-amplitude (12 kV) SWs followed 10 seconds later by the main SW dose, or 500 low-amplitude (12 kV) SWs followed 10 seconds later by the main SW dose. Data are shown as mean ± SD; * = P<0.05 from pre-SWL values.
Gross morphology and tissue histology
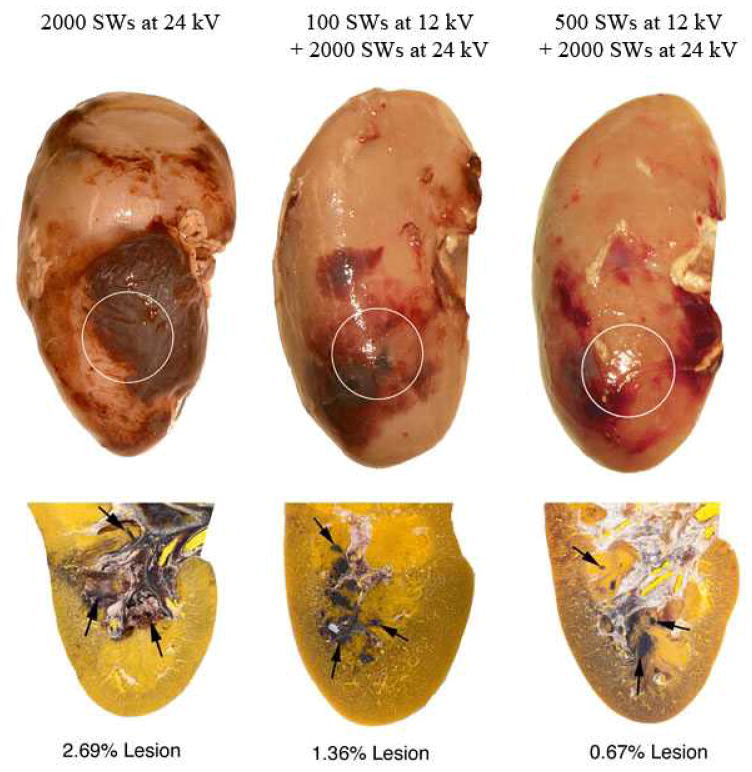
Figure 2 shows examples of the gross morphology of post-SWL treated kidneys showing the presence of subcapsular hematomas in pigs treated with the main dose of 2000 SWs all at high-amplitude (9 of 9 kidneys), 100 low-amplitude SWs plus the main SW dose (7 of 8 kidneys) and 500 low-amplitude SWs plus the main SW dose (6 of 8 kidneys). Histologic examination of all SW-treated kidneys revealed multiple, small, focal hemorrhagic lesions that were largely localized to one or more papillae in the region targeted by the focal volume of the lithotripter (Figure 2). Some kidneys had additional areas of intraparenchymal bleeding that involved the cortex. In a few kidney sections, damage extending from the medulla to the surface could be traced and likely accounted for the presence of subcapsular hematomas.
Figure 2.
Paired images show the posterior surface and a representative histology section of kidneys from different treatment protocols. The quantified hemorrhagic lesion size is shown below each paired image. Circles mark targeting of the focal zone to the lower renal pole. Arrowheads point to focal sites of hemorrhage within the parenchyma of several renal papillae located within and surrounding the lithotripter’s targeting zone.
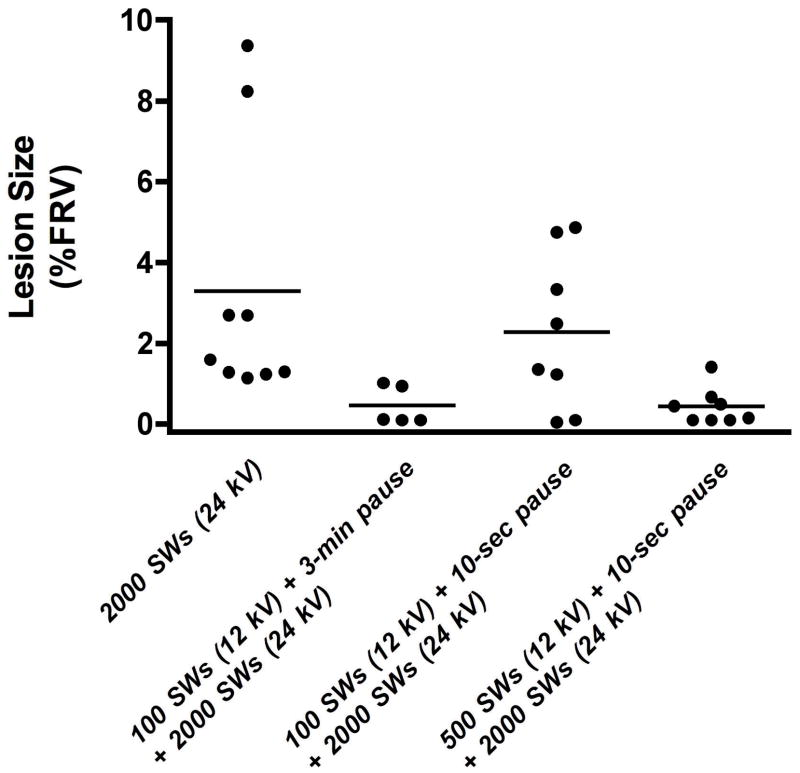
Table 2 and figure 3 show data related to quantification of the SWL-induced hemorrhagic lesion volume of the three groups, and for comparison purposes the renal lesion data from a previously published group of pigs are included in which a 3-min pause in treatment separated the initial 100 low-amplitude (12 kV) SWs from the main dose of 2000 high-amplitude (24 kV) SWs [7]. Mean lesion size was greatest for kidneys treated with the main dose of 2000 high-amplitude SWs. Treatment with 100 SWs at low amplitude followed by a 3-min pause preceding delivery of the main SW dose at high amplitude dramatically reduced lesion size by ~7-fold, but shortening the pause to just 10-sec failed to protect the kidney from injury. However, treatment with a larger dose of low amplitude SWs (500 SWs, 12 kV) immediately prior to the main SW dose significantly reduced the size of the lesion.
Table 2.
Renal lesion data (% FRV)
| Treatment | N | Minimum | Maximum | Mean | Median | SD | P value (vs. Group 1) | P value (vs. Group 2) | P value (vs. Group 3) | P value (vs. Group 4) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2000 SWs at 24 kV (Group 1) | 9 | 1.15 | 9.37 | 3.29 | 1.60 | 3.20 | - | 0.03 | 0.45 | 0.03 |
| 100 SWs at 12 kV + 3-min pause + 2000 SWs at 24 kV (Group 2) | 5 | 0.10 | 1.02 | 0.46 | 0.12 | 0.48 | 0.03 | - | 0.03 | 0.94 |
| 100 SWs at 12 kV + 10-sec pause + 2000 SWs at 24 kV (Group 3) | 8 | 0.05 | 4.87 | 2.27 | 1.93 | 1.91 | 0.45 | 0.03 | - | 0.03 |
| 500 SWs at 12 kV + 10-sec pause + 2000 SWs at 24 kV (Group 4) | 8 | 0.10 | 1.42 | 0.44 | 0.30 | 0.45 | 0.03 | 0.94 | 0.03 | - |
P values were derived from the comparison of mean lesion data. Mood’s median test of the median lesion data gave similar results with Group 3 vs. Group 4 being borderline significant (P=0.0528). Group 2 data was from a previously published study (ref. 7) that was carried out at our facility using the same lithotripter and pigs of the same size as the present experiments.
Figure 3.
Individual and mean (denoted by horizontal bar) renal hemorrhagic lesion sizes for each group of pigs undergoing SWL. Mean lesion size was greatest for kidneys treated only with the main dose of SWs (2000 SWs, 24 kV). Treatment with 100 SWs at low amplitude (12 kV) followed by a 3-min pause preceding delivery of the main SW dose at high amplitude (2000 SWs, 24 kV) dramatically reduced lesion size, but shortening the pause to just 10-sec failed to protect from injury. However, treatment with a larger dose of low amplitude SWs (500 SWs, 12 kV) immediately prior to the main dose significantly reduced the size of the lesion. Data for treatment using a 3-min pause comes from a previous study (ref. 7).
Figure 4 illustrates various two-step SW ramping treatment protocols that have been performed on pigs at our facility. Renal protection was apparent when either low-amplitude (12 kV) SWs were delivered continuously over ~4-min before switching to high-amplitude (24 kV) SW treatment, or there was a definitive pause between initial treatment with 100 SWs (12 kV, 18 kV or 24 kV) and resuming SW treatment at high-amplitude. All treatment groups that demonstrated renal protection from SW-induced injury had ~4-min interval between initiation of the two separate steps of SW treatment, whereas the protective response was lost upon reducing this time interval to ~1-min.
Figure 4.
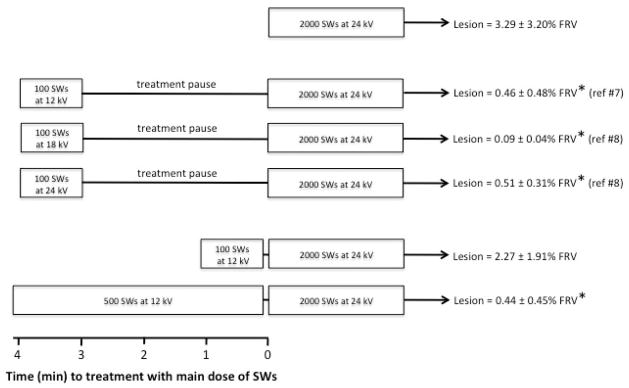
SWL protocol timelines illustrating the various step-wise treatment scenarios associated with renal protection. Lesion size was greatest for kidneys treated only with the main dose of SWs (2000 SWs at 24 kV). Interrupting treatment with a 3 min pause significantly reduced lesion size regardless of the voltage setting of an initial dose of 100 SWs (12 kV, 18 kV, 24 kV) given before the main SW dose. Adding a very short (~10-sec) pause between the initial dose (100 SWs, 12 kV) and main SW dose was not effective in reducing injury. However, extending the initial dose so that the duration of treatment at the low voltage setting (12 kV) was ~4-min (500 SWs, 120 SWs/min, 4.2 min) resulted in a significant reduction in lesion size. A common finding in all treatment protocols demonstrating renal protection was that the time between initiating the first set of SWs and the onset of the main SW dose was ~4-min. An asterisk (*) indicates that the size of the renal lesion is significantly (P<0.05) different from that measured in pigs treated with 2000 SWs all at 24 kV.
DISCUSSION
We have previously reported that delivery of 500 or 100 low-amplitude (12 kV) SWs to the pig kidney followed by a 3-min pause in treatment and then the administration of 2000 high-amplitude (24 kV) SWs dramatically reduced (~20-fold) the size of the resulting tissue hemorrhagic lesion compared to SWL with 2000 high-amplitude SWs alone [7]. Further studies demonstrated that the protective response to such SWL treatment protocols occurred even if the initial SW dose was 100 SWs at 18 kV or 24 kV [8]—that is, injury was reduced regardless of the amplitude of the initial SW dose as long as a 3-min pause was part of the protocol. Together, this implicated the 3-min pause, and not the amplitude of the initial dose, as being the critical factor in the development of the protective response. Such a conclusion is undoubtedly true when the kidney is exposed to only high-amplitude SWs since the degree of renal injury is positively correlated to SW number [1,2]. However, it is less certain whether the treatment pause is necessary for renal protection in the case of initial treatment with low-amplitude SWs. We have attempted to address this issue in the present study by removing the 3-min treatment pause in escalating SW amplitude treatment protocols that have been shown to protect the kidney from injury (see above). The renal lesion of 2.27% FRV in pigs initially treated with 100 low-amplitude SWs followed closely by 2000 high-amplitude SWs would certainly support the importance of a pause in treatment because an identical treatment protocol that included a 3-min pause during the transition from low- to high-amplitude SW delivery resulted in a 5-fold smaller tissue lesion of only 0.46% FRV. In contrast, extending the low-amplitude SW treatment to 500 SWs clearly demonstrated that the kidney was still protected from injury in the absence of a definitive pause in SW delivery (see Table 2). These new findings strongly suggest that protection from high-amplitude SW-induced injury is critically dependent upon the initial low-amplitude SW treatment conditions.
Our results also reveal that extending the period of continuous low-amplitude SW treatment from ~1-min (100 SWs, 120 SWs/min, 0.8-min) to ~4-min (500 SWs, 120 SWs/min, 4.2-min) allows the expression of the protective response. Figure 4 shows a variety of 2-step ramping SWL protocols that have been performed on pigs at our facility, and demonstrates that renal protection can be achieved by either delivering the initial dose of SWs at low-amplitude or including a definitive pause between the first SW dose and second SW treatment at high-amplitude. From the timing of the applied SWs in these experimental groups, it becomes clear that ~4-min elapsed between the initiation of the first and second set of SWs for renal protection to be apparent. That is not to say that this is the optimal time interval in a 2-step SW ramping protocol, but rather that such an interval between the two sets of SW treatment assures renal protection from tissue injury under animal study conditions done to date. Further studies are needed to identify the minimum number of SWs, minimum time interval between SW exposures, and how these factors interact in various SWL ramping protocols to allow the full expression of the renal protective response.
The present study demonstrates for the first time that a step-wise SW ramping protocol in which the initial dose of low-amplitude SWs are delivered continuously for ~4-min will initiate renal protection. This raises the real possibility that clinical SWL treatments that employ escalating SW power protocols with continuous delivery of SWs to the kidney may, at appropriate SW delivery settings, have the added benefit of reduced tissue injury. In this regard, a recent prospective, randomized clinical study by Lambert and colleagues demonstrated that renal stone patients treated continuously with SWs at increasing voltages (500 SWs at 14 kV + 1000 SWs at 16 kV + 1000 SWs at 18 kV; 60–80 SWs/min using the Doli 50 lithotripter) had less renal tissue injury than patients treated with a fixed SW voltage (2500 SWs at 18 kV; 60–80 SWs/min) [12]. Indicators of reduced renal injury were lower urinary levels of microalbumin and beta-2-microglobulin—markers of glomerular and tubule injury, respectively [12]. However, it is difficult to distinguish whether the reduced renal injury in such patients was due to the SW-voltage ramping protocol that began with 500 low-voltage SWs given over 6–8 minutes and/or the fact that 60% of the total administered SWs were at a lower voltage than the fixed SW voltage group—renal injury is greater at higher SW voltages [14,15]. Our experimental strategy of renal protection is different in that there is a two-step increase in SW voltage, with the initial treatment dose of low-amplitude SWs being added to, not substituted for, the fixed dose of high-amplitude SWs. Therefore, we are confident that the renal protection seen in our animal model is due to the initial low-amplitude SW treatment conditions.
We found no association between post-lithotripsy subcapsular hematomas or renal vasoconstriction and the degree of SWL-induced renal trauma, since nearly all pig kidneys exhibited subcapsular bleeding and a similar intensity of vasoconstriction after SW treatment, regardless of whether the measured volume of the parenchymal hemorrhagic lesion (a surrogate marker for renal tissue injury) was substantial or small. This was not altogether surprising given that subcapsular hemorrhage gives an incomplete picture of the degree of tissue injury and that renal vasoconstriction nearly always occurs after SWL irrespective of the number, strength, rate or sequence of administered SWs [1,7,10,14,16]. What is important to note is that the pre-SWL status of the renal vasculature in pigs treated with 100 or 500 low-amplitude SWs was similar (as reflected by equivalent blood pressures and renal hemodynamics), which implies that the resulting protection from tissue injury was solely related to extending low-amplitude SWs treatment from ~1-min to ~4-min.
The mechanism(s) involved in initiating the renal protective response when employing a two-step, SW amplitude ramping strategy is unknown, but we have shown that pretreatment of the kidney with 500 low-amplitude SWs and a 3-min pause in SW delivery will elicit a more robust vasoconstriction (Doppler measurements of resistive index in a blood vessel within the zone of SW treatment) during the subsequent application of 2000 high-amplitude SWs than without pretreatment [17]. This enhanced renal vasoconstriction occurs at a time when tissue injury would normally be detectable—that is, between 1000 to 2000 high-amplitude SWs [18]. A constricted blood vessel would be stiffer and likely be less susceptible to rupture by SW forces and there would be less bleeding within the parenchyma to support SW-induced cavitation activity—such factors could contribute to protecting the kidney from the damaging effects of SWL (discussed in ref. 17). If vasoconstriction is the mechanism of protection, then perhaps there must be sufficient time for the initial low-amplitude SW treatment to sensitize the renal vasculature and/or vasoconstrictor system(s) (e.g. neural, endocrine, paracrine, autocrine) for a robust renal vasoconstriction to occur upon subsequent high-amplitude SWL.
Acute injury to the microvasculature of the kidney and surrounding organs has been reported after SWL treatment, and in some cases can be severe [1]. There is debate as to whether such acute injury may have long-term adverse effects such as hypertension and diabetes [3,5,19]; or that multiple lithotripsies may drive the formation of brushite stone disease [4]. Considerable research has been focused on SWL treatment strategies to reduce these acute and chronic injurious complications of SWL, which have included pharmacological therapies (e.g. calcium channel lockers, anti-inflammatory agents, antioxidants) [2,20], and altering the manner in which SWs are applied (e.g. reductions in SW number, SW amplitude or SW rate) [2,6,21–24]. Our research group has reported that a two-step low- to high-amplitude SW treatment strategy with [7,8] or without (present study) a 3-min pause in the treatment protocol—or a SWL treatment that slows the rate at which SWs are delivered to the kidney [25]—can be an effective means to reduce acute tissue injury in animals. What is not known is whether a similar benefit also occurs in patients. Regardless, both SWL treatment strategies are being used in the clinic because 1) patients are more easily acclimatized to treatment when initially given low-voltage SWs, and 2) both escalating SW voltage and slow SW rate treatment strategies have been shown independently to enhance the efficiency of stone breakage and improve patient stone free rates [9,11,12,21–24,26–28]. Interestingly, Yong and colleagues recently reported that a combined approach of increasing SW amplitude at slow SW rate produced the best stone fragmentation efficiency in vitro [28], and presumably such a SWL treatment strategy would also lead to less tissue injury in vivo. Therefore, a major goal of SWL research continues to be optimization of SWL treatment protocols to achieve superior stone comminution with minimal tissue injury.
There remain many unknowns regarding escalating SW-amplitude treatment protocols. It is not know if such protection from tissue injury occurs in patients and whether the protective response will occur in lithotripters other than the Dornier HM3. The mechanism(s) responsible for the protection from SW-induced tissue injury is unknown, although we have shown that there is an association with renal vasoconstriction [17]. Some progress has been made in identifying requirements for the protective response to develop, which is dependent on the initial low-amplitude SW treatment settings [7,8, present study]. Herein, we report that 500 low-amplitude SWs given continuously for ~4-min prior to increasing SW amplitude can elicit renal protection. However, it is important to recognize that within the lithotripsy community there is ambiguity as to what constitutes low-amplitude SW treatment. The 12 kV pulses from the HM3 lithotripter generate relatively low pressures of ~25 MPa [29], and it remains to be determined whether an equivalent acoustic pressure on a different lithotripter gives comparable protection. These are some of the issues that need to be addressed to help ascertain whether treating patients with SWL ramping protocols will be a valuable strategy in the urologist’s arsenal for managing stone patients in a safer and more efficient manner.
Acknowledgments
Cynthia D. Johnson and Philip M. Blomgren assisted with the study. This work was supported by PHS grant P01-DK43881.
Abbreviations and Acronyms
- SWL
Shock wave lithotripsy
- SW
shock wave
- GFR
glomerular filtration rate
- ERPF
effective renal plasma flow
- FRV
functional renal volume
- kV
kilovolts
- MAP
mean arterial pressure
- HR
heart rate
- ANOVA
analysis of variance
- SD
standard deviation
References
- 1.Evan AP, Willis LR. Extracorporeal shock wave lithotripsy: complications. In: Smith AD, Badlani GH, Badley DH, et al., editors. Smith’s Textbook on Endourology. Hamilton, Ontario, Canada: BC Decker; 2007. pp. 353–365. [Google Scholar]
- 2.Handa RK, Evan AP. A chronic outcome of shock wave lithotripsy is parenchymal fibrosis. Urol Res. 2010;38:301–5. doi: 10.1007/s00240-010-0297-y. [DOI] [PubMed] [Google Scholar]
- 3.Janetschek G, Frauscher F, Knapp R, Hofle G, Perschel G, Bartsch G. New onset hypertension after extracorporeal shock wave lithotripsy: age related incidence and prediction by intrarenal resistive index. J Urol. 1997;158:346–51. doi: 10.1016/s0022-5347(01)64475-6. [DOI] [PubMed] [Google Scholar]
- 4.Parks JH, Worcester EM, Coe FL, Evan AP, Lingeman JE. Clinical implications of abundant calcium phosphate in routinely analyzed kidney stones. Kidney Int. 2004;66:777–85. doi: 10.1111/j.1523-1755.2004.00803.x. [DOI] [PubMed] [Google Scholar]
- 5.Krambeck AE, Gettman MT, Rohlinger AL, Lohse CM, Patterson DE, Segura JW. Diabetes mellitus and hypertension associated with shock wave lithotripsy of renal and proximal ureteral stones at 19 years of follow up. J Urol. 2006;175:1742–7. doi: 10.1016/S0022-5347(05)00989-4. [DOI] [PubMed] [Google Scholar]
- 6.Lingeman JE, McAteer JA, Gnessin E, Evan AP. Shock wave lithotripsy: advances in technology and technique. Nat Rev Urol. 2009;6:660–70. doi: 10.1038/nrurol.2009.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willis LR, Evan AP, Connors BA, Handa RK, Blomgren PM, Lingeman JE. Prevention of lithotripsy-induced renal injury by pretreating kidneys with low-energy shock waves. JASN. 2006;17:663–73. doi: 10.1681/ASN.2005060634. [DOI] [PubMed] [Google Scholar]
- 8.Connors BA, Evan AP, Blomgren PM, Handa RK, Willis LR, Gao S. Effect of initial shock wave voltage on shock wave lithotripsy-induced lesion size during step-wise voltage ramping. BJU Int. 2009;103:104–7. doi: 10.1111/j.1464-410X.2008.07922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demirci D, Sofikerim M, Yalcin E, Ekmekcioglu O, Gulmez I, Karacagil M. Comparison of conventional and step-wise shockwave lithotripsy in management of urinary calculi. J Endourol. 2007;21:1407–10. doi: 10.1089/end.2006.0399. [DOI] [PubMed] [Google Scholar]
- 10.Mitterberger M, Pinggera GM, Neururer R, et al. Multimodal evaluation of renal perfusional changes due to extracorporeal shock wave lithotripsy. BJU Int. 2008;101:731–5. doi: 10.1111/j.1464-410X.2007.07281.x. [DOI] [PubMed] [Google Scholar]
- 11.Honey RJD, Ray AA, Ghiculete D, Pace KT. Shock wave lithotripsy: A randomized, double-blind trial to compare immediate versus delayed voltage escalation. Urol. 2010;75:38–44. doi: 10.1016/j.urology.2008.12.070. [DOI] [PubMed] [Google Scholar]
- 12.Lambert EH, Walsh R, Moreno MW, Gupta M. Effect of escalating versus fixed voltage treatment on stone comminution and renal injury during extracorporeal shock wave lithotripsy: A prospective randomized trial. J Urol. 2010;183:580–4. doi: 10.1016/j.juro.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 13.Willis LR, Evan AP, Connors BA, Blomgren P, Fineberg NS, Lingeman JE. Relationship between kidney size, renal injury, and renal impairment induced by shock wave lithotripsy. JASN. 1999;10:1753–62. doi: 10.1681/ASN.V1081753. [DOI] [PubMed] [Google Scholar]
- 14.Connors BA, Evan AP, Willis LR, Blomgren PM, Lingeman JE, Fineberg NS. The effect of discharge voltage on renal injury and impairment caused by lithotripsy in the pig. JASN. 2000;11:310–8. doi: 10.1681/ASN.V112310. [DOI] [PubMed] [Google Scholar]
- 15.Rassweiler J, Kohrmann KU, Back W, et al. Experimental basis for shockwave-induced renal trauma in the model of the canine kidney. World J Urol. 1993;11:43–53. doi: 10.1007/BF00182171. [DOI] [PubMed] [Google Scholar]
- 16.Nazaroglu H, Akay AF, Bukte Y, Sahin H, Akkus Z, Bilici A. Effects of extracorporeal shock-wave lithotripsy on intrarenal resistive index. Scand J Urol Nephrol. 2003;37:408–12. doi: 10.1080/00365590310006354. [DOI] [PubMed] [Google Scholar]
- 17.Handa RK, Bailey MR, Paun M, et al. Pretreatment with low-energy shock waves induces renal vasoconstriction during standard shock wave lithotripsy (SWL): a treatment protocol known to reduce SWL-induced renal injury. J Urol. 2008;103:1270–4. doi: 10.1111/j.1464-410X.2008.08277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connors BA, Evan AP, Blomgren PM, et al. Reducing shock number dramatically decreases lesion size in a juvenile kidney model. J Endourol. 2006;20:607–11. doi: 10.1089/end.2006.20.607. [DOI] [PubMed] [Google Scholar]
- 19.Chew BH, Zavaglia B, Sutton C, et al. Twenty-year prevalence of diabetes mellitus and hypertension in patients receiving shock-wave lithotripsy for urolithiasis. BJU Int. 2011 doi: 10.1111/j.1464-410X.2011.10291.x. [DOI] [PubMed] [Google Scholar]
- 20.Sarica K, Yencilek F. Prevention of shockwave induced functional and morphological alterations: an overview. Arch Ital Urol Androl. 2008;80:27–33. [PubMed] [Google Scholar]
- 21.Madbouly K, El-Tiraifi AM, Seida M, El-Faqih SR, Atassi R, Talic RF. Slow versus fast shock wave lithotripsy rate for urolithiasis: a prospective randomized study. J Urol. 2005;173:127–30. doi: 10.1097/01.ju.0000147820.36996.86. [DOI] [PubMed] [Google Scholar]
- 22.Pace KT, Ghiculete D, Harju M. Shock wave lithotripsy at 60 or 120 shocks per minute: a randomized, double-blind trial. J Urol. 2005;174:595–9. doi: 10.1097/01.ju.0000165156.90011.95. [DOI] [PubMed] [Google Scholar]
- 23.Semins MJ, Trock BJ, Matlaga BR. The effect of shock wave rate on the outcome of shock wave lithotripsy: A meta-analysis. J Urol. 2008;179:194–7. doi: 10.1016/j.juro.2007.08.173. [DOI] [PubMed] [Google Scholar]
- 24.Honey RJD, Schuler TD, Ghiculete D, Pace KT. A randomized, double blind trial to compare shock wave frequencies of 60 and 120 shocks per minute for upper ureteral stones. J Urol. 2009;182:1418–23. doi: 10.1016/j.juro.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 25.Connors BA, Evan AP, Blomgren PM, et al. Extracorporeal shock wave lithotripsy at 60 shock waves/min reduces renal injury in a porcine model. BJU Int. 2009;104:1004–8. doi: 10.1111/j.1464-410X.2009.08520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Y, Cocks FH, Preminger GM, Zhong P. The effect of treatment strategy on stone comminution efficiency in shock wave lithotripsy. J Urol. 2004;172:349–54. doi: 10.1097/01.ju.0000132356.97888.8b. [DOI] [PubMed] [Google Scholar]
- 27.Maloney ME, Marguet CG, Zhou Y, et al. Progressive increase of lithotripter output produces better in-vivo stone comminution. J Endourol. 2006;20:603–6. doi: 10.1089/end.2006.20.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yong DZ, Lipkin ME, Simmons WN, et al. Optimization of treatment strategy used during shockwave lithotripsy to maximize stone fragmentation efficiency. J Endourol. 2011;25:1507–11. doi: 10.1089/end.2010.0732. [DOI] [PubMed] [Google Scholar]
- 29.Cleveland RO, Bailey MR, Fineberg N, et al. Design and characterization of a research electrohydraulic lithotripter patterned after the Dornier HM3. Rev Scientific Instr. 2000;71:2514–24. [Google Scholar]