The development and outcome of pregnancy-related group A streptococcal infections are dependent upon the stage of pregnancy or puerperium, epidemiologic factors of the patient's home and hospital environments, and virulence of the prevalent pathogens.
Keywords: Streptococcus pyogenes, pregnancy, postpartum sepsis
Abstract
Puerperal sepsis caused by group A Streptococcus (GAS) remains an important cause of maternal and infant mortality worldwide, including countries with modern antibiotic regimens, intensive care measures and infection control practices. To provide insights into the genesis of modern GAS puerperal sepsis, we reviewed the published cases and case series from 1974 to 2009, specifically seeking relationships between the likely source of pathogen acquisition, clinical signs, and symptoms at infection onset and patient outcomes that could provide clues for early diagnosis. Results suggest that the pathogenesis of pregnancy-related GAS infections in modern times is complex and not simply the result of exposure to GAS in the hospital setting. Additional research is needed to further explore the source of GAS, the specific M types involved, and the pathogenesis of these pregnancy-related infections to generate novel preventative and therapeutic strategies.
HISTORICAL PERSPECTIVE
Historically, epidemics of nosocomial puerperal sepsis were propagated by patient crowding and a general lack of hygienic conditions (reviewed in [1]). In the mid-19th century, Holmes and Semmelweis deduced that physicians transmitted childbed fever via their hands to pregnant women during labor and delivery, and showed that hand washing would largely interrupt this contagion [2]. With the added introduction of antiseptics and penicillin, maternal mortality was significantly reduced by the 1940s (reviewed in [3]). In the mid-1980s, however, invasive group A Streptococcus (GAS) infections reemerged worldwide (reviewed in [4]), including those associated with pregnancy and childbirth [5]. In the United States, the annual incidence of GAS postpartum infection is 6 per 100 000 live births, with approximately 2% maternal mortality [6]. Globally, puerperal sepsis causes approximately 75 000 maternal deaths per year (reviewed in [1]) with the highest maternal mortality in Asia (11.6%), Africa (9.7%), and Latin America/Caribbean (7.7%) [7].
Recent reports of pregnancy-related GAS infections emphasize demographics or the characteristics of the specific strains responsible. This review emphasizes the clinical presentation, complications, and timing of infection in relation to pregnancy and childbirth to provide important clinical clues for early diagnosis.
PREGNANCY-RELATED STREPTOCOCCUS PYOGENES INFECTIONS
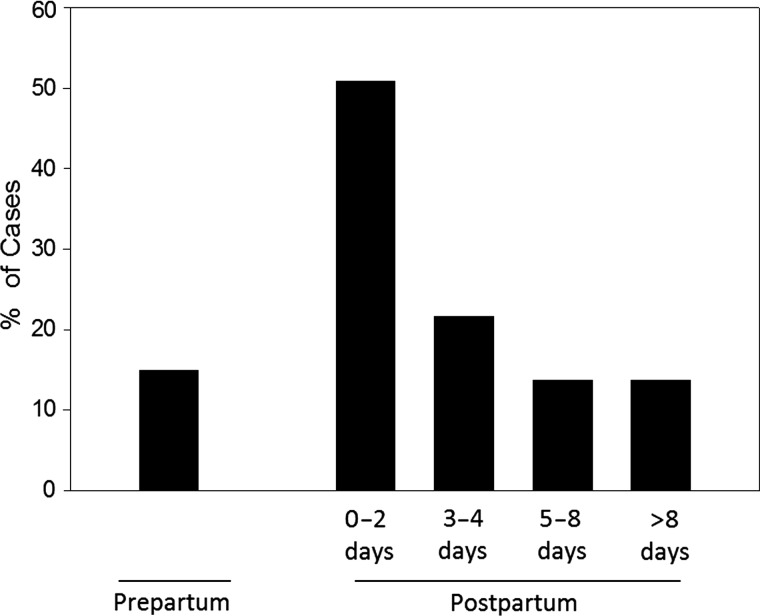
A PubMed database search was performed for the period 1974–2009 for English-language clinical reports of GAS infections occurring either during pregnancy or the postpartum period. The search produced 43 reports describing 67 patients (mean age, 28.7 years; Supplementary Table 1) from North America (50.7%), Europe (38.8%), Asia (5.9%), and Australia/New Zealand (4.5%). Pregnancy-related GAS infections occurred predominantly in the postpartum period (57/67; 85.0%). Of these, 84.4% followed vaginal delivery and the majority (72.5%) occurred within the first 4 days postpartum (Figure 1, Table 1, and Supplementary Table 1).
Figure 1.
Distribution of cases of pregnancy-associated Group A Streptococcus (GAS) infection. A literature search was performed for clinical reports of pregnancy-related GAS infections occurring either during pregnancy or the postpartum period using the PubMed database for the period 1974–2009. The search produced 43 reports describing 67 patients. Ten of these 67 patients (15%) developed GAS infection during pregnancy, 9 of whom were in the third trimester (weeks 28–42). Of the remaining 57 postpartum cases, the time of onset was reported in 51 cases.
Table 1.
Signs, Symptoms, and Clinical Characteristics of Pregnancy-Associated Group A Streptococcus Infections
| Characteristics of GAS Infection | No. (%) of Patients With the Indicated Featurea |
||||
|---|---|---|---|---|---|
| Prepartum |
Postpartum |
||||
| Third Trimesterb,c (n = 9) | 0–2 d (n = 26) | 3–4 d (n = 11) | 5–8 d (n = 7) | >8 d (n = 7) | |
| Method of birthd | |||||
| Cesarean delivery | 4 (57.1) | 4 (16.0) | 0 (0.0) | 0 (0.0) | 3 (75.0) |
| Vaginal delivery | 3 (42.9) | 21 (84.0) | 9 (100) | 7 (100.0) | 1 (25.0) |
| Not given | 2 | 1 | 2 | 0 | 3 |
| Initial symptoms | |||||
| Abdominal pain | 4 (44.4) | 15 (57.7) | 6 (54.5) | 5 (71.4) | 4 (57.1) |
| Chills | 2 (22.2) | 9 (34.6) | 3 (27.3) | 3 (42.9) | 2 (28.6) |
| Diarrhea, nausea, vomiting | 1 (11.1) | 8 (30.7) | 1 (9.1) | 2 (28.6) | 5 (71.5) |
| Pharyngitis or upper respiratory infection | 5 (55.6) | 3 (11.5) | 0 (0.0) | 2 (28.6) | 2 (28.6) |
| Clinical features | |||||
| Fever (≥38.0°C) | 7 (77.8) | 19 (73.1) | 8 (72.7) | 6 (85.7) | 6 (85.7) |
| Hypotention (systolic pressure ≤90 mm Hg) | 5 (55.6) | 9 (34.6) | 4 (36.4) | 2 (28.6) | 3 (42.9) |
| Tachycardia (≥100 beats per min) | 4 (44.4) | 7 (26.9) | 5 (45.5) | 2 (28.6) | 1 (14.3) |
| Leukocytosis (WBC count >11 000/mm3) | 2 (22.2) | 5 (19.2) | 4 (36.4) | 5 (71.4) | 4 (57.1) |
| Uterine tenderness | 1 (11.1) | 8 (30.8) | 3 (27.3) | 1 (14.3) | 1 (14.3) |
| Vaginal discharge | 0 (0.0) | 10 (38.5) | 4 (36.4) | 2 (28.6) | 3 (42.9) |
| Erythema | 2 (22.2) | 8 (30.8) | 2 (18.2) | 4 (57.1) | 0 (0.0) |
| Extremity pain | 1 (11.1) | 4 (15.4) | 3 (27.3) | 3 (42.9) | 0 (0.0) |
| Pharmacologic interventions | |||||
| Antibiotic treatment | 6 (66.7) | 21 (80.8) | 11 (100.0) | 6 (85.7) | 7 (100.0) |
| Surgical intervention | |||||
| Debridement, drainage, and/or amputations of extremities | 1 (11.1) | 2 (7.7) | 1 (9.1) | 3 (42.9) | 0 (0.0) |
| Exploratory surgery (laparotomy) | 1 (11.1) | 7 (26.9) | 3 (27.3) | 1 (14.3) | 3 (42.9) |
| Hysterectomy; salpingo-oophorectomy | 2 (22.2) | 8 (30.8) | 4 (36.4) | 0 (0.0) | 1 (14.3) |
| Surgical findings | |||||
| Ascites or pus in the peritoneal cavity | 0 (0) | 2 (7.7) | 1 (0.0) | 0 (0.0) | 3 (42.9) |
| Necrosis, inflammation, or exudate present in the uterus, ovaries, and/or fallopian tubes | 2 (22.2) | 8 (30.8) | 3 (27.3) | 0 (0.0) | 2 (28.6) |
| Normal placenta, uterus, and/or pelvic organs | 3 (33.3) | ||||
| Bacterial source(s) | |||||
| Genitourinary system | 3 (33.3) | 20 (76.9) | 7 (63.6) | 4 (57.1) | 3 (42.9) |
| Blood | 7 (77.8) | 17 (65.4) | 8 (72.7) | 4 (57.1) | 3 (42.9) |
| Oropharynx or respiratory system | 4 (44.4) | 2 (7.7) | 0 (0.0) | 0 (0.0) | 2 (28.6) |
| Peritoneum | 0 (0.0) | 2 (7.7) | 1 (9.1) | 2 (28.6) | 4 (57.1) |
| CNS system | 1 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (14.3) |
| Maternal outcomed | |||||
| Survived | 4 (44.4) | 18 (75.0) | 8 (88.9) | 5 (83.3) | 7 (100.0) |
| Died | 5 (55.6) | 6 (25.0) | 1 (11.1) | 1 (16.7) | 0 (0.0) |
| Not given | 0 | 2 | 2 | 1 | 0 |
| Fetal outcomed | |||||
| Survived | 2 (28.6) | 19 (95.0) | 8 (88.9) | 4 (100.0) | 5 (100.0) |
| Died | 5 (71.4) | 1 (5.0) | 1 (11.1)e | 0 (0.0) | 0 (0.0) |
| Not given | 2 | 6 | 2 | 3 | 2 |
Abbreviations: CNS, central nervous system; GAS, group A Streptococcus; WBC, white blood cell.
a Cases where the time of symptom onset was not reported were not included in calculations (Supplementary References 3, 10, 37).
b Calculations include 1 patient with infection at gestational week 27 (Supplementary Reference 16).
c Calculations exclude a patient who underwent dilation and curettage at gestational week 7 (Supplementary Reference 7).
d Cases in which the indicated feature was not reported were not included in calculations.
e Fetal demise was the result of an elective medical abortion (Supplementary Reference 42).
TEMPORALLY RELATED CLINICAL FEATURES OF PUERPERAL S. PYOGENES INFECTION
Third Trimester of Pregnancy
Ten of 67 patients (14.9%) developed GAS infection during pregnancy (Table 1). One patient presented during the first trimester with streptococcal toxic shock syndrome (StrepTSS) and had a spontaneous abortion, delivering a stillborn fetus. Of the remaining 9 patients, none had premature rupture of the membranes and all had normal pregnancies until the sudden onset of symptoms in the third trimester (weeks 28–42). Four patients required emergent cesarean delivery; of these, 2 died (50%). Both had necrosis and/or inflammation of the uterus at autopsy. One of the 2 survivors required bilateral salpingo-oophorectomy. Three patients delivered vaginally, and 2 of these mothers and their infants died. The method of delivery was not given in 2 patients; of these, 1 mother and her baby died. Surgical intervention was not performed in 4 patients likely due to hemodynamic instability, and of those, 3 patients (75.0%) died. Among cases where outcomes were stated, overall fetal and maternal mortalities were 75% and 60%, respectively.
Initial clinical signs and symptoms included fever (77.8%), hypotension (55.6%), abdominal pain (44.4%), and tachycardia (44.4%). A prodrome of sore throat or upper respiratory tract infection was reported in 55.6% of patients. GAS was cultured primarily from the blood (77.8%) and/or respiratory tract (44.4%). In 2 patients (22.2%), GAS was isolated from the myometrium/endometrium but without concomitant colonization of the vaginal vault. One patient had GAS isolated from the deep soft tissue of an extremity (Supplementary Reference 16). GAS was not cultured from the fetus and/or amniotic fluid of infants who died, but signs of infection (eg, placental inflammation, turbid amniotic fluid) were reported (Supplementary References 7 and 19). GAS isolated from the uteri of 2 fatal cases were M types 1 and 3 (Supplementary References 19 and 43) and are the most common M types associated with StrepTSS [8–11].
These data suggest that GAS infection during pregnancy does not originate from vaginal colonization but rather that upper respiratory infection/colonization precedes hematogenous seeding of the placenta/uterus, suggesting an important tropism of GAS for these tissues during pregnancy. Infections during this period resulted in disastrous outcomes for both mother and fetus.
Early Postpartum Period (<4 Days)
Of the 67 cases of pregnancy-associated GAS infection documented in our review, 57 (85%) occurred in the postpartum period. Of these, the time of onset was reported in 51 cases.
Days 0–2 Postpartum
Of 51 patients, 26 (50.9%) developed infection within 2 days of childbirth (Table 1). In 4 patients, symptoms of puerperal sepsis were evident within 1 hour of delivery. Clinical findings were consistent with ascending infection and included localized symptoms such as abdominal pain (57.7%), purulent vaginal discharge (38.5%), and uterine tenderness (30.8%). Gastrointestinal symptoms (diarrhea, nausea, vomiting) also occurred in 30.7% of cases. Systemic symptoms included fever (73.1%), chills (34.6%), and hypotension (34.6%); however, leukocytosis and tachycardia were not frequent findings. The highest postpartum maternal mortality was seen during this time (25.0%). Women who underwent abdominal surgery (30.8%) had necrosis of the ovaries, fallopian tubes, and/or uterus.
GAS was primarily isolated from the vagina or urinary tract (76.9%), suggesting that infection resulted from prior or hospital-associated vaginal colonization. Maternal-to-infant transmission was also reported (Supplementary References 11, 21, and 32), and infections in infants ranged from necrotizing fasciitis of the scalp to respiratory distress. The low incidences of documented vertical transmission (3/26, 11.5%) and infant mortality (5.0%) imply that most mothers likely acquired GAS after delivery.
These data suggest that a spectrum of GAS postpartum infections can develop rapidly—ranging from mild endomyometritis (absence of tachycardia and leukocytosis) to fulminant endomyonecrosis and death. Thus, patients should be closely monitored for possible GAS infection during the first 2 days after childbirth. Early and appropriate interventions should be instituted in women presenting with abdominal pain and fever during this time.
Days 3–4 Postpartum
Eleven of 51 patients (21.6%) developed GAS infection during this period. Clinical signs and symptoms as well as primary sites of bacterial isolation were consistent with ascending infection (Table 1). Patients had hypotension (36.4%) and fever (72.7%). Leukocytosis (36.4%) and tachycardia (45.5%) were more pronounced in this group though gastrointestinal symptoms were less prevalent. Exploratory surgery was performed in approximately one-third of cases. Surgical findings included necrosis, inflammation, and/or exudates of the ovaries, fallopian tubes, and/or uterus. One mother (11.1%) died; 1 fetus died as a result of elective medical abortion. These findings suggest that ascending infection in the day 3–4 postpartum period likely follows urogenital acquisition of GAS from either the hospital or home environments.
Mid–Postpartum Period (5–8 Days)
Seven patients (13.7%) developed infection during this time period, and had evidence of both ascending and descending (pharyngitis [28.6%]) infections (Table 1). Women presented with fever, hypotension, and leukocytosis. Diffuse erythema (scarlatina) or erythema associated with extremity pain were highest in this group. Further, 3 patients (42.9%) had debridement, drainage, or amputation of 1 or more extremity, suggesting spontaneous necrotizing fasciitis/myonecrosis and pyomyositis possibly related to antecedent soft tissue injury [12]. Cumulatively, these findings that suggest women with GAS infections at 5–8 days postpartum have greater systemic involvement with possible hematogenous seeding of the extremities. Given the timeframe of infection onset, it is likely that patients in this group acquired GAS from the home environment.
Late Postpartum Period (>8 Days)
Seven (13.7%) patients presented 2–5 weeks after childbirth (Table 1). Diverse clinical findings included a high incidence of gastrointestinal symptoms (71.5%), fever (85.7%), chills (28.6%), and abdominal pain (57.1%). Leukocytosis was present in 57.1%, hypotension in 42.9%, and vaginal discharge in 42.9%.
The peritoneum was the most common site of GAS isolation (57.1%). This is distinctly increased compared to GAS infection that developed earlier in the postpartum period. GAS was isolated from the respiratory tract (28.6%), blood (42.9%), and vagina or urinary tract (42.9%). One patient had GAS isolated from spinal fluid. Maternal survival was 100% and only 1 patient required hysterectomy (Supplementary Reference 8). Infant mortality was 0%, suggesting again that maternal acquisition of GAS occurred well after delivery. Although these cases meet the definition of postpartum GAS infection, the heterogeneity of signs/symptoms and sites of bacterial isolation in this group suggest that infections were not likely directly related to parturition.
PATHOGENESIS OF POSTPARTUM S. PYOGENES INFECTION
Host Factors Related to Pregnancy
A 2010 report demonstrated that postpartum women had a 20-fold increase in attack rate for invasive GAS infection compared to nonpregnant women [13]. This apparent increase in susceptibility could be due to (1) compromised mucosal or cutaneous barriers (eg, an open cervix, vaginal mucosal tears, episiotomy or cesarean delivery incisions); (2) a transiently more neutral vaginal pH after amniotic fluid release, which could favor growth of the organism [14]; and (3) suppressed innate immunity due to pregnancy per se. For example, alternatively activated M2 macrophages predominate in the postpartum uterus and are focused on uterine repair [15]; the relative absence of proinflammatory M1 macrophages in this setting could leave the host vulnerable to bacterial pathogens.
Genetic Susceptibility
Nooh and colleagues have shown that immunologic polymorphisms play a role in susceptibility to invasive GAS infection in general [16], although no studies have investigated this relative to postpartum infections specifically. An inherent resistance to GAS infection is suggested by the fact that a much larger proportion of women are colonized with GAS than actually develop infection. Specifically, Mead and Winn found that during the third trimester of pregnancy, 0.03% of women had GAS vaginal–rectal colonization [17]. If such colonization uniformly resulted in GAS infection postpartum, one would predict that among the 4.1 million live births in 2009, 123 000 women would develop infection (ie, 3000 cases per 100 000 live births). However, diagnosed GAS postpartum sepsis occurs with a frequency of 6 cases per 100 000 live births. These findings suggest that some level of innate or acquired mucosal immunity exists in most pregnant/postpartum women or that not all colonizing strains of GAS are capable of causing infection during pregnancy or after delivery.
Bacterial Factors
Development of pregnancy-related GAS infection may depend on the specific M type of GAS in the patient's environment. For example, a population-based study conducted from 1995 to 2000 by the US Centers for Disease Control and Prevention (CDC) showed that among postpartum GAS blood isolates, the most common M types were M28 (21%), M1 (14%), and types 4, 11, 12, and 13 (7%) [6]. A similar report of 18 cases from Utah demonstrated that severe GAS puerperal infection was solely associated with M types 1 and 28 [18]. Data presented here agree with these findings. Thus, despite the existence of >150 different M and emm types, only a handful of GAS strains are associated with invasive GAS infections in pregnant women. The importance of a specific M type or its associated toxins and virulence factors is yet to be determined.
CURRENT PERSPECTIVES ON DIAGNOSIS AND TREATMENT
Clinical Clues and Diagnosis
Diagnosis of GAS infections during pregnancy or early in the postpartum period is often difficult due to a low prevalence of these infections and to initial nonspecific symptoms. Because postpartum GAS infections remain relatively uncommon, physicians often misconstrue the pain of developing infection as typical postpartum discomfort. The widespread use of pain-relieving agents after childbirth can further mask the signs of incubating infection. Such factors can contribute to a delay in diagnosis with catastrophic outcomes [19].
Diagnosis is particularly problematic in pregnant females with premature rupture of the membranes, whether spontaneously or iatrogenically initiated. In this instance, numerous pathogens including group B streptococcus, Mycoplasma, Chlamydia, and various anaerobes are important causes of ascending endomyometrial infection. Nonetheless, the occurrence of fever, chills, and abdominal pain in a pregnant or early postpartum woman warrants an aggressive attempt to provide a definitive diagnosis. Leukocytosis, hypotension, and tachycardia are signs of developing StrepTSS and are associated with higher mortality.
From the clinical perspective, it is difficult to evaluate abdominal pain and fever in a woman who has just delivered a newborn. Computed tomography scans, magnetic resonance imaging, and ultrasounds will show an enlarged uterus with edema, but it is virtually impossible to distinguish an early GAS infection of the uterine cavity from a normal postpartum uterus. This is in contrast to postpartum infections caused by Clostridium perfringens or mixed aerobic/anaerobic pathogens that are clearly associated with gas in the tissues. For developing GAS infection, repeated laboratory tests may provide important clinical clues. For example, a mounting white blood cell count with a left shift or an elevated C-reactive protein level should increase suspicion of infection. Clostridium sordellii, a cause of postpartum- and abortion-related infections, is characterized by exceedingly high white blood cell counts (50–200 000 cells/µL) and hemoconcentration and can thus be distinguished from GAS infection [20].
Treatment
Surgical Intervention
Therapeutic surgical intervention is crucial in most cases but is sometimes delayed because surgeons may be reluctant to perform a hysterectomy on a woman in her prime child-bearing years and because patients with early onset of shock and organ failure may require stabilization before surgery can be undertaken. Source control is equally vital for postpartum women who present with nongynecologic GAS infections such as empyemas, necrotizing fasciitis of extremities, and pyomyositis.
Antibiotics
In the current review, 85%–100% of patients received antibiotic treatment, the most common being β-lactam antibiotics, clindamycin, and vancomycin. Reports of 3 intrapartum and 2 early postpartum cases (0–2 days) did not describe antibiotic usage, and mortality was 100% and 40%, respectively.
There is no human evidence-based information upon which to formulate antibiotic recommendations. Thus, current treatment guidelines are based largely on in vitro susceptibility data and results from animal studies. All GAS remain sensitive to β-lactam antibiotics in vitro. Because such susceptibility does not always predict efficacy and because protein synthesis inhibitors (eg, clindamycin) proved more efficacious in animal models of GAS infection, the Infectious Diseases Society of America (IDSA) recommends that patients with severe GAS infection receive penicillin (2–4 million units every 4–6 hours intravenously) plus clindamycin (600–900 mg/kg every 8 hours intravenously) for 10–14 days [21]. Alternatives for the penicillin-allergic patient include linezolid or the combination of clindamycin plus either vancomycin or daptomycin [21].
A recent survey of pharyngeal and invasive GAS isolates in the United States and Mexico found that approximately 5% were resistant to erythromycin [22]. In Europe, erythromycin resistance was 10%–12% [23]. Particularly disturbing is recent report from China that 97% of pharyngeal isolates were resistant to both erythromycin and clindamycin [24].
Intravenous Immune Globulin
Two patients in our series received intravenous immune globulin (IVIG) and both survived. Although there is ample evidence for the role of extracellular streptococcal toxins in invasive GAS infections, the most recent IDSA guidelines committee concluded that definitive clinical data are lacking and that additional studies are necessary before a recommendation can be made regarding use of IVIG for treatment of severe GAS infection [21].
Prevention
Hospital Infection Control Practices
Modern hospital-associated epidemics of postpartum GAS infection, though rare, still occur. Examples include (1) rectal colonization of an obstetrics and gynecology physician, which resulted in 9 postpartum cases [25], and (2) vaginal colonization of an operating room nurse, resulting in wound infections of 18 patients, of whom 13 were female and required major gynecologic or abdominal surgery [26]. The CDC now recommends in-depth epidemiologic investigations for 2 or more cases of invasive GAS infection occurring at an institution in a 6-month period, including screening of healthcare personnel [27].
Screening for GAS During Pregnancy
There are conflicting views on the value of screening for GAS in vaginal samples prior to delivery. One report in our series described 2 expectant mothers in whom routine prenatal screening revealed GAS colonization of the vagina. The patients were not treated, and both subsequently developed postpartum GAS infections (Supplementary Reference 25). However, most women in our series who developed intrauterine infection before childbirth had little to no evidence of GAS in the vaginal vault. This agrees with the findings of Mead and Winn, who concluded that due to the rarity of GAS colonization in the genital tract during pregnancy (0.03%), screening-based approaches are not necessary [17]. Yet, prenatal vaginal screening for GAS colonization could easily be done via culture or rapid strep test [28] during routine screening for group B streptococcus in the third trimester of pregnancy [29]. For GAS-positive patients, the M type of the colonizing strain and its association with postpartum infections may be of critical importance and may warrant attempts at decolonization before childbirth.
Risk Assessment of GAS Acquisition in the Home Environment
In the United States, most women are discharged from the hospital within 48 hours of delivery. The presence of small children in the home represents an unrecognized or underappreciated risk factor for maternal GAS acquisition, as pharyngeal GAS colonization rate is much higher in children (25%) than adults (2%–5%). Children with active streptococcal pharyngitis likely pose an even greater risk to the mother. It should be noted that toddlers (<4 years of age) with active pharyngitis may present atypically with nasal discharge or excoriations and abdominal pain, but without sore throat [30]. Symptomatic or not, such children have various degrees of close contact with their mother, who is anatomically and likely immunologically more susceptible to pathogens. Thus, pregnant women should be advised of the importance of GAS in the home environment during pregnancy and in the postpartum period after discharge from the hospital. Given the incidence of reported antecedent sore throat among women in our series (55.6% in the third trimester and 28.6% in both the 5–8 day and >8-day groups), pharyngeal screening for GAS may be warranted, particularly in those women whose family members include young children, especially those with active pharyngitis or upper respiratory illness.
CONCLUSIONS
Patients with group A streptococcal puerperal sepsis typically present with fever, abdominal pain, and hypotension with or without tachycardia or leukocytosis. Those with intrapartum or late postpartum infection are more likely to have had upper respiratory tract GAS infection prior to development of intrauterine infection. GAS infection during the third trimester is disastrous for both mothers and infants. In the postpartum period, maternal mortality is highest when infection develops within 4 days of delivery. However, only 13.9% of GAS postpartum infections are nosocomially acquired [31] largely due to improved infection control practices and shorter hospital stays after delivery. This suggests that acquisition of GAS in the home environment is increasingly important and could be a target for intervention or prevention. Specific M types of GAS are particularly virulent in pregnant women, and potential screening strategies should be directed at those strains. For the astute clinician, the temporal relationship between infection onset and stage of pregnancy or puerperium, plus the epidemiologic factors of the patient, have important implications.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support. This work was supported by the Office of Research and Development, Medical Research Service, US Department of Veterans Affairs (A. E. B. and D. L. S.), and the National Institutes of Health (grant number P20 RR0116454/GM103408).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Maharaj D. Puerperal pyrexia: a review. Part I. Obstet Gynecol Surv. 2007;62:393–9. doi: 10.1097/01.ogx.0000265998.40912.5e. [DOI] [PubMed] [Google Scholar]
- 2.Holmes OW. The writings of Oliver Wendell Holmes. In: Holmes OW, editor. Medical essays 1842–1882. Boston: Houghton, Mifflin & Co; 1891. [Google Scholar]
- 3.Charles D, Larsen B. Streptococcal puerperal sepsis and obstetric infections: a historical perspective. Rev Infect Dis. 1986;8:411–22. doi: 10.1093/clinids/8.3.411. [DOI] [PubMed] [Google Scholar]
- 4.Bisno AL, Stevens DL. Streptococcal infections in skin and soft tissues. N Engl J Med. 1996;334:240–5. doi: 10.1056/NEJM199601253340407. [DOI] [PubMed] [Google Scholar]
- 5.Schuitemaker N, van RJ, Dekker G, van DP, van GH, Gravenhorst JB. Increased maternal mortality in the Netherlands from group A streptococcal infections. Eur J Obstet Gynecol Reprod Biol. 1998;76:61–4. doi: 10.1016/s0301-2115(97)00155-3. [DOI] [PubMed] [Google Scholar]
- 6.Chuang I, Van BC, Beall B, Schuchat A. Population-based surveillance for postpartum invasive group A Streptococcus infections, 1995–2000. Clin Infect Dis. 2002;35:665–70. doi: 10.1086/342062. [DOI] [PubMed] [Google Scholar]
- 7.Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–74. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 8.Holm SE, Norrby A, Bergholm AM, Norgren M. Aspects of pathogenesis of serious group A streptococcal infections in Sweden, 1988–1989. J Infect Dis. 1992;166:31–7. doi: 10.1093/infdis/166.1.31. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz B, Facklam RR, Brieman RF. Changing epidemiology of group A streptococcal infection in the USA. Lancet. 1990;336:1167–71. doi: 10.1016/0140-6736(90)92777-f. [DOI] [PubMed] [Google Scholar]
- 10.Martin PR, Hoiby EA. Streptococcal serogroup A epidemic in Norway 1987–1988. Scand J Infect Dis. 1990;22:421–9. doi: 10.3109/00365549009027073. [DOI] [PubMed] [Google Scholar]
- 11.Gaworzewska E, Colman G. Changes in the patterns of infection caused by Streptococcus pyogenes. Epidemiol Infect. 1988;100:257–69. doi: 10.1017/s095026880006739x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamilton SM, Bayer CR, Stevens DL, Lieber RL, Bryant AE. Muscle injury, vimentin expression, and nonsteroidal anti-inflammatory drugs predispose to cryptic group A streptococcal necrotizing infection. J Infect Dis. 2008;198:1692–8. doi: 10.1086/593016. [DOI] [PubMed] [Google Scholar]
- 13.Deutscher M, Lewis M, Zell ER, Taylor TH, Jr., Van BC, Schrag S. Incidence and severity of invasive Streptococcus pneumoniae, group A Streptococcus, and group B Streptococcus infections among pregnant and postpartum women. Clin Infect Dis. 2011;53:114–23. doi: 10.1093/cid/cir325. [DOI] [PubMed] [Google Scholar]
- 14.Sitkiewicz I, Green NM, Guo N, Bongiovanni AM, Witkin SS, Musser JM. Adaptation of group A Streptococcus to human amniotic fluid. PLoS One. 2010;5:e9785. doi: 10.1371/journal.pone.0009785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Timmons BC, Fairhurst AM, Mahendroo MS. Temporal changes in myeloid cells in the cervix during pregnancy and parturition. J Immunol. 2009;182:2700–7. doi: 10.4049/jimmunol.0803138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nooh MM, Nookala S, Kansal R, Kotb M. Individual genetic variations directly effect polarization of cytokine responses to superantigens associated with streptococcal sepsis: implications for customized patient care. J Immunol. 2011;186:3156–63. doi: 10.4049/jimmunol.1002057. [DOI] [PubMed] [Google Scholar]
- 17.Mead PB, Winn WC. Vaginal-rectal colonization with group A streptococci in late pregnancy. Infect Dis Obstet Gynecol. 2000;8:217–9. doi: 10.1155/S1064744900000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byrne JL, Aagaard-Tillery KM, Johnson JL, Wright LJ, Silver RM. Group A streptococcal puerperal sepsis: initial characterization of virulence factors in association with clinical parameters. J Reprod Immunol. 2009;82:74–83. doi: 10.1016/j.jri.2009.06.126. [DOI] [PubMed] [Google Scholar]
- 19.Gallup DG, Freedman MA, Meguiar RV, Freedman SN, Nolan TE. Necrotizing fasciitis in gynecologic and obstetric patients: a surgical emergency. Am J Obstet Gynecol. 2002;187:305–10. [PubMed] [Google Scholar]
- 20.Aldape MJ, Bryant AE, Stevens DL. Clostridium sordellii infection: epidemiology, clinical findings, and current perspectives on diagnosis and treatment. Clin Infect Dis. 2006;43:1436–46. doi: 10.1086/508866. [DOI] [PubMed] [Google Scholar]
- 21.Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373–406. doi: 10.1086/497143. [DOI] [PubMed] [Google Scholar]
- 22.Villasenor-Sierra A, Katahira E, Jaramillo-Valdivia AN, et al. Phenotypes and genotypes of erythromycin-resistant Streptococcus pyogenes strains isolated from invasive and non-invasive infections from Mexico and the USA during 1999–2010. Int J Infect Dis. 2012;16:e178–81. doi: 10.1016/j.ijid.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richter SS, Heilmann KP, Dohrn CL, et al. Increasing telithromycin resistance among Streptococcus pyogenes in Europe. J Antimicrob Chemother. 2008;61:603–11. doi: 10.1093/jac/dkm525. [DOI] [PubMed] [Google Scholar]
- 24.Liang Y, Liu X, Chang H, et al. Epidemiological and molecular characteristics of clinical isolates of Streptococcus pyogenes collected between 2005 and 2008 from Chinese children. J Med Microbiol. 2012;61(Pt 7):975–83. doi: 10.1099/jmm.0.042309-0. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Nosocomial group A streptococcal infections associated with asymptomatic health-care workers—Maryland and California, 1997. JAMA. 1999;281:1077–8. [PubMed] [Google Scholar]
- 26.Stamm WE, Feeley JC, Facklam RR. Wound infections due to group A Streptococcus traced to a vaginal carrier. J Infect Dis. 1978;138:287–92. doi: 10.1093/infdis/138.3.287. [DOI] [PubMed] [Google Scholar]
- 27.Prevention of Invasive Group A Streptococcal Infections Workshop Participants. Prevention of invasive group A streptococcal disease among household contacts of case patients and among postpartum and postsurgical patients: Recommendations from the Centers for Disease Control and Prevention. Clin Infect Dis. 2002;35:950–9. doi: 10.1086/342692. [DOI] [PubMed] [Google Scholar]
- 28.Clegg HW, Dallas SD, Roddey OF, et al. Extrapharyngeal group A Streptococcus infection: diagnostic accuracy and utility of rapid antigen testing. Pediatr Infect Dis J. 2003;22:726–31. doi: 10.1097/01.inf.0000078835.72497.ab. [DOI] [PubMed] [Google Scholar]
- 29.Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease—revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010;59(RR-10):1–36. [PubMed] [Google Scholar]
- 30.Wannamaker LW. Perplexity and precision in the diagnosis of streptococcal pharyngitis. Am J Dis Child. 1972;124:352–8. doi: 10.1001/archpedi.1972.02110150050009. [DOI] [PubMed] [Google Scholar]
- 31.O'Brien KL, Beall B, Barrett NL, et al. Epidemiology of invasive group a streptococcus disease in the United States, 1995–1999. Clin Infect Dis. 2002;35:268–76. doi: 10.1086/341409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.