Chronic pulmonary aspergillosis (CPA) is an infectious disease that slowly destroys lung tissue. Using a standardized instrument, this study reported that CPA patients had substantial health status impairment. Antifungal therapy improved health status and prevented CPA progression in most patients.
Keywords: Aspergillus fumigatus, dyspnea, itraconazole, voriconazole, posaconazole
Abstract
Background. Chronic pulmonary aspergillosis (CPA) is an infectious disease that progressively destroys lung tissue. To date, no longitudinal data on the efficacy of antifungal treatment on health status in CPA patients exist.
Methods. Using the standardized St George's Respiratory Questionnaire, the health status of 122 patients with was assessed at baseline and quarterly over 12 months. The score range was 0–100, where higher score indicates worse heath status, and a change of ≥4 was deemed the minimal clinically important difference. Lung function, body mass index, Medical Research Council dyspnea scale, disease severity, and demographic data were reported.
Results. Mean age of patients was 59 years, and 45% were female. Overall, patients with CPA had substantial health status impairment at baseline. After treatment, 47%–50% gained substantial health improvement with a mean reduction of score of 14 at both 6 and 12 months, whereas 32% deteriorated with a mean rise of score of 11 and 14 after 6 and 12 months of treatment and observation, respectively, and 21% were not much different (stable).
Patients gained therapeutic benefit irrespective of their illness severity where >50% of those who had “poor” and “very poor” status at baseline improved with score reduction of ≥4 after 6 months of treatment. Replicating this analysis using a health status category, we found that at least 50% of patients with a “poor/very poor” health status category at baseline improved significantly to “fair” or “good/very good” categories. Side effects burdened health status considerably. In multivariate analysis, dyspnea and disease severity significantly defined health status impairment.
Conclusions. Antifungal therapy improved health status and prevented CPA progression in most patients.
Chronic pulmonary aspergillosis (CPA) is a chronic progressive infectious disease principally caused by Aspergillus fumigatus [1–3]. It usually results in multiple pulmonary cavities, fibrosis, and often, aspergilloma development [1, 3–5]. Occasionally, patients have single cavities or nodules with little or no cavitation [6]. Untreated, patients with CPA have a ≥50% 5-year mortality [7, 8] and many die early after clinical presentation [9]. Patients describe breathlessness, weight loss, fatigue, cough, and hemoptysis, and subsequently experience substantial physical capacity limitation and health status impairment [1].
Studies have increasingly shown that treatment with long-term antifungal medication improves symptoms such as cough, weight loss, and fatigue with improvement in daily life activities [2, 10, 11]. Prevention of progressive lung destruction is another key goal of treatment, as well as reducing mortality [10, 12]. Response to antifungal therapy is generally slow; most patients who respond have done so by 6 months [10, 11]. A recent study from our center in 79 patients with CPA found response (improvement or stability) to posaconazole in 61% at 6 months [10]. A more recent multicenter study found a 32% improvement rate in 41 patients with CPA after 6 months of treatment with voriconazole [11]. A comparative study of itraconazole against standard care (no antifungal therapy) for 6 months followed by 6 months of follow-up showed deterioration in 64% and 36% stable on no therapy, compared with only 24% deterioration and 35% improvement at 6 months on itraconazole [13]. Six months after stopping itraconazole, 30% had relapsed. However, assessing response has been based primarily on clinical assessment including weight gain and falling Aspergillus immunoglobulin G (IgG) titers as objective measures; radiological response is extremely slow. No studies have explored the effect of antifungal therapy on the health status of CPA patients using standardized and validated tools and is important for future therapeutic interventions in CPA.
The St George's respiratory questionnaire (SGRQ) [14] is a well-established, respiratory-specific measure that has been extensively used for assessing health status or therapeutic response in chronic obstructive pulmonary disease (COPD) [14–17] and other chronic pulmonary illnesses such as asthma [18], bronchiectasis[19], cystic fibrosis [20], and tuberculosis [21]. We have recently demonstrated that the SGRQ is a valid and reliable instrument for assessing health status in patients with CPA [22]. In that cross-sectional analysis with 88 CPA patients, SGRQ scores correlated significantly with the Medical Research Council (MRC) dyspnea scale and the Health Survey SF-36 components, and differentiated well between all different bands of disease severity [22, 23] as well as physician-rated disease severity [22]. The physician-rated disease severity was based on the main clinical manifestations of CPA and the characteristic radiological changes of pericavitary consolidation, pleural thickening, cavitary size and number, and presence or absence of fungal ball [10].
Here, we longitudinally assessed health status in patients with CPA every 3 months over a period of 12 months, and we investigated the effect of long-term antifungal treatment in improving or stabilizing health status in patients with CPA. We also compared characteristics of patients who improved or remained stable vs those who had worsening health status, and investigated factors associated with impaired health status in CPA.
METHODS
Study Design and Population
From >200 patients referred to the National Aspergillosis Centre (NAC) over 3 years, we present longitudinal prospectively collected data on 122 patients with CPA who fulfilled the selection criteria: (1) diagnosis of CPA (based on clinical, radiological, and laboratory findings) [1, 10, 24]; (2) completion of the SGRQ questionnaire every 3 months, until at least 6 months; (3) completion of SGRQ (no missing items) and adequate assessment criteria of CPA such as MRC dyspnea score and pulmonary function tests.
CPA severity and complexity was assessed using the NAC banding criteria: band 1, 2, or 3 (band 1 describes less severe and complex category [22, 23], as summarized in the Supplementary Data). The data were collected with the goal of establishing the usefulness and feasibility of adding a subjective health status measure to routine clinical assessment. This analysis will focus on data collected at baseline and at 3, 6, 9, and 12 months (±14 days).
Treatment
Typically patients start therapy with itraconazole, and voriconazole and posaconazole are second and third line of therapy, respectively. However, a small number of patients were given voriconazole or posaconazole as primary therapy [10, 25]. Continuous intravenous therapy was given to patients who failed or could not tolerate oral azole therapy, usually with multidrug resistance. All azole therapy was monitored with plasma therapeutic drug monitoring and doses were adjusted accordingly, and/or the preparation was switched in the case of itraconazole. We carefully recorded SGRQ scores and the completion date for each patient in each visit. Then, we reviewed the patient's note to identify the treatment that patient was taking in approximately the last 3 months before each SGRQ completion. Patients were routinely checked for Haemophilus influenzae and pneumococcal antibody status, and immunized if not protected.
The SGRQ and Its Minimal Clinically Important Difference
Patients first judge their overall health status by selecting 1 of 5 options: very poor, poor, fair, good, and very good; and then by responding to 50 items that cover symptoms, activity, and impact domains [14]. The scale is scored from 0 to 100, where the higher score indicates worse health status. More information on the SGRQ's structure and administration method can be seen in Jones et al [14], and on its validity in CPA in Al-shair et al [22]; a summary is provided in the Supplementary Data.
Jones reviewed several studies that aimed to estimate the minimal clinically important difference (MCID) of SGRQ using different methods [18, 26]. He concluded that change of score of ≥4 in the total SGRQ is a clinically significant difference [18, 26] (as summarized in the Supplementary Data). Response to therapy was defined as “improved” (score reduction of ≥4 in the total SGRQ), “deteriorated” (score increase of ≥4 in the total SGRQ), and “stable” (neither improved nor deteriorated). For further exploration, we used an arbitrary MCID of ≥10 units in the total SGRQ score. We also used a wider scale examining change in overall health status category: very poor, poor, fair, good, and very good at visits.
Statistical Analysis
Analysis of variance was used to examine the difference in means of parametric data (SGRQ symptom and impact dimensions and total scores) between overall heath status categories, CPA bands, and MRC dyspnea grades, whereas differences in the nonparametric data (activity dimension score) were examined using the Kruskal-Wallis test. The Wilcoxon test was used to compare total and dimensional SGRQ scores at visit 1 (baseline) vs 2, 3, 4, and 5. The χ2 test was used to investigate the categorical correlation between overall health status categories between visits. Univariate correlation between parametric variables was examined using the Pearson coefficient correlation, and Spearman coefficient correlation was used for nonparametric data. Multivariate linear regression analyses were used to investigate factors that are independently associated with health status. All aforementioned tests and plotted figure were performed using SPSS software, version 20 (IBM SPSS, Chicago, Illinois). Information on additional measurements and investigations are provided in the Supplementary Data.
RESULTS
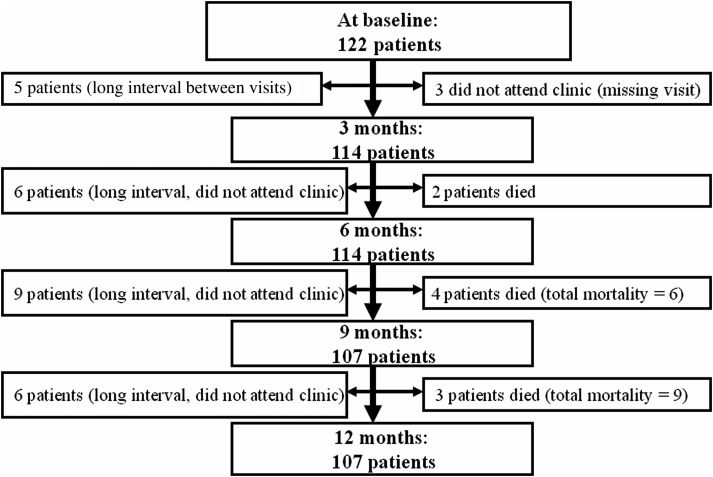
Health status of 122, 114, 114, 107, and 107 patients with CPA was longitudinally assessed at baseline and at 3, 6, 9, and 12 months, respectively (Figure 1).
Figure 1.
Total number of patients at visits, and reasons for exclusion compared to baseline visit.
At baseline, the mean age was 59 years; 45% of patients were women. The patients were categorized into different CPA severity bands where 43 (35%), 62 (51%), and 17 (14%) had CPA bands 1, 2, and 3 respectively. Overall, lung function was moderately poor, and patients were dyspneic and had to stop for breath when walking at their own pace on a level surface (MRC dyspnea grade 3). They had impairment in health status, reporting high scores of total (mean, 56 [range, 3–95]) and dimensional SGRQ scores (Table 1). Nine (7%) patients died between 3 and 12 months (Figure 1) and had generally worse disease at baseline (Table 1).
Table 1.
Baseline Characteristics and Demographic of the Study Population
| Variable | All Patients (n = 122) | Alive (n = 113) | Dead (n = 9) | P Value |
|---|---|---|---|---|
| Age, y | 59 (11.6) | 58.4 (11) | 64.9 (13) | .11 |
| Female sex, No. | 55 (45%) | 51 (93%) | 4 (7%) | .9 |
| FEV1, liters | 1.75 (0.77) | 1.8 (0.7) | 1.5 (0.9) | .5 |
| FEV1% of predicted | 63.2 (23) | 63.2 (23) | 61.5 (22) | .9 |
| FVC, liters | 2.87 (1) | 2.9 (1) | 2.5 (1.2) | .4 |
| FVC % of predicted | 83 (22.6) | 83.4 (23) | 81.5 (19) | .9 |
| FEV1/FVC | 62.6 (17.3) | 62.4 (17) | 61.4 (19) | .9 |
| Weight, kg | 64.6 (16) | 65.2 (16) | 56.6 (15) | .2 |
| BMI, kg/m2 | 22.5 (4.3) | 22.8 (4.5) | 20.9 (4) | .3 |
| MRC dyspnea scale | 2.9 (1.2) | 2.8 (1.2) | 3.6 (0.8) | .11 |
| CPA bands, No. | ||||
| Band 1 | 43 (35%) | 42 (37%) | 1 (11%) | .3 |
| Band 2 | 62 (51%) | 56 (50%) | 6 (67%) | |
| Band 3 | 17 (14%) | 15 (13%) | 2 (22%) | |
| SGRQ, symptom domain | 64 (23) | 64 (23) | 70.3 (18) | .4 |
| SGRQ, activity domain, median (IQR) | 73 (54–92) | 73 (53–92) | 86 (60–96) | .3 |
| SGRQ, impact domain | 48 (22) | 46.8 (21) | 59.1 (28) | .11 |
| SGRQ, total score | 56 (21) | 55.7 (21) | 66.4 (23) | .14 |
| Antifungal agent | ||||
| None | 13 (11%) | 13 (11%) | 0 | |
| Itraconazole | 48 (39%) | 42 (37%) | 6 (67%) | |
| Voriconazole | 26 (21.5%) | 26 (23%) | 0 | |
| Posaconazole | 32 (26%) | 29 (26%) | 3 (33%) | |
| Amphotericin B | 1 (0.8%) | 1 (1%) | 0 | |
| Micafungin | 2 (1.7%) | 2 (2%) | 0 |
Data are presented as mean (standard deviation) unless otherwise indicated. The P value is for differences between alive and dead patients.
Abbreviations: BMI, body mass index; CPA, chronic pulmonary aspergillosis; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; IQR, interquartile range; MRC, Medical Research Council; SGRQ, St George's Respiratory Questionnaire.
Overall Description of Health Status Throughout the Visits
Using the standard categories of overall health status at baseline, the majority of the patients judged their health status as fair (55 [46.6%]), followed by poor (34 [28.8%]), good (16 [13.6%]), very poor (10 [8.5%]), and very good (3 [2.5%]), respectively (Supplementary Table 1).
For all visits, total SGRQ scores correlated well with patients’ own health status assessment (P < .0001 for all the visits; Supplementary Table 1), so very poor scores were the highest with a range of 71–83, followed by poor (70–73), fair (52–57), good (31–36), and very good (5–44).
Overall Assessment of Treatment Efficacy
At visits at 3, 6, 9, and 12 months, we consistently found that total and domain scores were either lower (improved health status) or similar compared to baseline, with total SGRQ falling from baseline by approximately 3 units (Supplementary Table 2).
At 6 months compared to baseline, 54 (47.4%) patients reported improvement in total SGRQ score by ≥4; 24 (21.1%) remained stable, and 36 (31.5%) reported deterioration by a score of ≥4 (Figure 2). Between groups, the mean total SGRQ scores for the improved, stable, and deteriorated groups were 48 (SD, 20), 52 (SD, 26), and 63 (SD, 19), respectively (P = .004; Table 2).
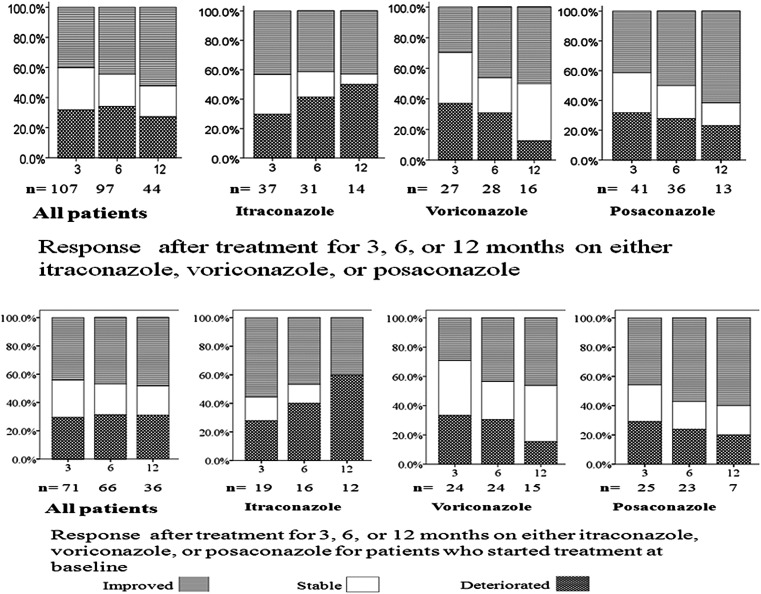
Figure 2.
Changes in clinical status for patients who continuously were on the same antifungal therapy: all, and then those on either itraconazole, voriconazole, or posaconazole alone at 3, 6, and 12 months. Two situations were demonstrated: patients who were already on treatment before completing the first St George's Respiratory Questionnaire (SGRQ) or those who completed the SGRQ and then started on treatment.
Table 2.
Total and Dimensional St George's Respiratory Questionnaire Scores of Patients Who Improved, Remained Stable, or Deteriorated at 6 and 12 Months of Follow-up Compared to Baseline
| SGRQ | At Baseline | At 6 mo | P Value | At Baseline | At 12 mo | P Value |
|---|---|---|---|---|---|---|
| Improved | ||||||
| SGRQ, symptom domain | 72.6 (20) | 61 (22) | <.0001 | 65.9 (22) | 52.8 (25) | <.0001 |
| SGRQ, activity domain | 70.5 (22) | 57.4 (26) | <.0001 | 68 (28) | 57.6 (31) | <.0001 |
| SGRQ, impact domain | 54.1 (20) | 39.2 (20) | <.0001 | 50.6 (22) | 35.5 (21) | <.0001 |
| SGRQ, total score | 62 (18) | 48 (20) | <.0001 | 58.5 (23) | 44.3 (23) | <.0001 |
| Stable | ||||||
| SGRQ, symptom domain | 55.5 (23) | 56.9 (24) | .6 | 55.9 (28) | 58.9 (28) | .2 |
| SGRQ, activity domain | 63 (33) | 62.7 (31) | .9 | 62.3 (33) | 60.3 | .4 |
| SGRQ, impact domain | 42 (25) | 43 (26) | .7 | 40.6 (23) | 39.7 (24) | .4 |
| SGRQ, total score | 51 (26) | 51.6 (26) | .15 | 49.4 (24) | 49.4 (24) | .8 |
| Deteriorated | ||||||
| SGRQ, symptom domain | 58.1 (23) | 71.8 (18) | <.0001 | 65 (22) | 70 (18) | .1 |
| SGRQ, activity domain | 65.7 (27) | 75.4 (25) | <.0001 | 66 (21) | 80 (18) | <.0001 |
| SGRQ, impact domain | 42 (19) | 51 (19) | <.0001 | 42 (19) | 60 (18) | <.0001 |
| SGRQ, total score | 52 (19) | 63 (19) | <.0001 | 53.1 (18) | 67.4 (15) | <.0001 |
The scale is scored from 0 to 100.
Abbreviation: SGRQ, St George's Respiratory Questionnaire.
From a baseline score of 62 (SD, 18), the total SGRQ score of the improved group at 6 months was substantially reduced to 48 (P < .0001); the stable group had almost the same score (P = .15), whereas the deteriorated group score rose by 11 units to 63 at 6 months (P < .0001) (Table 2). Similar changes were seen at 12 months: 53 (49.5%) of patients reported improvement in total score of ≥4, 20 (18.7%) remained stable, and 34 (31.8%) reported deterioration with a rise in total score of ≥4 (Table 2).
Total and dimensional SGRQ scores were consistent at both 6 and 12 months (Table 2). Additional data on contrasting response between 6 months with 12 months, and exploring overall response using overall health status categories, are shown in the Supplementary Data.
Effect of Therapy
As can been seen in Figure 2, 66%–68% of the patients who stayed on the same antifungal treatment for 3–6 months were either improved or remained stable (improvement of 40%–44%). Analyzing data for every antifungal agent separately, this was a consistent finding (Figure 2). As treatment extended from 3 to 6 months, the improved group on voriconazole and posaconazole increased by 13% and 8% and the deteriorated group decreased by 5% and 3%, respectively. Among those who deteriorated on itraconazole, 2 patients did not reach plasma therapeutic level. Two patients, 1 on amphotericin B and the other on micafungin, showed consistent improvement.
Forty-six patients (38%) experienced 1 or more side effect. Ten side effects were reported, and photosensitivity (due to voriconazole) and nausea were most frequent (Supplementary Figure 2); 9 of the patients needed to switch to another agent. Patients who experienced side effect(s) had significantly worse health status than those who had no side effects at 12 months, with a mean total SGRQ of 61 (SD, 22) vs 50 (SD, 23) (P = .01). Similar differences were observed in the other visits.
Moreover, those who were already on treatment for ≥3 months before completing their first SGRQ (our baseline) had slightly better health status than those (76 patients) who started treatment at baseline (Figure 2).
Patients on the Same Treatment for 12 Months
Forty-four patients remained on the same agent for 12 months (14, 16, and 13 on itraconazole, voriconazole, and posaconazole, respectively, and 1 on micafungin; Figure 3). We found that 23 (52%) improved, 9 (20%) remained stable, and 12 (30%) deteriorated. The mean total SGRQ score of the improved group at baseline was 58 (SD, 21) and fell to 45 (SD, 23) at 12 months (P < .001) (Supplementary Table 5).
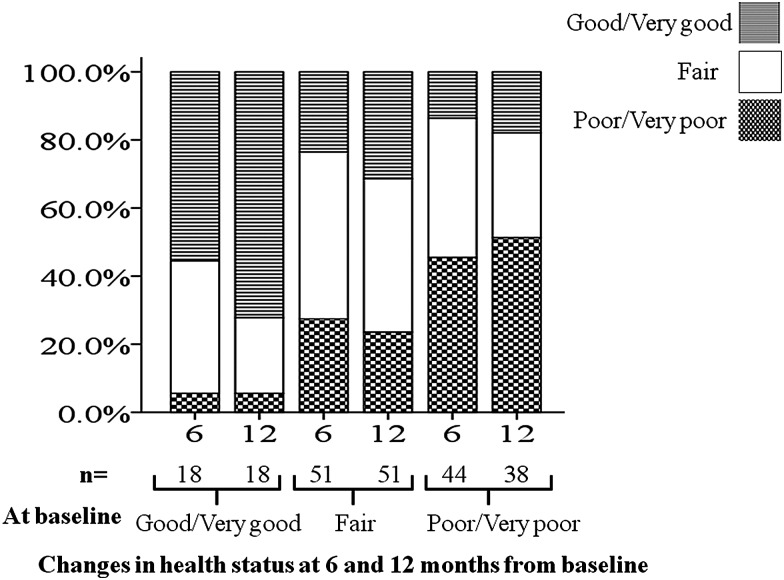
Figure 3.
Changes in overall health status at 6 and 12 months from baseline.
Most of the 12 patients who deteriorated were on itraconazole, and side effects may have contributed to “deterioration” (5 of 7 reported possible drug-related side effects; eg, ankle edema, headache, and skin rash), whereas the vast majority of patients on voriconazole or posaconazole improved as demonstrated in Figure 2. Note that patients who could not complete 6 months therapy were not included, so early discontinuation of therapy is not included in this assessment. Additionally, patients (n = 44) on the same agent for 12 months had better health status than those who either changed to different agents (n = 28) or those who had intermittent periods with no treatment (n = 35), but the difference did not reach statistical significance.
Major Improvement or Deterioration
To define the proportion of patients with major changes, we selected an arbitrary MCID of ≥10-unit improvement or deterioration of score. At 3, 6, and 12 months, 21 (20%), 25 (26%), and 11 (25%) had marked improvement (Supplementary Table 6). Conversely, 15 (14%), 20 (21%), and 6 (14%) had deteriorated significantly at the same time intervals. There were no striking differences between patients on itraconazole, voriconazole, or posaconazole.
Severity of Disease and Ability to Respond
Patients achieved benefit from treatment irrespective of their health status severity. We found that the majority of patients (>50%) who described their health status as poor or very poor at baseline reported improvement by a score reduction of ≥4 in total SGRQ at 6 months of follow-up (Supplementary Table 7).
Furthermore, by using a wider scale by examining changes in overall health status judgment, we found that approximately half of the patients who described their overall health status as poor or very poor at baseline improved considerably to either fair or good/very good categories at 6 and 12 months of follow-up (Figure 3). Moreover, among the 18 who described their overall health status as good or very good at baseline, only 1 patient deteriorated to poor/very poor at 6 and 12 months of follow-up. Most of the 51 patients who described their health status as fair at baseline remained stable over 6 and 12 months, with a slight increase in those who improved (4 patients) and fewer who deteriorated (2 patients) at 12 months compared to 6 months.
Variables Associated With Health Status Impairment in CPA
In univariate analysis, several factors were found to be associated with health status impairment (total SGRQ score; Table 3). However, using total SGRQ score as a dependent variable, multivariate analysis showed that only MRC dyspnea score and CPA band had significant correlation with health status impairment (Table 3); this module explained 48% of the variance of health status impairment.
Table 3.
Variables Associated With Health Status Impairment in Chronic Pulmonary Aspergillosis
| Univariate Analysis |
Multivariate Analysis |
|||
|---|---|---|---|---|
| Variable | Standardized Correlation Coefficient | P Value | Standardized Correlation Coefficient | P Value |
| MRC dyspnea score | 0.68 | <.0001 | 0.52 | <.0001 |
| CPA banding | 0.46 | <.0001 | 0.22 | .03 |
| BMI | −0.19 | .06 | −0.16 | .09 |
| FVC% | −0.11 | .29 | −0.17 | .2 |
| FEV1% | −0.2 | .05 | −0.15 | .24 |
| Age | 0.11 | .23 | 0.03 | .82 |
Abbreviations: BMI, body mass index; CPA, chronic pulmonary aspergillosis; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; MRC, Medical Research Council.
DISCUSSION
Using a standardized instrument, this is the first study in CPA patients to present subjective data documenting improvement in health status. The SGRQ has been widely used in assessing pharmacological and nonpharmacological therapeutic response in chronic progressive respiratory diseases, mainly COPD [15–17]. Overall, the change in health status in our patients with CPA was marginally better at 6 and 12 months, but this masks major changes for individual patients. Forty-seven percent to 50% of our cohort improved with a large reduction in score of 14 at both 6 and 12 months, representing a major health gain. Unfortunately, 32% of patients deteriorated with a rise in score of 11 and 14 after 6 and 12 months, respectively, of treatment and observation. One-fifth of the patients (21%) showed little difference (ie, were stable). One of the factors associated with better responses appeared to be the drug used; posaconazole appeared slightly better than voriconazole, which in turn was better than itraconazole; 50% and 62% of our patients were improved after 6 and 12 months, respectively, taking posaconazole, compared with 43% and 50% with voriconazole and 39% and 43% with itraconazole (Figure 2). These improvement rates are consistent with prior published data with posaconazole [10], voriconazole [11, 25], and itraconazole [12, 13]. This consistency is not surprising as we recently showed that SGRQ scores do differentiate between disease severity grades based on clinical, radiological, and laboratory findings [22].
An important observation is that patients gained therapeutic benefit irrespective of their illness severity. Indeed, we observed that >50% of those with poor and very poor health status at baseline improved with reduction of score of ≥4 after 6 months of treatment. Having replicating this analysis using much wider scale, we found that at least 50% of those who reported poor/very poor health status at baseline showed considerable improvement, changing to fair or good/very good categories (Figure 3). Moreover, as expected, better response was observed in those who reported better overall health status at baseline, suggesting the importance of early commencement of therapy and/or referral to a specialist center.
Drug side effects mitigated against good overall health gain. These were relatively common for all antifungal agents [1, 10, 27, 28]. Patients who experienced 1 or more side effects had significantly worse health status (with 11 scores more at 12 months) than those without side effects (P = .01), a consistent finding at all time points. Clinically, individual patients persisted with treatment despite side effects, and gained improvement in other aspect(s) of their illness (eg, reduced sputum production, hemoptysis, and weight gain). A common reason for stopping was peripheral neuropathy [6]. Posaconazole was the best-tolerated agent, but patients who responded to itraconazole or voriconazole without side effects did very well.
Another factor limiting improvement was coexisting pulmonary and systemic diseases [22]. We recently reported that underlying diseases such as COPD and bronchiectasis contributed substantially to poor health status in CPA [22]. Therapies for these conditions often have limited impact, especially when severe. In our multivariate analysis, only dyspnea followed by disease severity/complexity contributed significantly to the variability of health status, but other factors such as fatigue, psychological distress, and exacerbation probably contribute. Dyspnea is an independent prognostic factor in several chronic progressive respiratory illnesses including COPD [29]. Severity and complexity of underlying disease influenced health status in other settings [11, 30].
Regular assessment is fundamental to the management of CPA. Most studies assessed therapeutic response using clinical assessment combined with radiological and laboratory changes. Radiological improvement usually takes many months to be detectable, although failure can be sometimes be obvious earlier [1, 10, 25]. Quantitative serology (IgG or precipitin titer) is helpful in monitoring response and disease progression, but significant intrapatient variability can be a disadvantage [30, 31]. For many other conditions, standardized patient-reported outcomes are reliable and sensitive measures for assessing therapeutic response [17, 18, 32], but have not yet been applied to CPA. Validated quality-of-life tools add another reliable assessment option with advantages of more convenience to patients, better availability, and less consumption of time and healthcare resources. Our longitudinal study reported here adds to this body of data, although larger and longer studies are needed.
Fifteen patients did not complete 12 months of observation, as 6 either missed visits or had long intervals between visits, and 9 died. Exacerbations due to chest infections have significant impact on health status in chronic respiratory illnesses, for example, COPD [33, 34]; this topic as well as potential differences between more cavitating or more fibrosing CPA need to be addressed. We did try to minimize bacterial infection with preventive Haemophilus and pneumococcal immunization, as well as long-term antipseudomonal therapy, if required. Although our findings represent clinical reality, standardized assessments as undertaken in randomized clinical trials would be helpful. The SGRQ could be used as the primary endpoint in such a study, akin to that used to compare itraconazole and placebo in severe asthma with fungal sensitization [35].
In conclusion, antifungal therapy was reasonably effective in improving/maintaining health status of patients with CPA; however, more therapeutic approaches for this infectious progressive disease are urgently needed.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. The authors are grateful to Deborah Kennedy, Georgina Powell, and Marie Kirwan for helping with obtaining patients’ notes and ensuring the St George’s respiratory questionnaire scores were collected at each visit, and to the staff in the lung function laboratory, Wythenshawe Hospital, for helping with lung function measurement.
Author contributions. K. A.: study concept and design, data collection, entry, and analysis (performed the statistical analysis and drafted the manuscript), and manuscript preparation and writing. G. T. A.: study concept, writing of Web-based database and reporting application, data collection and entry, and manuscript review. C. H.: data collection and entry, and manuscript review. L. R.: data collection and manuscript review. P. J. N.: data collection and manuscript review. D. W. D.: study concept and design, data analysis, manuscript writing, editing, and review.
Potential conflicts of interest. D. W. D. holds founder shares in F2G Ltd, a University of Manchester spinout company; has current grant support from the National Institute of Allergy and Infectious Diseases, National Institute of Health Research, the European Union, and AstraZeneca; acts as an advisor/consultant to Myconostica (now part of Lab21 group) and T2Biosystems, as well as other companies over the last 5 years including Pfizer, Schering-Plough (now Merck), Nektar, Astellas, and Gilead; and has been paid for talks on behalf of Merck, Astellas, GSK, Novartis, Merck, Dainippon, and Pfizer. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Denning DW, Riniotis K, Dobrashian R, Sambatakou H. Chronic cavitary and fibrosing pulmonary and pleural aspergillosis: case series, proposed nomenclature change, and review. Clin Infect Dis. 2003;37(suppl 3):S265–80. doi: 10.1086/376526. [DOI] [PubMed] [Google Scholar]
- 2.Camuset J, Nunes H, Dombret MC, et al. Treatment of chronic pulmonary aspergillosis by voriconazole in nonimmunocompromised patients. Chest. 2007;131:1435–41. doi: 10.1378/chest.06-2441. [DOI] [PubMed] [Google Scholar]
- 3.Denning DW, Park S, Lass-Florl C, et al. High-frequency triazole resistance found In nonculturable Aspergillus fumigatus from lungs of patients with chronic fungal disease. Clin Infect Dis. 2011;52:1123–9. doi: 10.1093/cid/cir179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saraceno JL, Phelps DT, Ferro TJ, Futerfas R, Schwartz DB. Chronic necrotizing pulmonary aspergillosis: approach to management. Chest. 1997;112:541–8. doi: 10.1378/chest.112.2.541. [DOI] [PubMed] [Google Scholar]
- 5.Pendleton M, Denning DW. Multifocal pulmonary aspergillomas—a management challenge. NY Acad Sci. 2012;1272:58–67. doi: 10.1111/j.1749-6632.2012.06827.x. [DOI] [PubMed] [Google Scholar]
- 6.Baxter CG, Bishop P, Low SE, Baiden-Amissah K, Denning DW. Pulmonary aspergillosis: an alternative diagnosis to lung cancer after positive [18F]FDG positron emission tomography. Thorax. 2011;66:638–40. doi: 10.1136/thx.2010.155515. [DOI] [PubMed] [Google Scholar]
- 7.Jewkes J, Kay PH, Paneth M, Citron KM. Pulmonary aspergilloma: analysis of prognosis in relation to haemoptysis and survey of treatment. Thorax. 1983;38:572–8. doi: 10.1136/thx.38.8.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nam HS, Jeon K, Um SW, et al. Clinical characteristics and treatment outcomes of chronic necrotizing pulmonary aspergillosis: a review of 43 cases. Int J Infect Dis. 2010;14:e479–82. doi: 10.1016/j.ijid.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Tomlinson JR, Sahn SA. Aspergilloma in sarcoid and tuberculosis. Chest. 1987;92:505–8. doi: 10.1378/chest.92.3.505. [DOI] [PubMed] [Google Scholar]
- 10.Felton TW, Baxter C, Moore CB, Roberts SA, Hope WW, Denning DW. Efficacy and safety of posaconazole for chronic pulmonary aspergillosis. Clin Infect Dis. 2010;51:1383–91. doi: 10.1086/657306. [DOI] [PubMed] [Google Scholar]
- 11.Cadranel J, Philippe B, Hennequin C, et al. Voriconazole for chronic pulmonary aspergillosis: a prospective multicenter trial. Eur J Clin Microbiol Infect Dis. 2012;31:3231–9. doi: 10.1007/s10096-012-1690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dupont B. Itraconazole therapy in aspergillosis: study in 49 patients. J Am Acad Dermatol. 1990;23(3 Pt 2):607–14. doi: 10.1016/0190-9622(90)70263-h. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal R, Vishwanath G, Aggarwal AN, Garg M, Gupta D, Chakrabarti A. Itraconazole in chronic cavitary pulmonary aspergillosis: a randomised controlled trial and systematic review of literature. Mycoses. 2013 doi: 10.1111/myc.12075. doi:10.1111/myc.12075. [DOI] [PubMed] [Google Scholar]
- 14.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis. 1992;145:1321–7. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 15.Jones PW, Bosh TK. Quality of life changes in COPD patients treated with salmeterol. Am J Respir Crit Care Med. 1997;155:1283–9. doi: 10.1164/ajrccm.155.4.9105068. [DOI] [PubMed] [Google Scholar]
- 16.Karner C, Cates CJ. Long-acting beta(2)-agonist in addition to tiotropium versus either tiotropium or long-acting beta(2)-agonist alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;4:CD008989. doi: 10.1002/14651858.CD008989.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapman KR, Rennard SI, Dogra A, Owen R, Lassen C, Kramer B. Long-term safety and efficacy of indacaterol, a long-acting beta(2)-agonist, in subjects with COPD: a randomized, placebo-controlled study. Chest. 2011;140:68–75. doi: 10.1378/chest.10-1830. [DOI] [PubMed] [Google Scholar]
- 18.Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. Eur Respir J. 2002;19:398–404. doi: 10.1183/09031936.02.00063702. [DOI] [PubMed] [Google Scholar]
- 19.Wilson CB, Jones PW, O'Leary CJ, Cole PJ, Wilson R. Validation of the St. George's Respiratory Questionnaire in bronchiectasis. Am J Respir Crit Care Med. 1997;156(2 Pt 1):536–41. doi: 10.1164/ajrccm.156.2.9607083. [DOI] [PubMed] [Google Scholar]
- 20.Padilla A, Olveira G, Olveira C, et al. Validity and reliability of the St George's Respiratory Questionnaire in adults with cystic fibrosis. Arch Bronconeumol. 2007;43:205–11. doi: 10.1016/s1579-2129(07)60052-4. [DOI] [PubMed] [Google Scholar]
- 21.Pasipanodya JG, Miller TL, Vecino M, et al. Using the St. George respiratory questionnaire to ascertain health quality in persons with treated pulmonary tuberculosis. Chest. 2007;132:1591–8. doi: 10.1378/chest.07-0755. [DOI] [PubMed] [Google Scholar]
- 22.Al-shair K, Atherton GT, Kennedy D, Powell G, Denning DW, Caress A. Validity and reliability of the St. George's Respiratory Questionnaire in assessing health status in patients with chronic pulmonary aspergillosis. Chest. 2012 doi: 10.1378/chest.12-0014. doi:10.1378/chest.12-0014. [DOI] [PubMed] [Google Scholar]
- 23.Harrison E, Singh A, Morris J, et al. Mannose binding lectin genotype and serum levels in patients with chronic and allergic pulmonary aspergillosis. Int J Immunogenet. 2012;39:224–32. doi: 10.1111/j.1744-313X.2011.01078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohno S, Izumikawa K, Kakeya H, et al. Clinical efficacy and safety of micafungin in Japanese patients with chronic pulmonary aspergillosis: a prospective observational study. Med Mycol. 2011;49:688–93. doi: 10.3109/13693786.2011.561369. [DOI] [PubMed] [Google Scholar]
- 25.Sambatakou H, Dupont B, Lode H, Denning DW. Voriconazole treatment for subacute invasive and chronic pulmonary aspergillosis. Am J Med. 2006;119:527 e17–24. doi: 10.1016/j.amjmed.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 26.Jones PW. St. George's Respiratory Questionnaire: MCID. COPD. 2005;2:75–9. doi: 10.1081/copd-200050513. [DOI] [PubMed] [Google Scholar]
- 27.Saito T, Fujiuchi S, Tao Y, et al. Efficacy and safety of voriconazole in the treatment of chronic pulmonary aspergillosis: experience in Japan. Infection. 2012;40:661–7. doi: 10.1007/s15010-012-0322-x. [DOI] [PubMed] [Google Scholar]
- 28.Park WB, Kim NH, Kim KH, et al. The effect of therapeutic drug monitoring on safety and efficacy of voriconazole in invasive fungal infections: a randomized controlled trial. Clin Infect Dis. 2012;55:1080–7. doi: 10.1093/cid/cis599. [DOI] [PubMed] [Google Scholar]
- 29.Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121:1434–40. doi: 10.1378/chest.121.5.1434. [DOI] [PubMed] [Google Scholar]
- 30.Chishimba L, Niven RM, Cooley J, Denning DW. Voriconazole and posaconazole improve asthma severity in allergic bronchopulmonary aspergillosis and severe asthma with fungal sensitization. J Asthma. 2012;49:423–33. doi: 10.3109/02770903.2012.662568. [DOI] [PubMed] [Google Scholar]
- 31.Brown GD, Denning DW, Levitz SM. Tackling human fungal infections. Science. 2012;336:647. doi: 10.1126/science.1222236. [DOI] [PubMed] [Google Scholar]
- 32.Make B. How can we assess outcomes of clinical trials: the MCID approach. COPD. 2007;4:191–4. doi: 10.1080/15412550701471231. [DOI] [PubMed] [Google Scholar]
- 33.Sethi S. Molecular diagnosis of respiratory tract infection in acute exacerbations of chronic obstructive pulmonary disease. Clin Infect Dis. 2011;52(suppl 4):S290–5. doi: 10.1093/cid/cir044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–38. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 35.Denning DW, O'Driscoll BR, Powell G, et al. Randomized controlled trial of oral antifungal treatment for severe asthma with fungal sensitization: the Fungal Asthma Sensitization Trial (FAST) study. Am J Respir Crit Care Med. 2009;179:11–8. doi: 10.1164/rccm.200805-737OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.