Abstract
Hill’s plots of oxygen binding isotherms reveal the presence of a transition between two different oxygen affinities at the beginning and end of the isotherm. They correspond to the two conformations anticipated by the MWC model, namely the T and R conformations at the beginning and end of oxygen binding, when the lower affinity of the T form develops into the higher affinity of the R form. The difference between the binding Gibbs free energies changes of the two affinities (ΔGL) is the free energy of binding cooperativity. Notably ΔGL is positive in favor of the T form, that moves to a higher energy level upon oxygen release. Osmotic stress reveals a higher volume/surface ratio of deoxyHb, with a positive ΔGW also in favor of the T form . Increasing protein concentration shifts the isotherms to the right indicating the formation of intermediate polymeric forms. Enthalpy of the intermediates show a strong absorption of heat at the third oxygenation step due to polymers formation with quinary, and above, structures. The disassembly of intermediate polymers releases energy with a negative ΔG that compensates and allow the positivity of ΔGL. High energy polymers are the barrier preventing the relaxation of the T and R conformations into one another. The MWC allosteric model is the best justification of oxygen binding cooperativity .
Keywords: Hemoglobin function, oxygen binding cooperativity, intermediates of oxygenation, concentration dependence of oxygen affinity, osmotic stress of hemoglobin, hemoglobin polymers
Introduction
To explain the cooperativity of oxygen binding isotherms Monod et al. [1 ] proposed the presence of two Hb forms with low (T form) and high (R form) oxygen affinity. In the absence of oxygen the allosteric equilibrium constant Lo=To/Ro is in favor of To, while with increasing Hb saturation Ro becomes the favored species.
Consistent with the model, linear extrapolation of the very first and very last saturation data of Hill’s plots isotherms define the presence of two hyperbolic binding for the first and last oxygen bound to Hb [2]. They have a higher and lower P50 reflecting a lower and higher oxygen affinity respectively. It is easy to reconcile the initial low affinity (KT) with deoxyhemoglobin in the T form, and the final high affinity (KR) with oxyhemoglobin in the R form. The overall binding affinity of the isotherm is defined by the value of PO2 at 50% saturation of the isotherm (P50), or the value of Pm when available.
Thermodynamic implications of the MCW allosteric model
As mentioned above, Hill’s plots of cooperative isotherms clearly indicate the presence of two different hyperbolic oxygen affinities of hemoglobin with lower (KT) and higher (KR) oxygen affinity, respectively.
The difference between the respective binding free energy changes, ΔGT and ΔGR, is
| (1) |
where ΔGL defines the free energy change of cooperativity.
The lower affinity of the initial isotherm implies that ΔGT > ΔGR. Thus, in the difference, ΔGL has a positive value, indicating that during deoxygenation the system in the T form gains a ΔGL amount of free energy, so that the T form is at higher energy level than the R form. Implied in eq (1) is a scalar LL defined as :
| (2) |
which is equivalent to the allosteric constant, Lo, of the Monod model [1]. Iimplied in eq (2), we have
| (3) |
It should be stressed that the evolving equilibrium between the T and R forms during the binding event implies a barrier of energy that prevents their mutual collapsing into a single conformation system.
Also in eq (1) ΔGL is non zero and positive, while conformational equilibrium requires a zero balance of all free energy exchanges as in
| (4) |
In the equation ΔGcorr is the free energy that allows the relaxation of the R form into the higher energy level of the T form.
For the origin of ΔGcorr, a possible hypothesis is the presence of a third intermediate component(s) that upon formation absorbs heat and energy from the environment (positive ΔGon). Then, by degrading, would release the absorbed energy (negative ΔGoff) to the system and/or the environment. The ΔGbalance would be the net sum of positive and negative ΔGi’s related to assembly/disassembly of the third component(s) as in
| (5) |
At equilibrium it should be ΔGbalance = 0. A negative non zero balance would be the amount (ΔGcorr) released to the system, not to the environment, to compensate for the positive ΔGL in eq (4) . A positive balance would invalidate the hypotheses formulated above.
It should be stressed that the third intermediate component(s) must be at higher energy level than T and R forms, in order to degrade into the lower levels of the two forms. Thus, the third component(s) would also provide the barrier necessary to prevent the mutual relaxation of the T and R forms into a single conformation system.
The implication of intermediate third components has been proposed also by Ackers et al., Perrella et al. and Smith et al. from data of equilibrium, cryo-electrophoresis and kinetics [3-5]. Ackers concludes that the data (his words) “imply the existence (in the Hb system, authors note ) of at least three molecular structures; while a degeneracy of multiple structure into only a few dominant free energy levels (the T and R forms, authors note) is frequently to be expected, the reverse situation is extremely unlikely” [3].
This report
In order to explore the thermodynamics involved in the binding statistics of the MWC model we monitored the response of oxygen binding isotherms to challenges of temperature, osmotic stress, and protein concentration. In our laboratories temperature dependence has been already explored [6], and is used here as reference data.
Materials
Human Hb was prepared as described [6].
Sebacyl crosslinked Hb (DECA) was prepared as described [7].
All reagents used in the various manipulations were reagent grade and obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO).
Protocols
Measurements of OD changes were performed using a Varian 14 DS recording spectrophotometer.
Oxygen-binding isotherms were measured following the technique developed by Dolman and Gill, using a thin-layer Hb solution exposed to successive dilution of the initial PO2 with nitrogen. [8].
Osmotic dependence of the isotherms was measured at osmotic pressures varying from 1.0 to 80 Atm, using betaine concentrations up to 5 M. Buffer osmolality was measured using a Wescor osmometer (Logan, UT). Protein concentration was near 30 mg mL−1 in 0.2 M borate buffer at pH 9.0, at 30°C.
Protein-concentration dependence of the isotherms was measured at Hb concentrations varying from 5 to 50 mg mL−1 in 0.1 phosphate buffer at pH 7.4, at 37°C.
Numerical Analyses
Numerical analyses of the isotherms were based on the binding polynomial, Pw [2]
| (6) |
from which the Adair’s sequential binding equation is obtained
| (7) |
In these equations, X is the partial pressure of oxygen (PO2) and βi are the Adair’s binding parameters at subsequent binding steps, i, from 1 to 4. Y is the fractional saturation with oxygen. The intrinsic binding constant at each step was computed from
| (8) |
while the relative proportion, α, of each intermediate as function of PO2 was computed from
| (9) |
The median ligand activity was computed from
| (10) |
Alternatively, the value of P50 and extent of cooperativity was estimated from the midpoint of the isotherm, using the Hill equation
| (11) |
and its logarithmic transformation for the Hill’s plots. In the equation, Y is the fractional saturation of hemoglobin with oxygen and “n” is the cooperativity index. Due to the quasi symmetric shape of the isotherm, Pm and P50 are practically identical.
Fitting minimization
Isotherms were globally minimized, grouping together those exposed to various temperatures or osmotic stresses. This technique substantially reduced the correlation between the floating parameters, allowing estimations within a range close to 1% of the estimated value.
Results
Temperature dependence
These data were previously reported by Bucci et al. [6]. Isotherms were measured at 7 temperatures between 20°C and 37°C. Numerical analyses were based on the binding polynomial
| (12) |
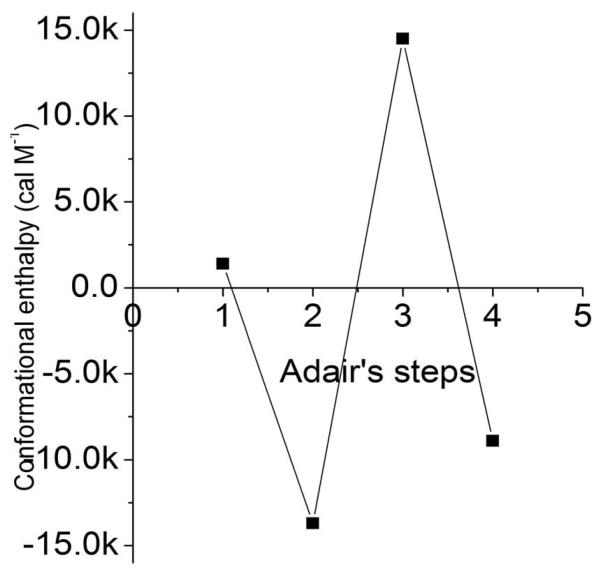
where the reference parameter βi,25 at 25°C is corrected for the enthalpy at each binding step. The enthalpy of conformational changes per se was obtained by subtracting the enthalpy of the binding of oxygen to heme, estimated to be ΔH = −14 Kcal/heme. The corrected data are plotted in Fig. 1. Notably, the enthalpy of the third step of oxygenation shows a large heat absorption, ΔH = 14 Kcal M−1 while the second and fourth oxygenation steps show large heat releases , ΔH = −13 and −8 Kcal M−1 respectively.
Fig 1.
Conformational enthalpy of the intermediates of oxygenation of hemoglobin. In 0.1 M borate buffer at ph 9.0. Adapted from [6].
Osmotic stress
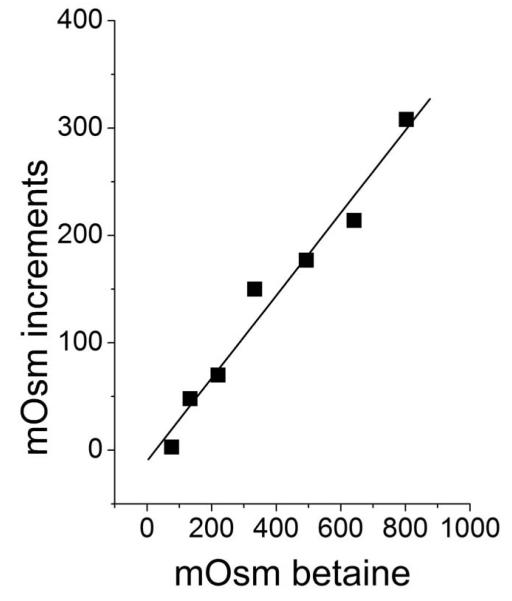
The osmolite of choice was betaine (trimethyl glycine MW 114.14 Da), which under the conditions of all experiments (0.2 M borate buffer at pH 9.0, at 30°C) did not bind to HbA, as is shown by the linearity of the increases of osmolality produced by increasing concentrations of betaine in the presence of Hb (Fig. 2).
Fig 2.
Increments of mOsm produced by increasing mOsm of betaine over a 240 mg ml−1 hemoglobin. Note the linearity with a slope = 0.99. In 0.2 M borate buffer at ph 9.0, 30°C.
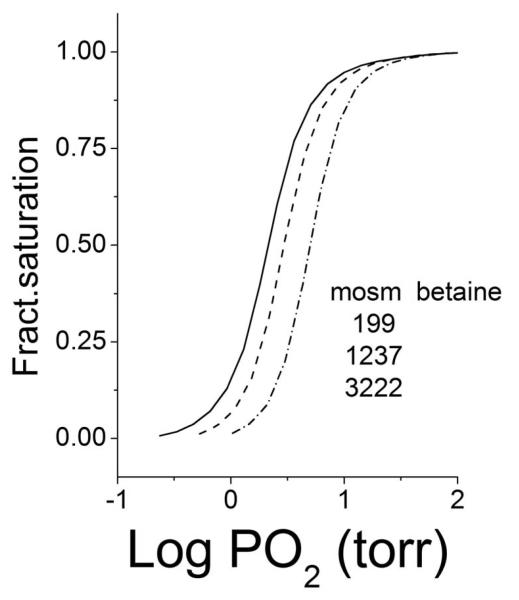
Isotherms at three different osmotic pressures are shown in Fig. 3. The presence of the osmolite right-shifts the isotherms, increasing their P50 indicating that the free energy of oxygen binding to Hb is increased by osmotic stress, as in:
| (13) |
where ΔV is a volume change produced by the osmolite, π = osm · RT is the osmotic pressure, and osm is the measured molality of the samples. The term πΔV is the free energy change, ΔGW, of the osmotic stress that would increase ΔG0 so reducing the oxygen affinity of Hb.
Fig 3.
Increasing right displacements of binding isotherms produced by the increasing osmolality of betaine listed in the graphic. In 0.2 M borate buffer at ph 9.0, 30°C.
Numerical analyses for estimating the ΔVi’s produced by the osmotic stress at each step of oxygen binding were based on the binding polynomial
| (14) |
where ΔVi’s are the volume change at each step.
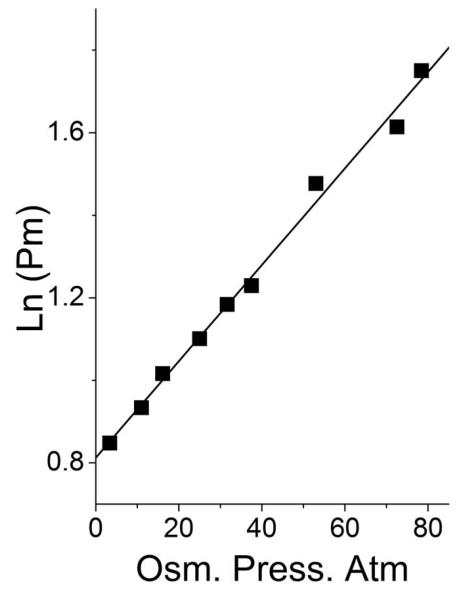
The overall volume change produced by the osmotic stress was also obtained from the slope of the plot of ln(P50) against the measured osmolality of the buffer, using:
| (15) |
as shown in Fig. 4.
Fig 4.
Dependence on osmotic pressure of the oxygen affinity (ln (Pm)) of hemoglobin. In 0.2 M borate buffer at ph 9.0, 30°C.
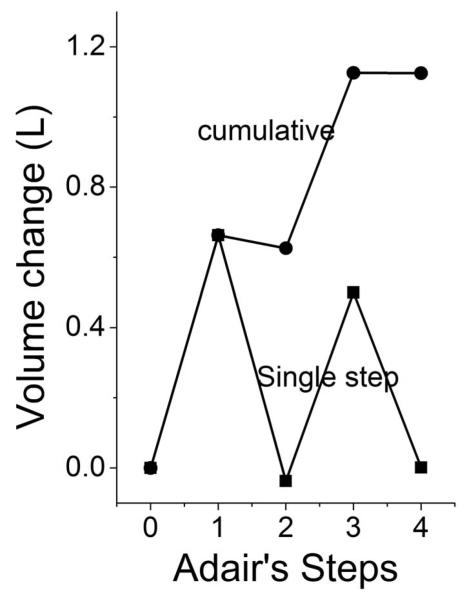
The numerical data obtained from these experiments show a very good correspondence between the cumulative and overall displaced volumes ΔVovr = Σ ΔVi = 1.2 L M−1 (Table 1). The step-by-step volume increases were not evenly distributed along the oxygen-binding steps (Fig. 5); they are evident only on the first and third steps.
Table 1.
)Vi values: overall and at individual Adair’s steps (1-4).
| )Vi (L M−1) cumulative i=1 to 4 |
)Vi (L M−1) indiv.step i=1 to 4 |
|---|---|
| 0.663 | 0.663 |
| 0.626 | −0.037 |
| 1.126 | 0.500 |
| 1.127 | 0.001 |
| Overall )V (L M−1) 1.10 | |
Fig 5.
ΔV increments and cumulus with oxygenation. In 0.2 M borate buffer at ph 9.0, 30°C.
Colombo et al.[9] were first to notice that addition of sugars not binding to Hb lowered its oxygen affinity. They interpreted this phenomenon as a solvation change involving 66 moles of water per mole of hemoglobin. The volume of 66 moles of water is near 1.2 L M−1, consistent with our ΔV values.
Concentration dependence
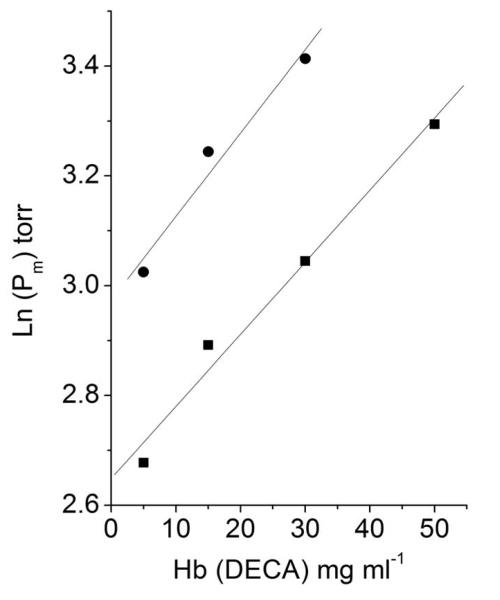
Experimental conditions were chosen for maximizing the effect of protein concentrations, similar to physiologic conditions: 0.1 M phosphate buffer at pH 7.4 and Hb concentrations between 5 and 50 mg mL−1 at 37°C. Sebacyl crosslinked Hb (DECA) was chosen because the intramolecular crosslink eliminates dimers formation. DECA is characterized by a P50 near 30 mmHg and a cooperativity with “n” above 2.0, very similar to the P50 and cooperativity of normal blood [7].
As shown in Fig. 6, the oxygen affinity of DECA decreases with increasing protein concentration, suggesting the association process of DECA tetramers into polymeric forms. The phenomenon can be described by:
| (16) |
where Kpol is a pseudo-association constant for polymer formation because it refers to the total Hb concentration CHb. The term Kpol CHb is the free energy changes, ΔGC , of polymer formation that would increase ΔG0 so reducing the oxygen affinity of Hb.
Fig 6.
Concentration dependence of oxygen affinity (ln (P50)) of DECA (sebacyl crosslinked hemoglobin [7]). In the presence (● ) and absence of 3.0 M betaine (■). In 0.1 M phosphate buffer at pH 7.4, 37°C.
In numerical analyses, the Adair’s parameter β3 was so low that it was difficult to estimate. Therefore, for the concentration-dependence data, we were unable to produce data for individual steps.
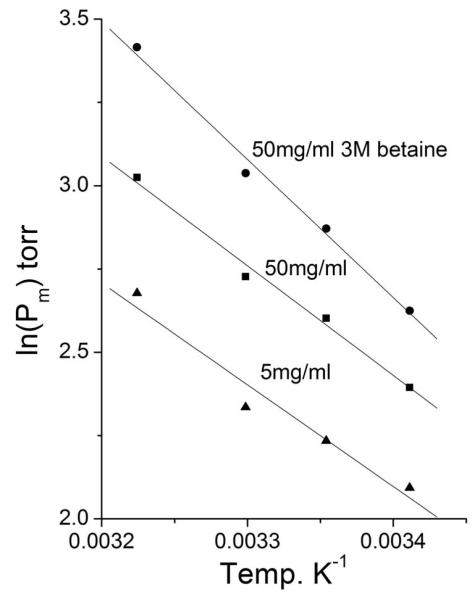
The negative slope (ΔH’s) of the van t’ Hoff plots at 5 mg mL−1 and 50 mg mL−1 hemoglobin concentration remained constant with ΔH = −6.1 kCal M−1 and −6.5 kCal M−1, respectively; only the intercept on the ordinates was higher at the higher Hb concentration. In the presence of 50 mg mL−1 Hb concentration, addition of 3 M betaine further increased the ordinates intercept of the sloping line and the enthalpy slightly decreased to ΔH = −8.2 kCal M−1. Fig. 7 shows the quasi-parallel upward displacement of the van t’Hoff lines under the three conditions.
Fig 7.
Van t’Hoff plots for DECA [7] at 5 mg ml−1(▴), at 50 mg ml−1(■) and at 50 mg ml−1 + 3M betaine (●). In 0.1 M phosphate buffer at pH 7.4.
Discussion
Osmotic stress
Based on eq (13), as mentioned above, the right shift of the isotherm in response to increasing π reveals the presence of a term
| (17) |
that decreases the oxygen affinity of the system.
ΔGW is the free energy change of the osmotic stress that, as proposed by Timasheff [10], is the expression of an increased solvent preferential exclusion from the protein surface that increases in size. In hemoglobin it is consistent with the increased hydrophobicity of the oxyHb surface, reported by Chothia [11].
The ΔVi data shown in Table 1 are positive, in favor of the deoxy T structure . Therefore ΔGW adds energy to the system in T form contributing to the free energy of cooperativity ΔGL. Assuming that the free energy changes ΔG1 and ΔG4 listed in Table 2, correspond to ΔGT and ΔGR respectively, a comparison between ΔGw, computed with eq (17), and ΔGL computed with eq (1) is shown in Table 3.
Table 2.
Osmotic data for Hb in 0.2 M borate buffer at pH 9.0 at 30E C. The four rows at each π value correspond to the 4 Adair’s steps (1-4) from top to bottom.
| π (Atm) | βi (torrBI) | Ki (mM−I) | ΔGi (cal M−1) |
|---|---|---|---|
| 11 | 0.236 | 190 | −7171 |
| 0.094 | 855 | −8059 | |
| 0.051 | 2620 | −8720 | |
| 0.023 | 5810 | −9189 | |
| 16 | 0.207 | 166 | −7094 |
| 0.077 | 798 | −8019 | |
| 0.045 | 2823 | −8764 | |
| 0.017 | 4866 | −9085 | |
| 25 | 0.144 | 115 | −6880 |
| 0.056 | 834 | −8045 | |
| 0.026 | 2242 | −8628 | |
| 0.012 | 5945 | −9203 | |
| 31.5 | 0.122 | 98 | −6782 |
| 0.061 | 990 | −8193 | |
| 0.019 | 1567 | −8369 | |
| 0.009 | 725 | −9181 |
Table 3.
Osmotic (ΔGW) and analytical (ΔGL) free energy changes for the conformational transition, with the corresponding scalars LW and LL, and the differences between analytical and osmotic values.
| ATM | ΔGW Kcal M−1 |
ΔGL Kcal M−1 |
LW | LL | ΔGL-ΔGW cal M−1 |
|---|---|---|---|---|---|
| 11 | 1.56 | 2.01 | 13 | 30 | 450 |
| 16 | 1.79 | 1.99 | 19 | 29 | 200 |
| 25 | 2.06 | 2.32 | 29 | 46 | 260 |
| 31.5 | 2.20 | 2.40 | 37 | 52 | 200 |
| 37.5 | 2.30 | 2.37 | 44 | 50 | 70 |
| Average 236 |
In Table 3 ΔGL is always higher than ΔGW by a few hundred Kcal M−1. The difference is better expressed by the respective values of the scalars LW and LL, eq (3). The differences are small and in the same direction. This may suggest that ΔGW refers only to the solvation of Hb, while ΔGL includes also the net sum of the positive and negative free energy changes resulting from the rearrangements of the T ↔ R conformation within the subunits and across their interfaces [12]. In other words ΔGW only detects the different solvation resulting from the conformational change included in ΔGL.
The third component(s) are polymeric
As shown in Fig. 6, the P50 of DECA has a distinct dependence on protein concentration, implying the presence of a reversible associating system that modulates the oxygen binding isotherm, as shown by eq (16). It is a general phenomenon in hemoglobin systems, previously reported from our laboratory for human and bovine hemoglobins[13].
In the experiments reported above, DECA is an intramolecularly crosslinked , non-dissociable tetrameric Hb[7]. In the absence of dimers, the association process could be only a polymerization of tetrameric Hb molecules.
Detailed step analyses of the concentration dependent isotherms are not available because, as mentioned above, the Adair’s parameter of the third step, β3, was so small to be undefined, suggesting that the tri-ligation fractional saturation of the isotherm results in the formation of components with very low oxygen affinity.
This phenomenon is concomitant with the strong heat absorption by the enthalpy of the third step of oxygenation, shown in Fig. 1 with ΔH3 near 14 Kcal M−1[6]. It can be proposed that the tri-ligation absorbs into the system the energy (ΔGon) necessary for the formation of the stereochemistry of new polymeric forms with high energy levels and very low oxygen affinity.
The free energy release by the negative enthalpies detected at the 2nd and 4th steps (Fig. 1), with ΔH2 near −13 Kcal M−1[6] and ΔH4 near −8 Kcal M−1[6], would result from the disassembly (ΔGoff) of unstable polymers relaxing into lower energy, stable, tetrameric structures like the T and R forms.
Our data proposing the presence of unstable polymeric intermediates dovetail with Ackers’ hypothesis of at least a third intermediate component degenerating into more stable forms[3].
Energetic relevance of the intermediates of oxygenation
Random-isodesmic aggregating polymers cannot be proposed because they would be at a lower energy level stabilizing intermediate forms, producing negative cooperativity, as described by Koshland et al. [14]. In fact, the equilibrium contribution of high energy components (the polymers) makes the average energy level of all of the intermediates higher than the levels of both the T and R forms. The high energy level of intermediates would be the barrier that prevents the T and R forms of Hb from relaxing into each other.
The high heat absorption at the third Adair’s step (ΔH3[6]) implies the formation of quinary, and above, polymeric forms. This hypothesis is supported by the fibers of HbS, which reveal the presence on the surface of tetrameric Hb of sites ready for the intermolecular contacts necessary to the self assembly and formation of perfectly designed quinary and higher order structures [15]. Similarly, also HbS fibers self assemble into a very low oxygen affinity. It is very tantalizing to propose that the HbS fibers are the mutation-stabilized form of an intermediate polymer. The history of measured isotherms in our laboratories and the data of Campbell et al [16] on fiber formation in vitro may support this proposition.
Osmotic stress offers a suggestion for the different shape of the R and T forms. A different solvation that involves 66 moles of water per mole of Hb, implies a larger surface/volume ratio of the molecule with a larger osmotic shell for oxyhemoglobin. Conversely the T form has a lower molecular surface/volume ratio. For the same mass, minimizing the surface gives to the T form a more compact and spherical shape, more spherical than that of the R structure with a larger surface/volume ratio.
The same reasoning can be applied to the hypothesis of Colombo et al.[9] that 66 molecules of water bind directly on the surface of hemoglobin. ΔGW would not distinguish between the equivalence of two hypotheses. The increased hydrophobicity of the system[11] would support the hydration shell proposal.
Distribution of the intermediates
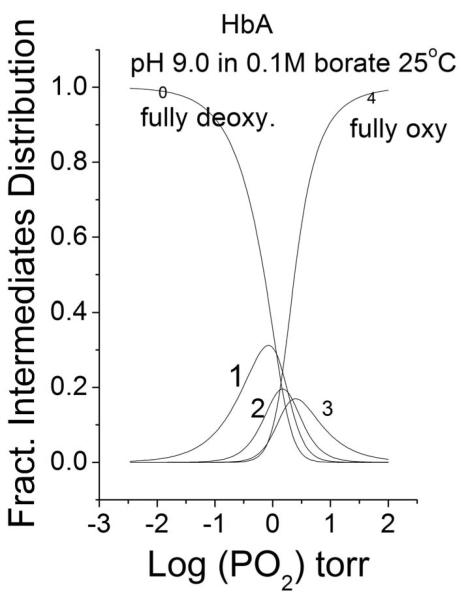
The Adair’s subsequent parameters βi are statistical binding constants, referred to all intermediates of ligation present along the evolving oxygen saturation of the isotherm. They describe the changing distribution of species increasingly saturated with oxygen, as shown in Fig 8. This is why we prefer to define the subsequent oxygenation steps as “Adair’s steps”.
Fig 8.
Distribution of liganded species (eq (9)) as function of increasing PO2. . In 0.1 M borate buffer at ph 9.0, 25°C . The numbers in the graphic reflect the Adair’s oxygenation steps.
Fig 5 shows the progress of ΔVi’s along the four Adair’s steps. The progress is not linear with the steps. The maxima at the first and third step are probably due to local prevalence of the most asymmetric species.
Similarly, for the enthalpies, as shown in Fig 1,, the non linear progress would be due to local prevalence of certain ligated species. The maximum heat absorption at the third Adair’s step is reached when tri-ligation makes of the polymers the dominant species.
Comparisons of Fig 1,5 and 8 support the hypothesis that there are large equilibrium conformational fluctuations among the intermediates of oxygenation, resulting into a multiple forms single system.
The correction in eq(4)
The enthalpy values shown in Fig 1 are data taken from Bucci et al.[6] obtained in the absence of betaine. They are the free energies either absorbed (ΔH3) or liberated (ΔH2,ΔH4) from and to the environment upon the T↔R conformational changes. As discussed, they would correspond to formation and degradation of polymeric forms. The free energy balance between absorption and release can be estimated by
| (18) |
where the residual −7.5 Kcal M−1 is the energy not released to the environment, because it is delivered to and absorbed by the intermediates of Hb oxygenation. The average, equally distributed over the four Adair’s steps, is near −1.9 Kcal M−1 close to the ΔGL’s in Table 3.
Apparently, it is formation and degradation of polymeric components that provides the ΔGcorr = −1.9 Kcal M−1 corr necessary to have ΔGeql = 0 in eq (4) that justifies the higher energy level of the T form.
The MCW model scenario
The energy necessary for the conformational change (ΔGL=2.0 KcalM−1) include the osmotic stress, ΔGW= 1.6 Kcal M−1 and the net free energy change near 300 Kcal M−1 of structural conformational changes; which, within the MWC model[1], can be considered allosteric effectors specific for the T form, that increase the energy level of the T form to a higher ΔGT values with lower oxygen affinity.
Reversibility implies the release of a - ΔGL free energy from the T form into the R structure. This decreases the energy level of the system and decreases the ΔGR value to a higher oxygen affinity.
It appears that the MWC model implies an extra packet of about 2000 Kcal M−1that is in turn either absorbed or released by the system modulating its oxygen affinity. The energy of the packet is provided by the release into the system of the energy absorbed from the environment by the enthalpies of the intermediates.
The data suggest that the T and R forms of Hb are separated at the two ends of a reversible thermal itinerary across the higher energy levels of the intermediates.
Besides providing the energy necessary for cooperativity, the high energy level of polymeric intermediates provide most of the barrier that prevents the T and R forms from relaxing into each other.
In this view the MWC allosteric model best describes a system where the evolving equilibrium between two conformations is regulated by intermediates all averaged by and included in the allosteric constant Lo.
Acknowledgments
Silvia Perozzi contributed her technical expertise to this research. The osmotic data are part of the master thesis of Henry Gering, a student of EB.
This manuscript is a late expression of NIH grant P48517 (EB). Partial support by the University of Maryland Medical School and University of Camerino is also acknowledged.
Abbreviations
- ΔH
enthalpy
- ΔGC
free energy change for polymer formation
- ΔGeql
equilibrium free energy change of cooperativity
- ΔGL
the free energy change of cooperativity
- ΔGoff
free energy of polymers disassembly
- ΔGon
free energy of polymers formation
- ΔGR
free energy change of oxygen binding to R form
- ΔGT
free energy change of oxygen binding to T form
- ΔGW
free energy change of osmotic stress
- DECA
sebacyl crosslinked hemoglobin
- LL
scalar of ΔGL
- LW
scalar of ΔGW
Appendix.
Initial and final parabolas
In eq (13,16) ΔGw and ΔGC are mass dependent extensive additive parameters that do not interfere with the intensive parameters of the system as shown in Fig. 7. Thus, referring to ΔG1 and ΔG4 (Table 2) as ΔGT and ΔGR, respectively, and from eq (13,16), for the hyperbolic binding of the T form we would have
| (19) |
where, according to eq(1), the positive ΔGL parameter specifically adds energy to the system to form the T structure and decreasing its oxygen affinity.
Regarding the R form, reversibility implies an opposite negative ΔGL in eq (1) , therefore for the R form we would have :
| (20) |
where a negative ΔGL specifically adds its negativity to ΔGR decreasing the energy level of the system into the R form and further increasing its oxygen affinity.
It is as if there is a fundamental hyperbolic oxygen binding of Hb
| (21) |
modulated by protein concentration, osmotic stress, and structural changes.
Governing equations
Fluctuations of the intermediates of oxygenation prevent their description with a universal equation. Only averages may be used. It is well known that the Hill’s equation (11) and its logarithmic transformation average in the scalar “n” all of the intermediates, and are very useful for estimating the overall oxygen affinity (P50) and presence of cooperativity (n>1.0) in the isotherm. Most useful are the statistical Adair’s parameters, eq (6-10) for following the equilibrium distribution of the intermediates and computing Pm.
Simultaneous numerical analyses of groups of isotherms are useful for reducing the correlations among the floating parameters. Di Cera and Gill [17,18] have developed numerical procedures for obtaining the Adair’s parameters and oxygen saturation fractions from single isotherms.
The MWC binding model adequately describes the system and is useful for simulations. It cannot be used for numerical analyses because, as shown in eq (3), there is a correlation equal 1.0 between the floating parameters Lo and either KR or KT (depending on the equation spelling). This kind of correlation cannot be reduced by simultaneous group analyses and prevents minimizations.
Significance for Biochemistry
Cooperativity is physiologically relevant. In fact, at the periphery, the acquired lower affinity helps the mass action of oxygen release, which corrects for the asymmetry of slow oxygen supply from red cells and fast mitochondrial consumption [19,20]. At the lungs the acquired higher affinity assures a full saturation of Hb exposed to a modest PO2.
It is tantalizing to present hemoglobin as a molecular machine that extracts oxygen from the lungs and expels it to the tissues under the pumping action of the assembly-disassembly of polymeric forms during the binding event. The MWC model best describe this mechanism with two switching conformations pumped by the allosteric constant Lo.
The thermodynamics of the T↔R conformational change finalize and justify the origin of the cooperativity of oxygen binding isotherms, after an almost century of search based on the binding statistics of non unique allosteric models.
Footnotes
This paper is dedicated to the late Gary C. Ackers. We miss a friend. Science is missing one of its major contributor.
References
- 1).Monod J, Wyman J, Changeaux JP. On the nature of allosteric transitions: a plausible model. J. Mol. Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 2).Wyman J, Gill SJ. Binding and Linkage: Functional Chemistry of Biological Macromolecules. University Science Books; Mill Valley, CA: 1990. [Google Scholar]
- 3).Ackers GK. The energetic of ligand linked subunit assembly in hemoglobin requires a third allosteric structure. Bipohys.Chem. 1990;37:371–373. doi: 10.1016/0301-4622(90)88036-r. [DOI] [PubMed] [Google Scholar]
- 4).Perrella M, Benazzi L, Shea MA, Ackers GK. Subunits hybridization studies of partially ligated cyanmethemoglobins using a cryogenic method. Biophys.Chem. 1990;35:97–103. doi: 10.1016/0301-4622(90)80064-e. [DOI] [PubMed] [Google Scholar]
- 5).Smith FR, Ackers GK. Experimental resolution of cooperative free energies for the ligation states of human hemoglobin. Proc.Natl. Acad.Sci.Usa. 1985;82:5347–5351. doi: 10.1073/pnas.82.16.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Bucci E, Fronticelli C, Gryczynski Z. Discontinuous release of heat at successive steps of oxygenation in human and bovine hemoglobin at pH 9.0. Biochemistry. 1991;30:3195–3199. doi: 10.1021/bi00227a006. [DOI] [PubMed] [Google Scholar]
- 7).Bucci E, Razynska A, Kwansa H, Matheson-Urbaitis B, O’Hearne M, Ularowski JA, Koehler RC. Production and characteristics of an infusable oxygen-carrying fluid based on hemoglobin intramolecularly cross-linked with sebacic acid. J.Lab.Clin.Med. 1996;128:146–153. doi: 10.1016/s0022-2143(96)90006-2. [DOI] [PubMed] [Google Scholar]
- 8).Dolman D, Gill SJ. Membrane-covered thin-layer optical cell for gas-reaction studies of hemoglobin. Annals Biochem. 1978;87:127–134. doi: 10.1016/0003-2697(78)90576-6. [DOI] [PubMed] [Google Scholar]
- 9).Colombo MF, Rau DC, Parsegian VA. Protein solvation in allosteric regulation: a water effect on hemoglobin. Science. 1992;256:655–659. doi: 10.1126/science.1585178. [DOI] [PubMed] [Google Scholar]
- 10).Timasheff SN. In disperse solution “osmotic stress”is a restricted case of preferential interactions. Proc.Nat,Acad,Sci.USA. 1998;95:7363–7367. doi: 10.1073/pnas.95.13.7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Chothia C, Wodak S, Janin J. Role of subunits interfaces in the allosteric mechanism of hemoglobin. Proc.Nat.Acad.SciencesUSA. 1976;73:5793–5797. doi: 10.1073/pnas.73.11.3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Perutz MF. Hemoglobin structure function and synthesis. Br.Med.Bull. 1976;32:193–194. doi: 10.1093/oxfordjournals.bmb.a071362. [DOI] [PubMed] [Google Scholar]
- 13).Kwansa HE, Arosio D, Bucci E. Adipyl crosslinked bovine hemoglobins as new models for allosteric transitions. Proteins: Structure, function and Genetics. 2000;39:166–169. [PubMed] [Google Scholar]
- 14).Koshland DE, Jr., Nemethy G, Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966;5:365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- 15).Cretegny I, Edelstein SJ. Double strand packing in hemoglobin S fibers. J.Mol.Biol. 1993;230:733–738. doi: 10.1006/jmbi.1993.1195. [DOI] [PubMed] [Google Scholar]
- 16).Campbell B, Bucci E. Dependence on pH of formation and oxygen affinity of hemoglobin S fibers in the presence and absence of phosphates and polyphosphates. Biophys Chem. 1987;28:215–223. doi: 10.1016/0301-4622(87)80092-3. [DOI] [PubMed] [Google Scholar]
- 17).DiCera E, Gill SJ. On the determination of species fractions from ligand binding data. Application to human hemoglobin. Biophys Chem. 1988;29:351–356. doi: 10.1016/0301-4622(88)85057-9. [DOI] [PubMed] [Google Scholar]
- 18).Gill SJ, Gaud HT. Analysis of ligand binding curves in terms of species fractions. Biophys Chem. 1978;8:53–59. doi: 10.1016/0301-4622(78)85022-4. [DOI] [PubMed] [Google Scholar]
- 19).Bucci E. Thermodynamic approach to oxygen delivery in vivo by natural and artificial oxygen carriers. Biophys.Chem. 2009;142:1–6. doi: 10.1016/j.bpc.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 20).Bucci E. Basic science offer a challenge for developing hemoglobin based oxygen carriers into therapeutic agents. Art. Cells, Blood Subst. Immobil. Biotechnol. 2011;39:206–213. doi: 10.3109/10731199.2010.538402. [DOI] [PubMed] [Google Scholar]