IL28B.rs12979860 CC genotype has been associated with spontaneous clearance of hepatitis C virus (HCV). This prospective study suggests a relationship between CC genotype, HCV-specific T-cell response, and acute disease resolution, thus suggesting a possible important role of cell-mediated immunity in favoring HCV clearance in CC patients.
Keywords: acute hepatitis C, cell-mediated immunity, interleukin-28B, prognostic factors
Abstract
Background. A single-nucleotide polymorphism (SNP; rs12979860) near the IL28B gene has been associated with spontaneous and treatment-induced hepatitis C virus clearance. We investigated predictors of spontaneous disease resolution in a cohort of patients with acute hepatitis C (AHC), analyzing epidemiological, clinical and virological parameters together with IL28B.rs12979860 genotypes and cell-mediated immunity (CMI).
Methods. Fifty-six symptomatic AHC patients were enrolled and followed prospectively. CMI was measured in 31 patients at multiple time points by interferon-γ enzyme-linked immunospot assay and was correlated to the IL28B.rs12979860 SNP.
Results. Eighteen patients had a self-limiting AHC that was associated with female sex (P = .028), older age (P = .018), alanine aminotransferase level >1000 U/L (P = .027), total bilirubin level >7 mg/dL (P = .036), and IL28B.rs12979860 genotype CC (P = .030). In multivariate analysis, only CC genotype was independently associated with self-limiting AHC (odds ratio, 5.3; 95% confidence interval, 1.1–26.5). Patients with the CC genotype with self-limiting AHC had a stronger (P = .02) and broader (P = .013) CMI than patients with the CT genotype with chronically evolving AHC. In patients with chronically evolving disease, CC genotype was associated with a broader CMI compared to CT genotype (P = .028). A negative CMI was more frequently associated with CT genotype among persistently infected patients (P = .043) and with persistent infection among CT patients (P = .033).
Conclusions. Self-limiting AHC was independently associated with CC genotype. The correlation between IL28B.rs12979860 genotypes and CMI is suggestive of a possible important role of CMI in favoring hepatitis C virus clearance in CC patients.
Between 50% and 80% of patients with acute hepatitis C (AHC) progress to chronic infection, which is a major cause of death from liver disease worldwide [1–3]. Viral and host factors have been associated with self-limiting AHC (spontaneous disease resolution) and an early, vigorous, and broad T-cell response seems critical in favoring hepatitis C virus (HCV) clearance [4–8]. Immunogenetic factors have also been investigated; the single-nucleotide polymorphism (SNP) rs12979860 near the interleukin 28B (IL28B) gene, encoding the type III interferon (IFN)-λ-3, has recently been associated with spontaneous and treatment-induced HCV clearance [9–14]. The mechanisms underlying the association between the IL28B.rs12979860 SNP and HCV clearance have not been elucidated and both innate and adaptive immunity could be involved [15–17].
Prospective cohort studies on AHC patients offer the opportunity to study in depth the potential viral and host determinants of disease outcome. The identification of reliable predictors of self-limiting AHC is essential to optimize therapy and to avoid costly and useless treatment and vaccine design.
In this prospective cohort study, we evaluated the natural AHC course by analyzing epidemiological, clinical, and virological parameters and the IL28B.rs12979860 SNP.
Because HCV-specific cell-mediated immunity (CMI) was previously measured in a sizeable subset of our patients’ cohort [7, 8], we had the unique opportunity to search for potential correlations between the IL28B.rs12979860 SNP and CMI.
PATIENTS AND METHODS
Study Population
Among patients with suspected AHC referred to 10 infectious disease clinics and 4 drug dependency services scattered throughout Italy during 1999–2005, 93 patients agreed to participate in this study. Fifty-six of them met the criteria to define diagnosis and outcome and were included in the study. The criteria for AHC diagnosis were sudden disease onset in previously healthy individuals, without history of hepatitis; no other cause of acute hepatitis; alanine aminotransferase (ALT) levels >10 times the upper limit of normal; serum HCV RNA positivity; HCV risk factors reported within 6 months before disease onset; evidence of anti-HCV seroconversion (anti-HCV seroconversion after disease onset and/or anti-HCV positivity at disease onset associated with an anti-HCV negative test in the previous 12 months and/or recombinant immunoblot assay [RIBA] seroconversion [increase in the number and intensity of RIBA reactive bands in follow-up sera] [18]); viral load parameters indicative of AHC (viral load fluctuations >1 log and/or HCV RNA level <100 000 IU/mL during the first 3-month follow-up [19, 20]).
Patients were included in the study if they met at least 5 of these criteria, of which at least 1 had to be the evidence of anti-HCV seroconversion or the presence of viral load parameters indicative of AHC. These 2 latter criteria were useful for excluding patients with exacerbation of chronic hepatitis C [18–20]. Individuals with concomitant immunological disorders or human immunodeficiency virus coinfection were excluded.
Biochemical and virological parameters were examined at disease onset (ie, the first patient visit), then monthly or bimonthly during the first months, quarterly from month 3 to month 12, and every 4 months thereafter until patients withdrew their participation or underwent antiviral therapy. Patients who withdrew or underwent antiviral therapy within 6 months after onset were excluded from the study.
Self-limiting AHC was defined by ALT normalization together with HCV RNA clearance within 6 months from disease onset and until follow-up ended. Two or more consecutive negative HCV RNA measurements, with an interval of at least 6 months between the 2 last negative determinations, were required to confirm self-limiting AHC in patients with <1 year of follow-up. Patients still viremic after 6 months of follow-up, irrespective of ALT, were considered to be persistently infected.
The study protocol conformed to the Helsinki Declaration and was approved by the Ethics Committee of the Istituto Superiore di Sanità.
HCV Testing
Anti-HCV antibodies were tested by Microparticle Enzyme Immunoassay HCV version 3 (AXSYM System, Abbott Diagnostics, Wiesbaden, Germany) and confirmed by second-generation RIBA (Deciscan, Diagnostic Sanofi Pasteur, Marnes-la-Coquette, France).
Until 2007, HCV RNA detection and quantitation were performed as described previously [7]. Thereafter, Abbott RealTime HCV assay (Abbott Molecular, Des Plaines, Illinois) was used.
Genotyping was performed using VERSANT HCV Genotype 2.0 assay (LiPA; Siemens Healthcare Diagnostics, Tarrytown, New York).
Assessment of IL28B.rs12979860 SNP
Nucleic acids extracted from serum or plasma by QIASYNPHONY automated system (Qiagen GmbH, Hilden, Germany) were used in the rs12979860 hu IL28B assay (Roche, Applied Science) following the manufacturer's instructions. Genotyping was performed in a blinded fashion relative to AHC outcome. Genotyping results were checked for Hardy-Weinberg equilibrium.
HCV-Specific T-Cell Response
Blood samples were collected at 3 representative time points from diagnosis (0–1 months [T0–T1]; 6 months [T6]; between 12 and 24 months [T12–T24]), and peripheral blood mononuclear cells (PBMCs) were purified and frozen. The HCV-specific T-cell response was measured by IFN-γ enzyme-linked immunospot (ELISpot) assay in longitudinal samples from 31 patients in 2002–2003.
T-cell response was measured against 7 peptide pools corresponding to core, NS3 protease, NS3 helicase, NS4, NS5a, and NS5b (divided into pool I and II) antigens and expressed as spot-forming cells (SFC)/106 PBMC. Breadth of response was calculated as the median number of peptide pools recognized; total anti-HCV response was calculated by adding up reactivity against the individual peptide pools. Three-fold over “mock” value (obtained by incubating PBMCs without peptides) plus at least 55 specific spots per million cells was used as positive cutoff. ELISpot assay on frozen cells was validated and positive controls were always included in the assay. PBMCs collected during 2002–2005 from the remaining 25 patients of our cohort were no longer viable once thawed in 2011.
Statistical Analysis
Mann-Whitney test was used for continuous variables to assess differences between distributions. The χ2 and Fisher exact tests were used for comparison of frequencies between groups. A P value of .05 was considered significant.
To assess independent associations, a multiple logistic regression model was built using stepwise selection technique. Only variables detectable at disease onset and in standard hospital settings were considered in the model. All statistical analyses were performed using Stata software, version 11.
RESULTS
Patients' Clinical Characteristics
Fifty-six patients met the criteria for inclusion in the study (Table 1). All of them fulfilled at least 1 of the 2 criteria useful to exclude patients with exacerbation of chronic hepatitis C. Fifty-four patients had evidence of anti-HCV seroconversion (6 seroconverted after disease onset, 38 had anti-HCV negative tests in the previous 12 months; 41 showed RIBA seroconversion during the follow-up) and 49 patients showed viral load parameters indicative of AHC during the first 3-month follow-up (36 showed viral load fluctuations >1 log, 49 had HCV RNA levels <100 000 IU/mL).
Table 1.
Characteristics of 56 Patients With Acute Hepatitis C
| Parameter | Total No. of Patients | Self-limiting | Chronic Evolution | P Value |
|---|---|---|---|---|
| Number | 56 | 18 (32) | 38 (68) | |
| Sex, M/F | 39/17 | 9/9 | 30/8 | .028 |
| Age, y, median (range) | 31 (19–78) | 45 (20–78) | 30.5 (19–62) | .018 |
| Risk factor | ||||
| Intravenous drug use | 23 | 4 | 19 | 0.172 |
| Invasive procedure | 13 | 4 | 9 | |
| Needle-stick injury | 2 | 1 | 1 | |
| Cohabitation with HCV-positive person | 1 | 1 | 0 | |
| Sexual contact | 6 | 3 | 3 | |
| Healthcare employment | 3 | 2 | 1 | |
| Other percutaneous exposure | 2 | 0 | 2 | |
| Undetermined | 6 | 3 | 3 | |
| ALT, U/L median (range) | 1233.5 (29–5917) | 1487 (29–5917) | 1164 (129–3276) | .118 |
| Peak total bilirubin, mg/dL, median (range) | 4.83 (0.30–30.37) | 7.84 (0.84–30.4) | 2.9 (0.30–209) | .024 |
| RNA titer, UI/mL, median (range) | 13 850 (960–23.8 × 105) | 20 850 (1630–23.8 × 105) | 13 850 (960–19.4 × 105) | .796 |
| Total bilirubin ≥7 mg/dL | 23 (41) | 11 (61) | 12 (32) | .036 |
| Total bilirubin <7 mg/dL | 33 (59) | 7 (39) | 26 (68) | |
| ALT ≥1000 U/L | 35 (62.5) | 15 (83) | 20 (53) | .027 |
| ALT <1000 U/L | 21 (37.5) | 3 (17) | 18 (47) | |
| Diagnostic criteria | ||||
| Sudden onset of disease | 56 (100) | 18 (100) | 38 (100) | … |
| No other causes of acute hepatitis | 56 (100) | 18 (100) | 38 (100) | … |
| ALT >10 × ULN | 53 (95) | 17 (94) | 36 (95) | 1.000 |
| HCV RNA positivity | 52 (93) | 16 (89) | 36 (95) | .587 |
| Risk factors | 50 (89) | 15 (83) | 35 (92) | .374 |
| Evidence of anti-HCV seroconversiona | 54 (96) | 17 (94) | 37 (97) | .544 |
| Viral load parameters revealing acute infectionb | 49 (87.5) | 17 (94) | 32 (84) | .409 |
| No. of fulfilled diagnostic criteria | ||||
| 7 | 38 (68) | 12 (67) | 26 (68) | 1.000 |
| 6 | 13 (23) | 4 (22) | 9 (24) | |
| 5 | 5 (9) | 2 (11) | 3 (8) | |
| HCV genotype | ||||
| 1a | 6 | 1 | 5 | .106 |
| 1b | 15 | 6 | 9 | |
| 2a/2c | 11 | 2 | 9 | |
| 2b | 1 | 0 | 1 | |
| 3a | 16 | 4 | 12 | |
| 4c/4d | 1 | 0 | 1 | |
| Undetermined | 6 | 5 | 1 | |
| Symptoms at the onset | 56 (100) | 18 (100) | 38 (100) | … |
| Jaundice at the onset | 35 (62.5) | 14 (77.8) | 21 (55) | .104 |
| Follow-up, mo, median (range) | 21 (6–117) | 24 (6–103) | 18 (6–117) | .168 |
| IL28B.rs12979860 | 54 | 17 | 37 | |
| Allele | ||||
| T | 23 (21.3) | 3 (8.8) | 20 (27) | .032 |
| C | 85 (78.7) | 31 (91.2) | 54 (73) | |
| Genotype | ||||
| TT | 2 (3.7) | 0 (0) | 2 (5.4) | .030c |
| CT | 19 (35.2) | 3 (17.7) | 16 (43.2) | |
| CC | 33 (61.1) | 14 (82.3) | 19 (51.4) | |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ALT, alanine aminotransferase; HCV, hepatitis C virus; ULN, upper limit of normal.
a Evidence of anti-HCV seroconversion included anti-HCV seroconversion after disease onset and/or anti-HCV positivity at disease onset associated with a documented anti-HCV-negative test in the previous 12 months and/or recombinant immunoblot assay seroconversion.
b Viral load parameters indicative of acute HCV infection included detection of viral load fluctuations >1 log and/or HCV RNA levels <100 000 IU/mL within 3 months from disease onset.
c CC versus non-CC.
All patients were symptomatic and 35 had jaundice.
RIBA seroconversion was observed in all patients HCV RNA negative at disease onset.
Intravenous drug use was the most frequently reported risk factor, and 3a and 1b were the prevalent HCV genotypes (Table 1). No significant differences were found regarding clinical and virological characteristics at disease onset among patients with different risk factors or genotypes (data not shown).
IL28B.rs12979860 Genotype Distribution and Clinical Characteristics
IL28B.rs12979860 genotype was determined in 54 patients: 33 had CC, 19 CT, and 2 TT (Table 1). Table 2 reports the distribution of IL28B.rs12979860 genotypes according to the main clinico-epidemiological characteristics at disease onset. The only significant association was found between ALT levels ≥1000 U/L and CC genotype.
Table 2.
Distribution of IL28B.rs12979860 Genotypes According to the Most Relevant Clinico-Epidemiological Parameters
| Characteristic | Total (N = 54), No. | CC (n = 33), No. | Non-CC (n = 21), No. | P Value |
|---|---|---|---|---|
| Sex | .333 | |||
| Male | 37 | 21 | 16 | |
| Female | 17 | 12 | 5 | |
| Risk factor | .573 | |||
| Intravenous drug use | 22 | 11 | 11 | |
| Invasive procedure | 13 | 9 | 4 | |
| Sexual contact | 6 | 4 | 2 | |
| Other exposures | 8 | 5 | 3 | |
| No risk factor | 5 | 4 | 1 | |
| Genotype | .421 | |||
| 3a | 15 | 9 | 6 | |
| 1b | 15 | 8 | 7 | |
| 2a/2c | 11 | 7 | 4 | |
| 1a | 6 | 3 | 3 | |
| Other | 2 | 1 | 1 | |
| Undetermined | 5 | 5 | 0 | |
| Alanine aminotransferase level | .015 | |||
| ≥1000 U/L | 34 | 25 | 9 | |
| <1000 U/L | 20 | 8 | 12 | |
| Total bilirubin level | .975 | |||
| ≥7 mg/dL | 23 | 14 | 9 | |
| <7 mg/dL | 31 | 19 | 12 | |
| Jaundice | .199 | |||
| Yes | 34 | 23 | 11 | |
| No | 20 | 10 | 10 |
Clinical Outcome
Clinical outcome was determined after a median follow-up of 21 months (Table 1). Eighteen patients (32.1%) experienced self-limiting AHC. Among them, HCV clearance and ALT normalization within 3 months after onset occurred in 15 (83.3%) and 13 patients (72.2%), respectively. Thirty-eight patients became chronically infected. Only 1 AHC-resolving patient (A26) and 10 persistently infected patients were followed for <1 year. No difference in median follow-up duration was found between patients with different outcomes.
Seventeen of 18 patients with self-limiting AHC (94.4%) showed at least 1 negative HCV RNA test during the first 3-month follow-up, whereas the same was observed in only 36.8% (14/38) of chronically evolving patients (P < .0001). A similar figure was obtained analyzing the first month of follow-up (9/18 vs 4/38, respectively; P = .002).
Four patients were HCV RNA negative at disease onset (2 with self-limiting AHC): 3 became viremic some weeks later and 1 remained negative until follow-up ended.
Concerning the clinico-epidemiological features at disease presentation (Table 1), self-limiting AHC was significantly associated with female sex, older age, and higher median peak bilirubin level. In addition, AHC-resolving patients showed ALT levels ≥1000 U/L and total bilirubin levels ≥7 mg/dL significantly more often than persistently infected patients. No significant differences were observed between these 2 groups as concerned the other clinical and virological characteristics.
Clinical Outcome and IL28B.rs12979860 Genotype
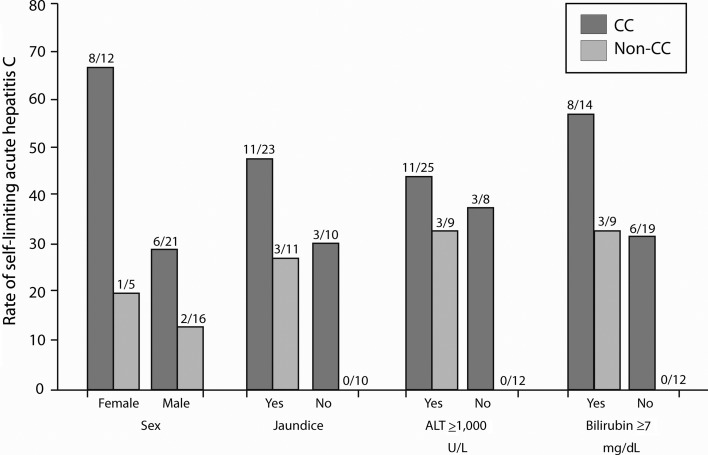
Seventeen of 54 patients tested for IL28B.rs12979860 spontaneously resolved AHC. Patients carrying the C allele or CC genotype achieved AHC resolution significantly more often than those with the T allele or non-CC genotype (Table 1). Figure 1 shows the effects of IL28B.rs12979860 genotype on the association between self-limiting AHC and some dichotomous variables at disease presentation. Approximately 67% of CC females and 29% of CC males resolved AHC, whereas only 20% of non-CC females and 12.5% of non-CC males did the same. Similar correlations were found between CC genotype and all other clinical characteristics examined, which, when present in CC patients, increased the probability of self-limiting AHC and, when absent in non-CC patients, nullified the chance of achieving AHC resolution.
Figure 1.
Spontaneous acute hepatitis C resolution and clinical features at disease onset by CC versus non-CC IL28B genotype. The fractional number on top of each column represents the proportion of patients with the clinical characteristic. Abbreviation: ALT, alanine aminotransferase.
Multivariate Analysis
After multivariate analysis, CC genotype was the only independent predictor of AHC resolution. CC patients were 5.3 times more likely to resolve AHC than non-CC patients (Table 3).
Table 3.
Univariate and Multivariate Analyses of Clinico-Epidemiological Characteristics at Disease Onset Associated With Self-limiting Acute Hepatitis C
| Variables at Disease Onseta | Univariate |
Multivariate |
||
|---|---|---|---|---|
| OR | (95% CI) | OR | (95% CI) | |
| IL28B.rs12979860-CC | 4.4 | (1.1–18.0) | 5.3 | (1.1–26.5) |
| Female sex | 3.7 | (1.1–12.6) | 2.9 | (.7–12.5) |
| Older age | 4.3 | (1.3–14.3) | 3.4 | (.8–14.9) |
| Bilirubin level >7 mg/dL | 3.4 | (1.1–11.0) | 3.2 | (.8–13.9) |
| ALT level >1000 U/L | 4.5 | (1.1–18.1) | …b | …b |
| Jaundice | 2.8 | (.8–10.2) | …b | …b |
Abbreviations: ALT, alanine aminotransferase; CI, confidence interval; OR, odds ratio.
a The adjusting variables were introduced in the logistic model because of their association (<0.11) in the univariate analysis.
b Factor removed by stepwise selection.
Relation Between IL28B.rs12979860 Genotype, CMI, and AHC Outcome
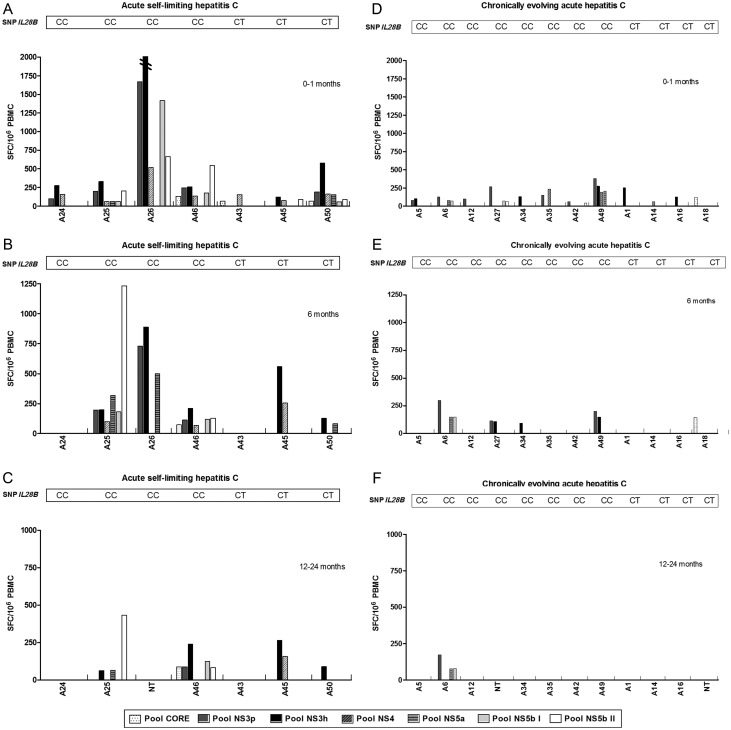
Thirty-one AHC patients (9 resolving and 22 chronically evolving) were analysed for HCV-specific CMI (Table 4) [7]. Among them, 16 were CC and 15 CT. Twelve of 31 patients always showed a negative CMI, whereas 19 patients scored CMI positive at T0–T1; 10 patients maintained a positive T-cell response at T6, and only 5 patients scored positive at T12–T24 (Table 4 and Figure 2). Also the strength and breadth of CMI decreased over time, especially among chronically infected patients (Figure 2).
Table 4.
IL28B.rs12979860 Genotype Distribution and Frequency of Hepatitis C Virus–Specific T-Cell Response in Patients With Self-limiting Acute Hepatitis C and in Those With Persistent Infection at Different Time Points During the Follow-up
| Self-limiting Infection |
Chronic Evolution |
||||||
|---|---|---|---|---|---|---|---|
| Time Point | Total | CC + CT | CC | CT | CC + CT | CC | CT |
| T0–T1 mo | |||||||
| Tested | 31 | 9 | 6 | 3 | 22 | 10 | 12 |
| T-cell response + | 19 | 7 | 4 | 3 | 12 | 8 | 4 |
| T-cell response − | 12 | 2 | 2 | 0 | 10 | 2 | 8 |
| T6 mo | |||||||
| Tested | 31 | 9 | 6 | 3 | 22 | 10 | 12 |
| T-cell response + | 10 | 5 | 3 | 2 | 5 | 4 | 1 |
| T-cell response − | 21 | 4 | 3 | 1 | 17 | 6 | 11 |
| T12–T24 mo | |||||||
| Tested | 27 | 8 | 5 | 3 | 19 | 8 | 11 |
| T-cell response + | 5 | 4 | 2 | 2 | 1 | 1 | 0 |
| T-cell response − | 22 | 4 | 3 | 1 | 18 | 7 | 11 |
Figure 2.
Analysis of the magnitude and breadth of the T-cell response in subjects with acute self-limiting infection (A–C) and subjects with chronic evolution (D–F). Peripheral blood mononuclear cell (PBMC) samples were tested by interferon-γ enzyme-linked immunospot assay (ELISpot) at 3 representative time points after disease onset (0–1 month, A and D; 6 months, B and E); between 12 and 24 months (C and F) against 7 peptide pools corresponding to core, NS3 protease, NS3 helicase, NS4, NS5a, and NS5b (pool I and II) antigens. To simplify, only responses above the threshold are shown. For all remaining subjects, ELISpot responses were negative at all time points tested. Numbers represent spot-forming cells per106 PBMCs. For each patient, IL28B genotype is reported. Abbreviations: NT, not tested; PBMC, peripheral blood mononuclear cell; SFC, spot-forming cell; SNP, single-nucleotide polymorphism.
Irrespective of IL28B.rs12979860 genotype, AHC-resolving patients always showed higher frequency, breadth, and strength of positive CMI than persistently infected patients. Significant differences in frequency, breadth and strength were detected at T12–T24 (4/8 vs 1/18; P = .0179; Table 3), at T0–T1 (median number of targeted peptide pools, 5 [range, 2–7] vs 1.5 [range, 1–4], respectively; P = .0037; Figure 2), and at T6 (564 SFC/106 PBMCs [range, 120–1223] vs 149.5 SFC/106 PBMCs [range, 18–670], respectively; P = .02), respectively.
Irrespective of disease outcome, CC patients always displayed higher frequency, breadth, and strength of positive CMI than did CT patients, although these differences were not significant. However, analyzing separately every possible combination between outcome, IL28B.rs12979860 genotype and CMI, we found a significant association between CT genotype and negative CMI in persistently infected patients at T0–T1 (2/10 and 8/12 in CC and CT patients, respectively; P = .043) and between persistent infection and negative CMI in CT patients at T12–T24 (negative CMI, 11/11 vs 1/3, in chronically infected and resolving patients, respectively; P = .033). A broader and stronger CMI in CC patients was particularly evident when CC-resolving patients were compared to persistently infected CT patients. In fact, at T0–T1 the median number of targeted peptide pools was significantly higher in the former group than in the latter one (5.5 [range, 3–6] vs 1 [range, 1–1], respectively; P = .013), and CMI was also stronger at all time points, although the difference was significant only at T6 (893.5 SFC/106 PBMCs [range, 204–1223] vs 122 SFC/106 PBMCs [range, 68–153], respectively; P = .02). In persistently infected patients, those with CC genotype showed a significant broader CMI compared to CT patients at T0–T1 (median numbers of targeted peptide pools, 4 [range, 3–5] vs 1 [range, 1–1], respectively; P = .028).
Overall, and irrespective of IL28B.rs12979860 genotype and AHC outcome, no significant differences were found in the ability to mount a specific CMI among patients harboring different HCV genotypes at any time point.
DISCUSSION
Standardized and stringent definitions for AHC diagnosis and outcome are essential for a reliable estimation of the rate of self-limiting AHC and for a nonbiased identification of its determinants [2, 3, 21]. In this study we adopted the best diagnostic criteria to exclude patients with exacerbation of chronic hepatitis C [18–20] and a more stringent definition of self-limiting AHC compared to many other studies [2]. In addition, the median follow-up duration for our AHC-resolving patients was 24 months and only 1 of them (A26) was followed for <1 year.
In our study, the rate of self-limiting AHC compares to that of previous studies on symptomatic patients [1–3, 7, 13, 22–24]. A high proportion (83.3%) of our AHC-resolving patients cleared HCV during the first 3-month follow-up period, as reported by others [1, 2, 22–24]. Furthermore, the detection of at least 1 negative HCV RNA test during the first 3-month follow-up period was significantly associated with self-limiting AHC.
In univariate analysis, self-limiting AHC was significantly associated with CC genotype, female sex, older age, higher median peak total bilirubin level, high ALT level (≥1000 U/L), and/or total bilirubin levels (≥7 mg/dL) at disease onset. Unlike other studies, in our study no association was found between AHC resolution and presence of jaundice at disease onset [1, 2, 11, 22, 23], probably because 62.5% of our symptomatic patients were jaundiced, whereas a marked elevation of biochemical parameters was likely a more accurate marker of strong antiviral immune response and disease resolution in our study cohort [11, 12]. The association between female sex and self-limiting AHC remains still unexplained [2, 13]. The Toll-like receptor 7, involved in recognition of viral products and activation of innate immunity, might underlie this association, as its stimulation determines higher IFN-α responses in females compared to males [25]. Finally, the association of self-limiting AHC with older age was already observed among Italian patients [26], whereas other studies reported an association with younger age or no association at all [1–3].
After multivariate analysis, only CC genotype was independently associated with self-limiting AHC, whereas only marginally significant associations were found for the other variables. However, the limited sample size might have negatively influenced the accuracy of this analysis, besides precluding reliable assessment of possible interactions between variables.
CC genotype increased the chance of self-limiting AHC in female patients and in those with marked elevations of biochemical parameters at onset. These findings help to outline the hypothetical profile of patients fated to different outcomes. For instance, a non-CC male patient, younger than 45 years, with low ALT and/or low bilirubin levels is unlikely to resolve AHC. Therefore, knowledge of host IL28B.rs12979860 genotype may help management of treatment. Currently, a 3-month observational period is recommended before starting AHC treatment, irrespective of symptoms [3, 27, 28]. However, some concerns exist on whether the delay in starting treatment may reduce response to therapy [22, 27–30]. Non-CC patients with the most unfavorable profile could be the best candidates for immediate treatment.
In our study, marked elevations of biochemical parameters were shown to be associated with both CC genotype and self-limiting AHC. This may suggest that CC patients are able to mount a more vigorous and effective immune response to HCV, leading to a more efficient clearance of infected hepatocytes, but also to a more severe liver injury.Type III IFNs can directly inhibit replication of most viruses, including HCV, and therefore may play an important role in innate immunity [15–17]. However, several studies have also demonstrated that type III IFNs are involved in both the regulation and development of adaptive immunity [15–17]. Under this respect, our study offers the unique opportunity to search for possible correlations between IL28B.rs12979860 genotype and HCV-specific CMI in influencing AHC outcome in humans.
We provided some evidence in favor of a correlation between IL28B.rs12979860 genotype and HCV-specific CMI, although the results were limited to a subset of patients, without exploring the full spectrum of CMI. In addition, these preliminary results suggest that CMI may possibly contribute to HCV clearance in CC patients. Even in persistently infected patients, those with CC genotype showed a significant broader CMI than CT patients. Thus, IL28B.rs12979860 genotype likely plays an important but not exclusive role, and a complex interplay exists between antiviral innate and adaptive immunity on one side, and host genetic factors on the other.
In this study, no significant differences were found in HCV genotype distribution with respect to the different clinical, immunological, and immunogenetic parameters analyzed. Other authors reported the associations between the IL28B SNP and some HCV genotypes [31, 32] were able to influence the response to antiviral therapy in chronic hepatitis patients [31]. However, these associations were not found for AHC patients [32]. We cannot completely exclude that the lack of associations between the IL28B SNP and HCV genotypes in our study was due to the limited sample size. Similar associations, if present, could bias the correlation between IL28B genotype, CMI, and outcome.
Our study has several limitations, the first of which is a limited statistical power, due to the small sample size. This is mainly due to the low incidence of AHC that often occurs in difficult to manage subjects. In addition, all patients were symptomatic; thus, they do not represent the overall spectrum of disease (mostly asymptomatic), and are more likely to clear HCV. Indeed, diagnosis of asymptomatic AHC is usually incidental or requires screening in people at risk for HCV; additionally, even some risk factors (eg, intravenous drug use, nosocomial exposure) have been associated with AHC resolution [33, 34]. The vast majority of our AHC patients (82%) were recruited from infectious diseases clinics and virtually all of them were symptomatic as asymptomatic infections are usually unrecognized in everyday clinical practice. Finally, we were unable to determine the contamination time in most, if not in all cases. This might be especially relevant for immunological investigation.
Despite the fact that our patients were recruited during 1999–2005, their clinico-epidemiological characteristics do not substantially differ from those of cases of symptomatic AHC notified to the Italian surveillance system in recent years [35]; thus, they are also representative of the current population of acutely HCV-infected patients.
In conclusion, our findings indicate that patients with symptomatic AHC have a high rate of spontaneous resolution that is independently associated with CC genotype. Furthermore, we provided for the first time evidence of a relationship between the IL28B.rs12979860 SNP, CMI, and AHC outcome, which warrants confirmation in larger prospective studies.
Notes
Other members of the Acute Hepatitis C Italian Study Group. Salvatore Buonocore, Gennaro Lettieri, Paola Pierri (Department of Infectious Diseases, Cotugno Hospital, Naples, Italy); Lucio Cosco, Teresa Ferraro (Infectious Diseases Unit, Pugliese Ciaccio Hospital, Catanzaro, Italy); Paola Scognamiglio, Maria Rosaria Capobianchi (National Institute of Infectious Diseases Lazzaro Spallanzani, Rome, Italy); Ubaldo Baldi (Infectious Diseases Unit, Umberto I Hospital, Nocera Inferiore, Salerno, Italy); Franco Montesano (Drug Addiction Service ASL 7, Soverato, Catanzaro, Italy); Giulia Audino (Drug Addiction Service ASL 7, Catanzaro, Italy); Caterina De Stefano (Department of the Dependencies ASL 11, Reggio Calabria, Italy); Antonio Caterini (AIDS Reference Center, Belcolle Hospital, Viterbo, Italy); Mario Cuccia (Epidemiology and Prevention Service, AUSL 3, Catania, Italy); Gabriella Girelli, Paola Perrone, Luca Laurenti (Department of Cell Biotechnology and Hematology, University La Sapienza, Rome, Italy); Enza Piccolella, Cristiano Scotta (Department of Cellular and Development Biology, University La Sapienza, Rome, Italy); Riccardo Cortese, Alfredo Nicosia, Alessandra Vitelli (Okairos , Rome, Italy).
Acknowledgments. The authors thank Luigina Ferrigno and Lorenzo Fantozzi for their technical assistance.
Financial support. This work was supported in part by the Viral Hepatitis Project, Istituto Superiore di Sanità (D. Leg.vo 30/12/1992 n. 502) and by grants from the ISS-NIH Collaborative Programme (“Novel strategies toward developing a prophylactic and therapeutic vaccine against hepatitis C virus”; fasc. 30F3).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Kamal SM. Acute hepatitis C: a systematic review. Am J Gastroenterol. 2008;103:1283–97. doi: 10.1111/j.1572-0241.2008.01825.x. [DOI] [PubMed] [Google Scholar]
- 2.Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13:34–41. doi: 10.1111/j.1365-2893.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55:245–64. doi: 10.1016/j.jhep.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 4.Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194:1395–406. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest. 2009;119:1745–54. doi: 10.1172/JCI39133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox AL, Mosbruger T, Lauer GM, Pardoll D, Thomas DL, Ray SC. Comprehensive analyses of CD8+ T cell responses during longitudinal study of acute human hepatitis C. Hepatology. 2005;42:104–12. doi: 10.1002/hep.20749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Folgori A, Spada E, Pezzanera M, et al. Early impairment of hepatitis C virus specific T cell proliferation during acute infection leads to failure of viral clearance. Gut. 2006;55:1012–9. doi: 10.1136/gut.2005.080077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spada E, Mele A, Berton A, et al. Multi-specific T-cell response and negative HCV RNA tests during acute HCV infection are early prognostic factors of spontaneous clearance. Gut. 2004;53:1673–81. doi: 10.1136/gut.2003.037788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas DL, Thio CL, Martin MP, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 11.Tillmann HL, Thompson AJ, Patel K, et al. A polymorphism near IL28B is associated with spontaneous clearance of acute hepatitis C virus and jaundice. Gastroenterology. 2010;139:1586–92. doi: 10.1053/j.gastro.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Beinhardt S, Aberle JH, Strasser M, et al. Serum level of IP-10 increases predictive value of IL28B polymorphisms for spontaneous clearance of acute HCV infection. Gastroenterology. 2012;142:78–85. doi: 10.1053/j.gastro.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 13.van den Berg CH, Grady BP, Schinkel J, et al. Female sex and IL28B, a synergism for spontaneous viral clearance in hepatitis C virus (HCV) seroconverters from a community-based cohort. PLoS One. 2011;6:e27555. doi: 10.1371/journal.pone.0027555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rau M, Baur K, Geier A. Host genetic variants in the pathogenesis of hepatitis C. Viruses. 2012;4:3281–302. doi: 10.3390/v4123281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balagopal A, Thomas DL, Thio CL. IL28B and control of hepatitis C virus infection. Gastroenterology. 2010;139:1865–76. doi: 10.1053/j.gastro.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallagher G, Megjugorac NJ, Yu RY, et al. The lambda interferons: guardians of the immune-epithelial interface and the T-helper 2 response. J Interferon Cytokine Res. 2010;30:603–15. doi: 10.1089/jir.2010.0081. [DOI] [PubMed] [Google Scholar]
- 17.Pagliaccetti NE, Robek MD. Interferon-λ in HCV infection and therapy. Viruses. 2010;2:1589–602. doi: 10.3390/v2081589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu SN, Tung HD, Chen TM, et al. Is it possible to diagnose acute hepatitis C virus (HCV) infection by a rising anti-HCV titre rather than by seroconversion? J Viral Hepat. 2004;11:563–70. doi: 10.1111/j.1365-2893.2004.00533.x. [DOI] [PubMed] [Google Scholar]
- 19.McGovern BH, Birch CE, Bowen MJ, et al. Improving the diagnosis of acute hepatitis C virus infection with expanded viral load criteria. Clin Infect Dis. 2009;49:1051–60. doi: 10.1086/605561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamili S, Drobeniuc J, Araujo AC, Hayden TM. Laboratory diagnostics for hepatitis C virus infection. Clin Infect Dis. 2012;55(suppl 1):S43–8. doi: 10.1093/cid/cis368. [DOI] [PubMed] [Google Scholar]
- 21.Amin J, Law MG, Micallef J, et al. Potential biases in estimates of hepatitis C RNA clearance in newly acquired hepatitis C infection among a cohort of injecting drug users. Epidemiol Infect. 2007;135:144–50. doi: 10.1017/S0950268806006388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerlach JT, Diepolder HM, Zachoval R, et al. Acute hepatitis C: high rate of both spontaneous and treatment-induced viral clearance. Gastroenterology. 2003;125:80–8. doi: 10.1016/s0016-5085(03)00668-1. [DOI] [PubMed] [Google Scholar]
- 23.Lewis-Ximenez LL, Lauer GM, Schulze Zur Wiesch J, et al. Prospective follow-up of patients with acute hepatitis C virus infection in Brazil. Clin Infect Dis. 2010;50:1222–30. doi: 10.1086/651599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santantonio T, Medda E, Ferrari C, et al. Risk factors and outcome among a large patient cohort with community-acquired acute hepatitis C in Italy. Clin Infect Dis. 2006;43:1154–9. doi: 10.1086/507640. [DOI] [PubMed] [Google Scholar]
- 25.Berghöfer B, Frommer T, Haley G, Fink L, Bein G, Hackstein H. TLR7 ligands induce higher IFN-alpha production in females. J Immunol. 2006;177:2088–96. doi: 10.4049/jimmunol.177.4.2088. [DOI] [PubMed] [Google Scholar]
- 26.Santantonio T, Sinisi E, Guastadisegni A, et al. Natural course of acute hepatitis C: a long-term prospective study. Dig Liver Dis. 2003;35:104–13. doi: 10.1016/s1590-8658(03)00007-0. [DOI] [PubMed] [Google Scholar]
- 27.Santantonio T, Fasano M, Sinisi E, et al. Efficacy of a 24-week course of PEG-interferon alpha-2b monotherapy in patients with acute hepatitis C after failure of spontaneous clearance. J Hepatol. 2005;42:329–33. doi: 10.1016/j.jhep.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 28.Grebely J, Matthews GV, Dore GJ. Treatment of acute HCV infection. Nat Rev Gastroenterol Hepatol. 2011;8:265–74. doi: 10.1038/nrgastro.2011.32. [DOI] [PubMed] [Google Scholar]
- 29.Corey KE, Mendez-Navarro J, Gorospe EC, Zheng H, Chung RT. Early treatment improves outcomes in acute hepatitis C virus infection: a meta-analysis. J Viral Hepat. 2010;17:201–7. doi: 10.1111/j.1365-2893.2009.01167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deterding K, Grüner N, Buggisch P, et al. Delayed versus immediate treatment for patients with acute hepatitis C: a randomised controlled non-inferiority trial. Lancet Infect Dis. 2013;S1473–3099:70059–8. doi: 10.1016/S1473-3099(13)70059-8. [DOI] [PubMed] [Google Scholar]
- 31.Sarrazin C, Susser S, Doehring A, et al. Importance of IL28B gene polymorphisms in hepatitis C virus genotype 2 and 3 infected patients. J Hepatol. 2011;54:415–21. doi: 10.1016/j.jhep.2010.07.041. [DOI] [PubMed] [Google Scholar]
- 32.Neukam K, Nattermann J, Rallón N, et al. Different distributions of hepatitis C virus genotypes among HIV-infected patients with acute and chronic hepatitis C according to interleukin-28B genotype. HIV Med. 2011;12:487–93. doi: 10.1111/j.1468-1293.2011.00912.x. [DOI] [PubMed] [Google Scholar]
- 33.Shores NJ, Maida I, Soriano V, Núnez M. Sexual transmission is associated with spontaneous HCV clearance in HIV-infected patients. J Hepatol. 2008;49:323–8. doi: 10.1016/j.jhep.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 34.Martínez-Bauer E, Forns X, Armelles M, et al. Hospital admission is a relevant source of hepatitis C virus acquisition in Spain. J Hepatol. 2008;48:20–7. doi: 10.1016/j.jhep.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 35.Spada E, Mele A, Mariano A, Zuccaro O, Tosti ME. Risk factors for and incidence of acute hepatitis C after the achievement of blood supply safety in Italy: results from the national surveillance system. J Med Virol. 2013;85:433–40. doi: 10.1002/jmv.23485. [DOI] [PubMed] [Google Scholar]