ABSTRACT
The management of orbital fractures is one of the most interesting and difficult areas in facial trauma. The consequences of an orbital fracture are sometimes dramatic. They include varying types of defects from a loss of vision to diplopia, loss of an eye, epiphora, a disturbing loss of facial sensation, or even an unacceptable appearance of the eye and the hard and soft tissues around it. The controversies surrounding orbital fractures, and orbital floor fractures in particular include the following: the timing of primary surgery (early or late), bone grafting versus alloplastic materials, the management and prevention of diplopia, the prevention of enophtalmos, the management of infraorbital nerve numbness or dysesthesia.
Keywords: Orbital floor fracture, titanium preformed implants, diplopia
INTRODUCTION
The orbital floor is one of the parts of the maxillofacial skeleton that is frequently damaged in facial trauma, because of its ability to fracture selectively similar to a safety valve, allowing the dissipation of energy when orbit is struck. Can be considered as an evolutionary masterpiece, contributing to the orbit`s primary role of protecting the globe in combination with the strength of its lateral walls (1).
Orbital floor fractures occur in both adult and pediatric populations. However the characteristics of the fracture, physical and radiographic findings, and course of recovery differ. Linear nondisplaced orbital floor fractures with inferior rectus muscle entrapment occur in pediatric population, with minimal trauma and few external signs of injury. All affected eyes demonstrate significant limitation to elevation associated with diplopia, and despite prompt surgical repair, limited elevation and depression occur in the early postoperative period, possibly due to a compartment syndrome with raised pressure in the muscle sheath, hemorrhage and ischemia. Recovery of normal ocular motility may take weeks or month. CT evidence may be minimal in these cases (2-4).
In adult population – orbital blow-out fractures are characteristic and result in orbital volume expansion, with the orbital contents sagging into the maxillary sinus. Initially, enophthalmos (or sunken eye) and diplopia may not be apparent but, with clearance of hemorrhage and resolution of edema, the condition may be manifested. The decision to undertake surgical repair or reconstruction of these defects is based on functional limitations (impaired ocular motility, infraorbital anesthesia) or cosmetic deformity. Defects of 25% or less of the surface area, without entrapment, generally heal uneventfully without intervention. Repair of intermediate defects of 25% to 50% is based on degree of displacement, amount of volume expansion and any co-existing enophthalmos, even with edema. Larger or comminuted defects (greater than 50%) with significant disruption are best treated with early repair (within 7 days) because some degree of enophthalmos or diplopia is the norm when left unrepaired (5).
The main goal of orbital floor fracture repair is to restore the orbit to its preoperative status, which involves repositioning the herniated orbital tissues in the orbit and repairing the traumatic defect while preserving the orbital volume. The three-dimensional shape of the orbital walls in combination with a weak bony framework and close proximity to delicate anatomic structures define the challenges of the reconstructive procedure (6,7).
Principles of surgical treatment
Patients with isolated orbital floor fractures can be followed clinically initially. If surgery is needed, it is usually planned for 7-14 days after the trauma. Waiting allows time for spontaneous improvement, precise surgical planning, and resolution of swelling associated with the initial trauma because a too edematous orbit cannot allow an effective repair. Delaying surgery for over 14 days results in increased scarring of the orbit.
Specific indications for surgical repair:
restrictive strabismus
enophtalmus >2 mm
CT evidence of muscle entrapment
Oculocardiac reflex
Hypo-ophtalmos
Large floor fracture >50%, based on CT estimate of fracture size
Principles of surgical repair:
Exposure
Isolate the orbital soft tissue
Restore orbital volume
Provide a stable platform for soft tissue to rest
Usually, the fracture of the orbital floor is comminuted and bone fragments are missing, therefore one is reconstructing missing bone rather than reducing bone fragments.
There is hardly any anatomic region in the human body that is so controversial in terms of appropriate material used for fracture repair (8,9). There are different preferences of implant material depending on variations in schools of teaching, socio-economic factors but there is a paucity of evidence to support the ideal choice of an orbital implant.
In the past, the use of autogenic bone has been the gold standard in orbital wall reconstruction. Usually calvarial bone was used due to maximal biocompatibility and low cost. However, the harvesting of autogenous bone is associated with donor site morbidity, variable degree of resorption, prolonged total operating time, postoperative pain, scaring. Also autologous bone grafts are usually rigid and cannot be bend to match the concave-convex shape of the orbital floor, and provide less drainage from the orbit than with titanium (10-12).
Porous polyethylene sheets (PPE) – presents the advantage of easy contouring and smooth edges, but are not radiopaque and can present a lack of rigidity in heavily displaced fractures with massive tissue herniation into the maxillary sinus. However, infection is the most disastrous complication with the use of the PPE in the orbital wall reconstruction (13).
Composite of porous polyethylene and titanium (Medpor) are increasingly being used to prevent postoperative enophthalmus, but that are recent reports that show the risk of late complications such as infection, diplopia and implant migration associated with them.
An alternative resorbable material consisting of stiff sheets of pure polyglactin – polydioxanone (PDS) implants (Ethysorb) to bridge small-to-moderate defects has been described. After resorption of the implant, the resulting scar might not be stiff enough in all cases to provide adequate support for the globe and to prevent sagging of the periorbita into the maxillary sinus. Another drawback of pure PDS was that degradation may lead to an inflammatory reaction involving the surrounding periorbita, with possible scar formation and consecutive functional motility disturbances of the globe, leading to recurrence of diplopia (14).
Preformed titanium meshes. Orbits reconstructed with titanium mesh show better results than those reconstructed with bone grafts (15-18). It is malleable and therefore easily adapted to the shape of the orbital defect and it is the most biocompatible of all available material. Because of the mesh structure (Figure 1), connective tissue can grow around and through the implant and prevent migration. It is also preferred in significant fractures with large defects. However this has the potential disadvantage of making the implant very difficult to remove if required.
Figure 1. Titanium preformed floor plate.

Case report
A 54 year old man sustained a left orbital fracture after a human assault. Immediately after the injury, the patient noticed diplopia and discomfort in all fields of gaze. At the initial examination, there was important periorbital edema, ecchymosis, marked limitation of infraduction and supraduction of the right eye and associated pain and diplopia in all fields of gaze, complete anesthesia in the left V2 nerve territory. In addition, there was 2.5 mm of right enophtalmos – measured with Hertel exophtalmometer.
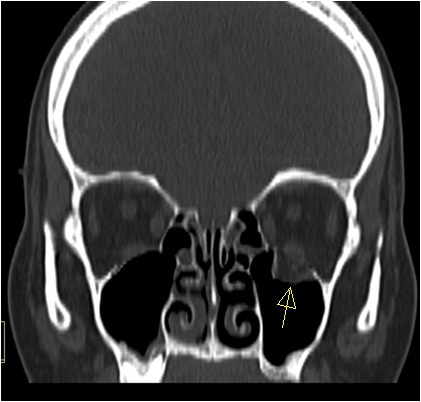
A preoperative computed tomography (Figure 2) scan showed an extensive fracture of the right orbital floor with major herniation of the orbital soft tissue in the maxillary sinus, of pure type. The clinical and radiographic findings supported the diagnosis of entrapment of the inferior rectus muscle.
Figure 2. CT.
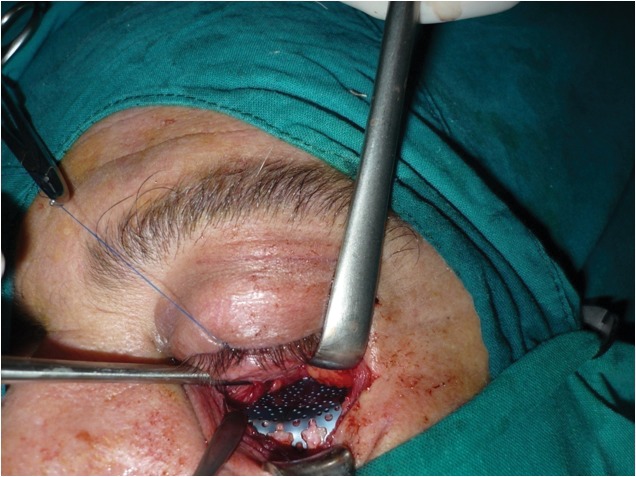
At surgery, the orbital wall was approached subtarsally, with a dissection carried in a preseptal plane. After subperiosteal dissection to expose the orbital floor, the herniated tissue was repositioned within the orbit and a 25 mm x 16 mm x 0.85 mm titanium floor plate was positioned over the bony defect (Figure 3). An intraoperative forced duction test was performed to ensure the release of the incarcerated inferior rectus muscle. The periosteum was meticulously sutured at the orbital rim.
Figure 3. Orbital floor reconstruction.
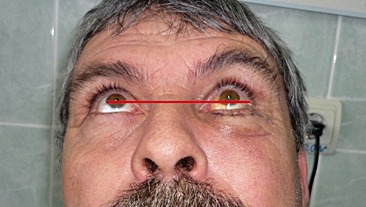
Postoperatively, the patient received intravenous antibiotics (Cefuroxime 1g IV every 12h for 5 days), systemic corticosteroids (Dexamethasone 4mg/ml per day for 5 days). The patient still had diplopia during the first week of follow-up, perhaps owing to the initial muscle and soft tissue edema, thereafter, the ductions of the right eye normalized, and the diplopia resolved by the end of the first month of follow up (Figure 4, Figure 5).
Figure 4. Preoperative aspect.
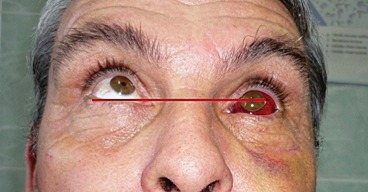
Figure 5. Postoperative 7 days.
The follow-up was extended 6 months after the titanium mesh was implanted, and the clinical assessment demonstrated good biocompatibility, good overall support of the orbital contents without the relapse of enophtalmus and no signs of local inflammatory reaction.
Surgical repair was performed using a subtarsal approach, and preformed titanium orbital floor implants (Stryker-Leibinger) secured with titanium screws at the infraorbital rim.
The lower eyelid offers numerous incision opportunities for exposure, based on skin creases at this level. They include – subciliary, subtarsal, and infraorbital incisions. Except for the infraorbital incision, the other two offer an exceptional healing, with almost no chance of hypertrophic scar.
After the transection of the orbicularis muscle, the dissection is performed in a preseptal orbital plane in order to avoid the herniation of the orbital fat in the operating field. At the infraorbital rim level, the periosteum is incised and the raised so that wide access to orbital floor is obtained.
The preformed titanium implants offer the advantage of minimal contouring and stability. The implant is trimmed off to protect the soft tissues, and contoured to achieve the required shape in order to accommodate key anatomical structures (nasolacrimal duct, infraorbital nerve and optic nerve). It is advisable not to extend the implant further posterior than 1 cm anterior to the optic canal entrance. Under adequate retraction of the intraorbital soft tissue, the mesh has to be positioned so that proper and stable recontouring of the orbital wall results. Care has to be taken that neither orbital fat nor muscles are entrapped. During the insertion process, the mesh may require rotation in order to be properly positioned. Once in place, the mesh is fixed over the orbital rim to prevent migration. After the implant insertion it is imperative to perform a forced duction test in order to assure that the implant has not created a decrease in ocular motility (19,20). ❑
DISCUSSION
Orbital floor fractures are one of the most common injuries observed in the middle facial region. One of the most important points of reconstruction of orbital wall fractures is restoration of normal orbital volume.
Numerous articles have been published on the subject of orbital tissue reconstruction. The ideal material for orbital reconstruction remains controversial. Numerous materials are available at present including lyophilized dura, polyethylene or polydoxanone sheets, titanium mesh and autogenous one graft. The more elastic materials are not capable of withstanding the dynamic stresses of large bony orbital defects; resorbable implants are prone to produce foreign body reactions, implant exposure may occur and only fibrous connective tissue remaining after resorption. The disadvantages of autologous bone graft include minimal contourability and donor site defects.
Titanium mesh has o long track record in the reconstruction of large orbital defects and correction of globe malposition. Advantages of titanium mesh plates are availability, easy intraoperative contouring and rigid fixation (21). Disadvantages include difficulties with ease of insertion, as rough or irregular edges on the mesh tend to catch on adjacent soft tissues. Intraoperative bending and repetitive removal of titanium mesh for orbital reconstruction may cause damage to the orbital soft tissue. Using preformed implants for orbital reconstruction in larger defects, avoids the time consuming and traumatic intraoperative bending and adjustment of the implants.
Four patients with pure orbital floor fractures and diplopia were treated by osteosynthesis of the fractures using preformed titanium implant between January 2011 and January 2012. All four patients had double vision particularly on extreme downward and upward gaze. Two patients were operated on immediately after the injury and two after approximately 7 days because of the extensive periorbital swelling on admission. All presented extensive fractures with multiple fragments projected into the maxillary sinus cavity. The orbital floor was exposed via a subtarsal approach, and the infraorbital nerve was located and protected before plating (Table 1).
Table 1.
| Preoperative | Postoperative 7 days | Postoperative 1 month | Postoperative 6 months | |
|---|---|---|---|---|
| Diplopia | 4 patients | Unchanged 1 Improved 3 Worse 0 |
Unchanged upper gaze 2 Normal 2 |
Persistant extreme upper gaze 1 Normalized 3 |
| Enophtalmus >2 mm | 2 patients | Improved 4 | Absent 4 | - |
| V2 anesthesia | 4 patients | Unchanged 2 Worse 2 |
Improved 4 | Persistant at the upper lip 2 Normalized 2 |
| Hypoglobus | 1 patient | Improved | Absent 4 | - |
Postoperative clinical, RX and CT scan verified the anatomical reduction of the orbital floor. None of the patients had diplopia or enophtalmos postoperatively and there was no displacement or resorption of the orbital floor or loosening of the screws 6 months after the operation, and no modifications in the visual acuity.
Nowadays computer assisted methods by creating an orbital model based on the data of the virtual orbital reconstruction, permit precise performing of individual titanium mesh implants for a 3D reconstruction of the orbital cavity (with or without the use of a stereolitographical resin based model) (22-24). The ideal form of the injured orbital cavity is recalculated using the mirror image of the unaffected side. This method facilitates the quick subperiosteal placement of the implants, without the repetitive trial fitting of the titanium mesh, therefore, the procedure is less traumatic to the periorbital tissues. ❑
CONCLUSION
The placement of preformed orbital implants for the reconstruction of extensive orbital defects proved to be less time consuming and more precise compared to "free hand" reconstruction efforts of orbital injuries using titanium mesh, calvarial or iliac grafts, and also the time and costs are significantly lower compared with the use of stereolitographyc models. ❑
CONFLICT OF INTEREST
none declared.
FINANCIAL SUPPORT
none declared.
References
- 1.Burm JS, Chung CH, Oh SJ. Pure Orbital Blowout Fracture, New Concepts and Importance of Medial Orbital Blowout Fracture. Plast Reconstr Surg. 1999;103:1839–1849. doi: 10.1097/00006534-199906000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Burm JS. Internal Fixation in Trapdoor-Type Orbital Blowout Fracture. Plast Reconstr Surg. 2005;116:962–970. doi: 10.1097/01.prs.0000178046.71684.fe. [DOI] [PubMed] [Google Scholar]
- 3.Burnstine MA. Clinical Recommendations for Repair of Orbital Facial Fractures. Curr Opin Ophthalmol. 2003;14:236–240. doi: 10.1097/00055735-200310000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Chang EL, Bernardino CR. Update on Orbital Trauma. Curr Opin Ophthalmol. 2004;15:411415–411415. doi: 10.1097/01.icu.0000137854.37950.fb. [DOI] [PubMed] [Google Scholar]
- 5.Hammer B, Prein J. Correction of Post-Traumatic Orbital Deformities: Operative Techniques and Review of 26 Patients. J.Craniomaxillofac. Surg. 1995;23(2):81–90. doi: 10.1016/s1010-5182(05)80453-6. [DOI] [PubMed] [Google Scholar]
- 6.Nagasao T, Hikosaka M, Morotomi T, et al. Analysis of the Orbital Floor Morphology. J Craniomaxillofac Surg. 2007;35:112–119. doi: 10.1016/j.jcms.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Parsons GS, Mathog RH. Orbital Wall and Volume Relationships. Arch Otolaryngol Head Neck Surg. 1988;114:743–747. doi: 10.1001/archotol.1988.01860190047020. [DOI] [PubMed] [Google Scholar]
- 8.Potter JK, Ellis E. Biomaterials for Reconstruction of the Internal Orbit. J Oral Maxillofac Surg. 2004;62:1280–1297. doi: 10.1016/j.joms.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann J, Cornelius CP, Groten M, et al. Orbital Reconstruction with Individually Copy-Milled Ceramic Implants. Plast Reconstr Surg. 1998;101:604–612. doi: 10.1097/00006534-199803000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg RA, Garbutt M, Shorr N. Oculoplastic Uses of Cranial Bone Grafts. Ophthalmic Surg. 1993;24:190–196. [PubMed] [Google Scholar]
- 11.Habal MB. Bone Grafting The Orbital Floor for Posttraumatic Defects. J Craniofac Surg. 1992;3:175–180. doi: 10.1097/00001665-199211000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Kelly CP, Cohen AJ, Yavuzer R, et al. Cranial Bone Grafting for Orbital Reconstruction: Is It Still the Best? J Craniofac Surg. 2005;16:181–185. doi: 10.1097/00001665-200501000-00039. [DOI] [PubMed] [Google Scholar]
- 13.Lee S, Maronian N, Most SP, et al. Porous High-Density Polyethylene for Orbital Reconstruction. Arch Otolaryngol Head Neck Surg. 2005;131:446–450. doi: 10.1001/archotol.131.5.446. [DOI] [PubMed] [Google Scholar]
- 14.Buchel P, Rahal A, Seto I, et al. Reconstruction of Orbital Floor Fracture with Polyglactin 910/Polydioxanon Patch (Ethisorb): Retrospective Study. J Oral Maxillofac Surg. 2005;63:646–650. doi: 10.1016/j.joms.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Ellis III E, Tan Y. Assessment of Internal Orbital Reconstructions for Pure Blowout Fractures: Cranial Bone Grafts versus Titanium Mesh. J Oral Maxillofac Surg. 2003;61:442–453. doi: 10.1053/joms.2003.50085. [DOI] [PubMed] [Google Scholar]
- 16.Kuttenberger JJ, Hardt N. Long-term Results Following Reconstruction of Craniofacial Defects with Titanium Micro-Mesh Systems. J Craniomaxillofac Surg. 2001;29:75–81. doi: 10.1054/jcms.2001.0197. [DOI] [PubMed] [Google Scholar]
- 17.Gear AJL, Lokeh A, Aldridge JH, et al. Safety of Titanium Mesh for Orbital Reconstruction. Ann Plast Surg. 2002;48:901–910. doi: 10.1097/00000637-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Kuttenberger JJ, Hardt N. Long-term Results Following Reconstruction of Craniofacial Defects with Titanium Micro-Mesh Systems. J Craniomaxillofac Surg. 2001;29:75–81. doi: 10.1054/jcms.2001.0197. [DOI] [PubMed] [Google Scholar]
- 19.Metzger MC, Schön R, Weyer N, et al. Anatomical 3-Dimensional Pre-bent Titanium Implant for Orbital Floor Fractures. Ophthalmology. 2006;113:1863–1868. doi: 10.1016/j.ophtha.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 20.Schon R, Metzger MC, Zizelmannn C, et al. Individually Preformed Titanium Mesh Implants for True-to-Original Repair of Orbital Fractures. Int J Oral Maxillofac Surg. 2006;35:990–995. doi: 10.1016/j.ijom.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 21.Scolozzi P, Momjian A, Heuberger J, et al. Accuracy and Predictability in Use of AO Three-Dimensionally Preformed Titanium Mesh Plates for Posttraumatic Orbital Reconstruction: a Pilot Study. J Craniofac Surg. 2009;20:110–13. doi: 10.1097/SCS.0b013e3181abb44b. [DOI] [PubMed] [Google Scholar]
- 22.Eufinger H, Wittkampf AR, Wehmoller M, et al. Single-Step Fronto-Orbital Resection and Reconstruction with Individual Resection Template and Corresponding Titanium Implant: a New Method of Computer-Aided Surgery. J Craniomaxillofac Surg. 1998;26:373–378. doi: 10.1016/s1010-5182(98)80070-x. [DOI] [PubMed] [Google Scholar]
- 23.Lauer G, Pradel W, Schneider M, et al. Efficacy of Computer Assisted Surgery in Secondary Orbital Surgery. J Craniomaxillofac Surg. 2006;34:299–305. doi: 10.1016/j.jcms.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Luebbers HT, Messmer P, Obwegeser JA, et al. Comparison of Different Registration Methods for Surgical Navigation in Cranio-Maxillofacial Surgery. J Craniomaxillofac Surg. 2008;36:109–116. doi: 10.1016/j.jcms.2007.09.002. [DOI] [PubMed] [Google Scholar]


