ABSTRACT
Breast cancer represents the most frequent form of neoplasia in women worldwide, being responsible of 1.6% of annual deaths. Therefore, it is a major public health issue and research in this field should be a priority. Chemoterapics drugs are extremly potent tools, which alone or in association to radiotherapy, increase survival and lower the reccurrence rate of cancer, but their use can be limited by cardiotoxicity. Cardiotoxicity can appear early or late after therapy, and may vary from subclinical myocardial dysfunction to irreversible heart failure. Currently, cardiac dysfunction induced by chemotherapy is diagnosed through classical echocardiographic parameters. However, these cannot detect subtle, early changes of cardiac structure and function. Consequently, description of new methods, which could detect cardiac dysfunction in an early stage, becomes essential for detecting the group of patients at risk for irreversible heart failure and for monitoring the treatment.
Keywords: chemotherapy, cardiotoxicity, non-invasive methods
INTRODUCTION
Breast cancer represents the most frequent form of cancer in women worldwide, comprising 16% off all cancers. A report of the World Health Organization showed that 1 from 6 cancers is determined by breast cancer and this form of neoplasm causes 1.6% of all annual deaths in the world (1). The incidence of breast cancer is increasing, over 1.1 million of women being newly diagnosed with cancer every year (1). Therefore, breast cancer is currently a major public health and economical issue and research on new therapies, as well as monitoring their safety use, should be a priority. Many studies showed that, due to new chemotherapy, breast cancer can be considered a curable disease. And indeed, use of a multidisciplinary approach - surgery, radiotherapy, chemotherapy - has conducted to a significant reduction in the mortality (2). As a consequence of increased life expectancy, it becomes essential that specific oncologic therapies should be safe (3).
Cancer treatment has improved significantly in recent years and it has been proved to increase significantly rate of cure in breast cancer, as well as to reduce recurrences. However, the applicability of these drugs is limited by the risk of cardiotoxicity (4). Cardiotoxicity is one of the most important adverse reactions of chemotherapy, leading to an important increase of morbidity and mortality (5,6). Cardiotoxicity can appear early or late in the course of the disease, and may vary from subclinical myocardial dysfunction to irreversible heart failure or even death (7). Data on the mechanism of the appearance of cardiac dysfunction during chemotherapy and the susceptibility of patients to develop cardiotoxicity are scarce (4,8). Some studies suggest that patients without known cardiovascular history may develop symptomatic heart failure in direct connection to the cumulative dose received, affirmation which has led to the use of reduced doses of chemotherapy and, therefore, to a reduction in their efficiency (9). But also under these circumstances, there is a risk of cardiotoxicity induced by chemotherapy, risk which cannot be foreseen by the cumulative dose. Moreover, the cardiac alteration is very frequently subclinical and it can appear early (during therapy), late (during the first year after therapy) or very late (more than one year after finishing therapy) (10). Consequently, better understanding of pathophysiology and early diagnosis of subclinical cardiac dysfunction in patients with breast cancer under chemotherapy, as well as the close cardiac monitoring during antineoplastic treatment is essential in order to reduce cardiotoxicity.
Overtime, recommendations of diagnosis of cardiac dysfunction induced by chemotherapy used functional and structural parameters of conventional echocardiography, such as left ventricular (LV) ejection fraction (EF), fractional shortening (FS), as well as diameters and volumes (11,12). However, these conventional measurements allow only the late diagnosis of cardiac dysfunction, which might be already irreversible. Therefore, there is major need for other accurate and reproducible parameters, able to detect early, subclinical, LV dysfunction and, thus, able to identify patients at risk for rapid progression toward irreversible cardiac failure, who can benefit from early therapeutic measures. Therefore, this article reviews the definition and mechanisms of cardiotoxic effects of chemotherapy. It also emphasizes the importance of early detection of cardiac dysfunction in the course of the disease, with special focus on the imagistic methods. ❑
DEFINITION OF CHEMOTERAPY-INDUCED CARDIOTOXICITY
Cardiotoxicity is a general term used to define "toxicity that affects the heart" (13). A growing body of researches is now studying cardiovascular events associated with chemotherapy (14), however a clear definition of cardiotoxicity and the certain mechanisms involved are lacking. This definition refers to a direct effect of the chemotherapy on the entire cardiovascular system, but also to an indirect effect due to a thrombogenic status or to a hemodynamic flow alteration (3). A committee of the cardiac review and evaluation supervising trastuzumab clinical trials clinically defined chemotherapy-induced cardiotoxicity as one or more of the following: 1) reduction of LVEF, either global or specific in the interventricular septum; 2) symptoms or signs associated with heart failure (HF); 3) reduction in LVEF from baseline ≤ to 5% to <55% in the presence of signs or symptoms of HF, or a reduction in LVEF ≥10% to <55% without signs or symptoms of HF (15). Cardiac dysfunction associated with chemotherapy in breast cancer can be acute, subacute or chronic side effect (3). Acute or subacute cardiotoxicity develops any time from the initiation of treatment up to 2 weeks after the completion of therapy, and it can be characterized by different type of arrhythmias, abnormalities in ventricular repolarization and QT intervals, acute coronary syndromes, or pericardial reaction and alteration in myocardial function (3). Chronic cardiotoxicity refers to the side effects that can appear within 1 year after the completion the treatment – early cardiotoxicity, or more than 1 year after the chemotherapy – late cardiotoxicity. Since the most typical sign of chronic cardiotoxicity is a subclinical, asymptomatic systolic or diastolic cardiac dysfunction that can leads to irreversible heart failure and even death (16), the ideal definition of cardiotoxicity is lacking, and therefore intensive analysis on cardiotoxicity should be perform.
A study from 1979, performed prior to the development of modern treatment of HF and to the implementation of LVEF screening during chemotherapy, reported a prevalence of clinical HF of 2.2% in a large cohort of more than 4.000 patients who received anthracyclines, with a mortality attributed to HF of 71% (17). However, a more recent study of patients who received more than 500 mg/m2 of anthracyclines, reported a 63% prevalence of LV dysfunction after 10 years of follow-up, in contrast to an 18% prevalence in those who received less than 500 mg/m2 of anthracyclines. Despite modern treatment of HF, 45% of patients with anthraciclynes-induced cardiac dysfunction showed no improvement of LV function in a study published in 2010 (5). On contrary, cardiotoxicity related to trastuzumab may be reversible, if the drug is discontinued and the treatment for HF is initiated (18). Therefore, cardiotoxicity may be defined also based on the underlying mechanisms and reversibility, this characterization having the ability to predict irreversible HF and to monitor the specific management (Table 1) (10,19). ❑
Table 1.
| Type I (anthracycline-like) | Type II (trastuzumab-like) | |
|---|---|---|
| Cellular mechanism | Cells death | Cells dysfunction |
| Dose related | Cumulative | Not-cumulative |
| Reversibility | Permanent | Reversible |
Table 1 shows the response of GT to clonidine administration in multiple sclerosis patients when it was chosen a cut off value of EDSS of 3. ❑
MECHANISMS OF CHEMOTHERAPY-INDUCED CARDIOTOXICTY
Numerous studies demonstrated that the type of chemotherapeutic drugs plays an essential role in cardiotoxicity development (2, 3,20). Thus, the hypothetical mechanisms involved in chemotherapy-induced cardiotoxicity are: 1) direct cellular toxicity, with a cumulative myocardial injury, resulting in both diastolic and systolic dysfunction; 2) effects on the coagulation system, resulting in ischemic events, thrombogenesis and vascular toxicity; 3) arrhythmogenic effects; 4) hypertensive effects; 5) myocardial and/or pericardial inflammation associated with myocardial dysfunction or pericardial sequels (2,3). ❑
DIRECT EFFECTS ON THE HEART
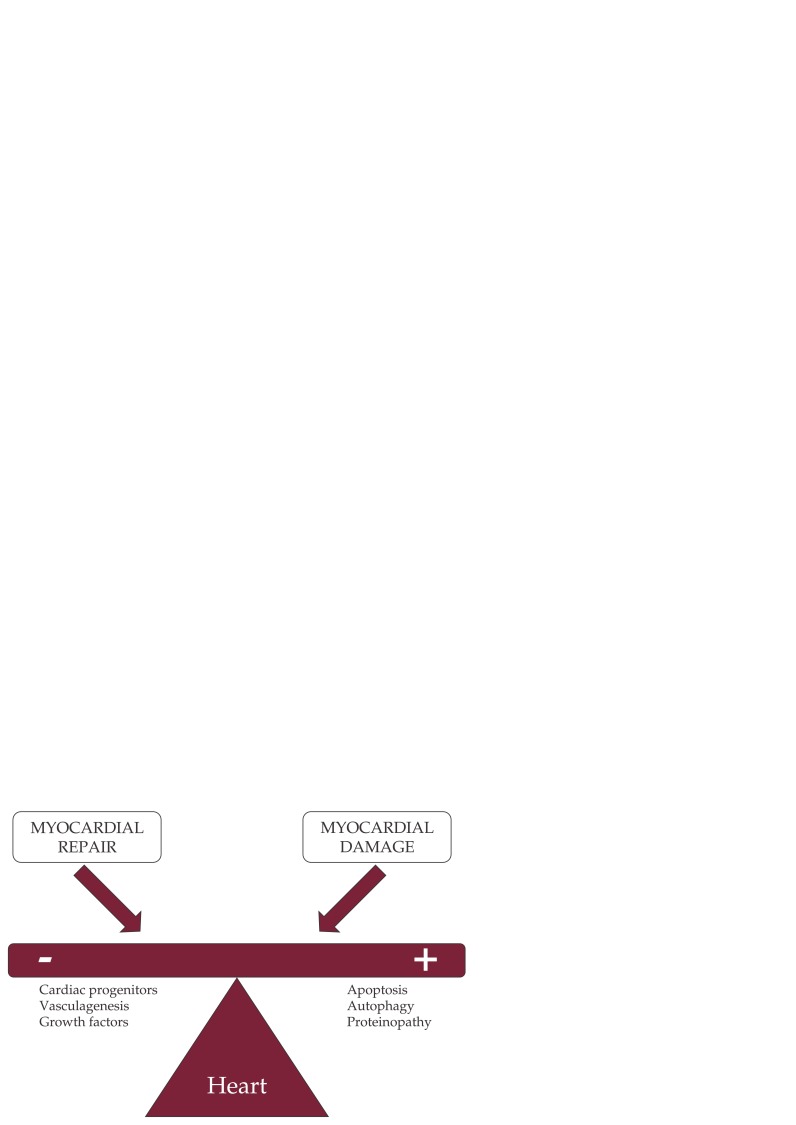
Several chemotherapeutical drugs induce rapid apoptosis or necrosis, growth deprivation and angiogenesis suppression, or a compromise in repair capacity, not only in the proliferating cancer cells, but also in the myocardium, leading to the cardiotoxicity (Figure 1) (8,21). Anthracyclines, a widely used chemotherapeutics, induce mitochondrial damage, changes in ATP production, and cellular apoptosis, along with increase production of free radical species that affects cellular membrane (22). Trastuzumab is direct cardiotoxic or potentiate the cardiotoxic effect of anthracyclines, due to an effect on ErbB2 receptors, expressed on the myocardium, where they have a protective role on cardiac function (23). On the other hand, taxanes-induced cardiotoxicity could be associated with myocardial damage via effects on subcellular organelles (24), or to massive histamine release, resulting in conduction disturbances and arrhythmias (8). 5-Fluorouracil has direct toxic effects on vascular endothelium, leading to coronary spasm and endothelial-independent vasoconstriction via protein-kinase C (25). However, whether the observed cardiomyocytes damage has a clinical importance is still controversial, along with the role of protooncogene abl in the development of cardiotoxicity (26,27). ❑
Figure 1. The direct effects on the myocardium of the chemotherapeutical drugs leading to cardiotoxicity (8,21).
EFFECTS ON THE ANTICOAGULATION SYSTEM
Chemotherapy can induce blood clotting, thrombosis and thromboembolic events, leading to cardiovascular and cerebrovascular ischemia (8). Furthermore, chemotherapy can cause injury to the endothelial layer and disruption to the endothelial cells, activating the coagulation cascade (8). Particularly, cisplatin can activate the platelets aggregation and thromboxane formation, increasing the thrombogenesis. The risk of thromboembolic events increases also in patients with established risk factors and in those with metastatic disease (8). ❑
ARRHYTHMOGENIC EFFECTS
Taxanes, in particular paclitaxel, is a prototype of pro-arrhythmogenic drug, having a chronotropic effect either indirectly through histamine release or directly on the Purkinje system (28). The most important pro-arrhythmogenic effect of chemotherapy remains the QT interval prolongation, which can be explained by the interaction of anticancer drugs with HERG K channels, allowing the rapid decreasing of potassium inflow into the cell (29). Atrial fibrillation is another important arrhythmogenic side effect of chemotherapy, which can complicate the outcome of patients with cancer (30). It may be induced by drugs such as Docetaxeil, 5-fluorouracil, cisplatin, etoposide, or by high doses of corticosteroids, probably due to inflammation process associated, since 18.3% of patients with history of cancer had atrial fibrillation compared with 5.6% of those without history of cancer (31). ❑
HYPERTENSIVE EFFECT
Hypertension is a common side effect of several anticancer drugs, like antiangiogenic therapy. The related mechanism is the inhibition of NO-synthase activity, and the decrease in NO production, with a significant increase in vasoconstriction and peripheral vascular resistance and blood pressure (32). Hypertension can coexist with cancer, and sometime may be exacerbated by chemotherapy, with direct effect on ventricular hypertrophy and heart failure (8). ❑
CLINICAL MANIFESTATIONS OF EFFECTS OF CHEMOTHERAPY ON THE CARDIOVASCULAR SYSTEM
Numerous studies demonstrated the cardiotoxicity of specific classes of chemotherapeutic drugs (33), although recently there is a global analysis of the different classes together (28). In summary, congestive heart failure and left ventricular dysfunction are associated with use of anthracyclines, a cumulative-dose reaction, more frequently seen in women, in those with previous cardiac diseases, and after mediastinal irradiation (33). Trastuzumab (Herceptin), a monoclonal antibody for the HER 2 protein, rises the risk of cardiotoxicity if administrated concomitantly with antracyclines (34). 5-fluorouracil, another widely used chemotherapeutical drug, may induce myocardial ischemia and electrocardiogram alteration of the repolarization phase (3). Antimicrotubule molecules, such as vincalkaloids or taxanes, may produce cardiac heart failure, rhythm and conduction disturbances, and ischemia (33). Neurohumoral activation without direct cardiotoxicity may induce heart failure if cyclophosphamide or mitomycin is used. The use of Tamoxifen, a selective estrogen receptor modulator, is associated with alteration of cholesterol metabolism, with a significant increased risk of thromboembolic disease and stroke (3). The anti-vascular growth factor antibody, bevacizumab, is also related with thromboembolic risk, hypertension, and even pulmonary edema (35). Moreover, left ventricular dysfunction or even heart failure, hypertension, and arrhythmias may developed in patients treated monoclonal antibodies, or interferon – α, a biological agents additional used in cancer treatment (33). Table 2 describes in summary the cardiovascular side effects produced by chemotherapy (8). ❑
Table 2.
Cardiovascular manifestations of different classes of chemotherapeutical drugs (modified from 3).
| Chemoterapeutical drug | Cardiovascular manifestations |
|---|---|
| Anthracyclines | LVD, HF, myocarditis, arrhythmia |
| 5-fluorourcil | Ischemia, HF, pericarditis, cardiogenic shock |
| Taxanes (paclitaxel), vinca alkaloids | Sinus bradicardia, ventricular tachycardia, atrioventricular block, HF, ischemia |
| Cyclophosphamide | HF (neurohumoral activation), mitral regurgitation |
| Trastuzumab | HF, LVD, arrhythmia |
| Tamoxifen | Thromboembolism, cholesterol metabolism anomalies |
| Bevacizumab | Hypertension, thromboembolism |
| COX-2 specific inhibitors | Thromboembolism |
LVD: left ventricular dysfunction; HF: heart failure
NON-INVAZIVE METHODS FOR MONITORING CHEMOTHERPY-INDUCED CARDIOTOXICITY
In order to prevent the occurrence of cardiotoxicity during chemotherapy in cancer patients, although monitoring of cardiovascular function could be time-consuming and expensive, it is highly recommended to perform it before, during, and after completion the treatment. A series of diagnostic and prognostic methods have been suggested – clinical, imagistic, serological, or molecular – for detection of cardiotoxicity and initiation the specific therapeutic measures. According to recent guidelines, cardiotoxicity is defined as a reduction of the EF ≥5% to <55% with symptoms or heart failure, or an asymptomatic reduction of the EF ≥10% to <55% (36). However, these cut-off limits are arbitrary and frequently dysfunction, systolic or diastolic, develops during chemotherapy even when EF or FS are within normal limits. This form may lead to severe and irreversible cardiomyopathy and even to death and, therefore, its diagnosis is crucial (16). Consequently, diagnosis of subclinical cardiac dysfunction in patients with cancer treated with epirubicin is extremely important.
Overtime, recommendations of diagnosis of cardiac dysfunction induced by chemotherapy used functional and structural parameters of conventional echocardiography, such as LVEF, FS, as well as diameters and volumes (37,38). However, these conventional measurements allow only the late diagnosis of cardiac dysfunction, which might be already irreversible. Therefore, there is major need for accurate and reproducible parameters, able to detect early, subclinical, LV dysfunction and, thus, able to identify patients at risk for rapid progression toward irreversible cardiac failure, who can benefit from early therapeutic measures. We emphasize the role of imagistic methods and biological measurements in assessment of chemotherapy-induced cardiotoxicity. ❑
IMAGISTIC METHODS
Radionuclide ventriculography (RVG), Positron Emission Tomography (PET) and Cardiac Magnetic Resonance (CMR), along with echocardiography, have been the proposed methods for monitoring changes in cardiac structure and function during chemotherapy.
Radionuclide ventriculography it has been considered to be the gold standard for cardiotoxicity screening (39), but the single largest study involving RVG for monitoring of cardiotoxicity was conducting in almost 1500 patients receiving doxorubicin (40). Using this method, 19% of patients receiving a cumulative dose of doxorubicin of 450g/m2 will be at risk to develop cardiac dysfunction (40). However, a more recent meta-analysis showed that LVEF measured using RVG is not accurate enough for prediction of heart failure (41), due to the fact that often RVG overestimating LVEF, associating errors in volumes measurements. Moreover, the method is expensive, with low temporal and spatial resolution, and with a high risk of irradiation. Thus, RVG cannot be used in currently medical practice in present.
Positron emission tomography. The utility of PET in cancer patients was focused on the detection of metastatic lesion and response to the chemotherapy (10). Moreover, this method is capable to evaluate also the pericardial metastasis (42). However, PET has limited indications in cardiac dysfunction monitoring in cancer patients receiving chemotherapy (10).
Cardiac magnetic imaging. Currently, CMR is recognized by the ACC/AHA guideline as a technique to screen chemotherapy-induced cardiotoxicity, offering complete information regarding myocardial performance, valvular and pericardial involvement (43). Even this method has several advantages, particularly in obese patients with a suboptimal images quality, and it has been considered to be the golden standard to measure the LVEF, having the unique possibility to demonstrate myocardial edema seen in acute myocardial injury (10), it is less used for routine screening and monitoring of cardiotoxicity, in part probably due to the widespread availability to the echocardiography.
Echocardiography is probably the most readily available method for assessment to monitor cardiotoxicity, with serial measurements of the LVEF or LVSF. Furthermore, it can provide other information regarding multiple cardiac effects of chemotherapy, such as valvular and pericardial involvement, and through the newest echocardiographic techniques, it can be used to detect early, subclinical myocardial injury.
Current guidelines recommend measurement at baseline, and then regular assessment of cardiac function in order to detect cardiotoxicity, using conventional parameters such as EF or FS (36). Even if echocardiography has become the standard method for the cardiac evaluation in cancer patients, being non-invasive, cost-effective and widely available, there are some important limitations of these parameters. Firstly, these are image-quality and operator dependent parameters. Secondly, using LVEF assessment, only global LV function is quantified, whereas regional function is not evaluated accurate enough. And finally, global parameters are load-dependent, and therefore insensitive to detect subtle changes of myocardial function. Bi (44), Mercuro (7), and Sawaya (12) also reported a decrease of EF late in the course of the disease. Diastolic parameters were significantly affected after the 3rd cycle and persistent after a cumulative dose of epirubicin of 270 g/m2, however their accuracy was low for prediction of EF reduction. Other study reported significant changes in LV diastolic function only after a dose of 300 g/m2 of epirubicin, with no changes in EF (7). These results provide evidence that subtle impairment in cardiac function may be detected in patients with breast cancer treated with epirubicin, suggesting a need for other parameters that might be able to identify even better early changes in myocardial function.
Measurement of long-axis function by tissue velocity imaging, a reliable and reproducible method, has been used extensively to detect subclinical LV dysfunction. Thus, in patients with breast cancer treated with anthracycline, Lotrionte et al found that systolic velocities assessed after a minimum of 6 months of chemotherapy were significantly lower compared to baseline, and able to predict further LV dysfunction (45). Meanwhile, Mangina-Tassan et al demonstrated a reduction of myocardial systolic velocities very late, after 3.5 years from the completion of treatment with anthracycline (46). However, assessment of velocities has several limitations, such as translational motion and ultrasound beam angle dependence.
Speckle tracking imaging assesses myocardial deformation independently of cardiac translation or the insonation angle. It uses myocardial speckles that represent tissue markers that can be tracked frame-to-frame throughout the cardiac cycles. Although the golden standard for the measurements of LV deformation and rotation is tagged cardiac magnetic resonance (CMR), several studies validated STI by reference with CMR, proving its accuracy (47). Myocardial deformation (strain) and the rate of deformation (strain rate) reflect intrinsic contractility of the myocardium. Sawaya et al demonstrated that regional myocardial strain (longitudinal, radial, and circumferential) is significantly decreased in patients treated with anthracycline and trastuzumab before decrease of EF, and also that can predict further changes in EF (12). Similarly, Bi et al demonstrated a significant early reduction of longitudinal strain after treatment with epirubicin (44). However, other study failed to reveal a decrease of myocardial strain after chemotherapy (48). These suggest that deformation parameters are a sensitive tool to detect early changes of contractile function after epirubicin. STI also offers the unique opportunity to assess rotational deformation of the LV, with good agreement with tagged CMR (49). Therefore, it has been demonstrated the usefulness of new echocardiographic techniques, such as TVI and STI, for early detection of impairment of contractile myocardial function, before alteration in global systolic LV function, suggesting that these can be used in clinical practice in order to identify patients at risk for development of irreversible cardiac failure and to implement special preventive and therapeutic measures in patients with breast cancer treated with chemotherapy. These parameters should be now incorporated into clinical protocols in order to optimize the monitoring of chemotherapy - induced cardiac toxicity. ❑
BIOLOGICAL MEASUREMENTS
The use of other non-invasive methods, such as biological markers for monitoring cardiotoxicity has been investigated. Biomarkers may provide important information regarding the mechanism of cardiac dysfunction and may identify patients at to develop irreversible heart failure, therefore can be used in diagnosis of chemotherapy-induced cardiotoxicity and in monitoring cancer therapy. Several biological markers, such as the different isoforms of troponin, can detect damage of the cardiomyocytes, and may be useful in detecting the acute cardiotoxicity that is mediated by therapeutic agents that induce cell death (50). In present, strong data indicate that troponin offers the ability to detect chemotherapy-induced cardiotoxicity in its earliest phase, before the reduction in LVEF. Recently, increases in troponin levels have been observed in patients treated with standard doses of anthracyclines, as well as in patients treated with some of the newer antitumor agents (3). In particular, in trastuzumab-treated patients, troponin can identify patients who recover from cardiac dysfunction; this might help us to distinguish between reversible and irreversible cardiac damage (51). Thus, a prophylaxy with enalapril in patients with early increases in troponin level after chemotherapy, may prevent cardiovascular damage, not only in high-dose anthracycline-treated patients, but also in patients treated with standard-dose of anthracycline and trastuzumab (52). On the other hand, cardiac natriuretic peptides, markers of hemodynamic overload and increased wall stress, have been used as biomarkers in detecting cardiotoxicity, but definitive evidence is still lacking with regard to a diagnostic or prognostic role in predicting chemotherapy-induced cardiotoxicity (53). Other biomarkers used to monitor cardiovascular damage, such as myeloperoxidase, should also be validated for clinical use in cardio-oncology. Genomics, proteomics, and/or recently identified oligoclonal B-cell repertoires may provide with genomic profiles and serological biomarkers for assessment of cardiotoxicity in the future. ❑
MANAGEMENT OF CHEMOTHERAPY-INDUCED CARDIOTOXICITY
Currently, there are no guidelines developed specifically for the treatment of chemotherapy-induced cardiotoxicity, however a few small studies support the use of neurohormonal antagonists in the treatment and prevention of this pathology. Large, multi-centers trials are needed to establish guidelines for chemotherapy-induced cardiotoxicity. Until then, we follow the American College of Cardiology/ American Heart Association (ACC/AHA) and Heart Failure Society of America (HFSA) guidelines for treatment cardiac failure. Moreover, a close collaboration between the cardiologist and oncologist is strongly recommended in order to establish a specific management for the patients.
In addition to decreasing the cumulative dose of anthracyclines, there are other approaches that may reduce the risk of developing cardiac cells death. The administration of anthracyclines as infusions rather than as boluses, or the liposomal encapsulation of doxorubicin are all measures which may help reducing cardiac toxicity (54). Dexrazoxane, an EDTA like chelator, may reduce the risk of cardiotoxicity in association with doxorubicin or epirubicin. However, its use is limited to patients who receive a cumulative dose of doxorubicin >300 mg/m2 (55). On the other hand, carvedilol, a beta-blocker with antioxidant properties, might reduce the risk of anthracyclines induced cardiotoxicity. Kalay et al demonstrated in 50 patients receiving anthracycline therapy and either carvedilol 12.5 mg once daily or placebo, that there was no change in the LVEF in the carvedilol after 6 months. However, LVEF significantly decreased in the placebo group. Due to the small size of this study, additional larger trials are needed (56). Cardinale et al randomized 114 high risk patients with elevated troponin I after receiving high dose anthracyclines, to receive either enalapril at a starting dose of 2.5 mg daily or placebo for one year. 43% of the control group had a decrease in the LVEF compared to 0% in the enalapril group (57). Overall, once the diagnosis of chemotherapy-induced cardiotoxicity is established, the oncologist and cardiologist should discuss the patient's prognosis, while weighing the risks of discontinuing the cardiotoxic agent. The initiation of standard heart failure treatment, as well as the discontinuation of the cardiotoxic agent will increase the recovery of LV function. There is an urgent need for large multicenter trials in order to validate some of the preliminary albeit promising research, already conducted in this field. ❑
CONCLUSIONS
The advances in non-invasive cardiac imaging methods to monitor chemotherapy-induced cardiotoxicity and to early detection of impairment of contractile myocardial function, before alteration in global systolic LV function, demonstrate that these can be used in clinical practice in order to identify patients at risk for development of irreversible cardiac failure and to implement special preventive measures in cancer patients. Thus, myocardial systolic velocities, assessed by tissue velocity imaging, or LV deformation assessed by speckle tracking can detect subclinical cardiac dysfunction and predict impaired LV function .These parameters should be now incorporated into clinical protocols in order to optimize the monitoring of cardiotoxicity induced by chemotherapy. ❑
Abbreviations
- Arot
apical rotation
- AVO
aortic valve opening
- AVC
aortic valve closure
- ACC
American College of Cardiology
- AHA
American Heart Association
- CMR
Cardiac Magnetic Resonance
- EF
ejection fraction
- FS
fractional shortening
- HF
heart failure
- HFSA
Heart Failure Society of America
- LV
left ventricle
- NO
nitric oxide
- PET
Positron Emission Tomography
- RVG
Radionuclide ventriculography
- STI
speckle tracking imaging
CONFLICT OF INTEREST
none declared.
FINANCIAL SUPPORT
This work was supported by a grant of the Romanian National Authority for Scientific Research, CNCSIS - UEFISCDI, project number PN-II-ID-PCE-2011-3-0791, 112/27.0ct.2011.
References
- 1.http://www.who.int/cancer/detection/breastcancer/en/
- 2.Gillespie HS, McGann CJ, Wilson BD. Noninvasive diagnosis of chemotherapy related cardiotoxicity. Curr Cardiol Rev. 2011;7:234–244. doi: 10.2174/157340311799960672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albini A, Pannesi G, Donatelli F, et al. Cardiotoxicity of anticancer drugs: the need for cardio-oncology and cardio-oncological prevention. J Natl Cancer Inst. 2010;102:14–25. doi: 10.1093/jnci/djp440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brana I, Tabernero J. Cardiotoxicity. Ann Oncol. 2010;21:173–179. doi: 10.1093/annonc/mdq295. [DOI] [PubMed] [Google Scholar]
- 5.Cardinale D, Colombo A, Lamantia G, et al. Anthracycline-induced cardiomyopathy: Clinical relevance and response to pharmacologic therapy. JACC. 2010;55:213–220. doi: 10.1016/j.jacc.2009.03.095. [DOI] [PubMed] [Google Scholar]
- 6.Steinherz LJ, Steinherz PG, Tan CT, et al. Cardiac toxicity 4 to 20 years after completing anthracicline therapy. JAMA. 1991;266:1672–1677. [PubMed] [Google Scholar]
- 7.Mercuro G, Cadeddou C, Piras A, et al. Early epirubicin-induced myocardial dysfunction revealed by serial tissue Doppler echocardiography: correlation with inflammatory and oxidative stress markers. The Oncologist. 2007;12:1124–1233. doi: 10.1634/theoncologist.12-9-1124. [DOI] [PubMed] [Google Scholar]
- 8.Khakoo AY, Liu PP, Force T, et al. Cardiotoxicity due to cancer therapy. Tex Heart Inst J. 2011;38:253–256. [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen BV. Cardiotoxic consequences of anthracyline-containing therapy in patients with breast cancer. Semin Oncol. 2006;33:15–21. doi: 10.1053/j.seminoncol.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 10.Jiji RS, Kramer CM, Salerno M. Non-invasive imaging and monitoring cardiotoxicity of cancer therapeutic drugs. J Nucl Cardiol. 2012;19:377–388. doi: 10.1007/s12350-012-9512-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ewer MS, Ali MK, Mackay B, et al. A comparison of cardiac biopsy grades and ejection fraction estimations in patients receiving adriamycin. J Clin Oncol. 1984;2:112–117. doi: 10.1200/JCO.1984.2.2.112. [DOI] [PubMed] [Google Scholar]
- 12.Sawaya H, Sebag IA, Plana JC, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol. 2011;107:1375–1380. doi: 10.1016/j.amjcard.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.www.cancer.gov/dictionary/
- 14.www.clinicaltrials.gov
- 15.Seidman A, Hudis C, Pierri MC, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215–1221. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 16.Dolci A, Dominici R, Cardinale D, et al. Biochemical markers for prediction of chemotherapy-induced cardiotoxicity: systematic review of the literature and recommendations for use. Am J Clin Pathol. 2008;130:688–695. doi: 10.1309/AJCPB66LRIIVMQDR. [DOI] [PubMed] [Google Scholar]
- 17.Von Hoff DD, Layard MW, Basa P, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91:710–717. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- 18.Ewer MS, Vooletich MT, Durand J, et al. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol. 2005;23:7820–7826. doi: 10.1200/JCO.2005.13.300. [DOI] [PubMed] [Google Scholar]
- 19.Ewer SM, Ewer MS. Cardiotoxicity profile of trastuzumab. Drug Saf. 2008;31:459–467. doi: 10.2165/00002018-200831060-00002. [DOI] [PubMed] [Google Scholar]
- 20.Kang YJ. Molecular and cellular mechanisms of cardiotoxicity. Envirom Health Prospect. 2001;109:27–34. doi: 10.1289/ehp.01109s127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C, Zhang X, Ramil JM, et al. Juvenile exposure to anthracyclines impairs cardiac progenitor cell function and vascularization resulting in greater susceptibility to stress-induced myocardial injury in adult mice. Circulation. 2010;121:675–683. doi: 10.1161/CIRCULATIONAHA.109.902221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones RL, Swanton C, Ewer MS. Anthracycline cardiotoxicity. Expert Opin Drug Sat. 2006;5:1659–1672. doi: 10.1517/14740338.5.6.791. [DOI] [PubMed] [Google Scholar]
- 23.Crone SA, Zhao YY, Fan L, et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002;8:459–465. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- 24.Schimmel KJ, Richel DJ, van den Brink RB, et al. Cardiotoxicity of cytotoxic drugs. Cancer Treat Rev. 2004;30:181–191. doi: 10.1016/j.ctrv.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Alter P, Herzum M, Soufi M, et al. Cardiotoxicity of 5-fluorouracil. Cardiovasc Hematol Agents Med Chem. 2006;4:1–5. doi: 10.2174/187152506775268785. [DOI] [PubMed] [Google Scholar]
- 26.Kerkela R, Grazzete L, Yacobi R, et al. Cardiotoxicity and the cancer therapeutic agent imatinib metysilate. Nat Med. 2006;12:908–916. doi: 10.1038/nm1446. [DOI] [PubMed] [Google Scholar]
- 27.Gambacorti-Passerini C, Tornaghi L, Franceschino A, et al. In reply to "Cardiotoxicity and the cancer therapeutic agent imatinib metysilate". Nat Med. 2007;13:13–14. doi: 10.1038/nm0107-13b. [DOI] [PubMed] [Google Scholar]
- 28.Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis and management. J Am Coll Cardiol. 2009;53:2231–2247. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 29.Ederhy S, Cohen A, Dufaitre G, et al. QT interval prolongation among patients treated with angiogenesis inhibitors. Target Oncol. 2009;4:89–97. doi: 10.1007/s11523-009-0111-3. [DOI] [PubMed] [Google Scholar]
- 30.Lainscak M, Dagres N, Fillipatos GS, et al. Atrial fibrillation in chronic non-cardiac disease: where do we stand? Int J Cardiol. 2008;128:311–315. doi: 10.1016/j.ijcard.2007.12.078. [DOI] [PubMed] [Google Scholar]
- 31.Guzzetti S, Costantino G, Fundaro C. Systemic inflammation, atrial fibrillation, and cancer. Circulation. 2002;106:e40–e40. doi: 10.1161/01.cir.0000028399.42411.13. [DOI] [PubMed] [Google Scholar]
- 32.Dincer M, Altundag K. Angiotensin-converting enzymes inhibitors for bevacizumab-induced hypertension. Ann Pharmacother. 2006;40:2278–2279. doi: 10.1345/aph.1H244. [DOI] [PubMed] [Google Scholar]
- 33.Yeh ET, Tong AT, Lenihan DJ, et al. Cardiovascular complications of cancer therapy: diagnosis, pathogenesis, and management. Circulation. 2004;109:3122–3131. doi: 10.1161/01.CIR.0000133187.74800.B9. [DOI] [PubMed] [Google Scholar]
- 34.Popat S, Smith IE. Therapy insight: anthracycline and trastuzumab – the optimal management of cardiotoxic side effects. Nat Clin Pract Oncol. 2008;5:324–335. doi: 10.1038/ncponc1090. [DOI] [PubMed] [Google Scholar]
- 35.Elice F, Jacob J, Rickles FR, et al. Hemostatic complications of angiogenesis inhibitors in cancer patients. Am J Haematol. 2008;83:862–870. doi: 10.1002/ajh.21277. [DOI] [PubMed] [Google Scholar]
- 36.Martin M, Esteva FJ, Alba E, et al. Minimizing cardio-toxicity while optimizing treatment efficacy with trastuzumab: review and expert recommendations. The Oncologist. 2009;14:1–11. doi: 10.1634/theoncologist.2008-0137. [DOI] [PubMed] [Google Scholar]
- 37.Ewer MS, Ali MK, Mackay B, et al. A comparison of cardiac biopsy grades and ejection fraction estimations in patients receiving adriamycin. J Clin Oncol. 1984;2:112–117. doi: 10.1200/JCO.1984.2.2.112. [DOI] [PubMed] [Google Scholar]
- 38.Sawaya H, Sebag IA, Plana JC, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol. 2011;107:1375–1380. doi: 10.1016/j.amjcard.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Royen N, Jaffe CC, Krumholz HM, et al. Comparison and reproducibility of visual echocardiographic and quantitative radionuclide left ventricular ejection fractions. Am J Cardiol. 1996;77:843–850. doi: 10.1016/s0002-9149(97)89179-5. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz RG, McKenzie WB, Alexander J, et al. Congestive heart failure and left ventricular dysfunction complicating doxorubicin therapy. Am J Med. 1987;82:1109–1118. doi: 10.1016/0002-9343(87)90212-9. [DOI] [PubMed] [Google Scholar]
- 41.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97:2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 42.Weils LE, Arsos G, Baarslag HJ, et al. Pericardial involvement in a non-Hodgkin lymphoma patient: coregistered FDG-PET and CT imaging. Eur Heart J. 2007;28:2698–2698. doi: 10.1093/eurheartj/ehm218. [DOI] [PubMed] [Google Scholar]
- 43.ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR. Appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging. J Am Coll Cardiol. 2006;48:1475–1497. doi: 10.1016/j.jacc.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 44.Bi X, Deng Y, Zeng F, et al. Evaluation of epirubicin-induced cardiotoxicity by two-dimensional strain echocardiography in breast cancer patients. J Huazhong Univ Sci Technolog Med Sci. 2009;29:39–44. doi: 10.1007/s11596-009-0326-7. [DOI] [PubMed] [Google Scholar]
- 45.Lotrionte M, Palazzoni G, Natali R, et al. Assessment of left ventricular systolic dysfunction by tissue Doppler imaging to detect subclinical cardiomyopathy early after anthracicline therapy. Minerva Cardioangiol. 2007;55:711–720. [PubMed] [Google Scholar]
- 46.Tassan-Mangina S, Codorean D, Metivier M, et al. Tissue Doppler imaging and conventional echocardiography after anthracycline treatment in adults: early and late alteration of left ventricular function during a prospective study. Eur J Echocardiography. 2006;7:141–146. doi: 10.1016/j.euje.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 47.Becker M, Bilke E, Kuhl H, et al. Analysis of myocardial deformation based on pixel tracking in two-dimensional echocardiographic images enables quantitative assessment of regional left ventricular function. Heart. 2006;92:1102–1108. doi: 10.1136/hrt.2005.077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hale JL, Brown JK, Leano R, et al. Use of myocardial deformation imaging to detect preclinical myocardial dysfunction before conventional measures in patients undergoing breast cancer treatment with trastuzumab. Am Heart J. 2009;158:294–301. doi: 10.1016/j.ahj.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 49.Halle-Valle T, Crosby J, Edvardsen T, et al. Non-invasive method for assessment of left ventricular rotation: speckle tracking echocardiography. Circulation. 2005;112:3149–3156. doi: 10.1161/CIRCULATIONAHA.104.531558. [DOI] [PubMed] [Google Scholar]
- 50.Cardinale D, Sandri M, Colombo A, et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation. 2004;109:2749–2754. doi: 10.1161/01.CIR.0000130926.51766.CC. [DOI] [PubMed] [Google Scholar]
- 51.Kang YJ. Molecular and cellular mechanisms of cardiotoxicity. Environ Health Perspect. 2001;109:27–34. doi: 10.1289/ehp.01109s127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Filippatos TD, Liberopoulos EN, Pavlidis N, et al. Effects of hormonal treatment on lipids in patients with cancer. Cancer Treat Rev. 2009;35:175–184. doi: 10.1016/j.ctrv.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 53.Sandri MT, Salvatici M, Cardinale D, et al. N-terminal pro-B-type natriuretic peptide after high-dose chemotherapy: a marker predictive of cardiac dysfunction? Clin Chem. 2005;51:1405–1410. doi: 10.1373/clinchem.2005.050153. [DOI] [PubMed] [Google Scholar]
- 54.Saidi A, Alharethi R. Management of Chemotherapy Induced Cardiomyopathy. Current Cardiology Reviews. 2011;7:245–249. doi: 10.2174/157340311799960681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith LA, Cornelius VR, Plummer CJ, et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomized controlled trials. BMC Cancer. 2010;10:337–337. doi: 10.1186/1471-2407-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kalay N, Basar E, Ozdogru I, et al. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J Am Coll Cardiol. 2006;48:2258–2262. doi: 10.1016/j.jacc.2006.07.052. [DOI] [PubMed] [Google Scholar]
- 57.Cardinale D, Colombo A, Sandri MT, et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006;114:2474–2481. doi: 10.1161/CIRCULATIONAHA.106.635144. [DOI] [PubMed] [Google Scholar]